A Vero cell culture–derived seasonal influenza vaccine provides consistently high levels of protection against cell culture–confirmed infection over a complete influenza season. Influenza symptoms are also less severe and of shorter duration in individuals who become infected despite vaccination.
Abstract
Background. Current knowledge of the consistency of protection induced by seasonal influenza vaccines over the duration of a full influenza season is limited, and little is known about the clinical course of disease in individuals who become infected despite vaccination.
Methods. Data from a randomized double-blind placebo-controlled clinical trial undertaken in healthy young adults in the 2008–2009 influenza season were used to investigate the weekly cumulative efficacy of a Vero cell culture–derived influenza vaccine. In addition, the duration and severity of disease in vaccine and placebo recipients with cell culture–confirmed influenza infection were compared.
Results. Vaccine efficacy against matching strains was consistently high (73%–82%) throughout the study, including the entire period of the influenza season during which influenza activity was above the epidemic threshold. Vaccine efficacy was also consistent (68%–83%) when calculated for all strains, irrespective of antigenic match. Vaccination also ameliorated disease symptoms when infection was not prevented. Bivariate analysis of duration and severity showed a significant amelioration of myalgia (P = .003), headache (P = .025), and fatigue (P = .013) in infected vaccinated subjects compared with placebo. Cough (P = .143) and oropharyngeal pain (P = .083) were also reduced in infected vaccinated subjects.
Conclusions. A Vero cell culture–derived influenza vaccine provides consistently high levels of protection against cell culture–confirmed infection by seasonal influenza virus and significantly reduces the duration and severity of disease in those individuals in which infection is not prevented.
Clinical Trials Registration. ClinicalTrials.gov NCT00566345.
Annual immunization is the most effective intervention to prevent disease resulting from infection with seasonal influenza virus. The efficacy of currently licensed trivalent seasonal influenza vaccines (TIVs) in preventing laboratory-confirmed influenza infection has been demonstrated in a number of recent placebo-controlled trials [1–5]. However, influenza vaccine efficacy studies have rarely addressed the consistency of vaccine-induced protection; vaccine efficacy is generally reported as a point estimate for the entire study period, irrespective of the chronological distribution of breakthrough infections. Considering that a single vaccination is intended to protect against seasonal influenza epidemics that may occur from early autumn until late spring, further evaluation of the stability of the protection provided by seasonal influenza vaccines is warranted.
Influenza vaccine efficacy can be influenced by a number of factors, including the extent to which individuals are exposed to influenza virus and the degree of antigenic similarity between circulating virus strains and thoseincluded in the vaccine [6]; in clinical trials, laboratory-confirmed infections are reported in a proportion of subjects despite vaccination. However, little is known about the clinical course of disease in these individuals. Efficacy studies that use laboratory-confirmed influenza infection as a clinical endpoint seldom report the duration or severity of disease associated with breakthrough infections. A reduction in influenzalike illness (ILI) symptoms is frequently used as a less stringent clinical endpoint to demonstrate vaccine effectiveness, but, without laboratory confirmation, it is difficult to infer a causal relationship between vaccination, influenza infection, and disease status [7].
A recent phase III placebo-controlled trial demonstrated a novel Vero cell culture–derived seasonal influenza vaccine (VCIV) to be 78.5% efficacious against cell culture–confirmed influenza infection (CCII) with antigenically matched virus strains [1]. In the present study, we undertook additional analyses of data from this trial to assess the consistency of vaccine efficacy over a complete influenza season and to investigate the potential of the vaccine to ameliorate disease symptoms in individuals in which infection is not prevented.
METHODS
Study Design and Objectives
A randomized, double-blind placebo-controlled phase III trial investigating the efficacy of a VCIV has been described elsewhere [1]. This study was undertaken during the 2008–2009 Northern hemisphere influenza season at 36 centers throughout the United States in healthy young adults aged 18–49 years. The primary study objective was to demonstrate the efficacy of VCIV in preventing CCII due to influenza virus that was antigenically matched to 1 of the vaccine strains. Post hoc analyses were performed to assess the consistency of vaccine efficacy and to determine the effect of vaccination on the duration and severity of disease in infected individuals. The Sterling Institutional Review Board, Atlanta, GA, approved the study protocol and consent forms used in the study, which was conducted in compliance with Good Clinical Practice guidelines and the Declaration of Helsinki. The study employed a 4-member independent Data Monitoring Committee. Individuals who demonstrated an understanding of the study and provided written informed consent were accepted as potential study volunteers.
Vaccination and ILI Follow-up
Subjects were equally randomized to receive an intramuscular injection of 0.5 mL VCIV or placebo. VCIV is a trivalent split vaccine that was formulated to contain 15 μg of hemagglutinin antigen from each of the vaccine strains A/Brisbane/59/2007 (A/H1N1), A/Uruguay/716/2007 (A/H3N2), and B/Florida/4/2006. Vaccine viruses were egg-derived wild-type strains provided by the National Institute for Biological Standards and Control, Potters Bar, United Kingdom, which were subjected to 3 passages in Vero cell culture prior to vaccine production. Sequence analysis of the production viruses showed that typical mutations resulting from egg growth were retained following culture in Vero cells. Placebo was phosphate-buffered saline.
From 3 weeks postvaccination, subjects were instructed to return to the clinic for an ILI visit within 48 hours of onset of symptoms should they experience fever with cough, sore throat, muscle ache, headache, fatigue, nausea, or bloodshot eyes, or if they should experience any 2 of the listed symptoms in the absence of fever. At every ILI visit, nasopharyngeal swabs were obtained for culturing and typing of viruses.
Assessment of Disease Severity and Duration
ILI duration and severity data were obtained by post hoc review of subjects’ case report forms. Disease severity was investigator-assessed and classified as being mild, moderate, or severe. Symptoms that caused transient discomfort, which do not significantly interfere with normal function, and which resolve spontaneously or which may require minimal therapeutic intervention, were classified as mild. Symptoms that caused limited impairment of function, which could require therapeutic intervention but which caused no sequelae, were classified as moderate. Symptoms that resulted in marked impairment of function, which could lead to a temporary inability to resume normal life, and which produced sequelae that required prolonged therapeutic intervention, were classified as severe.
Laboratory Confirmation and Antigenic Typing of Influenza Viruses
Virus specimens collected using nasopharyngeal swabs at ILI visits were shipped to Bio Analytical Research Corporation, Lake Success, New York, for culture using traditional cell culture and Rapid R-Mix (Diagnostic Hybrids, Athens, Ohio) and virus typing via real-time polymerase chain reaction. The antigenic relatedness of virus isolates to vaccine strains was determined by hemagglutination inhibition analysis at the laboratory of the Influenza Division, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention (CDC). Genetic sequencing was done by GENEWIZ Inc, South Plainfield, New Jersey. Assessment of the genetic lineage of the B strains was performed at the Institute of Virology, Medical University of Vienna, Austria.
Statistical Analyses
Calculation of the cumulative weekly vaccine efficacy included all randomly assigned and immunized participants who stayed in the study for at least 21 days after vaccination. Cumulative vaccine efficacy (VE) was computed by the formula VE = (1 − RR) × 100, where RR is the ratio of influenza infection risk in the VCIV group compared with the risk in the placebo group. The 95% confidence interval (CI) of the RR was computed by the method proposed by Miettinen and Nurminen [8].
Analysis of the duration and severity of ILI symptoms was performed on all subjects with CCII. Subjects were analyzed as randomized; there were no randomization errors in this subset. All the Medical Dictionary for Regulatory Activities (MedDRA) preferred terms of adverse events (ILI symptoms) associated with influenza infection occurring within 3 days from the date of swab collection were listed. If a symptom occurred more than once in any subject, durations were added, whereas severity was considered to be the maximum of the severity scores. Symptoms with an incidence of <10% were not included in further analyses. To compare the joint distribution of duration and severity between the 2 treatment groups, a bivariate nonparametric permutation-based method (POSET test [9]) was applied, preceded by a partial ordering procedure on the pairs of data (severity, duration) in order to assign ranks to each pair. No adjustments were made for multiplicity. For each P value, 40 000 permutations were generated in order to obtain a standard error of around 0.001.
RESULTS
Study Participants
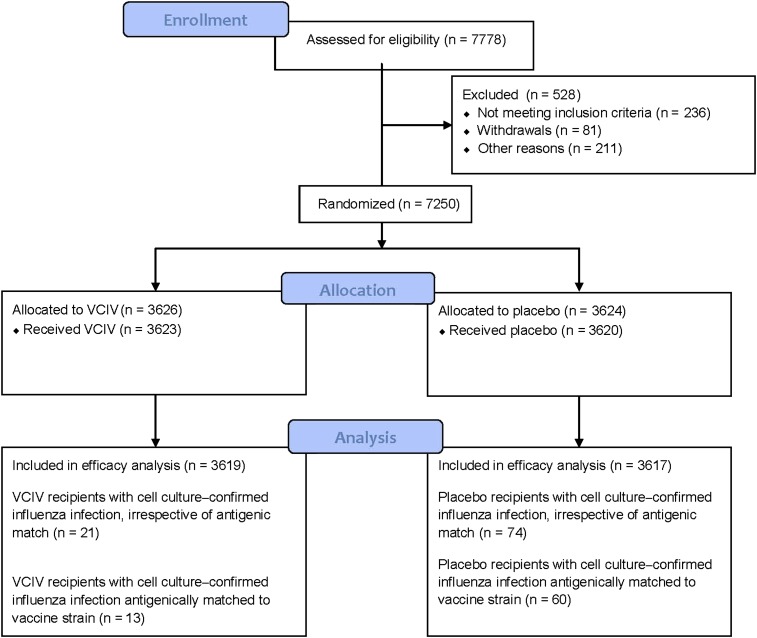
The flow of participants through the study is shown in Figure 1. All study subjects were vaccinated between 1 and 15 December 2008. The baseline characteristics of participants who received either VCIV or placebo is shown in Table 1. A total of 7243 participants received either VCIV (3623) or placebo (3620). The VCIV and placebo groups were evenly balanced by age and sex. The age range in both groups was 18–49 years, and the median age was 31 and 30 years for the VCIV and placebo groups, respectively. Analysis of swab specimens obtained at ILI visits continued until 15 May; no cell culture–confirmed influenza infections were recorded for specimens collected after the week ending 9 May.
Figure 1.
Flow of participants through the study. Abbreviation: VCIV, Vero cell culture–derived influenza vaccine.
Table 1.
Baseline Characteristics of Participants Receiving Vero Cell Culture--Derived Influenza Vaccine or Placebo
| Characteristics | VCIV (n = 3623) | Placebo (n = 3620) | Overall (n = 7243) |
| Male, No. (%) | 1823 (25.2) | 1865 (25.7) | 3688 (50.9) |
| Female, No. (%) | 1800 (24.9) | 1755 (24.2) | 3555 (49.1) |
| Age, years, mean (SD) [median] | 32.2 (9.7) [31.0] | 32.1 (9.7) [30.0] | 32.1 (9.7) [31.0] |
| Age range, years | 18–49 | 18–49 | 18–49 |
Abbreviations: SD, standard deviation; VCIV, Vero cell culture–derived influenza vaccine.
Consistency of Vaccine Efficacy Throughout an Entire Influenza Season
To investigate the consistency of vaccine efficacy on a week-by-week basis over the duration of the influenza season, the cumulative frequency of infections occurring in subjects receiving either vaccine or placebo were analyzed. Up to the week ending 17 January 2009, only 7 subjects reporting with ILI were shown to be infected with influenza virus. Six of these subjects had received placebo, with only a single vaccine recipient becoming infected during this period. However, due to the low number of infections, no statistical analysis of the consistency of vaccine efficacy could be carried out until the week ending 24 January 2009.
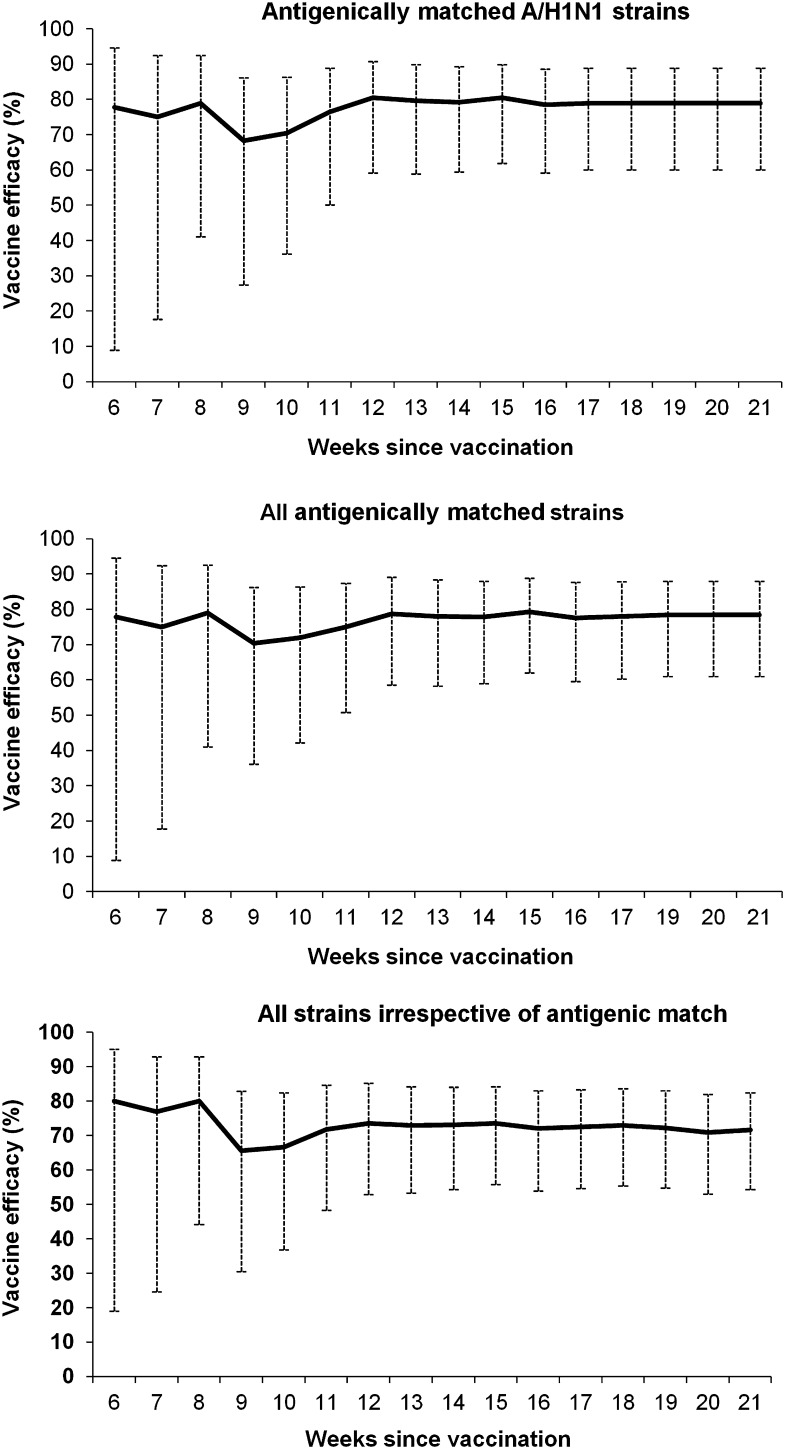
Figure 2 shows the cumulative efficacy of VCIV in preventing CCII from the week ending 24 January to the week ending 9 May 2009. These data show that vaccine efficacy is consistently high, including for the duration of the entire period from the end of January through mid-March during which influenza activity was above the epidemic threshold [10]. The weekly cumulative point estimate for vaccine efficacy was 71%–82% against cell culture–confirmed infection with antigenically matched A/H1N1 strains (Figure 2A), 73%–82% for cell culture–confirmed infections with all antigenically matched strains (Figure 2B), and 68%–83% for all cell culture–confirmed infections, irrespective of antigenic similarity to the vaccine strains (Figure 2C). The wider 95% CIs for the point estimates of vaccine efficacy during the first weeks of the observation period are a consequence of the low cumulative number of infections during this period (a total of only 14 infections up to the week ending 31 January), reflecting the fact that the seasonal influenza activity in the United States remained below the national baseline until the end of January and did not peak until mid-February [10].
Figure 2.
Weekly cumulative vaccine efficacy against cell culture–confirmed influenza infection. Vaccine efficacy could be calculated from 24 January 2009, ∼6 weeks after vaccination of the last subject on 15 December. Analysis of specimens from influenzalike illness visits continued until 15 May; the last laboratory-confirmed influenza infection was recorded in the week ending 9 May 2009.
Vaccine efficacy was at least as high in the later stages of the study compared with earlier stages; the weekly cumulative vaccine efficacy from the beginning of March onward was >78% for antigenically matched A/H1N1 strains, >77% for all antigenically matched strains, and >70% for all strains, irrespective of antigenic match. The consistency of vaccine efficacy over time was also investigated by dividing the study into 2 periods and analyzing vaccine efficacy in the first 3 months of the study compared with the period thereafter. In the first period, 10 of 3619 VCIV recipients had antigenically matched CCII compared with 40 of 3617 placebo recipients, such that vaccine efficacy was estimated to be 75.0% (95% CI, 50.8%–87.3%). In the second period, 3 of 3532 VCIV recipients had antigenically matched CCII compared with 20 of 3530 placebo recipients, resulting in vaccine efficacy of 85.0% (95% CI, 52.8%–95.2%). This analysis demonstrates that vaccine efficacy was similar during both periods.
Duration and Severity of ILI Symptoms in VCIV and Placebo Recipients With Cell Culture–Confirmed Influenza Infection
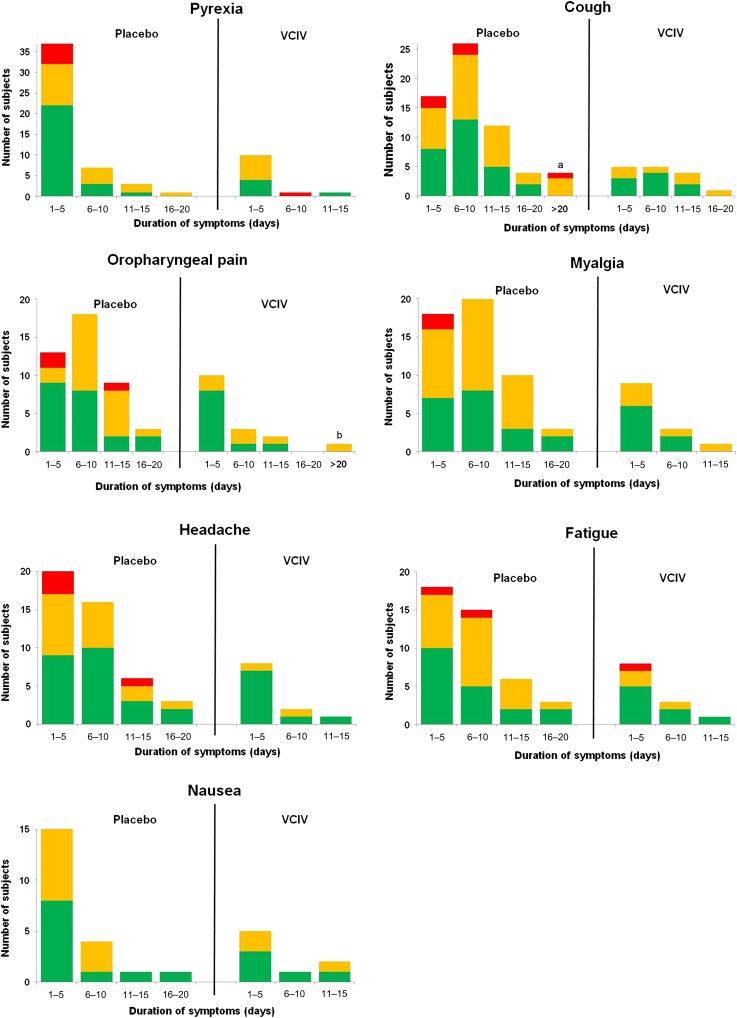
Despite the high efficacy of VCIV in preventing influenza infection, over the duration of the study a total of 21 vaccinated individuals were nevertheless infected with influenza virus as determined by CCII. To investigate the potential of vaccination to ameliorate influenza disease symptoms in subjects in which infection is not prevented, we compared the severity and duration of ILI symptoms in all placebo and VCIV recipients with CCII. For the majority of symptoms, VCIV recipients had a reduced proportion of cases rated moderate or severe and a reduced proportion of cases of longer duration (Figure 3). VCIV recipients experienced no severe cough, oropharyngeal pain, myalgia, headache, or nausea, and only single cases of severe pyrexia or fatigue. The mean and median duration of all ILI symptoms were reduced for VCIV recipients with the exception of nausea, and the mean severity score of all symptoms was reduced for all VCIV recipients with the exception of pyrexia (Table 2). Bivariate statistical analyses of the joint benefit of reduction in disease severity and duration show that this is significant for myalgia (P = .003), headache (P = .025), and fatigue (P = .013). There were also reductions in the duration and severity of cough (P = .143) and oropharyngeal pain (P = .083) for VCIV recipients, although these are not statistically significant.
Figure 3.
Amelioration of disease symptoms in subjects with cell culture–confirmed influenza infection. Symptoms are rated as severe (red), moderate (orange), or mild (green). aFour placebo recipients had cough for >20 days. Three were rated as moderate (31, 35, and 51 days duration), and 1 was rated as severe (22 days duration). bOne recipient of Vero cell culture–derived influenza vaccine had oropharyngeal pain for 31 days, rated moderate. Abbreviation: VCIV, Vero cell culture--derived influenza vaccine.
Table 2.
Bivariate Analysis of the Severity and Duration of Influenzalike Illness Symptoms Among Subjects With Cell Culture–Confirmed Influenza Infection
| No. (%) of Subjects With Symptoms |
|||||||
| Symptoms | Total | Mild | Moderate | Severe | Mean Severity Scorea | Duration, Days, Mean (Median) | P Valueb |
| VCIV (n = 21) | |||||||
| Pyrexia | 12 (57) | 5 (23.8) | 6 (28.6) | 1 (4.8) | 1.7 | 3.6 (3.0) | .491 |
| Cough | 15 (71) | 9 (42.9) | 6 (28.6) | 0 | 1.4 | 8.8 (7.0) | .143 |
| Oropharyngeal pain | 16 (76) | 10 (47.6) | 6 (28.6) | 0 | 1.4 | 7.4 (5.0) | .083 |
| Myalgia | 13 (62) | 8 (38.1) | 5 (23.8) | 0 | 1.4 | 4.2 (3.0) | .003 |
| Headache | 11 (52) | 9 (42.9) | 2 (9.5) | 0 | 1.2 | 5.6 (5.0) | .025 |
| Fatigue | 12 (57) | 8 (38.1) | 3 (14.3) | 1 (4.8) | 1.4 | 4.9 (3.5) | .013 |
| Nausea | 8 (38) | 5 (23.8) | 3 (14.3) | 0 | 1.4 | 6.9 (6.0) | .479 |
| Placebo (n = 74) | |||||||
| Pyrexia | 48 (65) | 26 (35.1) | 17 (23.0) | 5 (6.8) | 1.6 | 4.4 (3.5) | … |
| Cough | 63 (85) | 28 (37.8) | 30 (40.5) | 5 (6.8) | 1.6 | 10.0 (8.0) | … |
| Oropharyngeal pain | 43 (58) | 21 (28.4) | 19 (25.7) | 3 (4.1) | 1.6 | 8.1 (7.0) | … |
| Myalgia | 51 (69) | 20 (27.0) | 29 (39.2) | 2 (2.7) | 1.6 | 7.8 (7.0) | … |
| Headache | 45 (61) | 24 (32.4) | 17 (23.0) | 4 (5.4) | 1.6 | 7.1 (6.0) | … |
| Fatigue | 42 (57) | 19 (25.7) | 21 (28.4) | 2 (2.7) | 1.6 | 7.4 (6.0) | … |
| Nausea | 21 (28) | 11 (14.9) | 10 (13.5) | 0 | 1.5 | 5.1 (5.0) | … |
Abbreviations: ILI, influenzalike illness; VCIV, Vero cell culture–derived influenza vaccine.
Symptoms rated as mild were given a score of 1, moderate symptoms a score of 2, and severe symptoms a score of 3.
P value (POSET test) of the reduction in severity and duration of symptoms in VCIV recipients compared with placebo recipients.
DISCUSSION
We investigated the consistency of protection induced by a novel Vero cell culture–derived seasonal influenza vaccine in the United States in 2008–2009. Only low levels of influenza activity were detected up until mid-January 2009, but activity increased in mid-January, peaking in mid-February, and remaining high until mid-March [10]. The Vero-derived vaccine was consistently highly efficacious in preventing laboratory-confirmed influenza infection over this entire period (Figure 2). Importantly, vaccine efficacy did not wane as the influenza season progressed; the weekly cumulative vaccine efficacy from the beginning of March onward was >78% for antigenically matched A/H1N1 strains, >77% for all antigenically matched strains, and >70% for all strains, irrespective of antigenic match. This is also demonstrated by the similarly high estimates of vaccine efficacy for antigenically matched strains in the first 3 months of the study (75.0%) compared with the period thereafter (85.0%). Because subjects were vaccinated in December, our study does not allow us to be certain that vaccine efficacy would be maintained at equally high levels to the end of an influenza season if vaccination were to be initiated at an earlier time point, as recommended by the CDC [11]. However, as there was no indication of a reduction in vaccine efficacy up to 5 months after vaccination, it seems likely that earlier vaccination would also provide season-long protection.
Other studies assessing the longevity or consistency of vaccine efficacy against infection with seasonal influenza virus are limited. Historically, monovalent vaccines were reported to provide extended protection against the Hong Kong [12, 13] and Russian pandemic strain viruses [14]. More recent studies have reported a trivalent live-attenuated influenza vaccine (LAIV) to provide stable levels of protection against CCII for up to 12 months in children [15], and, in comparative studies, LAIV was relatively more efficacious than TIV in preventing CCII >4–8 months postvaccination compared to 0--4 months postvaccination [16]. To our knowledge, the consistency of the protective efficacy of modern split or subunit TIVs compared with placebo has not previously been demonstrated in any population.
The cumulative vaccine efficacy estimates and efficacy up to or after 3 months reported here for the Vero-derived vaccine are similar to those determined for the entire study period, where vaccine efficacy was estimated to be 79% for antigenically matched A/H1N1 strains, 78.5% for all matched strains, and 71.5% for all strains, irrespective of antigenic match [1]. It is difficult to compare absolute vaccine efficacy between independent studies due to a number of factors that cause heterogeneity between different studies, such as differences in virus strains, attack rate of circulating viruses, and clinical endpoints used; however, these efficacy estimates are in the upper range of those reported in other recent studies of seasonal influenza vaccines [2–5, 17, 18] and similar to or higher than those reported in recent meta-analyses of TIV efficacy [19, 20].
A proportion of individuals were infected with influenza virus despite vaccination. However, our results demonstrate that the benefit of vaccination extends beyond the prevention of infection, because the majority of ILI symptoms in subjects with CCII were both milder and of shorter duration in vaccine recipients compared with placebo recipients (Figure 3). In the cases of myalgia (P = .003), headache (P = .025), and fatigue (P = .013), the joint reduction in disease duration and severity was statistically significant (Table 2). The demonstration of statistical significance of VCIV in amelioration of disease is noteworthy in the context of the high efficacy of the vaccine in preventing influenza infection. This resulted in a small sample size for infected VCIV recipients and, consequently, a lower power of analysis, such that a larger effect is necessary to demonstrate statistical significance of disease amelioration than would be the case for a less efficacious vaccine. Amelioration of cough (P = .143) and oropharyngeal pain (P = .083) was also observed. The reduction in the severity and duration of these symptoms may also be clinically relevant, although the small sample size precluded the calculation of a statistically significant benefit.
Previous investigations into the potential of influenza vaccines to ameliorate disease in subjects with laboratory-confirmed influenza infection have been limited to a restricted selection of ILI symptoms, and few statistically significant reductions in the severity or duration of disease symptoms have been reported. A small number of studies have reported significant reductions in fever for TIV [21] or LAIV [22] recipients, or have shown a significant benefit of LAIV over TIV for the reduction of fever in children [23] or older adults [24]. Both TIV and LAIV have also been reported to reduce the rate of acute otitis media (AOM) in children [25]; we did not investigate the occurrence of AOM because our study was of young healthy adults, and AOM is not a common symptom of influenza infection in this population. Prevention of infection and disease in vaccinated individuals also has the potential to benefit nonvaccinated contacts by preventing or reducing virus transmission. In this respect, the level of virus shedding in individuals who were infected despite vaccination would also be of interest. Our study did not include a quantitative assay for detection of virus in nasopharyngeal specimens; however, other studies have suggested that virus shedding may be less effectively reduced in recipients of TIV compared with recipients of LAIV [26–28].
The conclusions of our study are subject to the limitations that are inherent to any post hoc analyses of study data. In addition, because no study arm with an egg-derived vaccine was included, we cannot make a direct comparison of vaccines produced in Vero cell culture or using standard technology. Other limitations are that due to the nature of the 2008–2009 influenza season, data are primarily for the A/H1N1 strain and only for adults aged 18–49 years because vaccination was recommended for all other populations in the United States at the time of the study. However, the potential clinical and socioeconomic benefits of vaccination in younger adults are also substantial [29–31], as reflected in the global drive to increase vaccine coverage beyond traditional high-risk groups. In the United States, annual vaccination is now recommended for all individuals above the age of 6 months [32], and it is the aim of the US Healthy People 2020 goals to achieve 80%–90% vaccine coverage [33]. Improvements in influenza vaccine supply may be required to achieve these goals, and the availability of modern, robust, and flexible manufacturing technologies will facilitate this process [34, 35]. The present study makes an important contribution to a growing body of evidence supporting the use of cell culture technology for vaccine manufacture.
Notes
Author contributions.
The study was designed by H. J. E., J. S., G. B., S. F., G. A., M. K. H., W. E. A., O. K., and P. N. B. Data were collected by G. B., G. A., M. K. H., and W. E. A., and analyzed by H. J. E., J. S., G. B., S. F., G. A., D. P., M. K. H., W. E. A., O. K., and P. N. B. All authors contributed to preparation and editing of the manuscript and approved the final version of publication.
Acknowledgments.
We would like to thank the following investigators at 36 study sites in the United States: Richard Beasley (Health Concepts, Rapid City, SD); Bruce Berwald (Radiant Research, St. Louis, MO); James Borders (Central Kentucky Research Associates, Lexington); Donald Brandon (California Research Foundation, San Diego); Laurence Chu (Benchmark Research Austin, Austin, TX); Arsenio Columbie (Pharmax Research Clinic, Miami, FL); Matthew Davis (Rochester Clinical Research, Rochester, NY); Victor Elinoff (Regional Clinical Research, Endwell, NY); Robert Epstein (Benchmark Research, San Francisco, CA); John Ervin (Center for Pharmaceutical Research, Kansas City, MO); Brandon Essink (Meridian Clinical Research, Omaha, NE); Charles Fogarty (Spartanburg Medical Research, Spartanburg, SC); David Fried (Omega Medical Research, Warwick, RI); Frederic Garner (PI-COOR Clinical Research, Burke, VA); Jeffrey Geohas (Radiant Research, Chicago, IL); Larry Gilderman (University Clinical Research, Pembroke Pines, FL); Wayne Harper (Wake Research Associates, Raleigh, NC); Darrell Herrington (Benchmark Research, San Angelo, TX); Steven Hull (Vince and Associates Clinical Research, Overland Park, KS); Jeff Jacqmein (Jacksonville Centre for Clinical Research, Jacksonville, FL); Robert Jeanfreau (Benchmark Research, Metairie, LA); Casey Johnson (Johnson County Clin-Trials, Lenexa, KS); Judith Kirstein (Advanced Clinical Research, West Jordan, UT); John Gettigan (Quality of Life Medical and Research Centre, Tucson, AZ); Leslie Moldauer (Radiant Research, Denver, CO); Michael Noss (Radiant Research, Cincinnati, OH); Gilbert Podolsky (Jean Brown Research, Salt Lake City, UT); Ivan Rarick (Benchmark Research, Sacramento, CA); Jeffrey Rosen (Clinical Research of South Florida, Coral Gables); John Rubino (Triangle Medical Research Associates, Raleigh, NC); Nathan Segall (Clinical Research Atlanta, Stockbridge, GA); William Seger (Benchmark Research, Fort Worth, TX); Stephan Sharp (Clinical Research Associates, Nashville, TN); Eric Sheldon (Miami Research Associates, South Miami, FL); Larkin Wadsworth (Sundance Clinical Research, St Louis, MO); Duane Wombolt (Clinical Research Associates of Tidewater, Norfolk, VA).
We thank the members of the Baxter research and development team (Roman Bobrovsky, Julie McLaren, Borislava Pavlova, Miranda Schneider, Leopold Grillberger, Keith Howard, Wolfgang Mundt, Manfred Reiter, Helga Savidis-Dacho, Christa Tauer, and Walter Wodal) for their role in this study, and members of the DynPort Vaccine Company (Cheryl Main, Aparna Kolhekar, and Patti Postal) for their support of this study; acknowledge the support of Alexander Klimov, Xiyan Xu, and Nancy Cox at CDC, Atlanta, GA, for the virus typing by use of the HI assay, and Franz X Heinz, Monika Redlberger-Fritz, and Therese Popow-Kraupp at the Institute of Virology, Medical University Vienna, Austria, for assessment of genetic lineage of the B strains.
Financial support.
This work was supported by federal (US government) funds from the Department of Health and Human Services, Office of the Assistant Secretary for Preparedness and Response, Biomedical Advanced Research and Development Authority (contract HHS0100200600013C) to DynPort Vaccine Company LLC (DVC), a CSC company. The content of this publication does not necessarily reflect the views or policies of the United States Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Potential conflicts of interest.
H. J. E., J. S., G. B., S. F., G. A., D. P., O. K., and P. N. B. report being employed by Baxter. H. J. E., J. S., G. B., S. F., G. A., O. K., and P. N. B. report having an equity interest in the company, and O. K. and P. N. B. hold patents on influenza vaccines derived from Vero cell cultures. M. K. H. and W. E. A. report being employed by DVC, and M. K. H. reports having an equity investment in CSC, the parent company of DVC.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Barrett PN, Berezuk G, Fritsch S, et al. Efficacy, safety, and immunogenicity of a Vero-cell-culture-derived trivalent influenza vaccine: a multicentre, double-blind, randomised, placebo-controlled trial. Lancet. 2011;377:751–9. doi: 10.1016/S0140-6736(10)62228-3. [DOI] [PubMed] [Google Scholar]
- 2.Frey S, Vesikari T, Szymczakiewicz-Multanowska A, et al. Clinical efficacy of cell culture-derived and egg-derived inactivated subunit influenza vaccines in healthy adults. Clin Infect Dis. 2010;51:997–1004. doi: 10.1086/656578. [DOI] [PubMed] [Google Scholar]
- 3.Monto AS, Ohmit SE, Petrie JG, et al. Comparative efficacy of inactivated and live attenuated influenza vaccines. N Engl J Med. 2009;361:1260–7. doi: 10.1056/NEJMoa0808652. [DOI] [PubMed] [Google Scholar]
- 4.Beran J, Vesikari T, Wertzova V, et al. Efficacy of inactivated split-virus influenza vaccine against culture-confirmed influenza in healthy adults: a prospective, randomized, placebo-controlled trial. J Infect Dis. 2009;200:1861–9. doi: 10.1086/648406. [DOI] [PubMed] [Google Scholar]
- 5.Ohmit SE, Victor JC, Rotthoff JR, et al. Prevention of antigenically drifted influenza by inactivated and live attenuated vaccines. N Engl J Med. 2006;355:2513–22. doi: 10.1056/NEJMoa061850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beran J, Wertzova V, Honegr K, et al. Challenge of conducting a placebo-controlled randomized efficacy study for influenza vaccine in a season with low attack rate and a mismatched vaccine B strain: a concrete example. BMC Infect Dis. 2009;9:2. doi: 10.1186/1471-2334-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nichol KL. Heterogeneity of influenza case definitions and implications for interpreting and comparing study results. Vaccine. 2006;24:6726–8. doi: 10.1016/j.vaccine.2006.05.064. [DOI] [PubMed] [Google Scholar]
- 8.Miettinen O, Nurminen M. Comparative analysis of two rates. Stat Med. 1985;4:213–26. doi: 10.1002/sim.4780040211. [DOI] [PubMed] [Google Scholar]
- 9.Hothorn T, Hornik K, van de Wiel MA, Zeileis A. A Lego system for conditional inference. Am Stat. 2006;60:257–63. [Google Scholar]
- 10.CDC. Update: influenza activity—United States, September 28, 2008–April 4, 2009, and composition of the 2009–10 influenza vaccine. MMWR Morb Mortal Wkly Rep. 2009;58:369–74. [PubMed] [Google Scholar]
- 11. CDC. Key facts about influenza (flu) and flu vaccine. Available at: http://www.cdc.gov/flu/keyfacts.htm. Accessed 23 August 2011.
- 12.Foy HM, Cooney MK, McMahan R, Bor E, Grayston JT. Single-dose monovalent A2-Hong Kong influenza vaccine: Efficacy 14 months after immunization. JAMA. 1971;217:1067–71. [PubMed] [Google Scholar]
- 13.Foy HM, Cooney MK, McMahan R. A Hong Kong influenza immunity three years after immunization. JAMA. 1973;226:758–61. [PubMed] [Google Scholar]
- 14.Clark A, Potter CW, Jennings R, et al. A comparison of live and inactivated influenza A (H1N1) virus vaccines: 2. Long-term immunity. J Hyg (Lond) 1983;90:361–70. doi: 10.1017/s0022172400028990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ambrose CS, Yi T, Walker RE, Connor EM. Duration of protection provided by live attenuated influenza vaccine in children. Pediatr Infect Dis J. 2008;27:744–8. doi: 10.1097/INF.0b013e318174e0f8. [DOI] [PubMed] [Google Scholar]
- 16.Ambrose CS, Wu X, Belshe RB. The efficacy of live attenuated and inactivated influenza vaccines in children as a function of time postvaccination. Pediatr Infect Dis J. 2010;29:806–11. doi: 10.1097/INF.0b013e3181e2872f. [DOI] [PubMed] [Google Scholar]
- 17.Jackson LA, Gaglani MJ, Keyserling HL, et al. Safety, efficacy, and immunogenicity of an inactivated influenza vaccine in healthy adults: a randomized, placebo-controlled trial over two influenza seasons. BMC Infect Dis. 2010;10:71. doi: 10.1186/1471-2334-10-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ohmit SE, Victor JC, Teich ER, et al. Prevention of symptomatic seasonal influenza in 2005–2006 by inactivated and live attenuated vaccines. J Infect Dis. 2008;198:312–17. doi: 10.1086/589885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Osterholm MT, Kelley NS, Sommer A, Belongia EA. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:36–44. doi: 10.1016/S1473-3099(11)70295-X. [DOI] [PubMed] [Google Scholar]
- 20.Jefferson T, Di Pietrantonj C, Rivetti A, Bawazeer GA, Al-Ansary LA, Ferroni E. Vaccines for preventing influenza in healthy adults. Cochrane Database Syst Rev. 2010 doi: 10.1002/14651858.CD001269.pub4. (7):CD001269. [DOI] [PubMed] [Google Scholar]
- 21.Kamada M, Nagai T, Kumagai T, et al. Efficacy of inactivated trivalent influenza vaccine in alleviating the febrile illness of culture-confirmed influenza in children in the 2000–2001 influenza season. Vaccine. 2006;24:3618–23. doi: 10.1016/j.vaccine.2006.01.067. [DOI] [PubMed] [Google Scholar]
- 22.Belshe RB, Mendelman PM, Treanor J, et al. The efficacy of live attenuated, cold-adapted, trivalent, intranasal influenza virus vaccine in children. N Engl J Med. 1998;338:1405–12. doi: 10.1056/NEJM199805143382002. [DOI] [PubMed] [Google Scholar]
- 23.Yang CF, Belshe RB, Kemble G, et al. Genetic sequence analysis of influenza viruses and illness severity in ill children previously vaccinated with live attenuated or inactivated influenza vaccine. Vaccine. 2010;28:5128–34. doi: 10.1016/j.vaccine.2010.02.109. [DOI] [PubMed] [Google Scholar]
- 24.Forrest BD, Steele AD, Hiemstra L, Rappaport R, Ambrose CS, Gruber WC. A prospective, randomized, open-label trial comparing the safety and efficacy of trivalent live attenuated and inactivated influenza vaccines in adults 60 years of age and older. Vaccine. 2011;29:3633–9. doi: 10.1016/j.vaccine.2011.03.029. [DOI] [PubMed] [Google Scholar]
- 25.Block SL, Heikkinen T, Toback SL, Zheng W, Ambrose CS. The efficacy of live attenuated influenza vaccine against influenza-associated acute otitis media in children. Pediatr Infect Dis J. 2011;30:203–7. doi: 10.1097/INF.0b013e3181faac7c. [DOI] [PubMed] [Google Scholar]
- 26.Clements ML, Betts RF, Murphy BR. Advantage of live attenuated cold-adapted influenza A virus over inactivated vaccine for A/Washington/80 (H3N2) wild-type virus infection. Lancet. 1984;1:705–8. doi: 10.1016/s0140-6736(84)92222-0. [DOI] [PubMed] [Google Scholar]
- 27.Clements ML, Murphy BR. Development and persistence of local and systemic antibody responses in adults given live attenuated or inactivated influenza A virus vaccine. J Clin Microbiol. 1986;23:66–72. doi: 10.1128/jcm.23.1.66-72.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sears SD, Clements ML, Betts RF, Maassab HF, Murphy BR, Snyder MH. Comparison of live, attenuated H1N1 and H3N2 cold-adapted and avian-human influenza A reassortant viruses and inactivated virus vaccine in adults. J Infect Dis. 1988;158:1209–19. doi: 10.1093/infdis/158.6.1209. [DOI] [PubMed] [Google Scholar]
- 29.Nichol KL, Lind A, Margolis KL, et al. The effectiveness of vaccination against influenza in healthy, working adults. N Engl J Med. 1995;333:889–93. doi: 10.1056/NEJM199510053331401. [DOI] [PubMed] [Google Scholar]
- 30.Nichol KL, Mallon KP, Mendelman PM. Cost benefit of influenza vaccination in healthy, working adults: an economic analysis based on the results of a clinical trial of trivalent live attenuated influenza virus vaccine. Vaccine. 2003;21:2207–17. doi: 10.1016/s0264-410x(03)00029-x. [DOI] [PubMed] [Google Scholar]
- 31.Molinari NA, Ortega-Sanchez IR, Messonnier ML, et al. The annual impact of seasonal influenza in the US: measuring disease burden and costs. Vaccine. 2007;25:5086–96. doi: 10.1016/j.vaccine.2007.03.046. [DOI] [PubMed] [Google Scholar]
- 32.Fiore AE, Uyeki TM, Broder K, et al. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Recomm Rep. 2010;59:1–62. [PubMed] [Google Scholar]
- 33. US Department of Health and Human Services. Healthy people 2020: topics and objectives index. Available at: http://www.healthypeople.gov/2020/topicsobjectives2020/pdfs/HP2020objectives.pdf. Accessed 23 August 2011.
- 34.Barrett PN, Mundt W, Kistner O, Howard MK. Vero cell platform in vaccine production: moving towards cell culture–based viral vaccines. Expert Rev Vaccines. 2009;8:607–18. doi: 10.1586/erv.09.19. [DOI] [PubMed] [Google Scholar]
- 35.Glezen WP. Cell-culture-derived influenza vaccine production. Lancet. 2011;377:698–700. doi: 10.1016/S0140-6736(11)60174-8. [DOI] [PubMed] [Google Scholar]