In a large North American cohort study, anal cancer incidence rates were substantially higher for HIV-infected men who have sex with men, other men, and women compared with HIV-uninfected individuals. Rates increased from 1996–1999 to 2000–2003 but plateaued by 2004–2007.
Abstract
Background. Anal cancer is one of the most common cancers affecting individuals infected with human immunodeficiency virus (HIV), although few have evaluated rates separately for men who have sex with men (MSM), other men, and women. There are also conflicting data regarding calendar trends.
Methods. In a study involving 13 cohorts from North America with follow-up between 1996 and 2007, we compared anal cancer incidence rates among 34 189 HIV-infected (55% MSM, 19% other men, 26% women) and 114 260 HIV-uninfected individuals (90% men).
Results. Among men, the unadjusted anal cancer incidence rates per 100 000 person-years were 131 for HIV-infected MSM, 46 for other HIV-infected men, and 2 for HIV-uninfected men, corresponding to demographically adjusted rate ratios (RRs) of 80.3 (95% confidence interval [CI], 42.7–151.1) for HIV-infected MSM and 26.7 (95% CI, 11.5–61.7) for other HIV-infected men compared with HIV-uninfected men. HIV-infected women had an anal cancer rate of 30/100 000 person-years, and no cases were observed for HIV-uninfected women. In a multivariable Poisson regression model, among HIV-infected individuals, the risk was higher for MSM compared with other men (RR, 3.3; 95% CI, 1.8–6.0), but no difference was observed comparing women with other men (RR, 1.0; 95% CI, 0.5–2.2). In comparison with the period 2000–2003, HIV-infected individuals had an adjusted RR of 0.5 (95% CI, .3–.9) in 1996–1999 and 0.9 (95% CI, .6–1.2) in 2004–2007.
Conclusions. Anal cancer rates were substantially higher for HIV-infected MSM, other men, and women compared with HIV-uninfected individuals, suggesting a need for universal prevention efforts. Rates increased after the early antiretroviral therapy era and then plateaued.
With the dramatic decline in human immunodeficiency virus (HIV)-related mortality resulting from advances in combination antiretroviral therapy (ART) and the longer life expectancy and aging of HIV-infected persons, there is growing concern about an increased burden of certain cancers, particularly infection-related cancers such as anal cancer. Meta-analyses have reported that anal cancer incidence rates are 30 times as high for HIV-infected individuals compared with the general population [1, 2], although incidence rate estimates from individual studies have varied widely from 18 to 149 cases per 100 000 person-years [3–10].
Understanding the factors that contribute to the variability in anal cancer incidence rates in HIV-infected persons is critical for targeting screening and prevention efforts to appropriate high-risk groups. In general, HIV-infected men who have sex with men (MSM) have been observed to have the highest rates [9, 11, 12]. HIV-infected heterosexual men also have been reported to have higher rates than HIV-infected women [12–14], although some studies have indicated no differences by sex [2, 4]. Finally, it is unclear how improvements in immune function and extension of life with effective ART use have affected the incidence of anal cancer in HIV-infected persons. In the current study, we have used data from a multicohort collaboration in the United States and Canada to assess key factors contributing to the heterogeneity in anal cancer incidence.
METHODS
Participants and Data Sources
We analyzed data from 13 cohort studies (10 from the United States and 3 from Canada) that are included in the North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) for the years 1996–2007. Cohorts were included if they provided data on anal cancers and sexual orientation. Study methods for NA-ACCORD have been described elsewhere [15]. Study protocols were approved by the local institutional review board for each cohort.
Eleven of the 13 participating cohorts were clinic-based, and data were obtained from medical records. The remaining 2 participating cohorts (Multicenter AIDS Cohort Study [MACS], and Women’s Interagency HIV Study [WIHS]) were interval-based, and data were obtained as part of a research protocol at scheduled 6-month study visits. All contributing cohorts used standardized methods of data collection, and submitted demographic, treatment, clinical, laboratory, and vital status data on enrolled HIV-infected individuals. In addition, 3 cohorts (MACS, WIHS, and Kaiser Permanente Northern California [KPNC]) contributed data on HIV-uninfected individuals. Because sexual orientation was unknown for the majority of HIV-uninfected individuals, we were unable to stratify men in the comparison group by MSM status.
Incident anal cancer diagnoses were ascertained separately by each cohort from medical records, patient interviews (confirmed by medical record reviews), or linkage with a cancer registry. Cases comprised all anal cancer diagnoses, regardless of histology, because histological information was not provided by all cohorts. Individuals with prevalent anal cancer were excluded.
Statistical Methods
The primary objective was to estimate anal cancer incidence overall and over time among HIV-infected MSM, HIV-infected other men (ie, heterosexual, injection drug use [IDU], or other HIV risk factor), and HIV-infected women. We compared incidence rates to the reference groups of HIV-uninfected men and women. For each cohort member, the start of follow-up (ie, baseline) was defined as the cohort entry date, or 1 January 1996, whichever came last. Individuals were followed until there was an anal cancer diagnosis, loss to follow-up, death, or up to 31 December 2007, whichever occurred first.
We initially computed anal cancer incidence rates over the entire study period. Adjusted rate ratios (RRs) were calculated for HIV-infected MSM and other men compared with HIV-uninfected individuals (reference group) using Poisson models with terms for calendar era (1996–1999, 2000–2003, and 2004–2007), age at entry into a calendar era by 5-year age groups, race/ethnicity (white, black, other), and cohort. Multiple imputation [16] was used to impute missing race/ethnicity on the basis of an individual’s age, HIV status, calendar period of entry into cohort, anal cancer status, and cohort. We also computed anal cancer incidence rates for HIV-infected and uninfected women, although adjusted RRs were not computed because no cases were observed among HIV-uninfected women.
We then computed cohort-specific anal cancer incidence rates for HIV-infected individuals. Using linear regression, the cohort-specific incidence rate was modeled as a function of each cohort’s prevalence of MSM. Each of the 13 observations representing an individual cohort was weighted according to the amount of person-time contributed by that cohort. We report the R2 as a measure of the amount of variability in cohort-specific rates explained by the cohort-specific prevalence of MSM.
In subsequent analyses, we evaluated whether anal cancer incidence rates have changed over time (1996–1999, 2000–2003, and 2004–2007) for HIV-infected MSM, other men, and women. Denominators for these rates were obtained by dividing each subject’s person-time into the corresponding calendar eras; to obtain numerators, anal cancer diagnoses made during a given era were assigned to that era. P values comparing anal cancer incidence rates by era (2000–2003 as reference) were obtained from a Poisson regression model, and a global P value was based on the likelihood ratio statistic. Multivariable Poisson models were then used to evaluate changes in RRs over time for HIV-infected MSM and other men compared with HIV-uninfected men, adjusting for race/ethnicity, age at entry into calendar era in 5-year groups, and cohort. Because only 3 of the 13 cohorts contributed data on HIV-uninfected individuals, we also report standardized incidence ratios (SIRs) for HIV-infected MSM, other men, and women, adjusting for age and race/ethnicity, using national US Surveillance, Epidemiology and End Results (SEER) program rates for 1996–2005 [17].
Finally, for HIV-infected individuals, we computed adjusted RRs in a multivariable Poisson model with terms for MSM/other men/women, calendar era, race/ethnicity, age at entry into calendar era, baseline CD4+ T-cell count, and cohort.
RESULTS
Thirteen NA-ACCORD cohorts contributed a total of 34 189 HIV-infected individuals (55% MSM, 19% other men, and 26% women; Table 1). Three of these cohorts also contributed 114 260 HIV-uninfected individuals (90% men). Median age by HIV infection status was similar among men and women. Among those with known race/ethnicity, most HIV-infected MSM and HIV-uninfected individuals were white, whereas most HIV-infected other men and women were black. The most common HIV transmission risk was heterosexual sex for both HIV-infected other men (59%) and women (61%).
Table 1.
Baseline Characteristics, Anal Cancer Rates, and Rate Ratios by HIV Status, Men Who Have Sex With Men, and Sex, North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD), Years 1996–2007
| HIV-Infected |
HIV-Uninfected |
||||
| MSM | Other Men | Women | Men | Women | |
| Baseline characteristics | |||||
| No. | 18 855a | 6492a | 8842 | 102 607 | 11 653 |
| Median age (IQR) | 38 (33–44) | 40 (35–46) | 36 (30–43) | 40 (34–47) | 37 (31–44) |
| Race/ethnicity | |||||
| Black | 3383 (18%) | 3360 (52%) | 4770 (54%) | 6153 (6%) | 1678 (14%) |
| White | 11 951 (63%) | 1773 (27%) | 2051 (23%) | 32 915 (32%) | 4306 (37%) |
| Otherb | 1946 (10%) | 732 (11%) | 1269 (14%) | 19 631 (19%) | 3201 (27%) |
| Unknown | 1575 (8%) | 627 (10%) | 752 (9%) | 43 908 (43%) | 2468 (21%) |
| HIV risk group | |||||
| MSM | 18 855 (100%) | 0 (0%) | 0 (0%) | … | … |
| IDU | 0 (0%) | 2225 (34%) | 1595 (18%) | … | … |
| Heterosexual | 0 (0%) | 3820 (59%) | 5433 (61%) | … | … |
| Other | 0 (0%) | 447 (7%) | 464 (5%) | … | … |
| Unknown | 0 (0%) | 0 (0%) | 1355 (15%) | … | … |
| Median year start of follow-up (IQR) | 2000 (1997–2004) | 2000 (1997–2003) | 2000 (1996–2003) | 1998 (1996–2003) | 1998 (1996–2003) |
| Median baseline CD4 cells/μL (IQR)c | 320 (140–505) | 225 (65–418) | 336 (155–538) | … | … |
| Anal cancer rates and rate ratios | |||||
| Cases | 122 | 14 | 15 | 13 | 0 |
| Person-years | 93 063 | 30 570 | 49 676 | 585 049 | 67 942 |
| Median years follow-up (IQR) | 4.0 (1.6–7.8) | 3.9 (1.6–7.2) | 5.3 (2.0–8.8) | 4.7 (2.0–10.0) | 5.0 (2.1–9.9) |
| Incidence rate per 100 000 person-years (95% CI) | 131 (109–157) | 46 (25–77) | 30 (17–50) | 2 (1–4) | 0 (0–5) |
| Rate ratio (95% CI) | 80.3 (42.7–151.1)d | 26.7 (11.5–61.7)d | Undefinede | Reference | Undefinede |
Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus; IDU, injection drug use; IQR, interquartile range; MSM, men who have sex with men.
Excludes 2280 HIV-infected men with missing or unknown HIV risk group.
Other race/ethnicity category was predominantly Hispanic.
Closest CD4 test result within 1 year of start of follow-up; 0.8% had missing baseline CD4.
Rate ratios for HIV-infected MSM and other men compared with HIV-uninfected men from Poisson regression models adjusted for race/ethnicity, calendar era, age at entry into calendar era, and cohort.
Zero cases of anal cancer among the reference group of HIV-uninfected females.
The anal cancer incidence rate per 100 000 person-years for HIV-infected MSM, other men, and women was 131, 46, and 30, respectively (Table 1). The higher rate for HIV-infected MSM compared with both other men and women was statistically significant (both P < .001); incidence rates for HIV-infected other men and women did not significantly differ (P = .27). Among HIV-uninfected men, there were 13 anal cancer cases corresponding to an incidence rate of 2 cases per 100 000 person-years. With HIV-uninfected men as the reference group, adjusted RRs were 80.3 (95% CI, 42.7–151.1) for HIV-infected MSM and 26.7 (95% CI, 11.5–61.7) for HIV-infected other men. No anal cancer cases were observed among HIV-uninfected women.
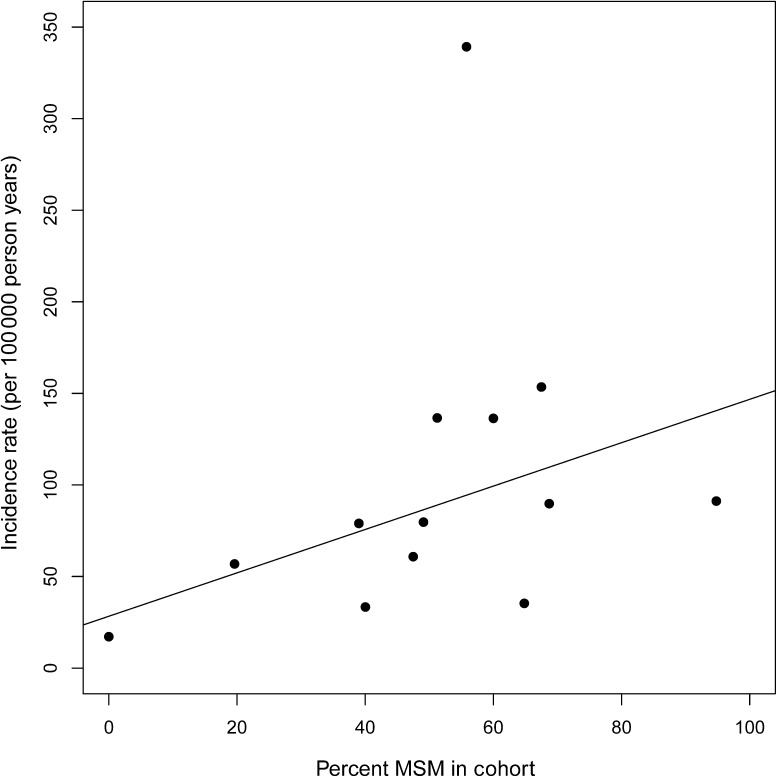
Figure 1 shows the anal cancer incidence rate per 100000 person-years for each of 13 cohorts as a function of each cohort’s MSM prevalence. The line represents the results of the weighted linear regression model with an intercept of 28.3 cases per 100 000 person-years, and slope of 1.18 cases per 100 000 person years per 1% increase (P = .047) in cohort-specific prevalence of MSM. Thus, the anticipated anal cancer incidence rate for a population with 0% MSM would be 28 cases per 100 000 person-years (eg, 28.3 + 1.18 × 0 = 28.3), whereas the incidence rate for a population with 100% MSM would be 146 cases per 100 000 person-years (eg, 28.3 + 1.18 × 100 = 146.3). Overall, the R2 from the model indicated that the cohort-specific prevalence of MSM explained 31% of the variability in incidence rates. Excluding the outlier cohort with a very high anal cancer incidence rate had little effect on results (data not shown) because the cohort contributed <0.5% of all HIV-infected person-time.
Figure 1.
Cohort-specific anal cancer incidence rates by cohort-specific prevalence of men who have sex with men (MSM), North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD), years 1996–2007. Each point represents 1 of 13 cohorts contributing data. The line represents the results of the weighted linear regression model with an intercept of 28.3 cases per 100 000 person-years, and slope of 1.18 cases per 100 000 person years per 1% increase in cohort-specific prevalence of MSM.
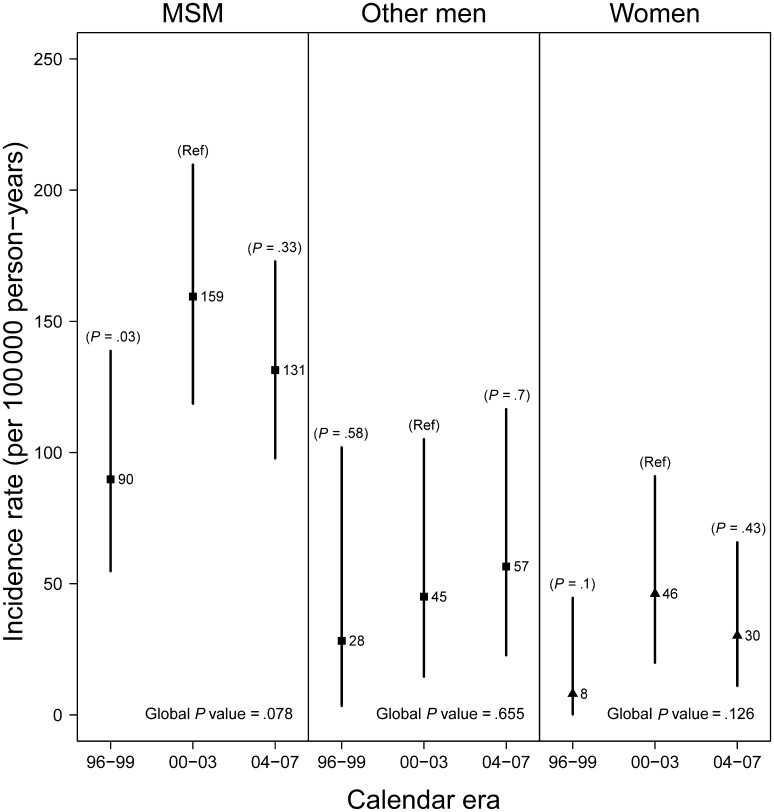
Figure 2 shows the change in crude incidence rates over time for HIV-infected MSM, other men, and women. For MSM, the increase in rate between 1996–1999 and 2000–2003 was significant (P = .03), whereas the difference in rates between 2000–2003 and 2004–2007 was not (P = .33). The pattern observed was similar for other men and women such that the incidence rate was lowest in the earliest era (1996–1999), although differences between eras were not significant. At each time point, the incidence rate for MSM was higher than for other men or women. The incidence rate was similar for other men and women at each time point, with substantial overlap of confidence intervals.
Figure 2.
Anal cancer incidence rates by calendar era for human immunodeficiency virus infected men who have sex with men (MSM), other men, and women, North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD), years 1996–2007. Vertical lines are 95% confidence intervals. P values from the Poisson regression model compare rates by calendar era with 2000–2003 as reference. The global P value for comparison of rates across eras is based on the likelihood ratio statistic.
Table 2 presents calendar era–specific, adjusted RRs for anal cancer for HIV-infected MSM and HIV-infected other men compared with HIV-uninfected men (reference). For HIV-infected MSM, the incidence rate compared with HIV-uninfected men was lowest in 1996–1999 with an adjusted RR of 60.8. The RR increased for HIV-infected MSM during 2000–2003 (P = .06) but did not continue to increase during 2004–2007 (P = .22). For HIV-infected other men, the incidence rate compared with HIV-uninfected men was also lowest in 1996–1999 with an adjusted RR of 18.2. Although slight increases in the RR were observed over time, differences between eras were not significant. SIRs for the same comparisons were of similar magnitude across time periods for HIV-infected MSM and other men (Table 2). Additionally, SIRs indicated a similar pattern for HIV-infected women with an increase in the SIR from 0 in 1996–1999 to 41.5 in 2000–2003 but a reduced SIR of 24.7 in 2004–2007.
Table 2.
Adjusted Anal Cancer Incidence Rate Ratios Between HIV-Infected Individuals Compared With HIV-Uninfected Individuals and With National US SEER Rates (Standardized Incidence Ratios), North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD), Years 1996–2007
| Rate Ratio (95% CI)a,b |
Standardized Incidence Ratio (95% CI)c |
||||
| Calendar Era | MSM | Other Men | MSM | Other Men | Women |
| 1996–1999 | 60.8 (28.3–130.3) | 18.2 (3.9–85.0) | 73.9 (44.5–110.7) | 17.4 (2.1–48.6) | 0 (0–0)d |
| 2000–2003 | 100.8 (51.7–196.5) | 27.3 (9.1–81.8) | 115.6 (86.1–149.5) | 24.8 (8.1–50.8) | 41.5 (16.7–77.4) |
| 2004–2007 | 78.8 (40.8–152.1) | 31.9 (11.9–85.4) | 78.7 (58.2–102.2) | 20.3 (6.6–41.5) | 24.7 (9.1–48.0) |
Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus; MSM, men who have sex with men.
Rate ratios for HIV-infected MSM and other men by calendar era compared with HIV-uninfected men (not stratified by era) from Poisson regression models adjusted for race/ethnicity (imputed for unknown), age at entry into calendar era, and cohort. The rate ratio was undefined for women because there were no cases in the reference group of HIV-uninfected women.
The P values for the rate ratios comparing 1996–1999 vs 2000–2003 and 2000–2003 vs 2004–2007 were P = .06 and P = .22, respectively, for MSM; and P = .63 and P = .79, respectively, for other men.
Age (<30, 30–39, 40–49, and ≥50) and sex and race/ethnicity (white, black, other) standardized incidence ratios computed using national US SEER rates from 1996 through 2005; individuals with missing race were dropped in the SIR analysis.
The SIR for women in 1996–1999 was 0 because the single female case diagnosed in 1996–1999 had missing race, and was therefore dropped in the SIR analysis.
Multivariable analysis of risk factors for anal cancer among HIV-infected individuals (Table 3) indicated that MSM were associated with a significantly greater incidence rate (RR, 3.3; 95% CI, 1.8–6.0) compared with HIV-infected other men, whereas no difference was found comparing women and other men (RR, 1.0; 95% CI, .5–2.2). The incidence rate during 1996–1999 was significantly lower than the incidence rate in 2000–2003 (RR, 0.5; 95% CI, .3–.9), but the incidence rate did not significantly change between 2000–2003 and 2004–2007. Other factors significantly associated with incident anal cancer included race/ethnicity (RR, 0.3; 95% CI, .1–.7 for other race/ethnicities compared with whites), age (RR per 10-year increase in age, 1.3; 95% CI, 1.1–1.5), and baseline CD4 count (RR, 0.2; 95% CI, .1–.3 for CD4 ≥500 compared with CD4 <200).
Table 3.
Rate Ratios for Anal Cancer in HIV-Infected Individuals, North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD), Years 1996–2007
| Univariate Modela |
Multivariable Modela |
|||||
| Variable | RR | (95% CI) | P Value | RR | (95% CI) | P Value |
| MSM status/sex | ||||||
| HIV-infected MSM | 2.8 | 1.6–4.9 | <.001 | 3.3 | 1.8–6.0 | <.001 |
| HIV-infected other men | 1.0 | Ref | … | 1.0 | Ref | … |
| HIV-infected women | 0.7 | 0.3–1.4 | .26 | 1.0 | 0.5–2.2 | .97 |
| Calendar era | ||||||
| 1996–1999 | 0.6 | 0.4–0.9 | .013 | 0.5 | 0.3–0.9 | .011 |
| 2000–2003 | 1 | Ref | … | 1.0 | Ref | … |
| 2004–2007 | 0.8 | 0.6–1.2 | .35 | 0.9 | 0.6–1.2 | .36 |
| Race/ethnicity | ||||||
| Black | 0.7 | 0.5–1.0 | .087 | 1.2 | 0.8–1.7 | .46 |
| White | 1.0 | Ref | … | 1.0 | Ref | … |
| Otherb | 0.3 | 0.1–0.6 | .002 | 0.3 | 0.1–0.7 | .004 |
| Age (per 10 years) | 1.4 | 1.2–1.7 | <.001 | 1.3 | 1.1–1.5 | .009 |
| Baseline CD4, cells/μL | ||||||
| <200 | 1.0 | Ref | … | 1.0 | Ref | … |
| 200–349 | 0.5 | 0.3–0.8 | .002 | 0.5 | 0.4–0.8 | .003 |
| 350–499 | 0.5 | 0.3–0.7 | .001 | 0.5 | 0.3–0.7 | <.001 |
| ≥500 | 0.2 | 0.1–0.3 | <.001 | 0.2 | 0.1–0.3 | <.001 |
Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus; MSM, men who have sex with men; Ref, reference; RR, rate ratio.
RRs obtained from Poisson regression models. Multivariable model adjusted for all variables in table and also for cohort.
Other race/ethnicity category was predominantly Hispanic.
DISCUSSION
In this large multicohort study, we confirmed that HIV-infected MSM experienced the greatest risk for anal cancer with incidence rates >80 times as high as HIV-uninfected individuals. Incidence rates differed widely across the 13 individual cohorts, with a large amount (31%) of the variation accounted for by the cohort-specific prevalence of MSM. We also found that both HIV-infected other men and women had substantially higher rates than HIV-uninfected men and women, and that HIV-infected other men and women had similar rates. Finally, we observed that rates of anal cancer for HIV-infected individuals increased after the early ART era and then plateaued in more recent years.
The substantially higher incidence rate of anal cancer among HIV-infected MSM was observed initially in the pre-ART era [18] and since has been confirmed by some studies [12, 19] but not others [4]. The French Hospital Database study indicated that HIV-infected MSM had an 8 times as high incidence rate of anal cancer compared with HIV-infected women [12], which is comparable to our finding of a 4 times as high rate comparing these same groups. Chaturvedi et al [19] reported that MSM had an SIR of 52, the highest of all risk groups examined. Finally, Dal Maso et al [4] reported that among Italians with AIDS, those with a history of IDU had the highest SIR (SIR, 85), followed by MSM (SIR, 47) and heterosexuals (SIR, 10).
We also observed that HIV-infected other men and women had similar anal cancer incidence, although both had elevated incidence rates compared with HIV-uninfected individuals. Piketty et al [12] reported a higher incidence of anal cancer for both HIV-infected MSM and other men compared with HIV-infected women. Chaturvedi et al [19], on the other hand, reported similar SIRs for heterosexual men (SIR, 14) and women (SIR, 15). Others have compared incidence rates by sex (ie, grouping MSM and other men together), with some indicating a higher incidence for anal cancer among men [13, 14], and others no difference by sex [2, 4, 12]. However, sex differences in the incidence rate of anal cancer are difficult to interpret without also knowing the sexual orientation among men.
Discrepant results for sex and MSM status across studies may reflect variation in sexual risk behaviors among populations, thus contributing to differences in the prevalence of anal human papillomavirus (HPV). Prior studies have indicated very high anal HPV prevalence, particularly for HIV-infected MSM (prevalence ranging from 85% to 95%) [20–22] but also for HIV-infected women (prevalence ranging from 76% to 90%) [23, 24], heterosexual men (prevalence of 60%) [20], and injection drug users (prevalence of 46%) [22]. D’Souza et al [11] reported that among MSM, a higher cumulative number of reported unprotected anal receptive partners at study baseline was associated with a higher incidence rate of anal cancer.
We also found that among HIV-infected persons, anal cancer incidence rates increased between 1996–1999 and 2000–2003, followed by stable rates. This pattern was similar for MSM, other men, and women and was independent of other factors, including age, race/ethnicity, and baseline CD4. Many studies, but not all [2, 4], have reported increases in anal cancer incidence rates over time in HIV-infected persons [5, 8, 9, 11, 12, 19, 25–28]; however, these studies compared rates between the pre-ART and ART eras. Among previous studies that evaluated trends over time during the ART era, some indicated continued increasing rates [8, 25, 26], whereas others found no increases during more recent years [10, 12]. However, of the 3 studies showing continued increases, 2 had a small sample size and few events in the most recent era [25, 26], and the third evaluated trends only through 2003 [8]. Similar to our results, the large French Hospital Database study observed an increase in anal cancer incidence rates for HIV-infected MSM, other men, and women through 1998, but no significant change in rates after 1999 [12].
Our finding of stabilizing rates in the ART era may mask opposing pressures on anal cancer risk. On one hand, the improved survival for HIV-infected individuals in the ART era results in longer exposure to HPV infection, which may result in increased risk of HPV-associated cancers [8, 12]. Crum-Cianflone et al [26], for example, observed that anal cancer was most common among HIV-infected patients with known duration of HIV infection of ≥15 years. On the other hand, anal cancer in HIV-infected patients may be related to immunosuppression [1, 11, 12, 29, 30], which was supported here by the observed association of baseline CD4 and anal cancer incidence rates. If true, then improvements in immune function over time with the use of ART may result in decreased anal cancer risk.
Our study had limitations. First, we lacked data on several factors, including HPV infection, sexual behavior, smoking, and anal cancer screening, which would help further elucidate differences between groups. Regarding anal cancer screening, there might have been more vigilance for anal cancer among HIV-infected MSM than among HIV-infected other men and HIV-infected women, and among HIV-infected persons than among HIV-uninfected persons. Such differences in screening could have biased results in either direction, as screening could result in diagnosis of both anal cancers and of precancerous lesions. Treatment of the latter could result in decreased anal cancer incidence, which might explain the observed plateau in anal cancer rates. To the extent that screening practices differ from cohorts included here from the United States and Canada, results may have limited generalizability to other populations.
An additional limitation was the lack of information on sexual orientation for HIV-uninfected individuals. Because MSM, whether or not they are HIV-infected, may be at increased risk for anal cancer [31], it is possible that confounding by MSM status contributed to the increased anal cancer risk we observed among HIV-infected MSM; however, few others have compared risk directly between HIV-infected and HIV-uninfected MSM to avoid this potential confounding [9, 11]. Also, among HIV-infected individuals, there may have been misclassification of sexual orientation, resulting in some MSM classified as other men. A further limitation was that anal cancer was rare in the HIV-uninfected population, limiting our statistical power and requiring us to use a single HIV-uninfected comparison group (not stratified by calendar era). Furthermore, there were no cases among women in the HIV-uninfected comparison group, precluding a direct comparison of anal cancer incidence rates by HIV status among women. Finally, neither the internal comparison group nor the external SEER rates included Canadians, even though our analysis included 3 HIV-infected Canadian cohorts.
Study strengths were evaluation of anal cancer rates for several well-defined groups of HIV-infected individuals (ie, MSM, other men, and women) and inclusion of an HIV-uninfected comparison group. The similarity of our results using this comparison group and the SEER rates as the comparison demonstrates the robustness of our findings. An additional strength was the very large sample size and the geographic and demographic diversity represented by the contributing cohorts.
Our finding of high anal cancer incidence rates in HIV-infected MSM, other men, and women suggests the need for enhanced primary and secondary prevention efforts among all HIV-infected persons, as opposed to a targeted approach. The HPV vaccine has been shown to be highly effective for prevention of anal cancer precursor lesions in women [32] and was recently approved for prevention of anal cancer [33]. Furthermore, vaccination of boys [34] or high-risk groups, such as MSM [35], can be highly cost effective. However, it remains to be seen how efficacious the vaccine will be for HIV-infected patients [36]. Anal dysplasia screening may also prove to be a cost-effective approach to preventing cancer in HIV-infected individuals [37, 38]. The New York State AIDS Institute guidelines for anal cancer screening of HIV-infected patients recommend annual digital rectal examinations for all patients, and targeted anal cytology for MSM, for individuals with a history of anogenital warts, and for women with a history of abnormal cervical or vulvar histology [39]. However, further research is needed to determine if such an approach will ultimately result in a decreased burden of anal cancer [40].
Notes
Acknowledgments.
We are grateful to all patients, physicians, investigators, and staff involved in the NA-ACCORD.
NA-ACCORD Participating cohorts (representatives): AIDS Link to the Intravenous Experience (Gregory D. Kirk); AIDS Clinical Trials Group Longitudinal Linked Randomized Trials (Constance A. Benson, Ronald J. Bosch, Ann C. Collier); HAART Observational Medical Evaluation and Research (Robert S. Hogg, Richard Harrigan, Julio Montaner); HIV Outpatient Study (John T. Brooks, Kate Buchacz)*; HIV Research Network (Kelly A. Gebo); Johns Hopkins HIV Clinical Cohort (Richard D. Moore)* ; John T. Carey Special Immunology Unit Patient Care and Research Database, Case Western Reserve University (Benigno Rodriguez)*; Kaiser Permanente Northern California (Michael A. Horberg, Michael J. Silverberg)*; Longitudinal Study of Ocular Complications of AIDS (Jennifer E. Thorne); Multicenter Hemophilia Cohort Study–II (James J. Goedert); Multicenter AIDS Cohort Study (Lisa P. Jacobson)*; Montreal Chest Institute Immunodeficiency Service Cohort (Marina B. Klein)*; Ontario HIV Treatment Network Cohort Study (Sean B. Rourke, Ann Burchell, Anita R. Rachlis)*; Southern Alberta Clinic Cohort (M. John Gill)*; Studies of the Consequences of the Protease Inhibitor Era (Steven G. Deeks, Jeffery N. Martin); University of Alabama at Birmingham 1917 Clinic Cohort (Michael S. Saag, Michael Mugavero, James Willig)*; University of North Carolina, Chapel Hill HIV Clinic Cohort (Joseph J. Eron, Sonia Napravnik)*; University of Washington HIV Cohort (Mari M. Kitahata and Heidi M. Crane)*; Veterans Aging Cohort Study (Amy C. Justice, Robert Dubrow, David Fiellin); Vanderbilt-Meharry CFAR Cohort (Timothy R. Sterling, Sam Stinette, David Haas)*; Women’s Interagency HIV Study (Stephen J. Gange, Kathryn Anastos)*.
*Indicates contribution of data to this analysis.
Executive Committee: Richard D. Moore, Michael S. Saag, Stephen J. Gange, Mari M. Kitahata, Amy C. Justice, Rosemary G. McKaig, Aimee M. Freeman.
Epidemiology/Biostatistics Core: Stephen J. Gange, Alison G. Abraham, Bryan Lau, Keri N. Althoff, Jinbing Zhang, Jerry Jing, Elizabeth Golub, Shari Modur, David Hanna, Peter Rebeiro, Adell Mendes.
Data Management Core: Mari M. Kitahata, Stephen E. Van Rompaey, Heidi M. Crane, Eric Webster, Liz Morton, Brenda Simon.
Financial support.
This work was supported by grants from the National Institutes of Health: U01-AI069918, U01-AA013566, U01-AI-35042, 5-M01-RR-00052 (General Clinical Research Centers [GCRC]), U01-AI-35043, U01-AI-35039, U01-AI-35040, U01-AI-35041, U01-AI38855: AIDS Clinical Trials Group Longitudinal Linked Randomized Trials (ALLRT); U01-AI38858; U01-AI68634; U01-AI68636; AI-69432; AI-69434, U01-AI-35004, U01-AI-31834, U01-AI-34994, U01-AI-34989, U01-AI-34993, and U01-AI-42590, U01- HD-32632, UL1-RR024131, P30-AI27757; K23-AI-61-0320, P30-AI27767, P30-AI50410; RR025747, P30-AI54999, R01-DA04334; R01-DA12568, R01-MH54907, R24-AI067039, N02-CP55504; Z01-CP010176, AHQ290-01-0012, K01-AI071754, K24-00432; R01-DA11602, K01-AI071725, R01 AG026250, and P30 AI027763. This work was also supported by the Canadian Institutes for Health Research (CIHR: TGF-96118; HCP-97105; CBR-86906; CBR-94036; KRS-86251; 169621) and the Canadian Trials Network (project 242). Finally, support was also provided by the Centers for Disease Control (CDC200-2006-18797); the findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Potential conflicts of interest.
M. J. S. received research grant support from Pfizer and Merck. G. D. K. has been a consultant to GlaxoSmithKline and Merck. G. D. has been a consultant to and received research grant support from Merck. M. B. K. has been a consultant to GlaxoSmithKline, Abbott, Pfizer, and Boehringer-Ingelheim, has received lecture fees from Abbott, Gilead, Tibotec, Bristol-Myers Squibb, and GlaxoSmithKline, and received research support from Schering Plough Canada. R. D. M. has been a consultant to Bristol-Myers Squibb and Merck. T. R. S. received research grant support from Pfizer and Bristol-Myers Squibb. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet. 2007;370:59–67. doi: 10.1016/S0140-6736(07)61050-2. [DOI] [PubMed] [Google Scholar]
- 2.Shiels MS, Cole SR, Kirk GD, Poole C. A meta-analysis of the incidence of non-AIDS cancers in HIV-infected individuals. J Acquir Immune Defic Syndr. 2009;52:611–22. doi: 10.1097/QAI.0b013e3181b327ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bedimo RJ, McGinnis KA, Dunlap M, Rodriguez-Barradas MC, Justice AC. Incidence of non-AIDS-defining malignancies in HIV-infected versus noninfected patients in the HAART era: impact of immunosuppression. J Acquir Immune Defic Syndr. 2009;52:203–8. doi: 10.1097/QAI.0b013e3181b033ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dal Maso L, Polesel J, Serraino D, et al. Pattern of cancer risk in persons with AIDS in Italy in the HAART era. Br J Cancer. 2009;100:840–7. doi: 10.1038/sj.bjc.6604923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diamond C, Taylor TH, Aboumrad T, Bringman D, Anton-Culver H. Increased incidence of squamous cell anal cancer among men with AIDS in the era of highly active antiretroviral therapy. Sex Transm Dis. 2005;32:314–20. doi: 10.1097/01.olq.0000162366.60245.02. [DOI] [PubMed] [Google Scholar]
- 6.Engels EA, Biggar RJ, Hall HI, et al. Cancer risk in people infected with human immunodeficiency virus in the United States. Int J Cancer. 2008;123:187–94. doi: 10.1002/ijc.23487. [DOI] [PubMed] [Google Scholar]
- 7.Long JL, Engels EA, Moore RD, Gebo KA. Incidence and outcomes of malignancy in the HAART era in an urban cohort of HIV-infected individuals. AIDS. 2008;22:489–96. doi: 10.1097/QAD.0b013e3282f47082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patel P, Hanson DL, Sullivan PS, et al. Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992–2003. Ann Intern Med. 2008;148:728–36. doi: 10.7326/0003-4819-148-10-200805200-00005. [DOI] [PubMed] [Google Scholar]
- 9.Seaberg EC, Wiley D, Martinez-Maza O, et al. Cancer incidence in the multicenter aids cohort study before and during the HAART era: 1984 to 2007. Cancer. 2011;116:5507–16. doi: 10.1002/cncr.25530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Silverberg MJ, Chao C, Leyden WA, et al. HIV infection and the risk of cancers with and without a known infectious cause. AIDS. 2009;23:2337–45. doi: 10.1097/QAD.0b013e3283319184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D’Souza G, Wiley DJ, Li X, et al. Incidence and epidemiology of anal cancer in the multicenter AIDS cohort study. J Acquir Immune Defic Syndr. 2008;48:491–9. doi: 10.1097/QAI.0b013e31817aebfe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Piketty C, Selinger-Leneman H, Grabar S, et al. Marked increase in the incidence of invasive anal cancer among HIV-infected patients despite treatment with combination antiretroviral therapy. AIDS. 2008;22:1203–11. doi: 10.1097/QAD.0b013e3283023f78. [DOI] [PubMed] [Google Scholar]
- 13.Clifford GM, Polesel J, Rickenbach M, et al. Cancer risk in the Swiss HIV Cohort Study: associations with immunodeficiency, smoking, and highly active antiretroviral therapy. J Natl Cancer Inst. 2005;97:425–32. doi: 10.1093/jnci/dji072. [DOI] [PubMed] [Google Scholar]
- 14.Newnham A, Harris J, Evans HS, Evans BG, Moller H. The risk of cancer in HIV-infected people in southeast England: a cohort study. Br J Cancer. 2005;92:194–200. doi: 10.1038/sj.bjc.6602273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gange SJ, Kitahata MM, Saag MS, et al. Cohort profile: the North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) Int J Epidemiol. 2007;36:294–301. doi: 10.1093/ije/dyl286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raghunathan TE, Lepkowski JM, Van Hoewyk J, Solenberger P. A multivariate technique for multiply imputing missing values using a sequence of regression models. Surv Methodol. 2001;27:85–95. [Google Scholar]
- 17. National Cancer Institute. Surveillance, epidemiology, and end results. Available at: http://www.seer.cancer.gov/. Accessed 14 September 2011.
- 18.Melbye M, Cote TR, Kessler L, Gail M, Biggar RJ. High incidence of anal cancer among AIDS patients. The AIDS/Cancer Working Group. Lancet. 1994;343:636–9. doi: 10.1016/s0140-6736(94)92636-0. [DOI] [PubMed] [Google Scholar]
- 19.Chaturvedi AK, Madeleine MM, Biggar RJ, Engels EA. Risk of human papillomavirus-associated cancers among persons with AIDS. J Natl Cancer Inst. 2009;101:1120–30. doi: 10.1093/jnci/djp205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brooks JT, Patel P, Kojic EM, et al. Program and abstracts of the XVIII International AIDS Conference, 2010 (Vienna, Austria). Geneva, Switzerland: International AIDS Society. Anal human papillomavirus (HPV) infection in HIV-infected men who have sex with men (MSM) compared with men who have sex with women (MSW) in the SUN study. [Google Scholar]
- 21.Palefsky JM, Holly EA, Ralston ML, Jay N. Prevalence and risk factors for human papillomavirus infection of the anal canal in human immunodeficiency virus (HIV)-positive and HIV-negative homosexual men. J Infect Dis. 1998;177:361–7. doi: 10.1086/514194. [DOI] [PubMed] [Google Scholar]
- 22.Piketty C, Darragh TM, Da Costa M, et al. High prevalence of anal human papillomavirus infection and anal cancer precursors among HIV-infected persons in the absence of anal intercourse. Ann Intern Med. 2003;138:453–9. doi: 10.7326/0003-4819-138-6-200303180-00008. [DOI] [PubMed] [Google Scholar]
- 23.Kojic EM, Cu-Uvin S, Conley L, et al. Human papillomavirus infection and cytologic abnormalities of the anus and cervix among HIV-infected women in the study to understand the natural history of HIV/AIDS in the era of effective therapy (the SUN study) Sex Transm Dis. 2011;38:253–9. doi: 10.1097/OLQ.0b013e3181f70253. [DOI] [PubMed] [Google Scholar]
- 24.Palefsky JM, Holly EA, Ralston ML, Da Costa M, Greenblatt RM. Prevalence and risk factors for anal human papillomavirus infection in human immunodeficiency virus (HIV)–positive and high-risk HIV-negative women. J Infect Dis. 2001;183:383–91. doi: 10.1086/318071. [DOI] [PubMed] [Google Scholar]
- 25.Crum-Cianflone N, Hullsiek KH, Marconi V, et al. Trends in the incidence of cancers among HIV-infected persons and the impact of antiretroviral therapy: a 20-year cohort study. AIDS. 2009;23:41–50. doi: 10.1097/QAD.0b013e328317cc2d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crum-Cianflone NF, Hullsiek KH, Marconi VC, et al. Anal cancers among HIV-infected persons: HAART is not slowing rising incidence. AIDS. 2010;24:535–43. doi: 10.1097/QAD.0b013e328331f6e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hessol NA, Pipkin S, Schwarcz S, Cress RD, Bacchetti P, Scheer S. The impact of highly active antiretroviral therapy on non-AIDS-defining cancers among adults with AIDS. Am J Epidemiol. 2007;165:1143–53. doi: 10.1093/aje/kwm017. [DOI] [PubMed] [Google Scholar]
- 28.Simard EP, Pfeiffer RM, Engels EA. Cumulative incidence of cancer among individuals with acquired immunodeficiency syndrome in the United States. Cancer. 2011;117:1089–96. doi: 10.1002/cncr.25547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guiguet M, Boue F, Cadranel J, Lang JM, Rosenthal E, Costagliola D. Effect of immunodeficiency, HIV viral load, and antiretroviral therapy on the risk of individual malignancies (FHDH-ANRS CO4): a prospective cohort study. Lancet Oncol. 2009;10:1152–9. doi: 10.1016/S1470-2045(09)70282-7. [DOI] [PubMed] [Google Scholar]
- 30.Reekie J, Kosa C, Engsig F, et al. Relationship between current level of immunodeficiency and non-acquired immunodeficiency syndrome-defining malignancies. Cancer. 2010;116:5306–15. doi: 10.1002/cncr.25311. [DOI] [PubMed] [Google Scholar]
- 31.Daling JR, Weiss NS, Hislop TG, et al. Sexual practices, sexually transmitted diseases, and the incidence of anal cancer. N Engl J Med. 1987;317:973–7. doi: 10.1056/NEJM198710153171601. [DOI] [PubMed] [Google Scholar]
- 32.Kreimer AR, Gonzalez P, Katki HA, et al. Efficacy of a bivalent HPV 16/18 vaccine against anal HPV 16/18 infection among young women: a nested analysis within the Costa Rica Vaccine Trial. Lancet Oncol. 2011;12:862–70. doi: 10.1016/S1470-2045(11)70213-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. US Food and Drug Administration news release: Gardasil approved to prevent anal cancer. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm237941.htm. Accessed 14 September 2011.
- 34.Kim JJ, Goldie SJ. Cost effectiveness analysis of including boys in a human papillomavirus vaccination programme in the United States. BMJ. 2009;339:b3884. doi: 10.1136/bmj.b3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim JJ. Targeted human papillomavirus vaccination of men who have sex with men in the USA: a cost-effectiveness modelling analysis. Lancet Infect Dis. 2010;10:845–52. doi: 10.1016/S1473-3099(10)70219-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilkin T, Lee JY, Lensing SY, et al. Safety and immunogenicity of the quadrivalent human papillomavirus vaccine in HIV-1-infected men. J Infect Dis. 2010;202:1246–53. doi: 10.1086/656320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goldie SJ, Kuntz KM, Weinstein MC, Freedberg KA, Welton ML, Palefsky JM. The clinical effectiveness and cost-effectiveness of screening for anal squamous intraepithelial lesions in homosexual and bisexual HIV-positive men. JAMA. 1999;281:1822–9. doi: 10.1001/jama.281.19.1822. [DOI] [PubMed] [Google Scholar]
- 38.Lampinen TM, Latulippe L, van Niekerk D, et al. Illustrated instructions for self-collection of anorectal swab specimens and their adequacy for cytological examination. Sex Transm Dis. 2006;33:386–8. doi: 10.1097/01.olq.0000204747.66265.2c. [DOI] [PubMed] [Google Scholar]
- 39. New York State Department of Health AIDS Institute. Neoplastic complications of HIV infection. Available at: http://www.hivguidelines.org/wp-content/uploads/2009/05/NEOPLACOMPLIC.pdf. Accessed 14 September 2011.
- 40.Chiao EY, Giordano TP, Palefsky JM, Tyring S, El Serag H. Screening HIV-infected individuals for anal cancer precursor lesions: a systematic review. Clin Infect Dis. 2006;43:223–33. doi: 10.1086/505219. [DOI] [PubMed] [Google Scholar]