Introduction
Craniocervical arterial dissection (CCAD) in childhood usually presents with symptoms of acute ischemic stroke (AIS) or transient ischemic attack (TIA). It occurs in 2.5 children per 100,000 per year [1]. Separation between the intimal layers of the vessel wall creates an area of damaged endothelium with exposure of collagen, activated tissue factor, and exposed von Willebrand factor. These factors generate secondary fibrin and platelet adhesion, leading to thrombus propagation. Once a clot has formed, ischemia occurs from vessel occlusion at the site of dissection or from clot embolus downstream [2]. Aneurysmal dilatation, which can occur secondary to impaired integrity of the vessel wall and persistent arterial pressure occlusion, frequently appears in the C1-C2 vertebral circulation in children [2,3].
Risk factors for dissection in children include head and neck injury, connective tissue disorders (such as Ehlers-Danlos syndrome), and male gender [4–6, Class IV]. Other well-known childhood AIS risk factors (e.g., thrombophilia) may theoretically contribute to risk of AIS in the presence of CCAD. Patients with arterial abnormalities have a high risk of recurrent AIS [7, Class III].
There are two types of CCAD in childhood: extracranial dissection and intracranial dissection. These entities have differing risk factors and management. Extracranial dissections account for 5% to 25% of childhood-onset AIS [4,8••, 9••] and are often preceded by trauma [4–6, Class IV]. Typically, anterior circulation dissection presents with focal neurologic symptoms such as hemiparesis or aphasia. Posterior circulation events from vertebral or basilar dissection are more challenging to diagnose because their symptoms and signs can range from dizziness to coma. Clues to this diagnosis include history of recent trauma and/or cranial nerve abnormalities. Early evidence suggests that dissection is more prevalent in the posterior than the anterior circulation of children with AIS [8••, Class IV]. Interestingly, although neck pain is a common sign of dissection in adult AIS, diffuse headache is more common in children [4,6].
Because of imprecise classification and challenges in definitive diagnosis, the prevalence of intracranial dissection in childhood is unknown. Recent literature in childhood AIS has focused upon intracranial focal cerebral arteriopathy, which occurs in up to 80% of previously healthy children with AIS [10, Class IV]. Although many of these transient narrowings within the intracranial cerebral vasculature are likely to be associated with an infectious or parainfectious phenomenon rather than dissections [11•, Class IV], recent case reports suggest that some of these lesions may indeed be dissections [12].
Diagnosis
Diagnosis of childhood CCAD and AIS relies upon the appropriate clinical suspicion. Improvement in clinical suspicion and community awareness will help address the concerning observation that median time to diagnosis for childhood-onset AIS is 25 hours from time of symptom onset [13].
The International Pediatric Stroke Study defines CCAD as “(1) angiographic double lumen, intimal flap, or pseudo aneurysm, or, on axial T1 fat saturation MRI images, a “bright crescent sign” in the arterial wall [as seen in Fig. 1, Case 1]; (2) cervical or cranial trauma, or neck pain, less than 6 weeks preceding angiographic findings of segmental arterial narrowing (or occlusion) located in the cervical arteries; (3) angiographic segmental narrowing (or occlusion) of the vertebral artery at the level of the C2 vertebral body, even without known traumatic history” [14, Class IV].
Figure 1.
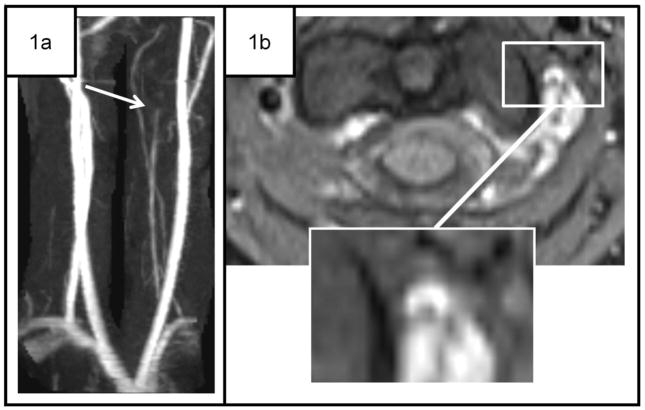
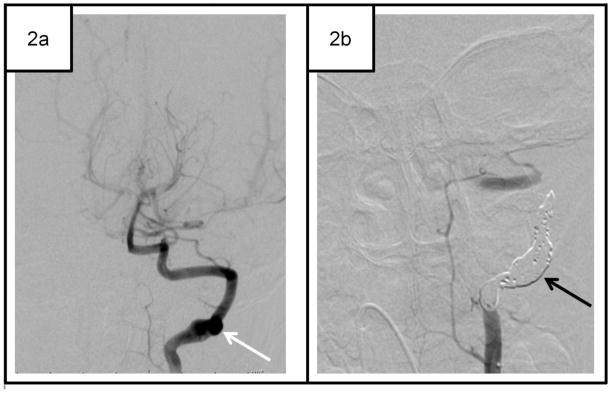
Selected cases of childhood spontaneous craniocervical arterial dissection (CCAD).
Case 1. 12-year-old girl with transient ischemic attack (TIA) and CCAD. One hour after incidental neck trauma, the patient had left facial droop that spontaneously resolved. 1a Maximum intensity projection (MIP) images using time of flight (TOF) magnetic resonance angiography (MRA), ordered at the initial neurology visit (1 month later) shows slow flow through a small left vertebral artery, with abrupt cutoff at C2-C3 (white arrow). 1b T1 fat saturation images reveal a “crescent sign” at C1–C2 (inset). MRI of the brain was normal.
Case 2. 4-year-old boy with dissecting aneurysm and right visual field cut. MRI/MRA and CT angiography showed left posterior cerebral artery infarct but were equivocal for the diagnosis of CCAD. 2a Conventional angiography revealed a dissecting aneurysm of the left vertebral artery at the C2-C3 level (white arrow). The patient failed medical management with aspirin and low-molecular-weight heparin. 2b He has been symptom-free for 14 months after interventional coiling of left vertebral artery (black arrow).
Spontaneous CCAD occurs with no preceding history of significant trauma, or after seemingly innocuous trauma (examples from our experience include neck torsion during rugby, chiropractor manipulation, climbing over a wall, landing on the head when playing on a trampoline, and falling off of a skateboard). At the same time, it is appreciated that many children without dissection also report a recent prior history of minor trauma, and some “background” level of minor trauma is expected in the healthy pediatric population. Well-designed case-control studies are lacking on this issue. In one series of CCAD in children, minor trauma occurred prior to presentation in 25% [4]. Most children with spontaneous CCAD present with nonspecific, often transient, neurologic symptoms including headache, vomiting, dizziness, vertigo, diplopia, confusion, and neck pain. Other presenting symptoms and signs include altered level of consciousness (25%), Horner’s syndrome, and/or seizures (12.5%) [15].
Traumatic CCAD, in contrast, occurs after generalized major trauma, such as a motor vehicle accident. It can present with similar signs and symptoms but is increasingly recognized via screening imaging studies in asymptomatic patients with major trauma.
The cornerstone of diagnosing CCAD is imaging of the cervical and intracranial vasculature. The imaging modalities available for diagnosis of CCAD—conventional angiography (CA), CT angiography (CTA), magnetic resonance angiography (MRA), and Doppler ultrasound (DUS)—possess complementary strengths and weaknesses. Our suggested algorithm for diagnostic evaluation is shown in Figure 2, but it should be recognized that individual clinical circumstances warrant careful, case-by-case consideration.
Figure 2.
Algorithm for the initial radiologic evaluation of suspected spontaneous craniocervical arterial dissection (CCAD). Evaluation begins with brain MRI with diffusion-weighted imaging (DWI), time-of-flight (noncontrast) MR angiography (MRA) of the head, and contrast-enhanced MRA of the neck with a T1 fat-saturated sequence. AIS arterial ischemic stroke; CA catheter angiography; CTA CT angiography; TIA transient ischemic attack.
In the past 10 to 20 years, advances in technology have made MRI/MRA and CT/CTA increasingly sensitive and specific, although CA remains the gold standard for equivocal cases. In general, imaging modalities can be divided into those that can analyze the arterial lumen (CA), and those that can depict both the lumen and mural arterial thrombus (MRA with MRI, CTA, and DUS).
Anatomic Site of Involvement
Some authors have hypothesized that the extracranial carotid and vertebral arteries are more vulnerable to dissection than other arteries in the body because of their mobility and proximity to bony projections of the cervical spine [16]. The vertebral artery is particularly vulnerable at its tortuous course around the C1-C2 lateral masses and through the transverse foramina [3]. As a result, two thirds of vertebral artery CCADs occur at these sites [17]. The most common location for carotid CCAD is 2 to 3 centimeters above the carotid bulb [16].
Conventional Angiography
CA is still widely considered the gold standard for diagnosis of adult and childhood CCAD, but the risks of this technique may outweigh its benefits in many clinical scenarios [18••]. CA depicts intraluminal findings of CCAD with very high spatial resolution through direct intra-arterial injection of contrast. The most common findings of extracranial CCAD on CA are arterial stenosis, aneurysm formation, or occlusion [19,20]. An intimal flap or double lumen is indicative of CCAD, but these findings are detected in fewer than 10% of dissected arteries, and less commonly in the vertebral arteries [19]. A relative drawback of CA is that intramural hematoma or periarterial findings cannot be directly visualized [21].
Increasingly, CA is supplanted by MRI/MRA for the primary diagnosis of CCAD [21–23]. This trend is due to increased availability of MRI/MRA, potential complications of CA (e.g. femoral hematoma, femoral arterial pseudoaneurysm, recurrent AIS, and radiation exposure), and the need for sedation in CA [22]. Additionally, as noninvasive techniques like MRI/MRA become more prevalent, fewer physicians are trained in CA, resulting in fewer experienced angiographers [24]. Although concern for CA complications is one of the primary factors in limiting CA, several recent papers have documented an excellent safety record for CA in children in the hands of experienced angiographers [25,26]. However, because CA carries a higher risk of complications in patients with connective tissue disease, especially Ehlers-Danlos or an undiagnosed collagen abnormality, CA should be used with caution in this population [27].
MRI and MRA
In most centers, MRI/MRA has become the first-line imaging modality for patients with suspected dissection [21,22]. MRI/MRA is noninvasive, uses no radiation, and simultaneously images for dissection and stroke.
Arterial luminal findings of CCAD on either time of flight (TOF) MRA or contrast-enhanced MRA are similar to findings of CA in both adults and children, including arterial stenosis, intimal flap, dissecting aneurysm, or occlusion [4,28,29]. A tapered stenosis (“flame sign”) or a thin, segmented stenosis (“string sign”) is a less common sign of CCAD [28,30]. Intimal flaps and dissecting aneurysms are two specific luminal findings for CCAD, but they are infrequently visualized on MRI/MRA [28,31,32].
Although CA is the gold standard for diagnosis, one advantage of MRI/MRA (TOF or contrast-enhanced) over CA is the ability to directly visualize the intramural hematoma with T1 or T2 fat-saturated imaging as a crescentic hyperintensity along the vessel wall [28,33] (as seen in Fig. 1, Case 1).
Intramural hematomas have been reported in up to 76% to 91% of dissected vessels [28,31,32]. On MRI, the appearance of the intramural hematoma in CCAD changes over time: it has been reported as isointense to nearby tissues for the first day or two, then T1 isointense and T2 hyperintense, and finally T1 hyperintense after several more days [21,28,33,34]. T1 and T2 hyperintensity in an intramural hematoma can persist for months [21,33]. The combination of mural hematoma and flowing blood on TOF MRA can lead to an apparent increase in vessel diameter on both source images and maximum intensity projection (MIP) images. This increase in vessel diameter on TOF MRA was 99% specific for CCAD in one study of carotid and vertebral CCAD [33].
Several studies of CCAD in adults that were published between 1994 and 2002 compared MRI/MRA(TOF) versus CA. MRI/MRA had 50% to 100% sensitivity and 29% to 100% specificity [33,35–39]. In a more recent study of CCAD in adults, contrast-enhanced MRA was 89% sensitive for detection of CCAD, versus 50% for TOF MRA [40]. This finding mimics our clinical experience. MRI/MRA can perform favorably compared with CA, and contrast-enhanced MRA is superior to TOF MRA. Compared with TOF MRA, contrast-enhanced MRA possesses several advantages in detecting arterial stenosis and dissection: imaging of the entire course of the cervical arteries in one acquisition [41], decreased overestimation of stenosis or occlusion due to slow flow [40], and fewer motion artifacts [42].
Limited studies in childhood AIS have demonstrated a 100% correlation between MRA (TOF) and CA in large-artery intracranial abnormalities [43, Class III]; other studies have shown that CA is more sensitive in intracranial abnormalities than extracranial abnormalities of the large vessels [44, Class IV]. Pediatric literature remains limited.
Several common artifacts can make the diagnosis of CCAD by MRA difficult, especially in the vertebral arteries. Examples include turbulent flow [45,46] and venous plexus artifact [47].
High-resolution MRI with specialized cervical surface coils is on the horizon for diagnosis of intramural hematoma in cases of CCAD in adults [48,49••,50, Class IV]. This promising technique could increase sensitivity for intramural hematoma and overcome the artifact related to confounding perivertebral venous plexus enhancement.
CT Angiography
Recent studies report a high sensitivity of CTA (98–100%) for diagnosis of spontaneous and traumatic CCAD in adults [51–53]. Other advantages of CTA include widespread availability, speed of examination, noninvasiveness, and ability to be performed as part of an initial trauma screening. The great disadvantage of CTA, especially for children, is the high radiation burden [54]. Furthermore, the diagnostic performance of CTA for CCAD in children has not been extensively studied.
The signs of dissection on CTA are similar to those on both MRA and CA. As in those modalities, the most common findings are stenosis or occlusion at a typical location [45,53,55,56]. The characteristic CCAD luminal findings of intimal flaps and dissecting aneurysms can also be visualized on CTA [55,56] more often than on MRA [45,57].
Artifacts associated with CTA include bone artifact near the skull base and artifact from dental amalgam [55,56]. High-quality CTA examinations also require excellent bolus timing and high injection rates, both of which can be more difficult to accomplish in children. References on CTA techniques in children have been published that address bolus timing and injection rates [58].
Duplex and Doppler Ultrasound
In adults, DUS in the diagnosis of spontaneous CCAD has reported sensitivities ranging from 66% to 96% [51,59–62]. Advantages of DUS are that it is relatively inexpensive, uses no radiation, and can be performed emergently at the bedside, but its sensitivity is highly dependent on the operator [63].
Compared with the anterior circulation, DUS is less sensitive in evaluating the vertebral arteries because of their location within the transverse foramina [64], their deeper and more irregular course, and their small diameter [63]. DUS also has reduced sensitivity for focal disease of the carotid arteries near the skull base because of the limited window [62].
Because of its limited utility in evaluating the vertebral arteries, DUS has not yet been widely studied in children with CCAD.
Diagnosis of Arterial Injury Following Trauma
Recent adult trauma literature cites a prevalence of blunt cervical arterial injury (BCAI) following major trauma as high as 1.1% to 1.2% [65,66], although a recent survey of the National Pediatric Trauma registry estimated the prevalence in pediatric trauma at 0.03%. Presumably, BCAI is either underdiagnosed in pediatric patients or its prevalence is lower than in adults [67].
Recent adult surgical and trauma literature advocates CTA as a screening tool for BCAI following major blunt trauma [65,66]. Controversy exists regarding the sensitivity of CTA in this setting, with sensitivities that range from 54% to 97.7% [52,68]. One pediatric study reported a sensitivity of 88% and a specificity of 100% in CTA in this setting [69].
Given the current recommendations in the trauma literature and the advantages of CTA in this setting (speed, 24/7 availability, and ability to scan multiple body parts at once), CTA is often used for BCAI screening in both children and adults, but given the relative lack of data on BCAI specifically in children and concerns over radiation exposure, MRI/MRA should be considered as an alternative screening tool when appropriate [70].
Treatment
Treatment of adult extracranial CCAD has become more controversial over the past 2 to 3 years, with some experts advocating less aggressive therapy such as aspirin, and others using more invasive techniques such as stenting. Although anticoagulation is the recommended (and the most commonly used) treatment for childhood AIS [18••, 71••, Class IV], there are no randomized controlled trials comparing antiplatelet therapy versus anticoagulation in adults or children with CCAD, and this practice is based upon less evidence in children with AIS than in adults. In the absence of an evidence-based treatment strategy, management relies largely upon consensus-based recommendations, patient education about risks and benefits, and physician experience.
Considerable variability of treatment recommendations is evident across international pediatric stroke centers and even within the United States [9••]. Recently published childhood stroke guidelines recommend treatment of extracranial CCAD with anticoagulation, such as unfractionated heparin (UFH), low-molecular-weight heparin (LMWH), or warfarin. The American Heart Association (AHA) Scientific Statement says, “In children with extracranial CCAD, it is reasonable to begin either UFH or LMWH as a bridge to oral anticoagulation. It is reasonable to treat a child with an extracranial [CAD] with either subcutaneous LMWH or warfarin for 3 to 6 months” [18••]. The American College of Chest Physicians suggests “for AIS secondary to dissection … anticoagulant therapy with LMWH or vitamin K antagonists for at least 6 weeks, with ongoing treatment dependent on radiologic assessment” [71••].
Recent adult literature suggests that antiplatelet therapy may be as effective as anticoagulation in preventing recurrent stroke, death, or disability in extracranial carotid artery dissection, but this conclusion is controversial. A Cochrane meta-analysis of 36 observational studies in 2010 concluded that there was no statistically significant difference between antiplatelet or anticoagulation therapy when considering recurrent stroke (OR, 0.63; 95% CI, 0.21–1.86) or death (OR, 2.02; 95% CI, 0.62–6.60) [72••, Class II]. In a subanalysis of 26 studies, however, there was a strong but nonsignificant trend favoring the use of anticoagulation in preventing death or disability (OR, 1.77; 95% CI, 0.98–3.22; P = 0.06) [72••]. Given the recent data, AHA guidelines suggest, “Alternatively [in children with CCAD], an antiplatelet agent may be substituted for LMWH or warfarin” [18••].
Interventional procedures to stent or balloon extracranial dissection are typically reserved for patients who fail medical therapies. AHA recommendations suggest that surgical procedures are reasonable in children who continue to have symptoms from an extracranial CCAD despite aggressive medical therapy [18••].
The use of anticoagulation in intracranial dissection is discouraged by AHA guidelines because of the risk of subarachnoid hemorrhage [18••].
Follow-up imaging is necessary for patients with CCAD, typically at 3 to 6 months after their initial presentation. In addition, children with vertebral dissections should be evaluated with appropriate neck imaging, to assess for a cervical skeletal abnormality [73,74].
Pharmacologic treatment
Antiplatelet therapy for CCAD is aimed at preventing arterial thrombus propagation. The most common antiplatelet agent is aspirin, typically dosed at 2 to 5 mg/kg per day. Clopidogrel is sometimes prescribed, although dosing in pediatric patients older than 2 years is not well established. With aspirin, contraindications include aspirin hypersensitivity and known bleeding disorder. Aspirin has few drug interactions, and its main side effect is an increased bleeding tendency, particularly risk of hemorrhage during trauma [71••]. Although rare, patients and their families also need to be aware of the risk of Reye syndrome, especially with concomitant influenza [75]. A yearly flu shot is recommended. Nasal flu vaccination is not recommended. The cost of aspirin is minimal.
Anticoagulation treatment for extracranial CCAD is also aimed at preventing clot propagation. Usually, UFH is the initial medication of choice for acute treatment, given its short half-life. At the Children’s Hospital Colorado, UFH is managed with the abbreviated protocol shown in Table 1. UFH should be administered by a physician experienced with its use.
A large area of ischemia (typically the size of more than one third to one half the territory of the middle cerebral artery) is a relative contraindication to anticoagulation, as is the presence of subarachnoid hemorrhage. Patients with known intracranial CCAD should be treated with anticoagulation only if other therapies have failed.
Side effects of anticoagulation include increased bleeding risk, with a variable incidence of 2% to 26% [76,77]. In our experience, clinically significant bleeds are limited (5% risk at a mean follow-up of 3 years) in patients with AIS and arteriopathy who are treated with anticoagulation [78].
Side effects of heparin include bleeding and heparin-induced thrombocytopenia (HIT). Well-designed, large prospective studies of HIT incidence in children are lacking, but the estimated frequency in non-neonates ranges from 0.5% to 2% and is likely to be higher following cardiac surgery [79,80].
When a patient is stable and beyond the high-risk period for hemorrhagic conversion or intracranial swelling, transition to LMWH or warfarin is considered.
According to the American College of Chest Physicians (ACCP) guidelines, target levels for LMWH are an anti-FXa level of 0.50 to 1.0 U/mL in a sample taken 4 to 6 hours after administration [71••]. Side effects of LMWH are similar to those of UFH, although the risk of HIT is thought to be lower with LMWH.
The ACCP suggests adjusting the warfarin dosage to attain a target International Normalized Ratio (INR) of 2.0 to 3.0. The main adverse effect of warfarin is bleeding. Some evidence suggests that long-term use may be associated with osteoporosis, but this link is by no means definitive [81].
Long-term management of LMWH and warfarin are best conducted with the assistance of a pediatric hematologist.
Table 1.
Protocol for the use of unfractionated heparin in children with acute ischemic stroke
|
(Protocol from Children’s Hospital Colorado, adapted from Monagle P, Chalmers E, Chan A, et al.; American College of Chest Physicians [71••].)
Interventional procedures
Endovascular therapies have been successful in multiple case reports in childhood AIS and specifically in CCAD [82,83]. The risk-benefit analysis of interventional therapies is uncertain. Adverse events have also been described, such as the failure of a Merci clot retrieval device in a 14-year-old with presumed intracranial dissection [84]. These adverse events are likely to be underreported [85•, Class IV]. For these reasons, patients should be selected for interventional therapies on a case-by-case basis, ideally with the input of a clinician with pediatric stroke expertise. In the setting of extracranial CCAD, interventional therapies such as stenting or selective occlusion with coils should be entertained only if patients fail aggressive medical management (as seen in Fig. 1, Case 2). Intra-arterial thrombolytic devices or devices that mechanically retrieve the clot are also controversial, and should be used (if at all) only within evidence-based adult time windows for treatment.
Opinion statement.
Diagnosis of craniocervical arterial dissection (CCAD) in children begins with a careful history and physical in a child with a transient ischemic attack (TIA) or arterial ischemic stroke (AIS). The extent of radiologic evaluation for suspected CCAD is based upon careful consideration of the risks associated with the best imaging techniques, weighed against the benefits of enhanced vascular imaging with better diagnostic sensitivity. Although conventional angiography (CA) and CT angiography (CTA) have a higher sensitivity than magnetic resonance angiography (MRA), they are accompanied by risks: for CA, femoral hematoma, femoral arterial pseudoaneurysm, recurrent AIS, and radiation exposure; for CTA, radiation.
For children (non-neonates) with suspected CCAD, MRI with MRA is recommended as the first-line imaging study. MRI usually includes diffusion-weighted, FLAIR, and T1 images of the brain, and T1 or T2 fat-saturation axial imaging through the neck. MRA should include 3D time-of-flight MRA of the head and neck (from the aortic arch through the circle of Willis). Contrast-enhanced MRA should be highly considered in neck imaging. If MRI/MRA is equivocal, CCAD is strongly suspected but not detected on MRI/MRA (especially in the posterior circulation), or the child has recurrent events, additional imaging of the craniocervical vasculature is likely warranted. Individual clinical circumstances warrant careful, case-by-case consideration.
Treatment of CCAD in children is challenging and differs for intracranial and extracranial dissections. In extracranial CCAD, we most commonly use anticoagulation for 6 weeks to 6 months in patients with TIA or AIS. Typically, unfractionated heparin is used in the acutely ill patient at heightened risk for bleeding (because of its short half-life), whereas low-molecular-weight heparin (LMWH) or warfarin are reserved for the stable patient. If the history is suspicious for dissection (head and neck trauma, recent cervical chiropractic manipulation, recent car accident, or neck pain), we consider treatment for dissection even with normal MRI/MRA. For patients with CCAD with a stroke size greater than one third to one half of the middle cerebral artery territory (or other bleeding risk factors) and extracranial CCAD, in whom there is concern about heightened risk for hemorrhagic conversion, we commonly use aspirin therapy during the acute phase. Regardless of their treatment in the initial weeks to months, we subsequently treat all patients with aspirin for 1 year after their event, and sometimes longer if they have other risk factors. Interventional techniques, such as extracranial cerebral arterial stent placement or selective occlusion, are understudied in children. Interventional techniques are typically reserved for patients who fail aggressive medical management and have recurrent TIA or AIS.
The diagnosis and treatment of intracranial dissection is extraordinarily challenging in children, in whom inflammatory intracranial arteriopathies are common. When intracranial arteriopathy is clearly associated with dissection, the clinician should look for the presence of subarachnoid hemorrhage and/or dissecting aneurysm. Treatment decisions should be made by a multidisciplinary pediatric stroke team, given the lack of data in this area. Intracranial cerebral artery stent placement carries high risk and is not recommended for intracranial CCAD in children.
Most importantly, we educate all children with CCAD and their parents about the paucity of evidence in the treatment of this disease, the risks of enhanced imaging techniques such as CTA or CA, and the challenges involved in weighing the risks of aggressive therapies and interventions against the costs of unclear diagnosis and potentially ineffective treatments. We also educate our patients with CCAD about the signs and symptoms of recurrence and the importance of emergent evaluation.
Footnotes
Disclosure
No potential conflicts of interest relevant to this article were reported.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Fullerton HJ, Johnston SC, Smith WS. Arterial dissection and stroke in children. Neurology. 2001;57:1155–1160. doi: 10.1212/wnl.57.7.1155. [DOI] [PubMed] [Google Scholar]
- 2.Tan MA, Armstrong D, MacGregor DL, et al. Late complications of vertebral artery dissection in children: pseudoaneurysm, thrombosis, and recurrent stroke. J Child Neurol. 2009;24:354–360. doi: 10.1177/0883073808324775. [DOI] [PubMed] [Google Scholar]
- 3.Fusco MR, Harrigan MR. Cerebrovascular dissections--a review part I: Spontaneous dissections. Neurosurgery. 2011;68:242–257. doi: 10.1227/NEU.0b013e3182012323. [DOI] [PubMed] [Google Scholar]
- 4.Rafay MF, Armstrong D, DeVeber G, et al. Craniocervical arterial dissection in children: clinical and radiographic presentation and outcome. J Child Neurol. 2006;21:8–16. doi: 10.1177/08830738060210010101. [DOI] [PubMed] [Google Scholar]
- 5.Brandt T, Orberk E, Weber R, et al. Pathogenesis of cervical artery dissections: association with connective tissue abnormalities. Neurology. 2001;57:24–30. doi: 10.1212/wnl.57.1.24. [DOI] [PubMed] [Google Scholar]
- 6.Tan MA, DeVeber G, Kirton A, et al. Low detection rate of craniocervical arterial dissection in children using time-of-flight magnetic resonance angiography: causes and strategies to improve diagnosis. J Child Neurol. 2009;24:1250–1257. doi: 10.1177/0883073809333539. [DOI] [PubMed] [Google Scholar]
- 7.Fullerton HJ, Wu YW, Sidney S, Johnston SC. Risk of recurrent childhood arterial ischemic stroke in a population-based cohort: the importance of cerebrovascular imaging. Pediatrics. 2007;119:495–501. doi: 10.1542/peds.2006-2791. [DOI] [PubMed] [Google Scholar]
- 8••.Ganesan V, Cox TC, Gunny R. Abnormalities of cervical arteries in children with arterial ischemic stroke. Neurology. 2011;76:166–171. doi: 10.1212/WNL.0b013e318205d4d5. This retrospective review of children with CCAD describes the radiographic characteristics of CCAD in children. [DOI] [PubMed] [Google Scholar]
- 9••.Goldenberg NA, Bernard TJ, Fullerton HJ, et al. Antithrombotic treatments, outcomes, and prognostic factors in acute childhood-onset arterial ischaemic stroke: a multicentre, observational, cohort study. Lancet Neurol. 2009;8:1120–1127. doi: 10.1016/S1474-4422(09)70241-8. This large international series of children with AIS from the International Pediatric Stroke Study reports treatments and early outcomes in childhood AIS. Children with arterial anomalies have worse early outcomes. [DOI] [PubMed] [Google Scholar]
- 10.Danchaivijitr N, Cox TC, Saunders DE, et al. Evolution of cerebral arteriopathies in childhood arterial ischemic stroke. Ann Neurol. 2006;59:620–626. doi: 10.1002/ana.20800. [DOI] [PubMed] [Google Scholar]
- 11•.Amlie-Lefond C, Bernard TJ, Sebire G, et al. Predictors of cerebral arteriopathy in children with arterial ischemic stroke: results of the International Pediatric Stroke Study. Circulation. 2009;119:1417–1423. doi: 10.1161/CIRCULATIONAHA.108.806307. This large international series of children with AIS from the International Pediatric Stroke Study demonstrates that preceding upper respiratory infection is a risk factor for intracranial arterial abnormalities in childhood AIS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nomazulu D, Mackay MT, Fullerton H, Freeman J, DeVeber G. Intracranial Dissection Mimicking Transient Cerebral Arteriopathy in Childhood Arterial Ischemic Stroke. American Heart Association International Stroke Conference; 2010. pp. 2–24. [Google Scholar]
- 13.Srinivasan J, Miller SP, Phan TG, et al. Delayed recognition of initial stroke in children: need for increased awareness. Pediatrics. 2009;124:e227–e234. doi: 10.1542/peds.2008-3544. [DOI] [PubMed] [Google Scholar]
- 14.Sebire G, Fullerton H, Riou E, et al. Toward the definition of cerebral arteriopathies of childhood. Curr Opin Pediatr. 2004;16:617–622. doi: 10.1097/01.mop.0000144441.29899.20. [DOI] [PubMed] [Google Scholar]
- 15.Golomb MR, Rafay M, Armstrong D, et al. Intra-arterial tissue plasminogen activator for thrombosis complicating cerebral angiography in a 17-year-old girl. J Child Neurol. 2003;18:420–423. doi: 10.1177/08830738030180060301. [DOI] [PubMed] [Google Scholar]
- 16.Schievink WI, Roiter V. Epidemiology of cervical artery dissection. Front Neurol Neurosci. 2005;20:12–15. doi: 10.1159/000088125. [DOI] [PubMed] [Google Scholar]
- 17.Arnold M, Bousser MG. Clinical manifestations of vertebral artery dissection. Front Neurol Neurosci. 2005;20:77–86. doi: 10.1159/000088152. [DOI] [PubMed] [Google Scholar]
- 18••.Roach ES, Golomb MR, Adams R, et al. Management of stroke in infants and children: a scientific statement from a Special Writing Group of the American Heart Association Stroke Council and the Council on Cardiovascular Disease in the Young. Stroke. 2008;39:2644–2691. doi: 10.1161/STROKEAHA.108.189696. This consensus statement from the American Heart Association about treatment of childhood stroke provides specific recommendations about children with CCAD. [DOI] [PubMed] [Google Scholar]
- 19.Houser OW, Mokri B, Sundt TM, Jr, et al. Spontaneous cervical cephalic arterial dissection and its residuum: angiographic spectrum. AJNR Am J Neuroradiol. 1984;5:27–34. [PMC free article] [PubMed] [Google Scholar]
- 20.Hart RG, Easton JD. Dissections of cervical and cerebral arteries. Neurol Clin. 1983;1:155–182. [PubMed] [Google Scholar]
- 21.Flis CM, Jager HR, Sidhu PS. Carotid and vertebral artery dissections: clinical aspects, imaging features and endovascular treatment. Eur Radiol. 2007;17:820–834. doi: 10.1007/s00330-006-0346-7. [DOI] [PubMed] [Google Scholar]
- 22.Mortazavi MM, Verma K, Tubbs RS, et al. Pediatric traumatic carotid, vertebral and cerebral artery dissections: a review. Childs Nerv Syst. 2011 doi: 10.1007/s00381-011-1409-x. [DOI] [PubMed] [Google Scholar]
- 23.Provenzale JM, Sarikaya B. Comparison of test performance characteristics of MRI, MR angiography, and CT angiography in the diagnosis of carotid and vertebral artery dissection: a review of the medical literature. AJR Am J Roentgenol. 2009;193:1167–1174. doi: 10.2214/AJR.08.1688. [DOI] [PubMed] [Google Scholar]
- 24.Kaufmann TJ, Huston J, III, Mandrekar JN, et al. Complications of diagnostic cerebral angiography: evaluation of 19,826 consecutive patients. Radiology. 2007;243:812–819. doi: 10.1148/radiol.2433060536. [DOI] [PubMed] [Google Scholar]
- 25.Ahlhelm F, Kaufmann R, Ahlhelm D, et al. Carotid artery stenting using a novel self-expanding braided nickel-titanium stent: feasibility and safety porcine trial. Cardiovasc Intervent Radiol. 2009;32:1019–1027. doi: 10.1007/s00270-009-9572-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wolfe TJ, Hussain SI, Lynch JR, et al. Pediatric cerebral angiography: analysis of utilization and findings. Pediatr Neurol. 2009;40:98–101. doi: 10.1016/j.pediatrneurol.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 27.Zilocchi M, Macedo TA, Oderich GS, et al. Vascular Ehlers-Danlos syndrome: imaging findings. AJR Am J Roentgenol. 2007;189:712–719. doi: 10.2214/AJR.07.2370. [DOI] [PubMed] [Google Scholar]
- 28.Oelerich M, Stogbauer F, Kurlemann G, et al. Craniocervical artery dissection: MR imaging and MR angiographic findings. Eur Radiol. 1999;9:1385–1391. doi: 10.1007/s003300050853. [DOI] [PubMed] [Google Scholar]
- 29.Leclerc C, Webb SE, Miller AL, et al. An increase in intracellular Ca2+ is involved in pronephric tubule differentiation in the amphibian Xenopus laevis. Dev Biol. 2008;321:357–367. doi: 10.1016/j.ydbio.2008.06.029. [DOI] [PubMed] [Google Scholar]
- 30.Leclerc X, Lucas C, Godefroy O, et al. Preliminary experience using contrast-enhanced MR angiography to assess vertebral artery structure for the follow-up of suspected dissection. AJNR Am J Neuroradiol. 1999;20:1482–1490. [PMC free article] [PubMed] [Google Scholar]
- 31.Arnold M, Bousser MG, Fahrni G, et al. Vertebral artery dissection: presenting findings and predictors of outcome. Stroke. 2006;37:2499–2503. doi: 10.1161/01.STR.0000240493.88473.39. [DOI] [PubMed] [Google Scholar]
- 32.Vertinsky AT, Schwartz NE, Fischbein NJ, et al. Comparison of multidetector CT angiography and MR imaging of cervical artery dissection. AJNR Am J Neuroradiol. 2008;29:1753–1760. doi: 10.3174/ajnr.A1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Levy C, Laissy JP, Raveau V, et al. Carotid and vertebral artery dissections: three-dimensional time-of-flight MR angiography and MR imaging versus conventional angiography. Radiology. 1994;190:97–103. doi: 10.1148/radiology.190.1.8259436. [DOI] [PubMed] [Google Scholar]
- 34.Kitanaka C, Sasaki T, Eguchi T, et al. Intracranial vertebral artery dissections: clinical, radiological features, and surgical considerations. Neurosurgery. 1994;34:620–626. doi: 10.1227/00006123-199404000-00008. [DOI] [PubMed] [Google Scholar]
- 35.Miller PR, Fabian TC, Croce MA, et al. Prospective screening for blunt cerebrovascular injuries: analysis of diagnostic modalities and outcomes. Ann Surg. 2002;236:386–393. doi: 10.1097/01.SLA.0000027174.01008.A0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoshimoto Y, Wakai S. Unruptured intracranial vertebral artery dissection. Clinical course and serial radiographic imagings. Stroke. 1997;28:370–374. doi: 10.1161/01.str.28.2.370. [DOI] [PubMed] [Google Scholar]
- 37.Mascalchi M, Bianchi MC, Mangiafico S, et al. MRI and MR angiography of vertebral artery dissection. Neuroradiology. 1997;39:329–340. doi: 10.1007/s002340050418. [DOI] [PubMed] [Google Scholar]
- 38.Auer A, Felber S, Schmidauer C, et al. Magnetic resonance angiographic and clinical features of extracranial vertebral artery dissection. J Neurol Neurosurg Psychiatry. 1998;64:474–481. doi: 10.1136/jnnp.64.4.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Biffl WL, Ray CE, Jr, Moore EE, et al. Noninvasive diagnosis of blunt cerebrovascular injuries: a preliminary report. J Trauma. 2002;53:850–856. doi: 10.1097/00005373-200211000-00008. [DOI] [PubMed] [Google Scholar]
- 40.Rizzo L, Crasto SG, Savio D, et al. Dissection of cervicocephalic arteries: early diagnosis and follow-up with magnetic resonance imaging. Emerg Radiol. 2006;12:254–265. doi: 10.1007/s10140-006-0476-x. [DOI] [PubMed] [Google Scholar]
- 41.Yang CW, Carr JC, Futterer SF, et al. Contrast-enhanced MR angiography of the carotid and vertebrobasilar circulations. AJNR Am J Neuroradiol. 2005;26:2095–2101. [PMC free article] [PubMed] [Google Scholar]
- 42.Mitra D, Connolly D, Jenkins S, et al. Comparison of image quality, diagnostic confidence and interobserver variability in contrast enhanced MR angiography and 2D time of flight angiography in evaluation of carotid stenosis. Br J Radiol. 2006;79:201–207. doi: 10.1259/bjr/72842752. [DOI] [PubMed] [Google Scholar]
- 43.Husson B, Rodesch G, Lasjaunias P, et al. Magnetic resonance angiography in childhood arterial brain infarcts: a comparative study with contrast angiography. Stroke. 2002;33:1280–1285. doi: 10.1161/01.str.0000014504.18199.0d. [DOI] [PubMed] [Google Scholar]
- 44.Rollins N, Dowling M, Booth T, et al. Idiopathic ischemic cerebral infarction in childhood: depiction of arterial abnormalities by MR angiography and catheter angiography. AJNR Am J Neuroradiol. 2000;21:549–556. [PMC free article] [PubMed] [Google Scholar]
- 45.Elijovich L, Kazmi K, Gauvrit JY, et al. The emerging role of multidetector row CT angiography in the diagnosis of cervical arterial dissection: preliminary study. Neuroradiology. 2006;48:606–612. doi: 10.1007/s00234-006-0100-5. [DOI] [PubMed] [Google Scholar]
- 46.Provenzale JM, Sarikaya B, Hacein-Bey L, et al. Causes of misinterpretation of cross-sectional imaging studies for dissection of the craniocervical arteries. AJR Am J Roentgenol. 2011;196:45–52. doi: 10.2214/AJR.10.5384. [DOI] [PubMed] [Google Scholar]
- 47.Bloem BR, Van Buchem GJ. Magnetic resonance imaging and vertebral artery dissection. J Neurol Neurosurg Psychiatry. 1999;67:691–692. doi: 10.1136/jnnp.67.5.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Naggara O, Oppenheim C, Toussaint JF, et al. Asymptomatic spontaneous acute vertebral artery dissection: diagnosis by high-resolution magnetic resonance images with a dedicated surface coil. Eur Radiol. 2007;17:2434–2435. doi: 10.1007/s00330-006-0524-7. [DOI] [PubMed] [Google Scholar]
- 49••.Oppenheim C, Naggara O, Touze E, et al. High-resolution MR imaging of the cervical arterial wall: what the radiologist needs to know. Radiographics. 2009;29:1413–1431. doi: 10.1148/rg.295085183. This article reports the use of a promising new technique (high-resolution MRI with specialized cervical surface coils) to detect CCAD in adults. [DOI] [PubMed] [Google Scholar]
- 50.Bachmann R, Nassenstein I, Kooijman H, et al. Spontaneous acute dissection of the internal carotid artery: high-resolution magnetic resonance imaging at 3.0 tesla with a dedicated surface coil. Invest Radiol. 2006;41:105–111. doi: 10.1097/01.rli.0000195836.57778.1f. [DOI] [PubMed] [Google Scholar]
- 51.Pugliese F, Crusco F, Cardaioli G, et al. CT angiography versus colour-Doppler US in acute dissection of the vertebral artery. Radiol Med. 2007;112:435–443. doi: 10.1007/s11547-007-0152-6. [DOI] [PubMed] [Google Scholar]
- 52.Eastman AL, Chason DP, Perez CL, et al. Computed tomographic angiography for the diagnosis of blunt cervical vascular injury: is it ready for primetime? J Trauma. 2006;60:925–929. doi: 10.1097/01.ta.0000197479.28714.62. [DOI] [PubMed] [Google Scholar]
- 53.Chen CJ, Tseng YC, Lee TH, et al. Multisection CT angiography compared with catheter angiography in diagnosing vertebral artery dissection. AJNR Am J Neuroradiol. 2004;25:769–774. [PMC free article] [PubMed] [Google Scholar]
- 54.Brenner D, Elliston C, Hall E, et al. Estimated risks of radiation-induced fatal cancer from pediatric CT. AJR Am J Roentgenol. 2001;176:289–296. doi: 10.2214/ajr.176.2.1760289. [DOI] [PubMed] [Google Scholar]
- 55.Sliker CW. Blunt cerebrovascular injuries: imaging with multidetector CT angiography. Radiographics. 2008;28:1689–1708. doi: 10.1148/rg.286085521. [DOI] [PubMed] [Google Scholar]
- 56.Rodallec MH, Marteau V, Gerber S, et al. Craniocervical arterial dissection: spectrum of imaging findings and differential diagnosis. Radiographics. 2008;28:1711–1728. doi: 10.1148/rg.286085512. [DOI] [PubMed] [Google Scholar]
- 57.Vertinsky AT, Schwartz NE, Fischbein NJ, et al. Comparison of multidetector CT angiography and MR imaging of cervical artery dissection. AJNR Am J Neuroradiol. 2008;29:1753–1760. doi: 10.3174/ajnr.A1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hellinger JC, Pena A, Poon M, et al. Pediatric computed tomographic angiography: imaging the cardiovascular system gently. Radiol Clin North Am. 2010;48:439–67. x. doi: 10.1016/j.rcl.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 59.Nebelsieck J, Sengelhoff C, Nassenstein I, et al. Sensitivity of neurovascular ultrasound for the detection of spontaneous cervical artery dissection. J Clin Neurosci. 2009;16:79–82. doi: 10.1016/j.jocn.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 60.Arnold M, Baumgartner RW, Stapf C, et al. Ultrasound diagnosis of spontaneous carotid dissection with isolated Horner syndrome. Stroke. 2008;39:82–86. doi: 10.1161/STROKEAHA.107.492652. [DOI] [PubMed] [Google Scholar]
- 61.Benninger DH, Georgiadis D, Gandjour J, et al. Accuracy of color duplex ultrasound diagnosis of spontaneous carotid dissection causing ischemia. Stroke. 2006;37:377–381. doi: 10.1161/01.STR.0000198811.65068.16. [DOI] [PubMed] [Google Scholar]
- 62.Dittrich R, Dziewas R, Ritter MA, et al. Negative ultrasound findings in patients with cervical artery dissection. Negative ultrasound in CAD. J Neurol. 2006;253:424–433. doi: 10.1007/s00415-005-0051-5. [DOI] [PubMed] [Google Scholar]
- 63.Baracchini C, Tonello S, Meneghetti G, et al. Neurosonographic monitoring of 105 spontaneous cervical artery dissections: a prospective study. Neurology. 2010;75:1864–1870. doi: 10.1212/WNL.0b013e3181feae5e. [DOI] [PubMed] [Google Scholar]
- 64.Sturzenegger M, Mattle HP, Rivoir A, et al. Ultrasound findings in spontaneous extracranial vertebral artery dissection. Stroke. 1993;24:1910–1921. doi: 10.1161/01.str.24.12.1910. [DOI] [PubMed] [Google Scholar]
- 65.Berne JD, Reuland KS, Villarreal DH, et al. Sixteen-slice multi-detector computed tomographic angiography improves the accuracy of screening for blunt cerebrovascular injury. J Trauma. 2006;60:1204–1209. doi: 10.1097/01.ta.0000220435.55791.ce. [DOI] [PubMed] [Google Scholar]
- 66.Schneidereit NP, Simons R, Nicolaou S, et al. Utility of screening for blunt vascular neck injuries with computed tomographic angiography. J Trauma. 2006;60:209–215. doi: 10.1097/01.ta.0000195651.60080.2c. [DOI] [PubMed] [Google Scholar]
- 67.Lew SM, Frumiento C, Wald SL. Pediatric blunt carotid injury: a review of the National Pediatric Trauma Registry. Pediatr Neurosurg. 1999;30:239–244. doi: 10.1159/000028804. [DOI] [PubMed] [Google Scholar]
- 68.Goodwin RB, Beery PR, Dorbish RJ, et al. Computed tomographic angiography versus conventional angiography for the diagnosis of blunt cerebrovascular injury in trauma patients. J Trauma. 2009;67:1046–1050. doi: 10.1097/TA.0b013e3181b83b63. [DOI] [PubMed] [Google Scholar]
- 69.Hogan AR, Lineen EB, Perez EA, et al. Value of computed tomographic angiography in neck and extremity pediatric vascular trauma. J Pediatr Surg. 2009;44:1236–1241. doi: 10.1016/j.jpedsurg.2009.02.039. [DOI] [PubMed] [Google Scholar]
- 70.Chamoun RB, Jea A. Traumatic intracranial and extracranial vascular injuries in children. Neurosurg Clin N Am. 2010;21:529–542. doi: 10.1016/j.nec.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 71••.Monagle P, Chalmers E, Chan A, et al. Antithrombotic therapy in neonates and children: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) Chest. 2008;133:887S–968S. doi: 10.1378/chest.08-0762. This consensus statement from the ACCP about the use of antithrombotic therapies in children provides specific recommendations about children with AIS. [DOI] [PubMed] [Google Scholar]
- 72••.Lyrer P, Engelter S. Antithrombotic drugs for carotid artery dissection. Cochrane Database Syst Rev. 2010:CD000255. doi: 10.1002/14651858.CD000255.pub2. This Cochrane Review of the treatment of CCAD (isolated to the carotids) reveals that (1) there are no randomized controlled trials with which to inform treatment of CCAD in adults, and (2) nonrandomized trials do not provide any evidence of a significant difference between anticoagulation and antiplatelet therapy in this disease. [DOI] [PubMed] [Google Scholar]
- 73.Dirik E, Yis U, Dirik MA, et al. Vertebral artery dissection in a patient with Wildervanck syndrome. Pediatr Neurol. 2008;39:218–220. doi: 10.1016/j.pediatrneurol.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 74.Sedney CL, Rosen CL. Cervical abnormalities causing vertebral artery dissection in children. J Neurosurg Pediatr. 2011;7:272–275. doi: 10.3171/2010.12.PEDS10106. [DOI] [PubMed] [Google Scholar]
- 75.Schror K. Aspirin and Reye syndrome: a review of the evidence. Paediatr Drugs. 2007;9:195–204. doi: 10.2165/00148581-200709030-00008. [DOI] [PubMed] [Google Scholar]
- 76.Andrew M, Marzinotto V, Massicotte P, et al. Heparin therapy in pediatric patients: a prospective cohort study. Pediatr Res. 1994;35:78–83. doi: 10.1203/00006450-199401000-00016. [DOI] [PubMed] [Google Scholar]
- 77.Kuhle S, Eulmesekian P, Kavanagh B, et al. A clinically significant incidence of bleeding in critically ill children receiving therapeutic doses of unfractionated heparin: a prospective cohort study. Haematologica. 2007;92:244–247. doi: 10.3324/haematol.10616. [DOI] [PubMed] [Google Scholar]
- 78.Bernard TJ, Goldenberg NA, Tripputi M, et al. Anticoagulation in childhood-onset arterial ischemic stroke with nonmoyamoya arteriopathy: findings from the Colorado and German (COAG) collaboration. Stroke. 2009;40:2869–2871. doi: 10.1161/STROKEAHA.109.550699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schmugge M, Risch L, Huber AR, et al. Heparin-induced thrombocytopenia-associated thrombosis in pediatric intensive care patients. Pediatrics. 2002;109:E10. doi: 10.1542/peds.109.1.e10. [DOI] [PubMed] [Google Scholar]
- 80.Risch L, Huber AR, Schmugge M. Diagnosis and treatment of heparin-induced thrombocytopenia in neonates and children. Thromb Res. 2006;118:123–135. doi: 10.1016/j.thromres.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 81.Barnes C, Newall F, Ignjatovic V, et al. Reduced bone density in children on long-term warfarin. Pediatr Res. 2005;57:578–581. doi: 10.1203/01.PDR.0000155943.07244.04. [DOI] [PubMed] [Google Scholar]
- 82.Kirton A, Wong JH, Mah J, et al. Successful endovascular therapy for acute basilar thrombosis in an adolescent. Pediatrics. 2003;112:e248–e251. doi: 10.1542/peds.112.3.e248. [DOI] [PubMed] [Google Scholar]
- 83.Chern JJ, Chamoun RB, Mawad ME, et al. Endovascular stenting of traumatic extracranial carotid artery dissections in the pediatric population: a case report. Cases J. 2009;2:171. doi: 10.1186/1757-1626-2-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Felker MV, Zimmer JA, Golomb MR. Failure of a clot retrieval device in an adolescent stroke patient. Pediatr Neurol. 2010;43:435–438. doi: 10.1016/j.pediatrneurol.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 85••.Amlie-Lefond C, DeVeber G, Chan AK, et al. Use of alteplase in childhood arterial ischaemic stroke: a multicentre, observational, cohort study. Lancet Neurol. 2009;8:530–536. doi: 10.1016/S1474-4422(09)70106-1. This large international series of children with AIS from the International Pediatric Stroke Study demonstrates that intravenous and intra-arterial thrombolysis is being used in childhood AIS, sometimes outside of established adult guidelines. [DOI] [PubMed] [Google Scholar]