Abstract
The Pneumonia Etiology Research for Child Health (PERCH) project is a 7-country, standardized, comprehensive evaluation of the etiologic agents causing severe pneumonia in children from developing countries. During previous etiology studies, between one-quarter and one-third of patients failed to yield an obvious etiology; PERCH will employ and evaluate previously unavailable innovative, more sensitive diagnostic techniques. Innovative and rigorous epidemiologic and analytic methods will be used to establish the causal association between presence of potential pathogens and pneumonia. By strategic selection of study sites that are broadly representative of regions with the greatest burden of childhood pneumonia, PERCH aims to provide data that reflect the epidemiologic situation in developing countries in 2015, using pneumococcal and Haemophilus influenzae type b vaccines. PERCH will also address differences in host, environmental, and/or geographic factors that might determine pneumonia etiology and, by preserving specimens, will generate a resource for future research and pathogen discovery.
The Pneumonia Etiology Research for Child Health (PERCH) project is a multi-country, standardized, and comprehensive evaluation of the etiologic agents causing severe and very severe pneumonia among children in developing countries. PERCH will be the largest and most comprehensive study of childhood pneumonia etiology thus far conducted. By rigorously updating and expanding our understanding of pneumonia pathogens, we expect that PERCH will directly lead to improved prevention strategies and therapies for one of the leading causes of child death and a major cause of serious illness and hospitalizations in children around the globe.
Our current understanding of pneumonia etiology was largely developed from research studies in the 1970s through the early 1990s. These findings showed that the majority of fatal pneumonia cases in children is due to 2 bacteria—Streptococcus pneumoniae and Haemophilus influenzae type b (Hib)—and that these deaths occurred primarily in communities with high levels of child mortality, typically impoverished settings that lack access to basic healthcare such as antibiotics and oxygen therapy [1, 2].
Substantial changes in disease burden, prevalence of comorbid conditions, or other factors that may affect the etiology of pneumonia have occurred since the last systematic set of etiology studies in children were undertaken. For example, most of the evidence on which current empiric treatment algorithms for pneumonia in developing countries are based was collected before the human immunodeficiency virus (HIV) pandemic and in the absence of antiretroviral treatment of HIV-infected children, before recent substantial successes in malaria control, and before the widespread deployment of Hib and pneumococcal conjugate vaccines. Furthermore, lower rates of very severe malnutrition and increased urbanization and crowding may also impact the etiology of pneumonia and make pneumonia etiology data obtained in the 1980s less relevant to policy today. Simply put, continued use of clinical algorithms based on the assumption that predominant causes of severe pneumonia are almost always H. influenzae and S. pneumoniae may lead to treatments that are not appropriate for a child’s actual infection and will miss the opportunity to guide vaccine development against important new or unrecognized pathogens.
STUDY DESIGN, TESTING, AND ANALYSIS
Although inferences regarding pneumonia etiology can be drawn from many different study designs, including cohort studies, case series, and treatment or intervention trials, the PERCH study has been designed as a case-control study that aims to enroll ∼6000 patients hospitalized for severe or very severe pneumonia and ∼6000 controls selected randomly from the community. The main reasons for this design are as follows: (1) the use of hospitalized patients, as opposed to those observed outside of facilities, is necessary to assure that we can safely collect a range of specimens from patients and that we gain the efficiency of testing only those with severe and very severe pneumonia; and (2) the inclusion of controls is important to guide the interpretation of results from the use of highly sensitive detection tests on upper respiratory tract specimens and to facilitate the identification of risk factors for pneumonia and/or specific etiologies. Although PERCH recognizes that pneumonia is an important illness in neonates and that important questions remain regarding the etiologies of serious pneumonia in this age group, it was considered sufficiently important and unique to require its own study, and thus PERCH focuses on children aged 1–59 months old.
PERCH will employ and evaluate innovative diagnostic techniques and analytic methods that were not available during previous etiology studies. The understanding that H. influenzae and S. pneumoniae are the main causes of fatal pneumonia in children is supported primarily by 2 types of evidence: first, laboratory diagnoses from a limited number of lung aspiration studies of severe pneumonia cases seen at hospital facilities, and second, trials of antibiotic therapy and Hib and pneumococcal vaccines. In the latter instance, the proportionate reduction in cases of pneumonia or fatal pneumonia is used as a proxy for the proportion of cases or deaths due to the targeted bacterium, or specifically, Hib and/or pneumococcus. In recent clinical trials, Hib and pneumococcal conjugate vaccines have prevented about one-fifth to one-third of x-ray confirmed pneumonia with alveolar consolidation [3–10].
Although current evidence suggests that fewer fatal pneumonia cases are due solely to viral infections, laboratory investigations of children in developing countries and elsewhere often show that respiratory syncytial virus, influenza virus, and parainfluenza virus are identified from a large proportion of hospitalized patients with lower respiratory tract illness [11]. In addition, there is increasing recognition of the importance of mixed infections and the interactions between viral and bacterial infections, for example, influenza virus infections that predispose to bacterial pneumonia [12]. Nevertheless, even in comprehensive studies of pneumonia etiology, between one-quarter and one-third of patients fail to yield an obvious etiology, suggesting that better, more sensitive laboratory methods are needed [1]. However, more sensitive tests may identify the presence of commensal pathogens that may not be the etiologic cause of the pneumonia episode being studied. Innovative or more rigorous epidemiologic methods are needed to establish the causal association between isolation of a potential pathogen and the pneumonia case being studied [11, 13].
Although a recent survey of the literature and the field indicates that >50 studies of pneumonia etiology in addition to PERCH are underway, the PERCH project aims to make a number of unique contributions [14]. The PERCH project includes 7 sites from diverse geographic areas and disease ecologies that are expected to represent the epidemiologic settings where most severe and fatal pneumonia will occur in the coming decades. It will also use standardized and innovative laboratory, clinical, specimen collection, and statistical techniques and comprehensive laboratory testing of body fluid and tissue specimens. By selecting sites that are geographically and epidemiologically diverse, PERCH aims to address differences in host, environmental, and/or geographic factors that might determine pneumonia etiology. By assuring rigorous adherence to standard laboratory, clinical, and epidemiologic protocols, PERCH will minimize the likelihood that any observed differences in etiology are artifacts that follow from differences in methods across sites. By employing the most recent laboratory and digital diagnostic methods (eg, digital auscultation recordings and digital radiographs of the chest) and preserving specimens for future research and pathogen discovery, the PERCH project will also generate a resource for pneumonia etiology research that will last beyond its immediate timelines.
Most pediatric pneumonia etiology studies attempt to ascribe a causative organism by collecting a limited number of diagnostic specimens, such as a blood and upper respiratory tract cultures. A few studies include a lower respiratory tract specimen obtained from bronchoalveolar lavage, lung aspirate, or occasionally, an induced sputum sample, and rarely, postmortem tissue specimens, and some more recent studies have included nucleic acid testing on specimens. PERCH will collect a comprehensive array of specimens, including nasopharyngeal, oropharyngeal, and induced sputum specimens from the respiratory tract, blood specimens, and urine specimens from all patients, and in some study sites, lung aspirates from patients with pneumonia and/or lung biopsies from deceased patients. Each of these specimens will be tested using a broad panel of diagnostic techniques that seek a large number of potential pathogens.
By collecting multiple specimens from the same patient and from community controls without pneumonia, PERCH will provide a comprehensive selection of pathogen data that can be used to validate (or refute) the results from other studies in which only 1 or 2 specimens are collected or in which only case patients are studied. For example, by comparing the findings from lung aspirates or pleural fluid, induced sputum, and the nasopharynx in the same patient, PERCH will be able to determine whether the concordance of results might allow future studies of pneumonia etiology to utilize induced sputum specimens as a complement to other more invasive, less frequently obtained specimens. Similarly, by comparing findings from multiplex polymerase chain reaction (PCR) in both patients with pneumonia and representative controls from the community without pneumonia, PERCH provides useful information on the strength of the association between a positive test result and pneumonia that would be absent in studies of only case patients.
PERCH is aiming both to study the association of positive tests with severe and very severe pneumonia and to determine the attributable fraction of pneumonia cases associated with an etiology or mixed infections. With multiple specimens and multiple tests on those specimens, the volume of data collected by PERCH therefore presents analytic challenges in interpreting detection of multiple pathogens and distinguishing infection from causation. For example, in cases a multiplex PCR test that can detect >30 unique pathogens will be applied on both nasopharyngeal and induced sputum specimens. Thus, PERCH is generating both large volumes of data and potentially correlated results that do not meet the standard assumption of ignorable correlation among regressors that generally underlie standard statistical methods used in the analysis of case-control studies (eg, logistic regression).
Thus, in PERCH we are aiming to develop an innovative statistical approach to model the attributable risk of pneumonia for each of these pathogens that can handle the complexity of the PERCH data set and address the correlation among pathogen infections in the presence of possible measurement errors. The proposed approach treats the multiple infectious agents as an unobserved or latent vector of variables, the values of which are informed by the pathogen measurements, pneumonia status, and covariate values. Specifically, the approach to statistical analysis being developed by PERCH aims to estimate (1) the prevalence of infection for each pathogen (or combination of pathogens) of interest among patients hospitalized with severe pneumonia and (2) the attributable risk for each pathogen as a putative cause of severe pneumonia in hospitalized patients. It can also estimate the proportion of cases with no pathogen identified, a group that will be a high priority for pathogen discovery efforts.
The research approaches used in PERCH were built on the experience of past pneumonia etiology studies such as the Board of Science and Technology in International Development (BOSTID) sponsored etiology projects of the 1980s, [15] the cumulative experience of study teams at Johns Hopkins Bloomberg School of Public Health (JHSPH) and the PERCH sites, and with expert input from the Pneumonia Methods Working Group (PMWG). The PMWG comprised 16 international experts from 10 different countries with backgrounds in clinical medicine, laboratory sciences, and epidemiology. The working group was charged with helping to resolve key methodologic issues in the design and conduct of pneumonia etiology research studies. The PERCH team provided the PMWG with 27 background documents and reviews on 20 topics and solicited feedback or recommendations on 90 strategic questions. The PMWG met twice in person and several times by teleconference during which they provided recommendations and, where needed, suggested additional data that needed to be collected in order to resolve any key uncertainties. The background documents to the PMWG and their recommendations are available on the PERCH Web site (http://www.jhsph.edu/ivac/perch.html).
In many ways, the BOSTID studies represent the last major, multisite effort to study pneumonia etiology around the world. This project included 12 sites around the world and collected an enormous amount of data, including information on both upper and lower respiratory tract infections [15]. However, despite annual meetings of investigators, there remained substantial heterogeneity across the sites in the laboratory and clinical methods used that complicates efforts to draw inferences by comparison across sites [16].
THE PERCH SITES
Representative sites and standardized study and analytic methods help us with extrapolating the results from PERCH sites to other areas. In PERCH, the study aims to include data from sites that are representative of the epidemiologic settings that we project will prevail in 2015 and onward. Specifically, PERCH assumes that from 2015 onward, with support from the Global Alliance for Vaccines and Immunization (GAVI) alliance, Hib and pneumococcal vaccines will likely be used in the routine childhood immunization programs of more than three-fourths of the world’s poorest countries. In addition, although malaria and HIV infection will continue to be important in pediatric populations in some geographic foci, the prevalence of these two co-infections will be low to moderate in most parts of the world as a consequence of both increasing access to malaria control interventions and expanded HIV prevention and treatment programs. Because these changes will affect pneumonia etiology, PERCH has selected sites with the intention that these sites will produce data by 2014 that will be representative for most areas from 2015 onward. All of the PERCH sites have begun official enrollment between August and December 2011. By 31 December 2013, therefore, PERCH aims to have completed 2 full years of enrollment in each of the 7 sites.
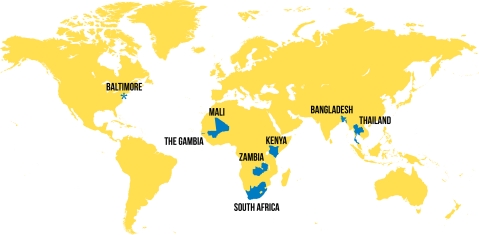
The 7 PERCH sites were selected by an open, competitive process with proposals reviewed by international experts and scored according to objective criteria. These sites represent areas where Hib and/or pneumococcal conjugate vaccines are routinely used (except Thailand), but they also represent a set of diverse epidemiologic settings that include the 2 geographic regions (Asia and Africa) (Figure 1) where the vast majority of the world’s severe and fatal pneumonia cases occur, a range of economic backgrounds (low-income, lower middle-income, and upper middle-income countries), urban and rural environments, variations in HIV infection and malaria prevalence, differences in altitude, and variations in the use of cooking and heating fuels (Table 1) Each of these factors potentially contributes to variations in the etiologic spectrum or burden of pneumonia in children.
Figure 1.
Map of study sites of the Pneumonia Etiology Research for Child Health project.
Table 1.
Pneumonia Etiology Research for Child Health Site Country Descriptions
| Country | 2009 Population, Thousandsa | 2009 GNI Per Capita, US$a | 2009 U5 Mortality Ratea | 2009 Infant Mortality Ratea | 2009 HIV Infection Prevalence Among Women 15–24 Years Olda | Malaria Incidence Rateb | Hib Vaccine Introduction Datec | PCV Introduction Date |
| Bangladesh | 162 221 | 590 | 52 | 41 | <0.1 | … | July 2009 | January 2013d |
| Gambia | 1705 | 440 | 103 | 78 | 2.4 | … | January 1997 | August 2009 |
| Kenya | 39 802 | 770 | 84 | 55 | 4.1 | 0.19 | January 2001 | February 2011 |
| Mali | 13 010 | 680 | 191 | 101 | 0.5 | 0.37 | January 2008 | March 2011 |
| South Africa | 50 110 | 5770 | 62 | 43 | 13.6 | 0.00 | January 1999 | November 2009 |
| Thailand | 67 764 | 3760 | 14 | 12 | … | … | Not routine | Not routine |
| Zambia | 12 935 | 970 | 141 | 86 | 8.9 | 0.33 | February 2004 | July 2012d |
Abbreviations: GNI, gross national income; Hib, Haemophilus influenzae type b; HIV, human immunodeficiency virus; PCV, pneumococcal conjugate vaccine; U5, under 5.
Source: United Nations Children’s Fund, State of the World’s Children 2011 (http://www.unicef.org/sowc2011).
Source: Roll Back Malaria Partnership (http://www.rollbackmalaria.org/partnership/wg/wg_monitoring/docs/annexes_ARFek4.pdf; accessed 30 November 2011).
Source: Vaccine Information Management System (http://www.jhsph.edu/ivac/vims.html; accessed 30 November 2011).
Forecasted.
Bangladesh Site
This Bangaldesh PERCH study represents a joint collaboration between the International Centre for Diarrhoeal Disease Research, Bangladesh (ICDDR,B) as an international research facility and the Government of Bangladesh, represented by the Institute of Epidemiology, Disease Control and Research. Two sites are involved, one urban and the other a rural center, each backed by well-defined catchment areas with ongoing population-based demographic and morbidity surveillance.
The sites where the PERCH study will recruit patients and controls includes 2 facilities: the Dhaka and Matlab hospitals of ICDDR,B (in the Dhaka and Chittagong divisions of Bangladesh, respectively); the former serves an urban population and the latter a rural population. The Dhaka hospital is a 350-bed facility supported by the Kamalapur urban field site, which has both active demographic and morbidity surveillance among a population of 350 000, of whom 11.5% are children <5 years old [17, 18]. Kamalapur has been involved in pneumonia studies since 1998 and has conducted pneumonia etiology surveillance since 2004 [19]. The Matlab hospital is a 140-bed facility and is supported by long-standing demographic and morbidity surveillance among a population of 114 000. Both hospitals have dedicated pneumonia wards. These sites represent both urban and rural communities typical of much of south Asia.
The Gambia Site
In the Gambia, the PERCH study will be conducted in the Basse field site of the UK Medical Research Council (MRC) Gambia, which has a long history of local and multicenter research of infectious diseases. The childhood pneumonia research program is well established, notably including large-scale conjugate Hib and pneumococcal vaccine field trials and a range of other clinical pneumonia studies. The Gambia was the first country in Africa to introduce conjugate Hib vaccine into its routine immunization schedule in 1998 and introduced conjugate pneumococcal vaccine in 2009. Ongoing efforts are underway to conduct comprehensive pneumococcal surveillance and assess the impact of the vaccine on pneumococcal disease and pneumonia rates. The MRC’s Basse site, which is a rural site in the eastern end of the country, has a well-established demographic surveillance system and a partnership with the Gambia government health services. This site will serve as the site for the PERCH project, thereby providing representation of a rural, low-HIV-seroprevalence, African setting with seasonal malaria transmission.
Kenya Site
Kenya was one of the first African countries to incorporate Hib and pneumococcal conjugate vaccines into its routine immunization program [20]. The PERCH site is the Kenya Medical Research Institute-Wellcome Trust Research Programme in Kilifi, on the Indian Ocean coast. This research program began in 1989 as a collaboration between the Kenya Medical Research Institute, the Wellcome Trust of Great Britain, and Oxford University. The program’s laboratories are located immediately adjacent to Kilifi District Hospital, and the clinical scientists at the program run the hospital’s pediatric service. A population of 250 000 people living around the hospital are followed in a demographic surveillance system (DSS) with 4-monthly household visits. The Kilifi site is currently monitoring the comprehensive impact of pneumococcal conjugate vaccine use on pneumococcal disease and pneumonia rates in persons of all ages.
The site is well positioned to conduct PERCH because the activities of the clinical service, the laboratories, and the DSS are linked at each point of contact by real-time data entry into a comprehensive relational database. Kilifi District Hospital, which is the only inpatient facility in Kilifi district, admits ∼4500 children per year, of whom 60% come from within the DSS area [21]. Ten years ago malaria was the dominant cause of morbidity, but now, with effective malaria treatment and prevention in place, pneumonia and neonatal illnesses are the commonest causes of admission [22]. The site shares many of the features common to rural populations in East Africa.
Mali Site
In Mali, the proposed site for conducting the PERCH case-control study is Hospital Gabriel Touré (HGT), where nearly all pediatric hospital admissions in Bamako take place. Each year, HGT treats ∼25 000 children as outpatients and admits ∼5000 others (most <5 years old). Most children admitted to HGT are very ill. A survey in 2000 revealed that 71% of all admissions to HGT among children <16 years old were for infectious diseases and 21% of all admitted children died [23]. The study will be conducted by investigators at Centre pour le Développement des Vaccins, Mali, a center established as a collaboration between the Ministry of Health of Mali and the University of Maryland School of Medicine in the United States, which is responsible for research and training in tropical diseases. With a high infant mortality rate, and a substantial burden of malaria but low to moderate HIV seroprevalence, this site represents large portions of West Africa.
South Africa Site
The PERCH study site is in Soweto, an urban low-income community with a diversity of ethnic backgrounds. Although the majority of households have access to running water, 25% of families live in informal settlements and use fossil fuels for heating and cooking. The community is severely affected by HIV, with one-third of mothers and at least 5% of children being HIV infected. The mortality rate of children <5 years old in South Africa, including the study site, increased from 60 to 69 deaths per 1000 live births from 1990 through 2005 [24]. Fifty-seven percent of all deaths occur in HIV-infected children, of which at least one-third are due to pneumonia even in the era of antiretroviral therapy [25].
There are 23 primary health care (PHC) clinics in the Soweto region and a single public hospital, the Chris Hani Baragwanath Hospital (CHBH), which is the sole referral hospital for all these PHC clinics. All immunization in the community occurs at 1 of the 23 PHC clinics and vaccines are provided free of charge, with minimal numbers of children being immunized through the private sector, where fees are charged. None of the PHC clinics admit children with severe pneumonia. All severe pneumonia cases are referred to CHBH, the largest hospital in the Southern Hemisphere, with 450 pediatric beds with an average occupancy rate of 80%. The hospital provides free secondary and tertiary care to the population of Soweto. An estimated 90% of all hospitalizations from the community occur at CHBH. All health care for children <6 years old, including diagnostic tests and treatment, provided at the hospital and PHC clinics are free of charge. There is, however, a very low threshold for referring children to the hospital for evaluation and further management of pneumonia. Travel to the hospital is supported either through readily available public transport or by ambulance if clinically indicated. Hib vaccine was introduced in 1999 and pneumococcal conjugate vaccine in 2009. There is an active, ongoing pneumococcal conjugate vaccine impact evaluation that will evaluate vaccine effectiveness for prevention of pneumonia and invasive pneumococcal disease. This site represents an urban, malaria-free setting with high HIV infection prevalence.
Thailand Site
In Thailand, the PERCH study will be implemented by the International Emerging Infections Program, part of a formal collaboration between the Thailand Ministry of Public Health and the US Centers for Disease Control and Prevention. The study will be conducted in 2 rural provinces, Sa Kaeo province is in eastern Thailand on the Cambodian border and Nakhon Phanom province, which borders Laos in northeastern Thailand. Active, population-based pneumonia surveillance is ongoing in all 20 hospitals in both provinces. Pneumonia epidemiology in the 2 provinces is similar except for the higher incidence of melioidosis in Nakhon Phanom, which is typical of northeastern Thailand. The PERCH study will be conducted in the 2 largest hospitals in each province. Unlike at the other PERCH sites, Hib and pneumococcal conjugate vaccines are available on the private market but are not routinely used for all children in the national immunization program. This site represents a rural/periurban region typical of much of Southeast Asia including Thailand and its immediate neighbors, many of whom are not currently using Hib and pneumococcal conjugate vaccines for routine immunization of all children.
Zambia Site
The Zambia PERCH study site will be located at University Teaching Hospital (UTH), a 1500-bed academic center and tertiary care facility with 425 pediatric inpatient beds located in the capital city, Lusaka, and the home of Zambia’s only medical school. In 2008 there were a total of 14 923 inpatient pediatric admissions, 3467 (23%) of which were for acute respiratory illness and 1035 (30%) of which occurred in children <5 years old. The case fatality rate of severe pneumonia was 25.8%. UTH serves the greater Lusaka district, an area of 70 square kilometers and a population of 1.3 million. Hib vaccine is routinely used and, with support from the GAVI alliance, pneumococcal conjugate vaccine is expected to be routinely used in Zambia by 2013. With relatively high rates of HIV infection, ongoing malaria in some areas, and a poor urban environment, Lusaka is representative of many parts of Africa.
THE CONDUCT OF PERCH
With >30 case report forms, an anticipated enrollment of >12 000 patients and controls, and a large number of patient specimens to track, PERCH has contracted with the EMMES corporation (Rockville, MD) to develop an electronic data system to capture all the data from each site and to manage and help analyze the data. Routine data management and regular reports to track the accrual of patients and quality indicators of study performance are generated by EMMES and shared with the PERCH core team at JHSPH and with the site investigators. The PERCH team, including the site investigators and the core team at JHSPH, are developing an overall analysis plan for PERCH. Epidemiologists and biostatisticians at JHSPH are available as a resource to perform these analyses.
To assure that the PERCH study is conducted in compliance with recognized ethical standards for research, the PERCH protocol has been reviewed by 10 institutional review boards (IRBs). The IRB at JHSPH reviewed the overall clinical protocol. The protocol was then adapted at each local site and reviewed by the local IRB. After local IRB approvals were received, these were sent back to the IRB at JHSPH as amendments to the PERCH protocol. Only after these amendments were received and approved at JHSPH—that is, after both JHSPH and local approvals had been obtained—was any enrollment conducted at the sites. This process has helped to assure that all appropriate IRBs have had a chance to review the protocol. In addition, regular reporting of patient status to the JHSPH IRB occurs throughout the course of the project.
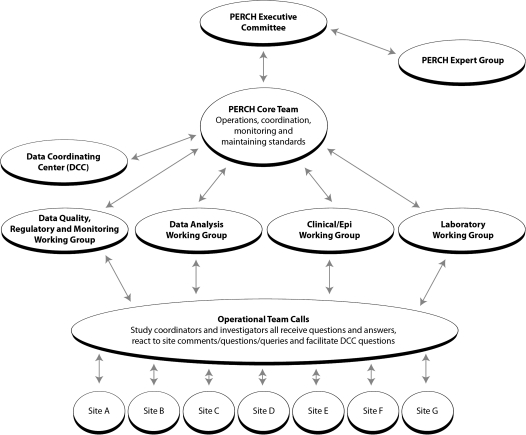
The PERCH project has defined a governance structure that allows for collective ownership of the study across sites while maintaining functional management systems. Table 2 and Figure 2 illustrates the roles and responsibilities of each group involved in study governance. The executive committee, comprising the principal investigator for each site plus 3 members of the PERCH core team, has responsibility for the overall governance of the study and for resolution of issues that cross sites. The PERCH expert group provides strategic advice and brings key expertise to the study. The 4 working groups meet regularly to address and/or resolve key issues with respect to the design of the study, and the operational team helps to monitor regular progress in the implementation of the protocol at each site and to identify and resolve issues that emerge.
Table 2.
Pneumonia Etiology Research for Child Health Governance Structures and Responsibilities
| PERCH Body | Responsibilities |
| Executive committee | Charged with overall governance of the study, including publication of findings, sharing of study results, reviewing and approving substudies, management of biorepository, resolving implementation issues, monitoring of site performance, and establishing and maintaining standards |
| Expert group | Primary responsibility is to provide sound, strategic advice during the conduct of the study; represents a “pre–peer review”; provides links with the external research community and provides a backward link with the Pneumonia Methods Working Group, which guided the preliminary phase of PERCH |
| Working groups (laboratory, clinical/epidemiology, data, and analysis) | Charged with operations and coordination; responsible for establishing, maintaining, and monitoring standardization across sites; will collaborate as needed, with questions from sites directed to the working group leaders; chair is responsible for organizing teleconferences and assuring that each meeting is productive and effective |
| Operational team | Responsible for regular monitoring of study progress at each site, clarification of operational issues that have been decided elsewhere, identification of issues that require attention of the working group, and assuring implementation of decisions of the working group at sites |
Figure 2.
Organizational diagram of the governance structures of the Pneumonia Etiology Research for Child Health project. Abbreviations: DCC, Data Coordinating Center; epi, epidemiologic; lab, laboratory; PI, principal investigator.
SUMMARY
We expect PERCH to provide important results to the scientific, public health, and policy communities that will influence treatment algorithms, diagnostic approaches, and vaccine development from 2015 onward. PERCH is designed to generate estimates of the distribution of severe and very severe pneumonia etiologies in children, of the incidence of severe and very severe pneumonia in different epidemiologic settings, and of the important environmental and behavioral risk factors for severe and very severe pneumonia. In addition, we anticipate that PERCH will influence pneumonia etiology research methods by revealing whether there is added value of induced sputum or lung biopsy specimens for detection of specific etiologies, and by enhancing our understanding of the sensitivity and specificity of new diagnostic tools. Finally, by building a repository of carefully collected biologic specimens and linking them with rigorously documented clinical and epidemiologic data, PERCH is expected to contribute to pathogen discovery in the future, too.
PERCH is a large etiology study, but it does not stand alone nor does it fill all the key research gaps in the field. Fortunately, after a period of relatively little research into pediatric pneumonia etiology in developing countries, the PERCH project now finds itself as just one of many ongoing or planned pneumonia studies that are contributing to a new evidence base on pneumonia etiology. PERCH is not an intervention study of either pneumonia treatment or pneumonia prevention strategies. In each of the study sites, local pneumonia case management is provided without standardization across the sites. PERCH is also not explicitly designed to evaluate interventions for pneumonia such as vaccines, improved cookstoves, or oxygen therapy. Immunization status and known or potential risk factors for pneumonia will be collected as part of the PERCH study, but the study does not include patients with meningitis, sepsis, or other syndromes potentially preventable by Hib and/or pneumococcal conjugate vaccines. In short, PERCH provides one key piece of the evidence base, but other studies are clearly needed.
As an observational etiology study, PERCH aims to provide a strong set of evidence to improve child survival and to do so in a way that is transparent and widely accessible. The PERCH protocol, standard operating procedures, and case report forms are all publicly available through the Internet by visiting http://www.jhsph.edu/ivac/perch.html. Through this series of papers, we aim to describe the issues that we debated in the course of designing the study and hope that by sharing these lessons with the pneumonia research community, we will help other investigators conducting pneumonia research elsewhere.
Notes
Disclaimer.
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the US Centers for Disease Control and Prevention.
Supplement sponsorship.
This article was published as part of a supplement entitled “Pneumonia Etiology Research for Child Health,” sponsored by a grant from The Bill & Melinda Gates Foundation to the PERCH Project of Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland.
Potential conflicts of interest.
All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Scott JA, Brooks WA, Peiris JS, Holtzman D, Mulhollan EK. Pneumonia research to reduce childhood mortality in the developing world. J Clin Invest. 2008;118:1291–300. doi: 10.1172/JCI33947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shann F. The management of pneumonia in children in developing countries. Clin Infect Dis. 1995;21(suppl 3):S218–25. doi: 10.1093/clind/21.supplement_3.s218. [DOI] [PubMed] [Google Scholar]
- 3.Shann F. Determining etiology of pneumonia. Pediatr Infect Dis J. 1995;14:920–1. [PubMed] [Google Scholar]
- 4.Sazawal S, Black RE Pneumonia Case Management Trials Group. Effect of pneumonia case management on mortality in neonates, infants, and preschool children: a meta-analysis of community-based trials. Lancet Infect Dis. 2003;3:547–56. doi: 10.1016/s1473-3099(03)00737-0. [DOI] [PubMed] [Google Scholar]
- 5.Shann F. Etiology of severe pneumonia in children in developing countries. Pediatr Infect Dis. 1986;5:247–52. doi: 10.1097/00006454-198603000-00017. [DOI] [PubMed] [Google Scholar]
- 6.Cutts FT, Zaman SM, Enwere G, et al. Efficacy of nine-valent pneumococcal conjugate vaccine against pneumonia and invasive pneumococcal disease in the Gambia: randomised, double-blind, placebo-controlled trial. Lancet. 2005;365:1139–46. doi: 10.1016/S0140-6736(05)71876-6. [DOI] [PubMed] [Google Scholar]
- 7.Klugman KP, Madhi SA, Huebner RE, et al. A trial of a 9-valent pneumococcal conjugate vaccine in children with and those without HIV infection. N Engl J Med. 2003;349:1341–8. doi: 10.1056/NEJMoa035060. [DOI] [PubMed] [Google Scholar]
- 8.Levine OS, Lagos R, Munoz A, et al. Defining the burden of pneumonia in children preventable by vaccination against Haemophilus influenzae type b. Pediatr Infect Dis J. 1999;18:1060–4. doi: 10.1097/00006454-199912000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Madhi SA, Levine OS, Hajjeh R, Mansoor OD, Cherian T. Vaccines to prevent pneumonia and improve child survival. Bull World Health Organ. 2008;86:365–72. doi: 10.2471/BLT.07.044503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mulholland EK, Adegbola RA. The Gambian Haemophilus influenzae type b vaccine trial: What does it tell us about the burden of Haemophilus influenzae type b disease? Pediatr Infect Dis J. 1998;17(9 suppl):S123–5. doi: 10.1097/00006454-199809001-00006. [DOI] [PubMed] [Google Scholar]
- 11.Berkley JA, Munywoki P, Ngama M, et al. Viral etiology of severe pneumonia among Kenyan infants and children. JAMA. 2010;303:2051–7. doi: 10.1001/jama.2010.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olsen SJ, Thamthitiwat S, Chantra S, et al. Incidence of respiratory pathogens in persons hospitalized with pneumonia in two provinces in Thailand. Epidemiol Infect. 2010;138:1811–22. doi: 10.1017/S0950268810000646. [DOI] [PubMed] [Google Scholar]
- 13.Fry AM, Lu X, Olsen SJ, et al. Human rhinovirus infections in rural Thailand: epidemiological evidence for rhinovirus as both pathogen and bystander. PLoS One. 2011;6:e17780. doi: 10.1371/journal.pone.0017780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilani Z, Kwong YD, Levine OS, et al. A literature review and survey of childhood pneumonia etiology studies: 2000 to 2010. Clin Infect Dis. 2012;54(Suppl 2):S102–108. doi: 10.1093/cid/cir1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Selwyn BJ. The epidemiology of acute respiratory tract infection in young children: comparison of findings from several developing countries. Coordinated data group of BOSTID researchers. Rev Infect Dis. 1990;12(suppl 8):S870–88. doi: 10.1093/clinids/12.supplement_s870. [DOI] [PubMed] [Google Scholar]
- 16.Scott JA. The global epidemiology of childhood pneumonia 20 years on. Bull World Health Organ. 2008;86:494–6. doi: 10.2471/BLT.08.052753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. National Statistics Office, Government of Thailand. Available at: http://www.nso.go.th. Accessed 3 August 2011.
- 18.Sirajul Islam M, Brooks A, Kabir MS, et al. Faecal contamination of drinking water sources of Dhaka city during the 2004 flood in Bangladesh and use of disinfectants for water treatment. J Appl Microbiol. 2007;103:80–7. doi: 10.1111/j.1365-2672.2006.03234.x. [DOI] [PubMed] [Google Scholar]
- 19.Brooks WA, Santosham M, Naheed A, et al. Effect of weekly zinc supplements on incidence of pneumonia and diarrhoea in children younger than 2 years in an urban, low-income population in Bangladesh: randomised controlled trial. Lancet. 2005;366:999–1004. doi: 10.1016/S0140-6736(05)67109-7. [DOI] [PubMed] [Google Scholar]
- 20.Cowgill KD, Ndiritu M, Nyiro J, et al. Effectiveness of Haemophilus influenzae type b conjugate vaccine introduction into routine childhood immunization in Kenya. JAMA. 2006;296:671–8. doi: 10.1001/jama.296.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moisi JC, Nokes DJ, Gatakaa H, et al. Sensitivity of hospital-based surveillance for severe disease: a geographic information system analysis of access to care in Kilifi district, Kenya. Bull World Health Organ. 2011;89:102–11. doi: 10.2471/BLT.10.080796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scott JA, Berkley JA, Mwangi I, et al. Relation between falciparum malaria and bacteraemia in Kenyan children: a population-based, case-control study and a longitudinal study. Lancet. 2011;378:1316–23. doi: 10.1016/S0140-6736(11)60888-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Campbell JD, Sow SO, Levine MM, Kotloff KL. The causes of hospital admission and death among children in Bamako, Mali. J Trop Pediatr. 2004;50:158–63. doi: 10.1093/tropej/50.3.158. [DOI] [PubMed] [Google Scholar]
- 24.Bryce J, Daelmans B, Dwivedi A, et al. Countdown Coverage Writing Group, Countdown to 2015 Core Group. Countdown to 2015 for maternal, newborn, and child survival: the 2008 report on tracking coverage of interventions. Lancet. 2008;371:1247–58. doi: 10.1016/S0140-6736(08)60559-0. [DOI] [PubMed] [Google Scholar]
- 25.Violari A, Cotton MF, Gibb DM, et al. Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med. 2008;359:2233–44. doi: 10.1056/NEJMoa0800971. [DOI] [PMC free article] [PubMed] [Google Scholar]