Abstract
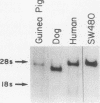
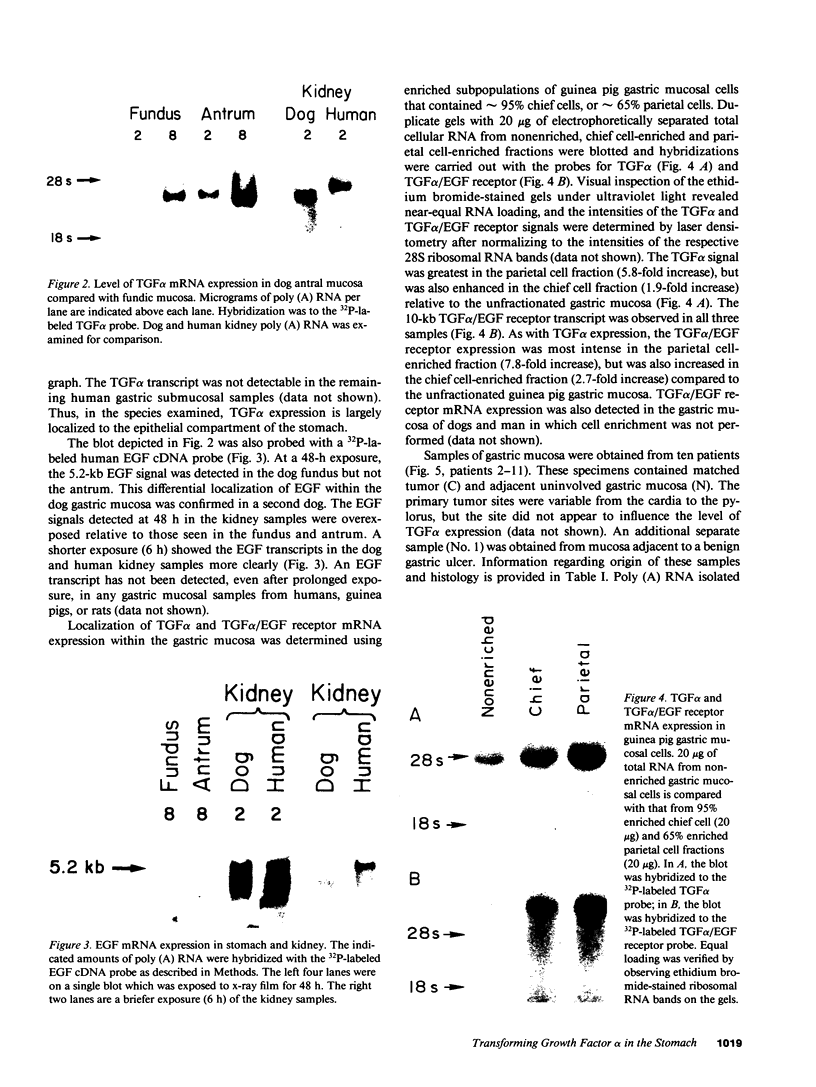
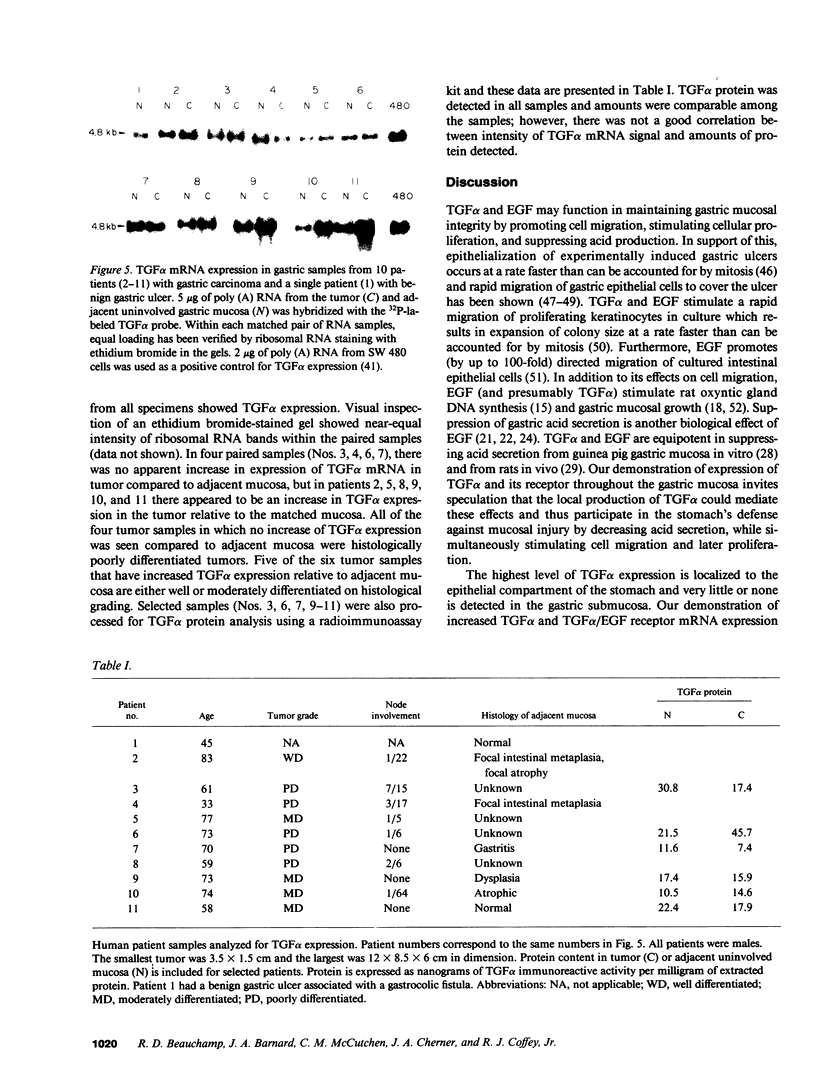
Transforming growth factor alpha (TGF alpha) shares with epidermal growth factor (EGF) structural homology (35%), a common cell-surface membrane receptor (TGF alpha/EGF receptor), and a nearly identical spectrum of biological activity, including inhibition of gastric acid secretion. Herein, we report expression of TGF alpha mRNA in normal gastric mucosa of the adult guinea pig, rat, and dog. TGF alpha mRNA was also detected in matched surgically resected gastric mucosa and adjacent gastric carcinoma from 10 patients, and in gastric mucosa adjacent to a benign ulcer from an additional patient. TGF alpha protein was quantitated by radioimmunoassay and was present in tumor and adjacent mucosa. TGF alpha/EGF receptor mRNA was also detected in gastric mucosa from all species studied. Localization of TGF alpha and TGF alpha/EGF receptor mRNA expression was examined in samples of unfractionated guinea pig gastric mucosa and from chief cell-enriched and parietal cell-enriched fractions. All samples exhibited TGF alpha and TGF alpha/EGF receptor expression. The TGF alpha signal was greatest in the parietal cell fraction (5.8-fold increase), but was also enhanced in the chief cell fraction (1.9-fold increase) relative to the unfractionated gastric mucosa. Like TGF alpha expression, TGF alpha/EGF receptor mRNA expression was most intense in the parietal cell-enriched fraction (7.8-fold increase), but was also increased in the chief cell-enriched fraction (2.7-fold increase) relative to the unfractionated guinea pig gastric mucosa. We conclude that TGF alpha and TGF alpha/EGF receptor genes are expressed in normal adult mammalian gastric mucosa. These findings, when interpreted in light of described actions of TGF alpha and EGF, provide evidence that local production of TGF alpha could play an important role in the regulation of acid secretion and mucosal renewal in the stomach.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrandon Y., Green H. Cell migration is essential for sustained growth of keratinocyte colonies: the roles of transforming growth factor-alpha and epidermal growth factor. Cell. 1987 Sep 25;50(7):1131–1137. doi: 10.1016/0092-8674(87)90179-6. [DOI] [PubMed] [Google Scholar]
- Bell G. I., Fong N. M., Stempien M. M., Wormsted M. A., Caput D., Ku L. L., Urdea M. S., Rall L. B., Sanchez-Pescador R. Human epidermal growth factor precursor: cDNA sequence, expression in vitro and gene organization. Nucleic Acids Res. 1986 Nov 11;14(21):8427–8446. doi: 10.1093/nar/14.21.8427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett C., Paterson I. M., Corbishley C. M., Luqmani Y. A. Expression of growth factor and epidermal growth factor receptor encoded transcripts in human gastric tissues. Cancer Res. 1989 Apr 15;49(8):2104–2111. [PubMed] [Google Scholar]
- Blay J., Brown K. D. Epidermal growth factor promotes the chemotactic migration of cultured rat intestinal epithelial cells. J Cell Physiol. 1985 Jul;124(1):107–112. doi: 10.1002/jcp.1041240117. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bringman T. S., Lindquist P. B., Derynck R. Different transforming growth factor-alpha species are derived from a glycosylated and palmitoylated transmembrane precursor. Cell. 1987 Feb 13;48(3):429–440. doi: 10.1016/0092-8674(87)90194-2. [DOI] [PubMed] [Google Scholar]
- Carpenter G., Stoscheck C. M., Preston Y. A., DeLarco J. E. Antibodies to the epidermal growth factor receptor block the biological activities of sarcoma growth factor. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5627–5630. doi: 10.1073/pnas.80.18.5627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey R. J., Jr, Derynck R., Wilcox J. N., Bringman T. S., Goustin A. S., Moses H. L., Pittelkow M. R. Production and auto-induction of transforming growth factor-alpha in human keratinocytes. 1987 Aug 27-Sep 2Nature. 328(6133):817–820. doi: 10.1038/328817a0. [DOI] [PubMed] [Google Scholar]
- Coffey R. J., Jr, Goustin A. S., Soderquist A. M., Shipley G. D., Wolfshohl J., Carpenter G., Moses H. L. Transforming growth factor alpha and beta expression in human colon cancer lines: implications for an autocrine model. Cancer Res. 1987 Sep 1;47(17):4590–4594. [PubMed] [Google Scholar]
- Coffey R. J., Jr, Sipes N. J., Bascom C. C., Graves-Deal R., Pennington C. Y., Weissman B. E., Moses H. L. Growth modulation of mouse keratinocytes by transforming growth factors. Cancer Res. 1988 Mar 15;48(6):1596–1602. [PubMed] [Google Scholar]
- Cohen S., Carpenter G. Human epidermal growth factor: isolation and chemical and biological properties. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1317–1321. doi: 10.1073/pnas.72.4.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchlow J., Magee D., Ito S., Takeuchi K., Silen W. Requirements for restitution of the surface epithelium of frog stomach after mucosal injury. Gastroenterology. 1985 Jan;88(1 Pt 2):237–249. doi: 10.1016/s0016-5085(85)80177-3. [DOI] [PubMed] [Google Scholar]
- Danielson P. E., Forss-Petter S., Brow M. A., Calavetta L., Douglass J., Milner R. J., Sutcliffe J. G. p1B15: a cDNA clone of the rat mRNA encoding cyclophilin. DNA. 1988 May;7(4):261–267. doi: 10.1089/dna.1988.7.261. [DOI] [PubMed] [Google Scholar]
- Dembiński A., Gregory H., Konturek S. J., Polański M. Trophic action of epidermal growth factor on the pancreas and gastroduodenal mucosa in rats. J Physiol. 1982 Apr;325:35–42. doi: 10.1113/jphysiol.1982.sp014133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derynck R., Roberts A. B., Winkler M. E., Chen E. Y., Goeddel D. V. Human transforming growth factor-alpha: precursor structure and expression in E. coli. Cell. 1984 Aug;38(1):287–297. doi: 10.1016/0092-8674(84)90550-6. [DOI] [PubMed] [Google Scholar]
- Derynck R. Transforming growth factor alpha. Cell. 1988 Aug 26;54(5):593–595. doi: 10.1016/s0092-8674(88)80001-1. [DOI] [PubMed] [Google Scholar]
- Derynck R. Transforming growth factor-alpha: structure and biological activities. J Cell Biochem. 1986;32(4):293–304. doi: 10.1002/jcb.240320406. [DOI] [PubMed] [Google Scholar]
- Dobner P. R., Kawasaki E. S., Yu L. Y., Bancroft F. C. Thyroid or glucocorticoid hormone induces pre-growth-hormone mRNA and its probable nuclear precursor in rat pituitary cells. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2230–2234. doi: 10.1073/pnas.78.4.2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finke U., Rutten M., Murphy R. A., Silen W. Effects of epidermal growth factor on acid secretion from guinea pig gastric mucosa: in vitro analysis. Gastroenterology. 1985 May;88(5 Pt 1):1175–1182. doi: 10.1016/s0016-5085(85)80077-9. [DOI] [PubMed] [Google Scholar]
- Forgue-Lafitte M. E., Kobari L., Gespach C., Chamblier M. C., Rosselin G. Characterization and repartition of epidermal growth factor-urogastrone receptors in gastric glands isolated from young and adult guinea pigs. Biochim Biophys Acta. 1984 Apr 10;798(2):192–198. doi: 10.1016/0304-4165(84)90303-9. [DOI] [PubMed] [Google Scholar]
- Frazier M. L., Mars W., Florine D. L., Montagna R. A., Saunders G. F. Efficient extraction of RNA from mammalian tissue. Mol Cell Biochem. 1983;56(2):113–122. doi: 10.1007/BF00227211. [DOI] [PubMed] [Google Scholar]
- González A., Garrido J., Vial J. D. Epidermal growth factor inhibits cytoskeleton-related changes in the surface of parietal cells. J Cell Biol. 1981 Jan;88(1):108–114. doi: 10.1083/jcb.88.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray J. S., Wieczorowski E., Ivy A. C. INHIBITION OF GASTRIC SECRETION BY EXTRACTS OF NORMAL MALE URINE. Science. 1939 May 26;89(2317):489–490. doi: 10.1126/science.89.2317.489. [DOI] [PubMed] [Google Scholar]
- Gregory H. Isolation and structure of urogastrone and its relationship to epidermal growth factor. Nature. 1975 Sep 25;257(5524):325–327. doi: 10.1038/257325a0. [DOI] [PubMed] [Google Scholar]
- Gregory H., Thomas C. E., Young J. A., Willshire I. R., Garner A. The contribution of the C-terminal undecapeptide sequence of urogastrone-epidermal growth factor to its biological action. Regul Pept. 1988 Aug;22(3):217–226. doi: 10.1016/0167-0115(88)90034-1. [DOI] [PubMed] [Google Scholar]
- Hingson D. J., Ito S. Effect of aspirin and related compounds on the fine structure of mouse gastric mucosa. Gastroenterology. 1971 Aug;61(2):156–177. [PubMed] [Google Scholar]
- Kasselberg A. G., Orth D. N., Gray M. E., Stahlman M. T. Immunocytochemical localization of human epidermal growth factor/urogastrone in several human tissues. J Histochem Cytochem. 1985 Apr;33(4):315–322. doi: 10.1177/33.4.3884705. [DOI] [PubMed] [Google Scholar]
- Konturek S. J., Brzozowski T., Piastucki I., Dembinski A., Radecki T., Dembinska-Kiec A., Zmuda A., Gregory H. Role of mucosal prostaglandins and DNA synthesis in gastric cytoprotection by luminal epidermal growth factor. Gut. 1981 Nov;22(11):927–932. doi: 10.1136/gut.22.11.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konturek S. J., Cieszkowski M., Jaworek J., Konturek J., Brzozowski T., Gregory H. Effects of epidermal growth factor on gastrointestinal secretions. Am J Physiol. 1984 May;246(5 Pt 1):G580–G586. doi: 10.1152/ajpgi.1984.246.5.G580. [DOI] [PubMed] [Google Scholar]
- Konturek S. J., Dembinski A., Warzecha Z., Brzozowski T., Gregory H. Role of epidermal growth factor in healing of chronic gastroduodenal ulcers in rats. Gastroenterology. 1988 Jun;94(6):1300–1307. doi: 10.1016/0016-5085(88)90667-1. [DOI] [PubMed] [Google Scholar]
- Konturek S. J., Radecki T., Brzozowski T., Piastucki I., Dembiński A., Dembińska-Kieć A., Zmuda A., Gryglewski R., Gregory H. Gastric cytoprotection by epidermal growth factor. Role of endogenous prostaglandins and DNA synthesis. Gastroenterology. 1981 Sep;81(3):438–443. [PubMed] [Google Scholar]
- MacDonald R. J., Swift G. H., Przybyla A. E., Chirgwin J. M. Isolation of RNA using guanidinium salts. Methods Enzymol. 1987;152:219–227. doi: 10.1016/0076-6879(87)52023-7. [DOI] [PubMed] [Google Scholar]
- Malden L. T., Novak U., Burgess A. W. Expression of transforming growth factor alpha messenger RNA in the normal and neoplastic gastro-intestinal tract. Int J Cancer. 1989 Mar 15;43(3):380–384. doi: 10.1002/ijc.2910430305. [DOI] [PubMed] [Google Scholar]
- Marquardt H., Hunkapiller M. W., Hood L. E., Todaro G. J. Rat transforming growth factor type 1: structure and relation to epidermal growth factor. Science. 1984 Mar 9;223(4640):1079–1082. doi: 10.1126/science.6320373. [DOI] [PubMed] [Google Scholar]
- Massagué J. Epidermal growth factor-like transforming growth factor. II. Interaction with epidermal growth factor receptors in human placenta membranes and A431 cells. J Biol Chem. 1983 Nov 25;258(22):13614–13620. [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes J. A., Tam J. P., Finke U., Saunders M., Bernanke J., Silen W., Murphy R. A. Transforming growth factor alpha inhibits secretion of gastric acid. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3844–3846. doi: 10.1073/pnas.83.11.3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A. B., Lamb L. C., Newton D. L., Sporn M. B., De Larco J. E., Todaro G. J. Transforming growth factors: isolation of polypeptides from virally and chemically transformed cells by acid/ethanol extraction. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3494–3498. doi: 10.1073/pnas.77.6.3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samsoondar J., Kobrin M. S., Kudlow J. E. Alpha-transforming growth factor secreted by untransformed bovine anterior pituitary cells in culture. I. Purification from conditioned medium. J Biol Chem. 1986 Nov 5;261(31):14408–14413. [PubMed] [Google Scholar]
- Schreiber A. B., Winkler M. E., Derynck R. Transforming growth factor-alpha: a more potent angiogenic mediator than epidermal growth factor. Science. 1986 Jun 6;232(4755):1250–1253. doi: 10.1126/science.2422759. [DOI] [PubMed] [Google Scholar]
- Simmen F. A., Gope M. L., Schulz T. Z., Wright D. A., Carpenter G., O'Malley B. W. Isolation of an evolutionarily conserved epidermal growth factor receptor cDNA from human A431 carcinoma cells. Biochem Biophys Res Commun. 1984 Oct 15;124(1):125–132. doi: 10.1016/0006-291x(84)90926-4. [DOI] [PubMed] [Google Scholar]
- Skinner K. A., Soper B. D., Tepperman B. L. Effect of sialoadenectomy and salivary gland extracts on gastrointestinal mucosal growth and gastrin levels in the rat. J Physiol. 1984 Jun;351:1–12. doi: 10.1113/jphysiol.1984.sp015227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner M. K., Coffey R. J., Jr Regulation of ovarian cell growth through the local production of transforming growth factor-alpha by theca cells. Endocrinology. 1988 Dec;123(6):2632–2638. doi: 10.1210/endo-123-6-2632. [DOI] [PubMed] [Google Scholar]
- Skinner M. K., Takacs K., Coffey R. J. Transforming growth factor-alpha gene expression and action in the seminiferous tubule: peritubular cell-Sertoli cell interactions. Endocrinology. 1989 Feb;124(2):845–854. doi: 10.1210/endo-124-2-845. [DOI] [PubMed] [Google Scholar]
- Starkey R. H., Cohen S., Orth D. N. Epidermal growth factor: identification of a new hormone in human urine. Science. 1975 Sep 5;189(4205):800–802. doi: 10.1126/science.1172293. [DOI] [PubMed] [Google Scholar]
- Svanes K., Ito S., Takeuchi K., Silen W. Restitution of the surface epithelium of the in vitro frog gastric mucosa after damage with hyperosmolar sodium chloride. Morphologic and physiologic characteristics. Gastroenterology. 1982 Jun;82(6):1409–1426. [PubMed] [Google Scholar]
- Taylor J. M., Illmensee R., Summers J. Efficeint transcription of RNA into DNA by avian sarcoma virus polymerase. Biochim Biophys Acta. 1976 Sep 6;442(3):324–330. doi: 10.1016/0005-2787(76)90307-5. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todaro G. J., Fryling C., De Larco J. E. Transforming growth factors produced by certain human tumor cells: polypeptides that interact with epidermal growth factor receptors. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5258–5262. doi: 10.1073/pnas.77.9.5258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twardzik D. R., Ranchalis J. E., Todaro G. J. Mouse embryonic transforming growth factors related to those isolated from tumor cells. Cancer Res. 1982 Feb;42(2):590–593. [PubMed] [Google Scholar]
- Yeomans N. D., Saint John D. J., de Boer W. G. Regeneration of gastric mucosa after aspirin-induced injury in the rat. Am J Dig Dis. 1973 Sep;18(9):773–780. doi: 10.1007/BF01070847. [DOI] [PubMed] [Google Scholar]