Abstract
Purpose
Neither the prognostic importance nor the appropriate management of central nervous system (CNS) involvement are known for patients with acute myeloid leukemia (AML) undergoing hematopoietic cell transplantation (HCT). We examined the impact of a CNS irradiation boost to standard intrathecal chemotherapy (ITC).
Methods and Materials
From 1995 to 2005, 648 adult AML patients received a myeloablative HCT: 577 patients were CNS negative (CNS−), and 71 were CNS positive (CNS+). Of the 71 CNS+ patients, 52 received intrathecal chemotherapy alone (CNS+ITC), and 19 received ITC plus an irradiation boost (CNS+RT).
Results
The CNS−, CNS+ITC, and CNS+RT, patients had 1 and 5 year relapse-free survivals (RFS) of 43% and 35%, 15% and 6%, 37% and 32%, respectively. CNS+ITC patients had a statistically significant worse RFS compared to CNS− patients, HR=2.65 (95% CI, 2.0-3.6), p<0.0001. CNS+RT patients had improved relapse free survival than CNS+ITC patients, HR=0.45 (95% CI, 0.2-0.8), p=0.01. The 1 and 5 year overall survivals (OS) of patients with CNS−, CNS+ITC, and CNS+RT, were 50% and 38%, 21% and 6%, and 53% and 42%, respectively. The survival of CNS+RT were significantly better than CNS+ITC patients, p=0.004. After adjusting for known risk factors, CNS+RT patients had a trend toward lower relapse rates and reduced non-relapse mortality.
Conclusion
CNS+ AML is associated with a poor prognosis. The role of a cranial irradiation boost to intrathecal chemotherapy appears to mitigate the risk of CNS disease, and needs to be further investigated to define optimal treatment strategies.
Keywords: acute myelgenous leukemia, intrathecal chemotherapy, central nervous system, cranial irradiation, hematopoietic cell transplantation
Introduction
Central nervous system (CNS) involvement in acute myeloid leukemia (AML) occurs in 2-4% of patients at diagnosis 1-3. Hematological parameters associated with an increased risk of CNS leukemia include high presenting white blood count, peripheral eosinophilia, elevated lactate dehydrogenase, French-American-British (FAB) subgroups M4 and M5, age less than 50 years, and extramedullary disease at presentation 4-7. Due to the infrequency of CNS involvement (CNS+) in patients with AML there is a paucity of literature on the optimal management of CNS+ disease. Prospective studies have shown no advantage to CNS prophylaxis in adult AML, but there is no consensus on treatment for CNS+ AML upon initial presentation, or during conditioning before hematopoietic cell (HCT) transplantation8.
ITC (intrathecal chemotherapy) and/or CNS irradiation (RT) are used to treat leukemic CNS disease. The National Cancer Care Network (NCCN) guidelines suggest that patients who present with CNS+ AML should be treated with itrathecal chemotherapy and cranial irradiation considered if symptomatic (e.g., NCCN guidelines)9. Conventional treatment of AML patients with CNS+ leukemia consists of intrathecal chemotherapy (ITC) with methotrexate (MTX) and/or cytosine arabinoside (Ara-C)10.
Currently, the standard of care for CNS+ AML patients in the setting of HCT remains controversial. Moreover, there is debate regarding the utility, and benefit of, adding a cranial irradiation boost to standard intrathecal chemotherapy. The purpose of this retrospective analysis was to evaluate the impact of CNS+ status on the outcome of HCT for AML, and to better understand the contribution of a cranial irradiation boost in addition to standard ITC.
Materials and Methods
Patients Characteristics
From 1995 to 2005, 648 consecutive patients aged ≥ 18 years of age underwent HCT for AML at the Fred Hutchinson Cancer Research Center. All patients provided informed consent for treatment according to transplantation protocols approved by the institutional review boards. In addition, separate institutional approval was obtained to retrospectively gather data from patient records and databases. Six hundred forty-eight patient charts were reviewed to determine CNS involvement as documented by positive cerebral spinal fluid (CSF) analysis, ITC administration, and cranial or craniospinal irradiation. All patients underwent routine diagnostic lumbar puncture for CSF sampling prior to transplantation, which is our institutional policy. 71 patients had positive CSF cytology. Five hundred seventy- seven patients did not have CNS involvement (CNS−). Of the 71 patients, 52 were treated with ITC alone (CNS+ITC), and 19 were treated with a radiation boost (CNS+RT). The median follow-up after HCT of CNS− patients were 36 months (range,1 – 124); CNS+ITC, 66 months (range, 37 – 106); and CNS+RT, 72 months (range, 25-107). The dates of transplant of CNS− patients were 1995-2005 while CNS+ patients were transplanted from 1995-2003. Therefore, CNS+ patients had a longer median follow-up.
Study Variables
The CNS− and CNS+ patients were similar with respect to sex, age, type of transplantation (HLA matched sibling or unrelated donor), and stage. Patients who had failed allogeneic or autologous HCT were excluded from the analysis. Patients were stratified according to relapse and remission status using the following criteria: 1) Group 1: first remission – less than 5% blasts in the marrow 2) Group 2: remission 2 and 3- less than 5% blasts in the marrow 3) Group 3 active AML or refractory anemia with excess blasts (RAEB), – all greater than 5% blasts in the marrow. In addition, patients were stratified as to the use of TBI in their conditioning regimen. Patient characteristics are shown in Table 1.
Table 1.
Patient characteristics
| CNS negative n=577 (% total) |
CNS positive n=71 (% total) |
|
|---|---|---|
| Female | 280 (49%) | 29 (41%) |
| Male | 297 (51%) | 42 (59%) |
| Median age years (range) | 43 (18-68) | 41 (18-58) |
| Group 1: First remission | 190 (33%) | 6 (8%) |
| Group 2: Second and Third Remission |
270 (47%) | 50 (70%) |
| Group 3: Primary refractory, relapse 1, 2, 3 |
117 (20%) | 15 (21%) |
| HLA matched sibling | 241 (42%) | 31 (44%) |
| Other related donor | 73 (13%) | 9 (13%) |
| Unrelated donor | 263 (46%) | 31 (44%) |
| No TBI | 240 (42%) | 10 (14%) |
| TBI | 337 (58%) | 61 (86%) |
Conditioning Regimen
Two hundred forty of the 577 (42%) CNS− patients, and 10 of the 71 (14%) of CNS+ positive patients received busulfan based containing regimen of busulfan/cyclophosphamide (BU-CY), or busulfan/fludarabine. The conditioning regimen employed for treatment was at the discretion of the treating physician. The BU-CY regimen consisted of 16 consecutive doses of BU at a dose of 1 mg/kg every 6 hours for 4 days followed by a total of 120 mg/kg of CY 60 mg/kg for 2 days. Patients received BU adjusted for targeted steady-state plasma levels of 600 to 900 ng/mL. This conditioning regimen included either oral or intravenous (iv) BU. Oral BU was given for 4 days (−7 to −4) 1mg/kg oral q 6 hours QID per day. IV BU was delivered on days −7 to −4 at 0.8mg/kg IV every 6 hours QID. The CY was given in both regimens on days −3 and −2 at 60mg/kg/day intravenously. For patients treated with a busulfan/fludarabine regimen, fludarabine 120 mg/m2 (30 mg/m2 for 4 days) was given with BU. The BU was adjusted for targeted steady-state plasma levels of 800 to 900 ng/mL. Phenytoin was administered to all patients who received BU.
CY/total body irradiation (TBI) conditioning was used in 337 of the 577 CNS− patients (58%), and in 61 of the 71 (86%) of the CNS+ patients. TBI was administered twice daily (BID) as 200 cGy fractions on days −3, −2, −1 days prior to marrow transplantation for a total dose of 1200 cGy. TBI was given via opposing Cobalt 60 sources from 1995-2000. The patient was placed in the supine position with knees flexed. Lung shielding was not employed. Since 2000, TBI was delivered using a linear accelerator with 6 – 18 MV photons using lung shielding. CY was then administered at a dose of 60 mg/kg adjusted ideal body weight/day on two consecutive days between days −5 and −4.
Intrathecal Chemotherapy
Patients were treated with ITC before and after transplantation. Although the number of ITC varied between the patients, the majority of CNS+ITC patients were treated with 2 sessions before, and 4 sessions after HCT.
The ITC regimens did not differ between CNS+ITC patients versus CNS+RT patients, nor did the number of ITC instillations.
There were 9 CNS− patients treated with ITC because of other high risk factors such as unexplained neurological deficits, or aggressive clinical course. These patients were included in the group of CNS− patients.
Radiation Treatment
CNS+RT patients were treated prior to transplantation, and none had a history of prior CNS radiotherapy. Nineteen CNS+ patients received either a cranial (n = 15) or craniospinal (n = 4) irradiation boost. The selection of patients for CNS boost was based on physician preference rather than on disease or patient characteristics. Cranial and/or craniospinal irradiations were performed as described by Kun11. The dose per fraction was 1.8 −2.0 Gy, prescribed to midplane. The cranial and craniospinal irradiation doses are shown in Table 2.
Table 2.
Cranial radiation dose (cGy) for patients treated with ITC and an irradiation boost
| Patient Number |
Cranial cGy/( # of fractions) | Craniospinal cGy/( # of fractions) |
Total cGy/(# of fractions) |
|---|---|---|---|
| 1 to 3 | 1000/(5) | ||
| 4 to 7 | 1080/(6) | ||
| 8 to 13 | 1200/(6) | ||
| 14 | 1440/(8) | ||
| 15 | 2000/(10) | ||
| 16 | 1200/(6) | 2000/(10) | 3200 (16) |
| 17 | 2400/(12) | 1400/(7) | 3800 (19) |
| 18 | 1200/(6) | 1080/(6) | 2280 (12) |
| 19 | 2400/(12) | 1200/(6) | 3600 (18) |
Statistical Methods
Overall and disease-free survivals were estimated by the Kaplan-Meier method. Cox regression analysis was used to estimate hazard ratios for these endpoints, with and without adjustment for disease status, donor relation, and the use of TBI in the conditioning regimen. All p-values were derived from hazard ratio (HR) analyses and were 2-sided.
Results
Relapse Free Survival
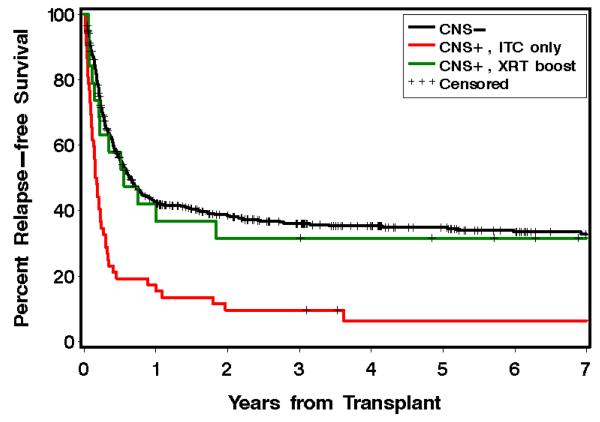
The 577 CNS− patients had 1 and 5 year relapse-free survivals (RFS) of 43% and 35%, respectively. The median RFS for this population was 230 days. The 52 CNS+ITC patients had 1 year and 5 year RFS of 15% and 6%, respectively. The median RFS was 58 days. The 19 CNS+RT patients had estimated 1 and 5 year RFS of 37% and 32%, respectively (Figure 1). The median RFS was 203 days. Patients treated with ITC alone had a statistically significant worse outcome compared to CNS− patients, HR=2.65 (95% CI, 2.0-3.6), p<0.0001. After adjusting for prognostic variables, such as disease status, donor relation, and the use of TBI in the conditioning regimen, this difference remained statistically significant, HR=1.91 (95% CI, 1.4-2.6), p < 0.0001. There was no difference between CNS− patients and CNS+RT, HR=1.17 (95% CI, 0.7-2.0), p=0.56. There was again no difference noted after adjusting for prognostic variables as mentioned previously, HR=0.87 (95% CI, 0.5-1.5), p=0.60. CNS+RT patients had better relapse free survival than CNS+ITC patients, HR=0.45 (95% CI, 0.2-0.8), p=0.01. After adjusting for prognostic variables the difference remained statistically significant, HR=0.44 (95% CI, 0.2-0.8), p=0.01. (Table 3)
Figure 1.
Kaplan-Meier curves for estimated relapse free survival of AML patients with CNS positive disease treated with ITC alone (red), versus CNS positive disease treated with ITC and an irradiation boost (green), versus CNS negative patients (black).
Table 3.
Analysis of relapse-free survival
| Survival | Unadjusted HR (95% CI) p |
Adjusted1 HR (95% CI) p |
||
|---|---|---|---|---|
| 1 year | 5 years | |||
| CNS– (n=577) | 43% | 35% | ||
| CNS+ / ITC (n=52) | 15% | 6% | vs CNS- 2.65 (2.0-3.6) p<0.0001 |
vs CNS− 1.91 (1.4-2.6) p<0.0001 |
| CNS+ / ITC+XRT (n=19) |
37% | 32% | 1.17 (0.7-2.0) p=0.56 | vs CNS− 0.87 (0.5-1.5) p=0.60 |
| CNS+ / ITC+XRT (n=19) |
37% | 32% | vs CNS+ / ITC 0.45 (0.2-0.8) p=0.01 |
vs CNS+ / ITC 0.44 (0.2-0.8) p=0.01 |
HR = hazard ratio for mortality + relapse
adjusted for stage of disease (3 groups), donor (unrelated vs related), TBI in conditioning regimen (yes/no)
Overall Survival
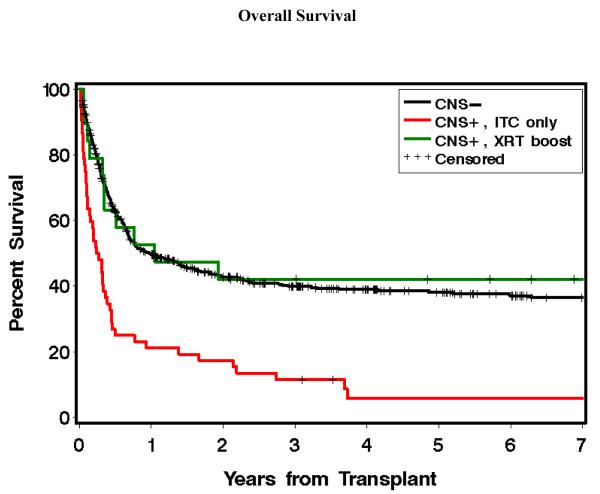
The 577 CNS− patients had 1 year and 5 year OS of 50% and 38%, respectively. The median OS was 358 days. The 52 CNS+ITC patients had 1 year and 5 year OS of 21% and 6%, respectively. The median OS was 86 days. The 19 CNS+RT patients had estimated OS of 53% at 1 year, and 42% at 5 years (Figure 2). The median OS was 383 days. CNS+ITC patients had a statistically significant worse outcome compared to CNS− patients, HR=2.59 (95% CI, 1.9-3.5), p<0.0001. The hazard ratios remained statistically significant after adjusting for prognostic variables such as stage of disease, donor type, and TBI conditioning, HR=1.86 (95% CI, 1.4-2.5), p<0.0001. CNS+RT patients had an estimated survival comparable to CNS− patients, HR=1.01 (95% CI, 0.6-1.8), p=0.98. This remained unchanged after adjusting for prognostic variables, HR=0.79 (95% CI, 0.4-1.4), p=0.43. CNS+RT patients compared to CNS+ITC patients had an increased OS, with a mortality HR of 0.37 (95% CI 0.2-0.7) p=0.004. The HR remained significant after adjusting for variables, HR=0.36 (95% CI, 0.2-0.7), p=0.003. (Table 4)
Figure 2.
Kaplan-Meier curves for estimated overall survival of AML patients with CNS positive disease treated with ITC alone (red), versus CNS positive disease treated with ITC and an irradiation boost (green), versus CNS negative patients (black).
Table 4.
Analysis of overall survival
| Survival | Unadjusted HR (95% CI) p |
Adjusted1 HR (95% CI) p |
||
|---|---|---|---|---|
| 1 year | 5 years | |||
| CNS− (n=577) | 50% | 38% | ||
| CNS+ / ITC (n=52) | 21% | 6% | vs CNS− 2.59 (1.9-3.5) p<0.0001 |
vs CNS− 1.86 (1.4-2.5) p<0.0001 |
| CNS+ / ITC+XRT (n=19) |
53% | 42% | vs CNS− 1.01 (0.6-1.8) p=0.98 |
vs CNS− 0.79 (0.4-1.4) p=0.43 |
| CNS+ / ITC+XRT (n=19) |
53% | 42% | vs CNS+ / ITC 0.37 (0.2-0.7) p=0.004 |
vs CNS+ / ITC 0.36 (0.2-0.7) p=0.003 |
HR = hazard ratio for mortality
adjusted for stage of disease (3 groups), donor (unrelated vs related), TBI in conditioning regimen (yes/no)
Relapse
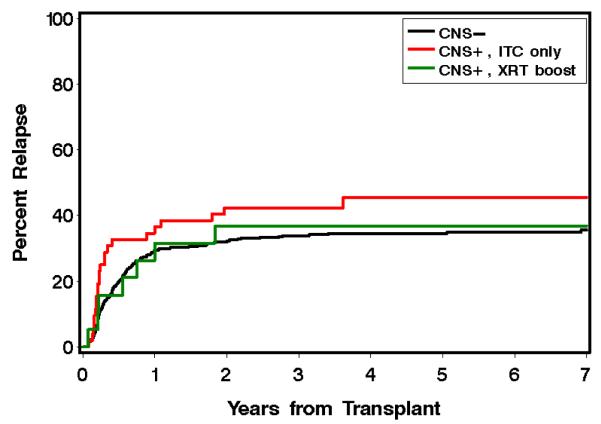
The 577 CNS− patients had relapse rates of 29% and 35% at 1 and 5 years, respectively. The CNS+ITC patients had 1 and 5 year relapse rates of 37% and 46%. These relapse rates were statistically worse among CNS+ITC patients, HR 2.75 (95% CI 1.8-4.3; p=<0.0001), even after adjusting for stage of disease, donor and TBI, (p=0.003). The 19 CNS+RT patients had 1 and 5 year relapse rates of 32% and 37%, which were lower compared to CNS+ITC patients, HR 0.42 (95% CI 0.2-1.0), p=0.05, even after adjusting for stage of disease, donor status and use of TBI in the conditioning regimen, (HR 0.48 (95% CI 0.2-1.1), P=0.09). Patients with CNS+RT had no statistically significant difference in relapse rates compared to CNS− patients, HR 0.85, (95% CI 0.4-1.8) p=0.67.
Non Relapse Mortality
Non relapse mortality was defined as death contributed to a cause other than AML, which included transplantation related mortality. The CNS− patients had a non-relapse mortality of 28% and 31% at 1 and 5 years. The CNS+ITC patients had a 48% 1 and 5 year non relapse mortality. Compared to the CNS− group, the CNS+ITC had increased non relapse mortality, HR 2.56 (95% CI 1.7-3.9), p<0.0001. If a radiation boost was given, there was no difference in non relapse mortality compared to the CNS− patients, HR 1.23 (95% CI 0.6-2.6), p=0.59. When adjusted for stage of disease, donor status, and TBI in the conditioning regimen, there was lower non-relapse mortality among the CNS+RT compared to CNS+ITC patients, HR 0.38, (95% CI 0.1-1.), p=0.05 .
TBI in Conditioning Regimen
CNS+ patients receiving TBI as part of the conditioning regimen (n=61) had a trend for worse OS then those who did not have TBI (n=10), adjusted HR=1.92 (95% CI, 0.9-4.3), p=0.11. The trend for OS was not statistically significant. Among CNS− patients, there were no differences in relapse free or overall survivals between those who received TBI conditioning (n=337) and those who did not (n=240).
Discussion
CNS involvement in AML is generally associated with a poor prognosis4,12,13. However, little is known about the prognostic significance of CNS involvement with AML before transplantation, or how best to treat it. In this investigation, 12% of AML patients who presented for HCT had CNS+ disease, reflecting the importance of treatment options in this patient population. This retrospective study describes the largest number of adult AML patients with CNS+ disease in the HCT setting. Patients with CNS involvement before transplant experience a poorer transplant outcome compared to CNS− patients, as illustrated by the dismal 6% 5 yr OS of CNS+ITC patients. Encouragingly, however, the addition of cranial irradiation to intrathecal chemotherapy appears to mitigate the risk of CNS disease, at least to some extent. This last finding is consistent with a report by Castagnola et al.4, which demonstrated improved survival in CNS+ patients treated with combined chemotherapy and cranial irradiation in the non transplant setting.
There is no current standard of care for CNS+ AML patients undergoing HCT. Our findings suggest a possible role for an irradiation boost as part of transplant conditioning regimens. In this study, the addition of RT showed a statistically significant DFS and OS benefit in patients with CNS involvement. The CNS+ITC patients had poor outcomes, with an estimated OS of 21% at 1 year, and 6% at 5 years. However, CNS+RT patients had overall 1 and 5 year OS rates of 53% and 42%, respectively. Furthermore, the addition of an irradiation boost was associated with RFS and OS comparable to CNS− patients. The OS mirrored the RFS for this patient cohort, which is not surprising in adult AML, as there are few salvage options available post transplantation.
Prognostic variables known to effect transplant outcome were examined. It has been established that patients in first remission have the most favorable prognosis15. The CNS+ patients were more likely to have primary refractory disease or were transplanted while in relapse. The statistical analysis accounted for the disease status according to historical prognosis status post transplantation. The outcome differences found between the CNS−, CNS+ITC, and CNS+RT patients, remained statistically significant after adjusting for other prognostic factors such as age, sex, unrelated donor, or presence of total body irradiation in the conditioning regimen. Nevertheless, some uncertainty remains since it was not clear from the retrospective chart review why a given attending physician chose ITC alone and another chose ITC and a CNS irradiation boost.
We also examined the role of TBI in CNS disease, given that the 12 Gy dose of TBI treats the entire craniospinal axis. There were no differences in RFS or OS observed between TBI containing regimens in the CNS+ versus CNS− patients. Furthermore, there was a trend for worse OS in CNS+ patients who received a TBI containing regimen compared to those who did not. Given the small number of CNS+RT patients, we are unable to draw conclusions on the effect of TBI and dose to control CNS disease. However, this OS difference may be suggestive that patients with a TBI containing regimen who had CNS+ITC, a TBI dose of 12 Gy may be inadequate to control CNS disease.
To better understand the observed striking differences in RFS and OS between the three treatment groups, we investigated relapse and non relapse mortality rates of our patient populations. There appeared to be both a possible decrease in relapse rates as well as a decrease in non-relapse mortality associated with CNS irradiation. The non relapse mortality rate in the CNS+ITC was 48%, which may contribute to the observed RFS and OS differences between the CNS+RT and CNS+ITC groups. CNS+RT patients had equivalent non relapse mortality rates compared to the CNS− patients. Given the retrospective nature of this analysis, it is possible that cranial irradiation was selectively administered to patients who were more likely to survive HCT, and selectively withheld from patients perceived by physicians to be at higher risk. Although more patients in the CNS+RT group were transplanted in 2nd/3rd remission and relapse, their performance status could have been superior than those in the CNS+ITC group, thus leading to a difference in the non-relapse mortality observed.
Our study showed a trend for decreased systemic relapse in the CNS+RT patients compared to the CNS+ITC patients. The radiation boost in this study may have led to improved RFS and OS benefits via a decrease in CNS relapse. Furthermore, patients with CNS+ITC could harbor occult CNS disease leading to systemic and bone marrow relapses. It has been established in the literature that patients with CNS+ disease have an increased risk of CNS relapse post transplantation16. This trend of decreased relapse in patients who are treated with CNS targeted therapy parallels relapse patterns in patients with CNS positive ALL treated with post transplant ITC 15. In an earlier report by our institution, patients with CNS positive ALL in the HCT setting were found to have higher rates of post transplant CNS failure than those who did not have involvement of the CNS15. Patients who had a history of, or active, CNS disease had a CNS relapse of 52% versus 17% if given post transplant ITC15. In our study, patients were not routinely accessed by lumbar puncture for CNS relapse in the post transplant setting, and we are unable to definitively comment on the impact of a radiation boost on CNS relapse.
Our study prompts unresolved questions. Due to the small number of patients receiving craniospinal radiation, we are unable to clearly define which patients would benefit from craniospinal versus cranial radiation. In addition, patients who were CNS+ or CNS− did not routinely undergo post transplantation lumbar puncture, so we are unable to report CNS specific death in our study. Also, we were not able to examine the potential differences of dose rate effects, particularly the low dose rate TBI versus the high dose rate cranial radiation boost, on CNS disease.
Neurocognitive outcomes have not been reported in adult CNS+ AML. However, our group previously reported on CNS toxicity, specifically, leukoencephalopathy, associated with CNS directed therapy prior to HCT in AML (15). In this paper, we reported that 7 of the 415 patients who received allogeneic transplantation for acute leukemia experienced leukecoencephalopathy. There was no statistically significant relationship between a history of CNS disease prior to transplantation and the development of leukoencephalopathy. All seven cases were among the 201 patients treated with CNS directed therapy prior to HCT, with either ITC or RT. Five of the seven cases were among the 57 patients that received 6 or more doses of IT-methotrexate. None of the patients that received that received post transplantation ITC without prior CNS therapy developed leukcoencephalopathy. Therefore, CNS directed therapy should be administered with caution due to the increased risk of CNS morbidity post transplantation. In our current report, we are unable to comment on the neurocognitive outcome or CNS morbidity of the CNS+ patients, given the lack of detailed neurocogitive follow up in our population.
In conclusion, CNS+ AML is associated with a poor prognosis. This result augments our recently published investigation on disease status based risk stratification on the overall outcome of AML after HCT17. The role of a CNS irradiation boost to standard ITC in HCT conditioning for CNS+ AML patients may potentially improve outcomes, but remains largely uncertain. Given the paucity of data in the literature regarding this subject, we recommend that if patients are to be treated with a radiation boost, it should precede HCT to allow for eradication of CNS disease prior to HCT. Whether there is a benefit to CSI over cranial RT in these patients remains unknown. Further, the cranial boost is given at a dose that is related to whether TBI is administered. With the addition of TBI, the majority of CNS+RT patients in this study were treated with a cranial boost to a total dose of 22-24 Gy. We are unable to further define the optimal cranial boost dose. Due to the added morbidity of CSI without data to support its inclusion in CNS+ AML, we recommend cranial irradiation instead of CSI.
Our study observed an improvement in RFS and OS with the addition of an irradiation boost, and a trend toward decreased relapse rates in the CNS positive patients who received a radiation boost. This investigation draws attention to CNS positive AML disease, and the role of a multimodality approach to CNS directed therapy. Furthermore, our study highlights the need for future prospective trials to further define optimal treatment strategies.
Figure 3.
Kaplan-Meier curves for estimated relapse of AML patients with CNS positive disease treated with ITC alone (red), versus CNS positive disease treated with ITC and an irradiation boost (green), versus CNS negative patients (black).
Acknowledgement
We sincerely thank the research and clinical transplantation team of the Fred Hutchinson Cancer Research Center and Seattle Cancer Care Alliance for their dedication and hard work.
Grant Support: CA18029, CA 15704, and HL 36444 from the National Institute of Health, Bethesda, MD. Presented as an oral presentation at ASTRO 2006, Philadelphia, PA.
Footnotes
Financial disclosure: The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest Notification: Actual or potential conflicts of interest do not exist.
References
- 1.Bassan R, Barbui T. Remission induction therapy for adults with acute myelogenous leukemia: towards the ICE age? Haematologica. 1995;80:82–90. [PubMed] [Google Scholar]
- 2.Holmes R, Keating MJ, Cork A, et al. A unique pattern of central nervous system leukemia in acute myelomonocytic leukemia associated with inv (16)(p13q22) Blood. 1985;65:1071–8. [PubMed] [Google Scholar]
- 3.Rees JK, Gray RG, Swirsky D, et al. Principal results of the medical research council’s 8th acute myeloid leukemia trial. Lancet. 1986;29:1236–41. doi: 10.1016/s0140-6736(86)92674-7. [DOI] [PubMed] [Google Scholar]
- 4.Castagnola C, Nozza A, Corso A, Bernasconi C. The value of combination therapy in adult myeloid leukemia with central nervous system involvement. Haematologica. 1997;82:577–580. [PubMed] [Google Scholar]
- 5.Cuttner J, Conjalka MS, Reilly M, et al. Association of monocytic leukemia in patients with extreme leucocytosis. Am J Med. 1980;69:55. doi: 10.1016/0002-9343(80)90467-2. [DOI] [PubMed] [Google Scholar]
- 6.Meyer RJ, Ferreria PP, Cuttner J, et al. Cytocentrifuge study of central nervous system leukemia at presentation in acute granulocytic leukemia: Evidence for a higher incidence in acute myelogenous leukemia. Proc Am Soc Clin Oncol. 1978;19:350. [Google Scholar]
- 7.Stewart DJ, Keating MJ, McCredie KB, et al. Natural history of central nervous system leukemia in adults. Cancer. 1982;47:184. doi: 10.1002/1097-0142(19810101)47:1<184::aid-cncr2820470130>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 8.Morrison FS, Kopecky KJ, Head DR, et al. Late intensification with POMP chemotherapy prolongs survival in acute myelogenous leukemia--results of a Southwest Oncology Group study of rubidazone versus adriamycin for remission induction, prophylactic intrathecal therapy, late intensification, and levamisole maintenance. Leukemia. 1992;6:708–14. [PubMed] [Google Scholar]
- 9.O’Donnell MR, Appelbaum FR, Baer MR, et al. NCCN guidelines for treating AML. 2009. http://www.nccn.org/professionals/physician_gls/PDF/aml.pdf. [Google Scholar]
- 10.Simone JV. Treatment of meningeal leukemia. J Clin Oncol. 1984;2:2357–8. doi: 10.1200/JCO.1984.2.5.357. [DOI] [PubMed] [Google Scholar]
- 11.Kun LE. Tumors of the Posterior Fossa and the Spinal Canal. In: Halperin EC, Constine LS, Tarbell NJ, et al., editors. Pediatric Radiation Oncology. ed 4th Lippincott Williams & Wilkins; Philadelphia: 2005. pp. 98–99. [Google Scholar]
- 12.Sanders KE, Ha CS, Cortes-Franco JE, et al. The role of craniospinal irradiation in adults with a central nervous system recurrence of leukemia. Cancer. 2004;100:2176–2190. doi: 10.1002/cncr.20280. [DOI] [PubMed] [Google Scholar]
- 13.Abott BL, Rubnitz JE, Tong X, et al. Clinical significance of central nervous system involvement at diagnosis of pediatric acute myeloid leukemia: a single institution’s experience. Leukemia. 2003;17:2090–2096. doi: 10.1038/sj.leu.2403131. [DOI] [PubMed] [Google Scholar]
- 14.Thomas EK, Forman SJ, Appelbaum FR, et al. In: Thomas’ Hematopoietic Cell Transplantation. ed 2nd Thomas E, editor. Blackwell Publishing; Malden, MA: 1999. [Google Scholar]
- 15.Thompson CB, Sanders JE, Flournoy N, et al. The risks of central nervous system relapse and leukoencephalopathy in patients receiving marrow transplants for acute leukemia. Blood. 1986;67:195–199. [PubMed] [Google Scholar]
- 16.Oshima K, Kanda Y, Yamashita T, et al. Central nervous system relapse of leukemia after allogeneic hematopoeitic stem cell transplantation. Biology of Blood and Marrow Transplantation. 2008;14:1100–1107. doi: 10.1016/j.bbmt.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 17.Sorror ML, Sandmaier BM, Storer BE, et al. Comorbidity and disease status based risk stratification of outcomes among patients with acute myeloid leukemia or myelodysplasia receiving allogeneic hematopoeitic stem cell transplantation. J of Clin Oncol. 2007;25:4246–4254. doi: 10.1200/JCO.2006.09.7865. [DOI] [PubMed] [Google Scholar]