In this randomized, double-blind, placebo-controlled trial of human immunodeficiency virus–infected youths aged 18–25, vitamin D3, 50000 IU once monthly for 3 months decreased parathyroid hormone in participants treated with tenofovir-containing antiretroviral regimens but not in those participants whose regimens did not contain tenofovir.
Abstract
Background. The study goal was to determine the effect of vitamin D (VITD) supplementation on tubular reabsorption of phosphate (TRP), parathyroid hormone (PTH), bone alkaline phosphatase (BAP), and C-telopeptide (CTX) in youth infected with human immunodeficiency virus (HIV) receiving and not receiving combination antiretroviral therapy (cART) containing tenofovir disoproxil fumarate (TDF).
Methods. This randomized, double-blind, placebo-controlled multicenter trial enrolled HIV-infected youth 18–25 years based on stable treatment with cART containing TDF (n = 118) or no TDF (noTDF; n = 85), and randomized within those groups to vitamin D3, 50 000 IU (n = 102) or placebo (n = 101), administered at 0, 4, and 8 weeks. Outcomes included change in TRP, PTH, BAP, and CTX from baseline to week 12 by TDF/noTDF; and VITD/placebo.
Results. At baseline, VITD and placebo groups were similar except those on TDF had lower TRP and higher PTH and CTX. At week 12, 95% in the VITD group had sufficient serum 25-hydroxy vitamin D (25-OHD; ≥20 ng/mL), increased from 48% at baseline, without change in placebo (P < .001). PTH decreased in the TDF group receiving VITD (P = .031) but not in the noTDF group receiving VITD, or either placebo group. The decrease in PTH with VITD in those on TDF occurred with insufficient and sufficient baseline 25-OHD (mean PTH change, −7.9 and −6.2 pg/mL; P = .031 and .053, respectively).
Conclusions. In youth on TDF, vitamin D3 supplementation decreased PTH, regardless of baseline 25-OHD concentration.
Clinical Trials Registration. NCT00490412.
Tenofovir disoproxil fumarate (TDF), a commonly used antiretroviral, is associated with decline in glomerular filtration rate (GFR) [1] and renal tubular phosphate wasting [2–8], measured as decreased tubular reabsorption of phosphate (TRP) [3, 4]. TDF use has been associated with alterations in bone mineralization and calcium balance, decreased bone mineral density (BMD) [9–13], and increased bone-specific alkaline phosphatase (BAP) [14], osteocalcin [13], and parathyroid hormone (PTH) (product label and [15]). TDF-induced decreases in BMD and increases in bone turnover may result from renal tubular phosphate wasting causing hypophosphatemia and secondary osteomalacia [14, 16], or through a TDF effect on PTH and calcium-phosphate balance [15, 16].
Vitamin D deficiency, common in youth with human immunodeficiency virus (HIV) infection in the United States [17], is also associated with renal tubular phosphate loss, hyperparathyroidism, and osteomalacia in non–HIV-infected populations [18]. In persons with vitamin D deficiency, vitamin D treatment increases TRP, decreases serum PTH, and improves bone health [18].
We hypothesized that independent of baseline vitamin D serum concentration, vitamin D supplementation in adolescents and young adults with HIV infection treated with TDF would increase TRP and decrease serum PTH and markers of bone turnover (BAP and C-telopeptide [CTX]) in a pattern similar to those with vitamin D deficiency not treated with TDF.
METHODS
The Adolescent Medicine Trials Network (ATN) for HIV/AIDS Interventions study 063 was a 12-week randomized, double-blind, placebo-controlled, multicenter trial performed between November 2007 and April 2010 at 16 ATN and 19 International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) sites in the United States and Puerto Rico. ATN predominately serves older adolescents and young adults with behaviorally acquired HIV infection (https://www.atnonline.org/public/purpose.asp). IMPAACT predominately serves children and youth with perinatally acquired HIV infection (https://impaactgroup.org). The study was approved by the local institutional review board of each participating center and required participants’ written informed consent prior to enrollment.
We enrolled persons aged 18–25 years with HIV-1 infection treated with combination antiretroviral therapy (cART) with ≥3 antiretrovirals (ARVs), unchanged for ≥90 days, and HIV-1 plasma RNA <5000 copies/mL within 60 days before entry. Subjects were excluded for renal diseases, current or recent pregnancy or lactation; use of medicines that affect BMD, interfere with vitamin D absorption or TDF excretion, or cause nephrotoxicity; use of vitamin D supplements >400 IU/day; or estimated GFR <70 mL/min/1.73 M2, serum calcium above the upper limit of normal (ULN), or urine calcium/creatinine ratio >0.20 mg/mg. Subjects taking standard multivitamins or calcium supplements coformulated with vitamin D could enroll. There was no exclusion based on pretreatment vitamin D status.
Enrollment was stratified based on current ARV treatment: cART containing TDF, or cART not containing TDF (noTDF). Within those groups (TDF and noTDF), participants were randomly assigned, in fixed blocks of 4 by sex, to treatment with vitamin D or placebo.
Participants received vitamin D3, 50000 IU (Bio-tech Pharmacal, Fayetteville, Arkansas or matching placebo gelatin capsule [19]. Capsule content was confirmed by independent analysis prior to use in the study. Treatment assignment was blinded to all study participants and personnel except the site pharmacist, with unblinding after prespecified data analysis. Directly observed oral treatment was administered at baseline and at weeks 4 and 8.
Baseline and week 12 measurements of main outcome variables were performed by batch analysis of stored samples at the US Department of Agriculture, Agricultural Research Service, Western Human Nutrition Research Center, Davis, California (USDA-ARS-WHNRC). All samples were collected after ≥4 hours of fasting. Spot urine samples for creatinine, calcium, and phosphate were collected upon arrival for the study visit. Spot urine samples for urine β-2 microglobulin (Uβ2MG) were collected from participants ≤1 hour after the first urine collection and after they drank a large glass of water.
Serum 25-hydroxy vitamin D (25-OHD) was measured by radioimmunoassay (RIA) (25-Dihydroxyvitamin D 125I RIA Kit, DiaSorin, Stillwater, Minnesota). Vitamin D status was categorized as deficient (25-OHD <12 ng/mL [<30 nmol/L]), insufficient (25-OHD 12–20 ng/mL [30–50 nmol/L]), sufficient (25-OHD ≥20 ng/mL [>50 nmol/L]), and excess [25-OHD >50 ng/mL [>125 nmol/L]) [20].
Serum 1,25 dihydroxy vitamin D (1,25-OHD) was measured by RIA (1,25-Dihydroxyvitamin D 125I RIA Kit, IDS, Fountain Hills, Arizona), with normal range of 48–150 pmol/L. Serum intact PTH and Uβ2MG were measured using a solid-phase, 2-site chemiluminescent enzyme-labeled immunometric assay (Intact-PTH, Siemens Medical Solutions Diagnostics, Tarrytown, New York), with normal range of 10–69 pg/mL for PTH and ULN of 300 ng/mL for Uβ2MG.
Serum BAP was measured by enzyme immunoassay (EIA; METRA BAP EIA kit 8012, Quidel Corp, San Diego, California), with a normal range of 11.6–29.6 U/L for premenopausal women 15.0–41.4 U/L for men. Serum CTX was measured by EIA (Serum Crosslaps, IDS, Fountain Hills, Arizona), with normal ranges of 860–5720 pmol/L and 891–5797 pmol/L for premenopausal women and men, respectively.
Urine calcium (UCa), phosphorus (UPO4), and creatinine (UCr) were measured by batch analysis at USDA-ARS-WHNRC. Serum sodium, potassium, chloride, bicarbonate/total CO2, calcium (SCa), phosphorus (SPO4), and creatinine (SCr) were measured at CLIA-certified local site laboratories. GFR was calculated by the Modification of Diet in Renal Disease formula [21]. Urine calcium to creatinine ratio (UCa/UCr) was used to estimate urinary calcium excretion, with normal <0.21 mg/mg. TRP percentage was calculated as (1 − [(UPO4 × SCr)/(SPO4 × UCr)]} × 100), with normal >96%. Calcium and vitamin D intake from diet and supplements were measured (Block Calcium/Vitamin D Screener [22], Nutritionquest, Berkeley, California).
Safety monitoring of SCa and UCa/UCr was performed in the clinical laboratory at each study site at baseline and every 4 weeks. For participants with SCa greater than ULN or UCa/UCr >0.20, repeat testing was required, and the study drug was discontinued for those with persistent elevations.
The study was designed to have 40 evaluable participants in each of 4 groups (TDF randomized to vitamin D or placebo; noTDF randomized to vitamin D or placebo). For participants taking TDF, the power to detect a vitamin D treatment effect was >80% for an increase in TRP of ≥5% between baseline and week 12, the magnitude of change previously associated with TDF [3].
Data are presented as mean (standard deviation) or median (range). Baseline analyses compared differences between participants in TDF and noTDF groups, and between randomized groups (vitamin D vs placebo), using an intent-to-treat analysis cohort (N = 203) for baseline data, and a per-protocol cohort for the data on change from baseline to week 12. Primary outcome measures were changes in TRP, BAP, CTX, and PTH. Safety was measured by change in GFR, Uβ2MG, UCa/UCr, and SCa.
Univariate analyses followed by multivariable models were used to measure the main effects of vitamin D and TDF, as well as the interaction between those 2 exposures, and to measure the effect of confounding variables. Statistical significance of differences was identified using Pearson χ2 test for categorical variables and Wilcoxon rank sum test for continuous variables, except as noted. Wilcoxon signed-rank test was used to test within group differences. Generalized linear models with normal errors and identity link were used for the univariate and multivariable models. A rank-based analysis was also performed due to the presence of outliers. Covariates for baseline analyses were chosen based on clinical significance (Table 1). Covariates for analyses comparing baseline to week 12 were chosen based on the GLMSELECT procedure in SAS software (version 9.2, SAS Institute, Cary, North Carolina) using stepwise selection and are listed in a footnote to Table 3.
Table 1.
Characteristics of the Study Populationa
| Randomized Group |
Randomized Group |
||||||
| Characteristic | Overall | Tenofovir | Vitamin D | Placebo | No Tenofovir | Vitamin D | Placebo |
| No. | 203 | 118 | 59 | 59 | 85 | 43 | 42 |
| Age, yearsb | |||||||
| Mean (SD) | 20.9 (2.0) | 21.1 (2.0) | 21.1 (2.1) | 21.1 (1.9) | 20.6 (2.0) | 20.5 (2.1) | 20.6 (2.0) |
| Median (range) | 21 (18–24) | 21 (18–24) | 21 (18–24) | 21 (18–24) | 20 (18–24) | 20 (18–24) | 21 (18–24) |
| Sex, No. (%)b,e | |||||||
| Male | 127 (63%) | 86 (73%) | 43 (73%) | 43 (73%) | 41 (48%) | 20 (47%) | 21 (50%) |
| Female | 76 (37%) | 32 (27%) | 16 (27%) | 16 (27%) | 44 (52%) | 23 (53%) | 21 (50%) |
| Race, No. (%)b | |||||||
| Black | 106 (52%) | 59 (50%) | 26 (44%) | 33 (56%) | 47 (55%) | 26 (60%) | 21 (50%) |
| White | 45 (22%) | 27 (23%) | 17 (29%) | 10 (17%) | 18 (21%) | 7 (16%) | 11 (26%) |
| Other or mixed | 52 (26%) | 32 (27%) | 16 (27%) | 16 (27%) | 20 (24%) | 10 (23%) | 10 (24%) |
| Hispanic, No. (%)b | 64 (32%) | 37 (31%) | 18 (31%) | 19 (32%) | 27 (32%) | 13 (30%) | 14 (33%) |
| BMI (kg/m2)b | |||||||
| Mean (SD) | 25.6 (7.0) | 25.5 (7.0) | 25.3 (7.3) | 25.6 (6.7) | 25.8 (7.0) | 27.7 (7.5) | 24.6 (6.4) |
| Median (range) | 23.7 (11.3–56.2) | 23.6 (16.6–56.2) | 23.4 (16.6–56.2) | 24.0 (17.3–56.2) | 23.7 (11.3–47.1) | 25.0 (16,1–47.1) | 22.8 (11.3–43.0) |
| Lifestyle, No. (%) | |||||||
| Smoke cigarettesb,e | 57 (28%) | 44 (37%) | 20 (34%) | 24 (41%) | 13 (15%) | 7 (16%) | 6 (14%) |
| Drink alcoholb,d | 114 (56%) | 76 (64%) | 34 (58%) | 42 (71%) | 38 (45%) | 21 (49%) | 17 (40%) |
| Use multivitamins | 43 (21%) | 21 (18%) | 11 (19%) | 10 (17%) | 22 (26%) | 12 (28%) | 10 (24%) |
| Total calcium intake (mg/day)b | |||||||
| Mean (SD) | 796 (523) | 795 (536) | 885 (567)e | 706 (493)e | 798 (507) | 795 (514) | 802 (505) |
| Median (range) | 619 (63–2832) | 606 (63–2832) | 803 (196–2832) | 568 (63–2571) | 650 (78–2737) | 666 (78–2737) | 637 (153–2143) |
| Inadequate calcium intake for age, No. (%)h | 150 (74%) | 86 (74%) | 40 (69%) | 46 (78%) | 64 (75%) | 33 (77%) | 31 (74%) |
| Total vitamin D intake (IU/day)b | |||||||
| Mean (SD) | 211 (199) | 205 (195) | 226 (208) | 184 (181) | 220 (204) | 227 (206) | 212 (204) |
| Median (range) | 131 (3–815) | 128 (3–815) | 177 (14–815) | 96 (3–750) | 134 (5–758) | 140 (14–758) | 128 (5–743) |
| HIV duration, yearsb,e | |||||||
| Mean (SD) | 6.6 (6.4) | 4.0 (4.8) | 4.2 (4.7) | 3.8 (5.0) | 10.2 (6.6) | 10.1 (7.1) | 10.3 (6.1) |
| Median (range) | 4 (<1–22) | 2 (<1–20) | 2 (<1–20) | 2 (<1–20) | 9 (<1–22) | 8 (<1–22) | 11 (1–21) |
| CDC disease stage, No. (%)b | |||||||
| A | 119 (59%) | 77 (65%) | 40 (68%) | 37 (63%) | 42 (49%) | 20 (47%) | 22 (52%) |
| B | 35 (17%) | 19 (16%) | 9 (15%) | 10 (17%) | 16 (19%) | 8 (19%) | 8 (19%) |
| C | 49 (24%) | 22 (19%) | 10 (17%) | 12 (20%) | 27 (32%) | 15 (35%) | 12 (29%) |
| CD4 cell count current (cells/uL)b,e | |||||||
| Mean (SD) | 587 (246) | 534 (204) | 515 (197) | 553 (211) | 660 (279) | 625 (249) | 696 (306) |
| Median (range) | 543 (72–1488) | 500 (191–1370) | 500 (191–1130) | 500 (197–1370) | 627 (72–1488) | 579 (229–1488) | 688 (72–1370) |
| Current viral load below quantitation limit, No. (%)b,e | 139 (68%) | 96 (81%) | 46 (78%) | 50 (85%) | 43 (51%) | 24 (56%) | 19 (45%) |
| Maximum ART exposure (months), No. (%)e | |||||||
| ≤6 | 30 (15%) | 25 (21%) | 10 (17%) | 15 (25%) | 5 (6%) | 4 (9%) | 1 (2%) |
| 6 to ≤24 | 62 (31%) | 53 (45%) | 25 (42%) | 28 (47%) | 9 (11%) | 4 (9%) | 5 (12%) |
| >24 | 111 (55%) | 40 (34%) | 24 (41%) | 16 (27%) | 71 (84%) | 35 (81%) | 36 (86%) |
| Antiretroviralse,g, No. (%) | |||||||
| PI | 112 (55%) | 54 (46%) | 28 (47%) | 26 (44%) | 58 (68%) | 31 (72%) | 27 (64%) |
| NNRTI | 76 (37%) | 60 (51%) | 29 (49%) | 31 (53%) | 16 (19%) | 8 (19%) | 8 (19%) |
| PI + NNRTI | 12 (6%) | 4 (3%) | 2 (3%) | 2 (3%) | 8 (9%) | 2 (5%) | 6 (14%) |
| Other | 3 (2%) | 0 | 0 | 0 | 3 (4%) | 2 (5%) | 1 (2%) |
| Efavirenz in ART, No. (%)b,e | 84 (41%) | 63 (53%) | 30 (51%) | 33 (56%) | 21 (25%) | 10 (23%) | 11 (26%) |
| Study site, No. (%)b,e | |||||||
| IMPAACT | 52 (26%) | 10 (8%) | 6 (10%) | 4 (7%) | 42 (49%) | 22 (51%) | 20 (48%) |
| ATN | 151 (74%) | 108 (92%) | 53 (90%) | 55 (93%) | 43 (51%) | 21 49%) | 22 (52%) |
| Season enrolled, No. (%)c | |||||||
| Winter | 44 (22%) | 27 (23%) | 12 (20%) | 15 (25%) | 17 (20%) | 11 (26%) | 6 (14%) |
| Spring | 62 (31%) | 43 (36%) | 23 (39%) | 20 (34%) | 19 (22%) | 8 (19%) | 11 (26%) |
| Summer | 53 (26%) | 23 (20%) | 12 (20%) | 11 (19%) | 30 (35%) | 15 (35%) | 15 (36%) |
| Fall | 44 (22%) | 25 (21%) | 12 (20%) | 13 (22%) | 19 (22%) | 9 (21%) | 10 (24%) |
| Tubular reabsorption of phosphate, %d | |||||||
| Mean (SD) | 92.5 (4.3) | 92.0 (3.8) | 91.4 (3.9) | 92.7 (3.7) | 93.3 (4.8) | 93.3 (5.3) | 93.2 (4.4) |
| Median (range) | 93.2 (71.4–99.6) | 92.4 (81.4–98.6) | 92.2 (83.0–98.5) | 92.6 (81.4–98.6) | 94.2 (71.4,99.6) | 94.0 (71.4–99.4) | 94.3 (80.9–99.6) |
| Bone alkaline phosphatase (U/L) | |||||||
| Mean (SD) | 36.2 (14.3) | 37.4 (15.3) | 36.9 (16.1) | 37.9 (14.7) | 34.6 (12.7) | 36.0 (14.7) | 33.1 (10.2) |
| Median (range) | 33.8 (11.2–102.7) | 35.1 (11.2–102.7) | 33.1 (14.3–102.7) | 37.5 (11.2–96.6) | 32.2 (14.0–70.7) | 34.0 (14.0–70.7) | 32.0 (15.0–54.0) |
| C-telopeptide (pmol/L)d | |||||||
| Mean (SD) | 5975 (2762) | 6310 (2411) | 6602 (2650) | 6019 (2128) | 5510 (3143) | 5827 (3741) | 5187 (2385) |
| Median (range) | 5456 (341,16,608) | 6088 (1604–15,268) | 6417 (1604–15,268) | 5665 (2077–11,385) | 4828 (341–16,608 | 4728 (341–16,608) | 4867 (1302–12,222) |
| Parathyroid hormone (pg/mL)e | |||||||
| Mean (SD) | 40.8 (24.5) | 47.7 (25.7) | 48.8 (25.6) | 46.6 (25.9) | 31.2 (19.1) | 30.0 (15.0) | 32.3 (22.7) |
| Median (range) | 35.5 (6.8–145.5) | 44.5 (7.5–145.5) | 46.9 (10.6–145.5) | 37.3 (7.5–32.5) | 25.9 (6.8–104.5) | 25.9 (7.7–74.7) | 24.8 (6.8–104.5) |
| Glomerular filtration rate (MDRD; mL/min/1.73 m2)d | |||||||
| Mean (SD) | 123 (26) | 119 (20) | 120 (22) | 117 (18) | 129 (31) | 130 (33) | 129 (29) |
| Median (range) | 121 (62–267) | 119 (62–174) | 119 (62–174) | 119 (82–166) | 126 (66–267) | 130 (66–213) | 124 (69–267) |
| Urine β-2 microglobulin (ng/mL)d | |||||||
| Mean (SD) | 169 (381) | 197 (398) | 237 (532) | 156 (176) | 130 (354) | 170 (492) | 89 (72) |
| Median (range) | 97 (4–3295) | 111 (4–3220) | 121 (4–3220) | 105 (6–997) | 87 (4–3295) | 92 (6–3295) | 78 (4–292) |
| Urine calcium/creatinine ratio (mg/mg)c | |||||||
| Mean (SD) | 0.06 (0.04) | 0.05 (0.04) | 0.05 (0.03) | 0.05 (0.04) | 0.07 (0.05) | 0.06 (0.05) | 0.07 (0.05) |
| Median (range) | 0.05 (0–0.23) | 0.04 (0.01–0.20) | 0.05 (0.01–0.15) | 0.04 (0.01–0.20) | 0.06 (0–0.23) | 0.05 (0–0.23) | 0.07 (0.01–0.20) |
| Anion gap (mEq/L)c | |||||||
| Mean (SD) | 10 (3) | 11 (3) | 11 (2) | 11 (3) | 10 (3) | 10 (3) | 10 (3) |
| Median (range) | 10 (2–18) | 10 (3–17) | 10 (7–17) | 11 (3–16) | 9 (2–18) | 9 (2–18) | 9 (5–17) |
| Serum 25-OH vitamin D (ng/mL) | |||||||
| Mean (SD) | 21.2 (12.3) | 20.8 (12.7) | 21.9 (14.1) | 19.7 (11.1) | 21.7 (11.8) | 20.8 (11.7) | 22.7 (12.0) |
| Median (range) | 18.5 (2.2–100.1) | 18.0 (2.2–100.1) | 19.0 (5.0–100.1) | 17.0 (2.2–54.2) | 20.0 (4.5–65.5) | 18.6 (4.5–56.6) | 22.0 (6.2–65.5) |
| Serum 25-OH vitamin D category, No. (%) | |||||||
| Deficient (<12 ng/mL) | 42 (21%) | 26 (22%) | 11 (19%) | 15 (25%) | 16 (19%) | 11 (26%) | 5 (12%) |
| Insufficient (12–<20) | 68 (34%) | 41 (35%) | 21 (36%) | 20 (34%) | 27 (32%) | 13 (30%) | 14 (33%) |
| Sufficient (>20–50) | 87 (43%) | 48 (41%) | 25 (42%) | 23 (39%) | 39 (46%) | 18 (42%) | 21 (50%) |
| Excess (>50) | 6 (3%) | 3 (3%) | 2 (3%) | 1 (2%) | 3 (4%) | 1 (2%) | 2 (5%) |
| Serum 1,25-OH(2) vitamin D (pmol/L) | |||||||
| Mean (SD) | 111.5 (47.7) | 113.7 (46.3) | 115.5 (47.3) | 111.8 (45.6) | 108.5 (49.8) | 105.3 (49.4) | 111.8 (50.6) |
| Median (range) | 104.6 (6.0–278.4) | 104.0 (19.1–278.4) | 103.4 (19.1–278.4) | 104.6 (34.5–228.7) | 106.0 (6.0–259.0) | 101.4 (6.0–259.0) | 108.8 (25.8–245.9) |
Abbreviations: ART, antiretroviral therapy; ATN, Adolescent Medicine Trials Network; BMI, body mass index; CDC, Centers for Disease Control and Prevention; HIV, human immunodeficiency virus; IMPAACT, International Maternal Pediatric Adolescent AIDS Clinical Trials; MDRD, Modification of Diet in Renal Disease; NNRTI, nonnucleoside reverse transcriptase inhibitor; PI, protease inhibitor; SD, standard deviation.
Participants were enrolled based on receiving or not receiving tenofovir disoproxil fumarate (TDF) as part of their combination ART and randomized within those groups to treatment intervention with vitamin D or placebo. Continuous data P values by Wilcoxon rank sum test. Categorical data P values by Pearson χ2 test.
Identifies variables used as covariates for multivariable modeling of baseline variables (see text; also included a measure of regular exercise).
.01 ≤ P < .05 for TDF versus noTDF groups.
.001 ≤ P < .01 for TDF versus noTDF groups.
P < .001 for TDF versus noTDF groups.
P < .05 in the TDF group only, for the comparison between randomized groups of total calcium intake at baseline. There were no other statistically significant differences between randomized groups at baseline.
Antiretroviral classes of the current regimen in addition to nucleoside reverse transcriptase inhibitor.
Total calcium intake was categorized as inadequate if <1300 mg (age ≤18 years) or <1000 mg (age ≥19 years) [20].
Table 3.
Changes in Renal Function, Calcium Homeostasis, and Bone Metabolism From Baseline to Week 12 by Tenofovir and Vitamin D Treatment Status: Multivariable Models
| Primary Model |
Model With Covariates |
|||
| Outcome Variable and Covariatesa | Coefficientb (SE) | P Value | Coefficient (SE) | P Value |
| Tubular resorption of phosphate (%) | ||||
| Vitamin D treatment | 1.4 (1.3) | .346 | 1.2 (1.3) | .140 |
| Tenofovir use | 0.1 ((1.2) | .936 | −0.6 (1.3) | .578 |
| Interaction | −0.9 (1.7) | .902 | 0.003 (1.6) | .437 |
| Duration of ART exposure >6 to <24 mo vs < 6 mo | −3.5 (1.2) | .002 | ||
| Duration of ART exposure >24 mo vs < 6 mo | −3.0 (1.2) | .001 | ||
| Serum calcium at baseline | −2.6 (1.1) | .007 | ||
| Ritonavir use | −2.5 (0.8) | .002 | ||
| BAP (U/L) | ||||
| Vitamin D treatment | −0.9 (1.8) | .296 | −0.9 (1.7) | .309 |
| Tenofovir use | 0.4 (1.7) | .995 | 0.5 (1.6) | .882 |
| Interaction | −0.6 (2.3) | .402 | 0.5 (2.3) | .414 |
| C-telopeptide (pmol/L) | ||||
| Vitamin D treatment | 890 (500) | .556 | 890 (500) | .556 |
| Tenofovir use | 713 (469) | .461 | 713 (469) | .461 |
| Interaction | −1428 (650) | .028 | −1428 (650) | .028 |
| PTH (pg/mL) | ||||
| Vitamin D treatment | 3.3 (4.4) | .087 | 2.5 (4.3) | .044 |
| Tenofovir use | 4.4 (4.1) | .449 | 4.2 (4.1) | .431 |
| Interaction | −11.8 (5.7) | .021 | −11.4 (5.6) | .023 |
| Glomerular filtration rate (ml/min/1.73 m2) | ||||
| Vitamin D treatment | 8 (5) | .748 | 7 (4) | .774 |
| Tenofovir use | 4 (5) | .462 | 1 (4) | .234 |
| Interaction | −10 (7) | .459 | −7 (5) | .747 |
| Ritonavir use | −9 (3) | .006 | ||
| Glomerular filtration rate at baseline | −0.4 (0.05) | <.001 | ||
| Supplemental calcium intake at baseline | 0.1 (0.0) | .010 | ||
| Urine β-2 microglobulin (ng/mL) | ||||
| Vitamin D treatment | −125 (397) | .354 | −198(388) | .645 |
| Tenofovir use | −39 (371) | .938 | −158 (389) | .354 |
| Interaction | −673 (516) | .132 | 671 (503) | .109 |
| Urine β-2 microglobulin at baseline | 1 (0.3) | .002 | ||
| UCa/UCr ratio (mg/mg) | ||||
| Vitamin D treatment | −0.01 (0.01) | .371 | −0.01 (0.01) | .876 |
| Tenofovir use | −0.01 (0.01) | .136 | −0.01 (0.01) | .427 |
| Interaction | 0.02 (0.02) | .315 | −0.02 (0.01) | .242 |
| Black race | −0.02 (0.01) | <.001 | ||
| Enrolled season spring vs winter | −0.00 (0.01) | .002 | ||
| Enrolled season summer vs winter | −0.01 (0.01) | .665 | ||
| Enrolled season fall vs winter | −0.03 (0.01) | .889 | ||
| Geographic latitude | −0.00 (0.00) | .002 | ||
| Baseline UCa/UCr | −0.54 (0.09) | <.001 | ||
| 25-OHD (ng/mL) | ||||
| Vitamin D treatment | 11.9 (2.8) | <.001 | 13.3 (2.4) | <.001 |
| Tenofovir use | 0.7 (2.7) | .110 | −2.6 (2.4) | .523 |
| Interaction | 3.0 (3.7) | .303 | 1.5 (3.1) | .318 |
| BMI at baseline | −0.4 (0.1) | .006 | ||
| ATN site | 6.2 (2.0) | .006 | ||
| Enrolled season spring vs winter | 7.6 (2.2) | .009 | ||
| Enrolled season summer vs winter | −2.9 (2.3) | .003 | ||
| Enrolled season fall vs winter | −3.2 (2.3) | .021 | ||
| CD4 percentage at baseline | 0.3 (0.1) | .001 | ||
| 25-OHD at baseline | −0.1 (0.1) | .002 | ||
| Baseline total vitamin D intake | −0.01 (0.00) | .009 | ||
| 1,25-OHD (pmol/L) | ||||
| Vitamin D treatment | 31.8 (13.3) | .006 | 31.1 (11.6) | .001 |
| Tenofovir use | 18.6 (12.5) | .325 | 19.0 (10.9) | .193 |
| Interaction | −23.5 (17.3) | .187 | −19.7 (15.1) | .202 |
| 1,25-OHD at baseline | −0.5 (0.08) | <.001 | ||
| Serum phosphate at baseline | 17.3 (6.8) | .003 |
Abbreviations: ART, antiretroviral therapy; ATN, Adolescent Medicine Trials Network; BAP, bone alkaline phosphatase; BMI, body mass index; HIV, human immunodeficiency virus; PTH, parathyroid hormone; SE, standard error; UCa/UCr, urine calcium/creatinine ratio.
Covariates listed in the table had P <.01 in multivariate analysis on ranks. Other significant covariates (.01 ≤ P <.05) were included in the models but are excluded from the table for clarity. The covariates used in the model selection were the following: age, sex, race, ethnicity, study site, BMI, multivitamin use, season, latitude, HIV duration, age of HIV acquisition (<9 vs ≥9 years), CDC stage, maximum treatment duration of ART; baseline values for: CD4 count, CD4 percentage, creatinine clearance, serum calcium, 25-OHD, 1,25-OHD, sodium, potassium, chloride, C02, anion gap, phosphorus, urine Ca/Cr and urine β-2 microglobulin; calcium intake (total, from diet, and from supplements), dietary and total vitamin D intake; and the use of the following antiretroviral drugs: abacavir, lamivudine, atazanavir, didanosine, emtricitabine, lopinavir, stavudine.
Coefficient and P values from generalized linear model. P values are fom likelihood ratio χ2 test.
RESULTS
Baseline Data
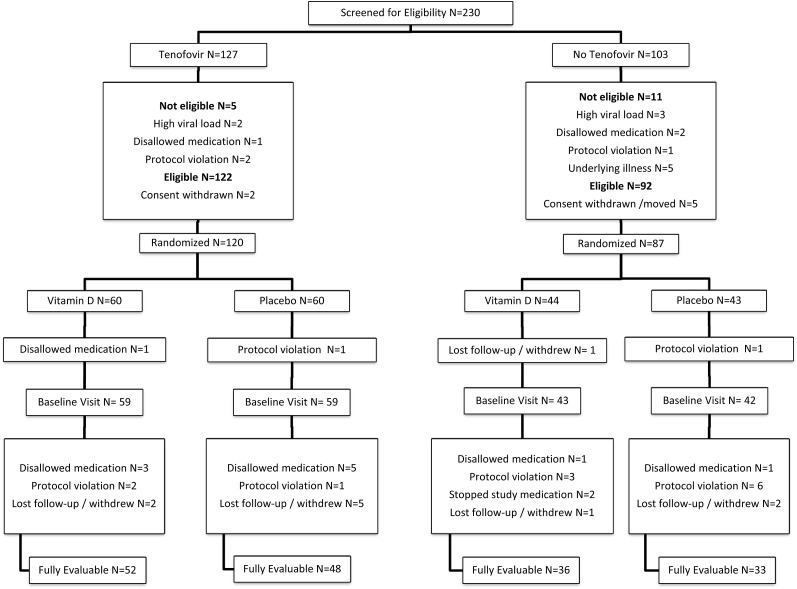
A total of 203 participants were randomized and completed the baseline visit (Figure 1). There were more males in the TDF group, reflecting the higher proportion of males at ATN sites, which disproportionately enrolled participants on TDF (Table 1). A higher proportion of participants in the noTDF group were from IMPAACT sites and were more likely to have acquired HIV early in life (44% vs 12%; P < .001). There was more use of tobacco and alcohol in the TDF group. The groups did not differ in self-reported exercise, geographic latitude, or serum phosphate or calcium (data not shown).
Figure 1.
Participant flow diagram. Of 217 subjects screened and eligible, 207 were randomized, 203 completed a baseline visit, and 169 completed 12 weeks of study with fully evaluable data and no missed doses of vitamin D. The tenofovir disoproxil fumarate (TDF) group had 118 participants at baseline, with 59 randomized to vitamin D (52 evaluable at week 12) and 59 to placebo (48 evaluable at week 12). The noTDF group included 85 participants at baseline, with 43 randomized to vitamin D (36 evaluable at week 12) and 42 to placebo (33 evaluable at week 12). Explanations of exclusions: underlying illnesses (1 each): hypercalcemia; renal stones, recent pregnancy; psychiatric illness; hepatitis B antigen positive. Disallowed medications: trimethoprim-sulfamethoxazole, amoxicillin, penicillin, lamotrigine, mometasone nasal.
At baseline, participants on TDF had lower GFR, TRP, and UCa/UCr and higher Uβ2MG, anion gap, CTX, and PTH than those on noTDF (Table 1). Serum levels of 25-OHD did not differ between groups. Multivariable analysis of the association of TDF use with baseline variables, after adjustment for demographic and HIV-related differences, showed that higher PTH (P < .001) and lower TRP (P = .017) remained strongly associated with TDF use, but the associations of TDF with lower GFR (P = .054) and higher Uβ2MG (P = .069) were less strong when covariates were considered (data not shown).
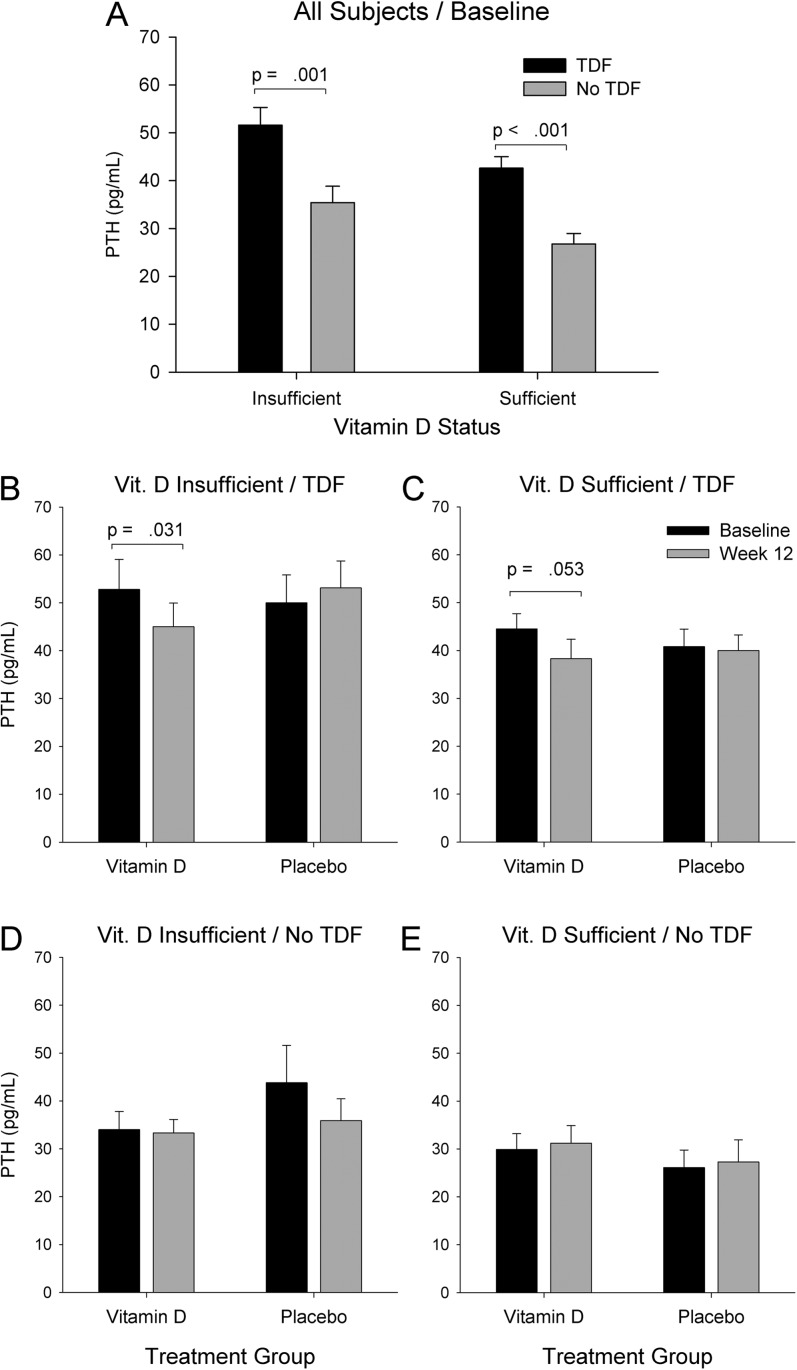
Among all participants, PTH was higher in those with insufficient 25-OHD, compared with those with sufficient 25-OHD (45.3 [28.4] and 35.5 [17.7] pg/mL, respectively; P = .024). PTH was higher in the TDF group compared with the noTDF group (47.7 [25.7] and 31.2 [19.1] pg/mL, respectively; P < .001), for both those with insufficient 25-OHD and those with sufficient 25-OHD (Figure 2A).
Figure 2.
Serum parathyroid hormone (PTH) concentration in all participants at baseline (A) categorized by vitamin D status (insufficient or sufficient) and combination antiretroviral therapy (tenofovir disoproxil fumarate [TDF] or noTDF); and PTH concentrations at both baseline and 12 weeks (B–E) further subdivided by randomized intervention group (vitamin D or placebo). At baseline (A) PTH was higher in participants using tenofovir (dark bars) compared with those not using tenofovir (light bars). This association of tenofovir with increased PTH was seen in those with vitamin D insufficiency (serum 25-OHD <20 ng/mL; A, left pair of bars) and in those with sufficient vitamin D status at baseline (A, right pair of bars). In the TDF group, vitamin D treatment led to a decrease in PTH from baseline to week 12, both in those with insufficient vitamin D (B) and sufficient vitamin D (C) at baseline. In the noTDF group, PTH did not change significantly in response to vitamin D treatment (D and E). Placebo treatment had no effect in any group (B–E).
In the TDF group, total calcium intake at baseline was higher in participants randomized to vitamin D compared with those randomized to placebo (Table 1). There were no other statistically significant baseline differences between randomized groups (vitamin D or placebo).
Effect of Vitamin D Treatment
Between baseline and week 12, mean 25-OHD and 1,25-OHD concentrations increased with vitamin D but not placebo in both the TDF and noTDF groups (Table 2). At week 12, 95% of all participants randomized to vitamin D had serum 25-OHD concentrations ≥20 ng/mL, increased from 48% at baseline, compared with placebo participants, in whom serum 25-OHD concentration ≥20 ng/mL was seen in 48% at baseline and 49% at week 12 (P < .001, placebo vs vitamin D). Vitamin D treatment in the TDF group decreased PTH and BAP while increasing UCa/UCr (Table 2). These changes in calcium balance and bone turnover were not seen with vitamin D in the noTDF group, or with placebo in either TDF or noTDF groups. There was no change in TRP with vitamin D treatment in either group. GFR increased with vitamin D but not placebo in the noTDF group but did not change in either arm of the TDF group. Serum calcium and phosphate (data not shown) and CTX (Table 2) did not change in any group.
Table 2.
Changes in Renal Function, Calcium Homeostasis, and Bone Metabolism From Baseline to Week 12 by Tenofovir and Vitamin D Treatment Status: All Participants With Baseline and Week 12 Data
| Outcome Variable | Intervention Group | No.a | Baselineb | Change | Pc Value |
| Overall | |||||
| Tubular reabsorption of phosphate (%) | Vitamin D | 85 | 92.2 (4.6) | −0.3 (5.6) | .313 |
| Placebo | 79 | 92.9 (4.0) | −1.0 (5.2) | .076 | |
| Bone alkaline phosphatase (U/L) | Vitamin D | 89 | 36.5 (15.4) | −0.93 (8.5) | .063 |
| Placebo | 81 | 35.9 (13.2) | 0.0 (6.5) | .771 | |
| C-telopeptide (pmol/L) | Vitamin D | 90 | 6275 (3163) | −45 (2321) | .811 |
| Placebo | 81 | 5373 (2264) | −74 (1835) | .699 | |
| Parathyroid hormone (pg/mL) | Vitamin D | 90 | 40.9 (23.6) | −4.0 (19.7) | .017 |
| Placebo | 81 | 40.7 (25.5) | −0.4 (17.1) | .518 | |
| Glomerular filtration rate (mL/min/1.73 m2) | Vitamin D | 88 | 124 (28) | 5 (23) | .146 |
| Placebo | 81 | 122 (24) | 1 (23) | .075 | |
| Urine β-2 microglobulin (ng/mL) | Vitamin D | 89 | 209 (514) | 287 (2270) | .437 |
| Placebo | 80 | 128 (145) | 14 (152) | .105 | |
| Urine calcium/creatinine ratio (mg/mg) | Vitamin D | 90 | 0.06 (0.04) | 0.01 (0.04) | .176 |
| Placebo | 80 | 0.06 (0.05) | 0.00 (0.06) | .932 | |
| Serum 25-OH vitamin D (ng/mL) | Vitamin D | 90 | 21.4 (13.1) | 13.9 (14.9) | <.001 |
| Placebo | 81 | 20.9 (11.5) | 0.4 (7.2) | .942 | |
| Serum 1,25-OH(2) vitamin D (pmol/L) | Vitamin D | 90 | 111.3 (48.2) | 23.4 (61.8 | <.001 |
| Placebo | 80 | 111.9 (47.5) | 3.3 (52.5) | .438 | |
| Tenofovir-containing | |||||
| Tubular reabsorption of phosphate (%) | Vitamin D | 49 | 91.4 (3.9) | −0.5 (4.6) | .614 |
| Placebo | 47 | 92.7 (3.7) | −1.0 (5.3) | .162 | |
| Bone alkaline phosphatase (U/L) | Vitamin D | 51 | 36.9 (16.1) | −1.3 (7.2) | .038 |
| Placebo | 48 | 37.9 (14.7) | 0.2 (6.7) | .984 | |
| C-telopeptide (pmol/L) | Vitamin D | 52 | 6602 (2650) | −322 (2211) | .442 |
| Placebo | 48 | 6019 (2128) | 216 (1772) | .328 | |
| Parathyroid hormone (pg/mL) | Vitamin D | 52 | 48.8 (25.6) | −7.1 (23.1) | .003 |
| Placebo | 48 | 46.6 (25.9) | 1.4 (16.0) | .273 | |
| Glomerular filtration rate (ml/min/1.73 m2) | Vitamin D | 50 | 120 (22) | 1 (17) | .921 |
| Placebo | 48 | 117 (18) | 3 (13) | .167 | |
| Urine β-2 microglobulin (ng/mL) | Vitamin D | 52 | 237 (532) | 547 (2922) | .227 |
| Placebo | 47 | 156 (176) | −2 (185) | .669 | |
| Urine calcium/creatinine ratio (mg/mg) | Vitamin D | 52 | 0.05 (0.03) | 0.01 (0.04) | .024 |
| Placebo | 47 | 0.05 (0.04) | 0.00 (0.05) | .868 | |
| Serum 25-OH vitamin D (ng/mL) | Vitamin D | 52 | 21.9 (14.1) | 15.6 (15.4) | <.001 |
| Placebo | 48 | 19.7 (11.1) | 0.7 (6.9) | .701 | |
| Serum 1,25-OH(2) vitamin D (pmol/L) | Vitamin D | 52 | 115.6 (47.3) | 19.3 (62.4) | .003 |
| Placebo | 47 | 111.9 (45.6) | 11.0 (54.0) | .118 | |
| Non-tenofovir-containing | |||||
| Tubular reabsorption of phosphate (%) | Vitamin D | 36 | 93.3 (5.3) | 0.1 (6.9) | .329 |
| Placebo | 32 | 93.2 (4.4) | −1.1 (5.1) | .330 | |
| Bone alkaline phosphatase (U/L) | Vitamin D | 38 | 36.0 (14.7) | −0.4 (10.1) | .583 |
| Placebo | 33 | 33.1 (10.2) | −0.2 (6.4) | .582 | |
| C-telopeptide (pmol/L) | Vitamin D | 38 | 5827 (3841) | 333 (2442) | .583 |
| Placebo | 33 | 5187 (2385) | −497 (1869) | .063 | |
| Parathyroid hormone (pg/mL) | Vitamin D | 38 | 30.0 (15.0) | 0.2 (13.0) | .876 |
| Placebo | 33 | 32.3 (22.7) | −2.9 (18.5) | .747 | |
| Glomerular filtration rate (mL/min/1.73 m2) | Vitamin D | 38 | 130 (33) | 10 (29) | .041 |
| Placebo | 33 | 129 (29) | −1 (32) | .312 | |
| Urine β-2 microglobulin (ng/mL) | Vitamin D | 37 | 170 (492) | −78 (514) | .853 |
| Placebo | 33 | 89 (72) | 37 (85) | .018 | |
| Urine calcium/creatinine ratio (mg/mg) | Vitamin D | 38 | 0.06 (0.05) | 0.00 (0.05) | .583 |
| Placebo | 33 | 0.07 (0.05) | 0.01 (0.07) | .747 | |
| Serum 25-OH vitamin D (ng/mL) | Vitamin D | 38 | 20.8 (11.7) | 11.5 (14.1) | <.001 |
| Placebo | 33 | 22.7 (12.0) | −0.0 (7.7) | .558 | |
| Serum 1,25-OH(2) vitamin D (pmol/L) | Vitamin D | 38 | 105.4 (49.4) | 29.1 (61.4) | .003 |
| Placebo | 33 | 111.9 (50.6) | −7.6 (49.0) | .500 |
Baseline and week 12 values represent all participants with a value at each of those time points. No. identifies the number of participants who had values at both time points, which allowed the calculation of the change from baseline to week 12.
Data are mean (standard deviation). For change, the change for each participant was calculated, and the mean of those values is reported.
P Values are from the Wilcoxon signed-rank test to test for within-group differences.
In multivariable regression analysis performed to identify treatment effects (vitamin D or placebo, TDF use, and the interaction between treatment and TDF use) on change in the primary outcomes, with further adjustment for baseline variables, the increases in 25-OHD and 1,25-OHD with vitamin D treatment occurred independently of TDF status (interaction P > .05), and confirmed that vitamin D treatment decreased PTH in those on TDF but not in the noTDF group (P = .023 for the vitamin D and TDF interaction; Table 3). The multivariable analysis showed a significant interaction between vitamin D treatment and TDF on CTX change (interaction P = .028). The increase in UCa/UCr and decrease in BAP with vitamin D treatment in the TDF group suggested by univariate analysis (Table 2) was not statistically significant in multivariable models.
Analysis of PTH concentrations stratified by TDF use and baseline serum 25-OHD concentration (<20 vs ≥20 ng/mL; Figure 2B–E) showed that in the noTDF group there was no change in PTH following vitamin D treatment, even in those with insufficient 25-OHD at baseline. In contrast, in participants on TDF who had insufficient baseline 25-OHD, PTH decreased significantly (−7.9 [28.1] pg/mL, P = .031) with vitamin D treatment. In participants in the TDF group who had baseline serum 25-OHD in the sufficient range, PTH also decreased significantly (−6.2 [15.9] pg/mL, P = .053) with vitamin D treatment. Despite these decreases in PTH with vitamin D treatment in the TDF group, week 12 PTH values remained significantly higher in the TDF group than in the noTDF group (44.5 [24.1] and 31.1 [16.5] pg/mL, respectively; P < .001).
Safety of this vitamin D regimen is supported by the absence of significant change in SCa or GFR, although there was a clinically insignificant but statistically significant change in UCa/UCr ratio in the TDF group (Table 2). One participant in the noTDF group and randomized to vitamin D treatment had persistent elevation in UCa/UCr >0.20 mg/mg at baseline and during the study. Fourteen others (4 TDF, 10 noTDF; 9 vitamin D, 5 placebo) had a single episode of UCa/UCr >0.20 mg/mg that reverted to normal when rechecked. All were included in the analyses. There were no clinical toxicities related to treatment.
DISCUSSION
At baseline, PTH was elevated in youth on TDF-containing antiretroviral regimens, suggesting a disorder of calcium balance. This elevated PTH was decreased by vitamin D supplementation only in those on TDF. The magnitude of decline in PTH with vitamin D treatment was similar in participants with and without vitamin D deficiency/insufficiency at baseline. Thus, our results confirmed our hypothesis that vitamin D treatment would reduce PTH in youth on TDF-containing regimens. In contrast and contrary to our hypothesis, we saw no consistently significant interactions between vitamin D supplementation and TDF use on markers of renal tubular or glomerular function, or on CTX or BAP.
TDF is associated with renal tubular toxicity [8] including renal phosphate loss [23], which has been postulated to cause secondary bone resorption and loss of BMD [14, 16]. However, not all studies of TDF-related bone changes have found a relationship between renal tubular dysfunction and markers of bone turnover [13]. Because high PTH can directly cause renal tubular phosphate losses [24], it is possible that TDF-associated renal phosphate wasting is directly related to high PTH, and not to a TDF effect on renal tubular function [25, 26]. Our findings of a decline in PTH not accompanied by an equal improvement in TRP suggest that a mixture of endocrine and renal tubular dysfunction may contribute to the biochemical changes associated with TDF use.
TDF-associated increases in PTH have been reported elsewhere (package insert; [15]. Elevated PTH enhances bone resorption and remodeling by stimulating osteoclasts and osteoblasts, resulting in increases in markers of bone turnover including BAP and CTX [27], actions that are controlled by vitamin D [28]. Hyperparathyroidism and vitamin D deficiency are both associated with increases in markers of bone turnover and decreases in BMD [18, 25]. Although the vitamin D treatment–related decline in PTH resulted in the increase in UCa/UCr ratio and a decline in BAP, we did not see a statistically significant decline in CTX. Perhaps in a longer study a further decline in markers of bone turnover would be evident.
TDF-associated elevations of PTH have been linked to vitamin D deficiency in cross-sectional studies of persons with HIV [15, 29]. Our study confirmed the association of TDF use with higher PTH concentrations, and identified the association of vitamin D insufficiency with baseline elevations in PTH. However, in participants on TDF, baseline PTH was elevated even among those with baseline 25-OHD in the sufficient range. Vitamin D treatment decreased PTH in participants on TDF but not in those on noTDF. Baseline 25-OHD concentration was not an important covariate in this effect: only the interaction of vitamin D treatment and TDF use were statistically significant. Stratified analysis of the effect of vitamin D treatment in vitamin D deficient/insufficient participants compared with those with sufficient vitamin D showed a statistically significant decrease in PTH only for those participants on TDF, and no effect of vitamin D in the noTDF group. Moreover, the magnitude of the effect of vitamin D treatment on PTH in the participants on TDF was similar in those with baseline 25OH-D concentrations in the sufficient and insufficient ranges.
It is unclear how vitamin D treatment may decrease PTH in persons taking TDF in the absence of measurable vitamin D deficiency. Because 1,25-OHD directly decreases PTH [25] and vitamin D treatment increased 1,25-OHD in this study, a direct effect of 1,25-OHD to decrease PTH is one possible mechanism. Impaired intestinal calcium absorption, found with other medications associated with bone toxicity [30, 31], may improve with vitamin D treatment [18]. Because TDF may have effects on other factors that directly increase PTH (eg, fibroblast growth factor 23 [32]), or effects on bone [33–35] that may indirectly lead to a secondary increase in PTH, treatment with vitamin D may correct a “functional vitamin D deficiency” associated with TDF use, even in those with normal serum 25-OHD concentrations.
The absence of consistent evidence of interactions of vitamin D supplementation and TDF use with changes in markers of bone formation (BAP) and resorption (CTX) may be from the relatively short follow-up time of the study, or from the differing time course of osteoblasts to differentiate into osteoclasts or osteogenic cells in response to vitamin D supplementation [36]. Further study with longer follow-up and direct measurements of BMD is needed to define the effect of vitamin D treatment on bone health in youth using TDF.
Major strengths of the study include the randomized, placebo-controlled design, and the use of a once-monthly formulation of vitamin D, which reduced the pill burden and allowed direct observation of participants taking each dose. This eliminated variability associated with poor adherence to study medication.
Limitations include enrollment of only young persons in whom renal clearance of TDF may be more rapid than in adults [37], thus potentially minimizing the role of renal abnormalities in TDF-induced bone changes. Calcium intake, an important factor in PTH concentration, was not controlled. The variability in vitamin D serum concentrations that occurs with once-monthly administration of a large dose of vitamin D [38] may be greater than daily administration of smaller doses, but intermittent therapy [39] may be easier for patients with HIV already challenged with daily pill-taking. TDF use was not randomized, and there were imbalances in TDF and noTDF groups. Multivariate analyses controlled for potential confounding and showed the interaction between TDF use, vitamin D treatment, and change in PTH over the 12 weeks of this study. Lack of association between TDF, vitamin D, and changes in other variables may be from lack of power of this study.
In summary, in this randomized, double-blind, placebo-controlled trial in HIV-infected youth, high-dose monthly vitamin D supplementation decreased PTH levels in those on TDF-containing cART but not in those on regimens not containing TDF. This effect occurred in those with both sufficient and insufficient baseline 25-OHD concentrations. These results suggest that vitamin D supplementation may offset a potential effect of TDF on regulation of calcium balance and bone metabolism.
Notes
Acknowledgments.
Vitamin D and placebo were supplied by Bio-tech Pharmacal. Laboratory assays were performed by Xiaowen Jiang, Melissa Zerofsky, Brian Piccolo, and Gertrud Schuster at the USDA-ARS-WHNRC.
The following ATN sites participated in this study: University of South Florida, Tampa (Emmanuel, Lujan-Zilberman, Julian); Children’s Hospital of Los Angeles (Belzer, Flores, Tucker); University of Southern California at Los Angeles (Kovacs, Homans, Lozano); Children’s National Medical Center (D’Angelo, Hagler, Trexler); Children’s Hospital of Philadelphia (Douglas, Tanney, DiBenedetto); John H. Stroger Jr Hospital of Cook County and the Ruth M. Rothstein CORE Center (Martinez, Bojan, Jackson); University of Puerto Rico (Febo, Ayala-Flores, Fuentes-Gomez); Montefiore Medical Center (Futterman, Enriquez-Bruce, Campos); Mount Sinai Medical Center (Steever, Geiger); University of California, San Francisco (Moscicki, Auerswald, Irish); Tulane University Health Sciences Center (Abdalian, Kozina, Baker); University of Maryland (Peralta, Gorle); University of Miami School of Medicine (Friedman, Maturo, Major-Wilson); Children’s Diagnostic and Treatment Center (Puga, Leonard, Inman); St Jude’s Children’s Research Hospital (Flynn, Dillard); and Children’s Memorial (Garofalo, Brennan, Flanagan).
The following IMPAACT sites participated in the study: Children’s Memorial–Chicago (Yogev, Sanders); Duke University Medical Center (Cunningham, Patil, Wilson); New York University School of Medicine (Borkowsky, Deygoo); University of Medicine and Dentistry of New Jersey (Dieudonne, Bettica, Monti); Bronx-Lebanon Hospital (Purswani, Vachon, Chittalae); Baylor College of Medicine (Shearer, Cooper, McMullen-Jackson); Boston Medical Center (Cooper, McLaud, Tucker); University of Colorado/Children’s Hospital of Denver (McFarland, Chambers, Katai); San Juan Hospital (Acevedo, Gonzalez, Angeli); Seattle Children’s Hospital (Frenkel, Venema-Weiss, Bowen); University of California, San Diego (Spector, Viani, Manning); State University of New York–Stony Brook (Nachman, Puccio, Ferraro); Howard University (Rana,Yu); Miller Children’s Hospital (Chen, Michalik, Jackson-Alvarez); Metropolitan Hospital–New York (Bamji, Paul, Riley); Jacobi Medical Center (Wiznia, Kassen, Burey); Harbor–UCLA Medical Center (Keller, Hayes, Gonzalez); Children’s Hospital of Los Angeles (Rodier, Rockwood); University of Miami (Scott, Falk, Florenz); and Columbia University (LaRussa, Higgins).
Financial support.
Eight of these sites used their General Clinical Research Center (GCRC)/Pediatric Clinical Research Center (PCRC) for the study; the centers were supported by grants from the GCRC Program of the National Center for Research Resources, National Institutes of Health, Department of Health and Human Services, as follows: Children’s National Medical Center, M01RR020359; University of Pennsylvania/Children’s Hospital of Philadelphia, NCRRUL1-RR-024134; University of California, San Francisco, UL1 RR024131; Seattle Children’s Hospital, UL1-RR025014; Texas Children’s Hospital and Baylor College of Medicine, M01-RR00188; Boston University Medical Center, UL1-RR02517; and SUNY Stony Brook, M01-RR10710. The Tulane University Health Sciences Center used its Clinical and Translational Research Center (CTRC) for the study; the center was supported in whole or in part by funds provided through the Louisiana Board of Regents RC/EEP (RC/EEP-06).
This work was supported by the ATN from the National Institutes of Health (U01 HD 040533 and U01 HD 040474) through the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) (B. Kapogiannis), with supplemental funding from the National Institute on Drug Abuse (N. Borek) and the National Institute of Mental Health (NIMH) (P. Brouwers, S. Allison). The protocol was coendorsed by the IMPAACT Group. Support for IMPAACT was provided by the National Institute of Allergy and Infectious Diseases, NICHD, and NIMH (U01 A1068632). The study was scientifically reviewed by the ATN’s Therapeutic Leadership Group. Network, scientific and logistical support was provided by the ATN Coordinating Center (C. Wilson, C. Partlow) at the University of Alabama at Birmingham. Network operations and analytic support was provided by the ATN Data and Operations Center at Westat, Inc (J. Korelitz, B. Driver). We thank the ATN Community Advisory Board and the youth who participated in the study.
Potential conflicts of interest.
All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Cooper RD, Wiebe N, Smith N, Keiser P, Naicker S, Tonelli M. Systematic review and meta-analysis: renal safety of tenofovir disoproxil fumarate in HIV-infected patients. Clin Infect Dis. 2010;51:496–505. doi: 10.1086/655681. [DOI] [PubMed] [Google Scholar]
- 2.Judd A, Boyd KL, Stohr W, et al. Effect of tenofovir disoproxil fumarate on risk of renal abnormality in HIV-1-infected children on antiretroviral therapy: a nested case-control study. AIDS. 2010;24:525–34. doi: 10.1097/QAD.0b013e3283333680. [DOI] [PubMed] [Google Scholar]
- 3.Kinai E, Hanabusa H. Renal tubular toxicity associated with tenofovir assessed using urine-beta 2 microglobulin, percentage of tubular reabsorption of phosphate and alkaline phosphatase levels. AIDS. 2005;19:2031–3. doi: 10.1097/01.aids.0000194130.05264.83. [DOI] [PubMed] [Google Scholar]
- 4.Kinai E, Hanabusa H, Kinai E, Hanabusa H. Progressive renal tubular dysfunction associated with long-term use of tenofovir DF. AIDS Res Hum Retroviruses. 2009;25:387–94. doi: 10.1089/aid.2008.0202. [DOI] [PubMed] [Google Scholar]
- 5.Rodriguez-Novoa S, Labarga P, Soriano V, et al. Predictors of kidney tubular dysfunction in HIV-infected patients treated with tenofovir: a pharmacogenetic study. Clin Infect Dis. 2009;48:e108–16. doi: 10.1086/598507. [DOI] [PubMed] [Google Scholar]
- 6.Buchacz K, Brooks JT, Tong T, et al. Evaluation of hypophosphataemia in tenofovir disoproxil fumarate (TDF)-exposed and TDF-unexposed HIV-infected out-patients receiving highly active antiretroviral therapy. HIV Med. 2006;7:451–6. doi: 10.1111/j.1468-1293.2006.00407.x. [DOI] [PubMed] [Google Scholar]
- 7.Essig M, Duval X, Kaied FA, et al. Is phosphatemia the best tool to monitor renal tenofovir toxicity? J Acquir Immune Defic Syndr. 2007;46:256–8. doi: 10.1097/QAI.0b013e3181142f31. [DOI] [PubMed] [Google Scholar]
- 8.Labarga P, Barreiro P, Martin-Carbonero L, et al. Kidney tubular abnormalities in the absence of impaired glomerular function in HIV patients treated with tenofovir. AIDS. 2009;23:689–96. doi: 10.1097/QAD.0b013e3283262a64. [DOI] [PubMed] [Google Scholar]
- 9.Gallant JE, Staszewski S, Pozniak AL, et al. Efficacy and safety of tenofovir DF vs stavudine in combination therapy in antiretroviral-naive patients: a 3-year randomized trial. JAMA. 2004;292:191–201. doi: 10.1001/jama.292.2.191. [DOI] [PubMed] [Google Scholar]
- 10.Gafni RI, Hazra R, Reynolds JC, et al. Tenofovir disoproxil fumarate and an optimized background regimen of antiretoviral agents as salvage therapy: impact on bone mineral density in HIV-infected children. Pediatrics. 2006;118:e711–18. doi: 10.1542/peds.2005-2525. [DOI] [PubMed] [Google Scholar]
- 11.Hazra R, Gafni RI, Maldarelli F, et al. Tenofovir disoproxil fumarate and an optimized background regimen of antiretroviral agents as salvage therapy for pediatric HIV infection. Pediatrics. 2005;116:e846–54. doi: 10.1542/peds.2005-0975. [DOI] [PubMed] [Google Scholar]
- 12.Martin A, Bloch M, Amin J, et al. Simplification of antiretroviral therapy with tenofovir-emtricitabine or abacavir-lamivudine: a randomized, 96-week trial. Clin Infect Dis. 2009;49:1591–601. doi: 10.1086/644769. [DOI] [PubMed] [Google Scholar]
- 13.Stellbrink H-J, Orkin C, Arribas JR, et al. Comparison of changes in bone density and turnover with abacavir-lamivudine versus tenofovir-emtricitabine in HIV-infected adults: 48-week results from the ASSERT study. Clin Infect Dis. 2010;51:963–72. doi: 10.1086/656417. [DOI] [PubMed] [Google Scholar]
- 14.Fux CA, Rauch A, Simcock M, et al. Tenofovir use is associated with an increase in serum alkaline phosphatase in the Swiss HIV Cohort Study. Antivir Ther. 2008;13:1077–82. [PubMed] [Google Scholar]
- 15.Rosenvinge MM, Gedela K, Copas AJ, et al. Tenofovir-linked hyperparathyroidism is independently associated with the presence of vitamin D deficiency. J Acquir Immune Defic Syndr. 2010;54:496–9. doi: 10.1097/qai.0b013e3181caebaa. [DOI] [PubMed] [Google Scholar]
- 16.Woodward CL, Hall AM, Williams IG, et al. Tenofovir-associated renal and bone toxicity. HIV Med. 2009;10:482–7. doi: 10.1111/j.1468-1293.2009.00716.x. [DOI] [PubMed] [Google Scholar]
- 17.Stephensen CB, Marquis GS, Kruzich LA, Douglas SD, Aldrovandi GM, Wilson CM. Vitamin D status in adolescents and young adults with HIV infection. Am J Clin Nutr. 2006;83:1135–41. doi: 10.1093/ajcn/83.5.1135. [DOI] [PubMed] [Google Scholar]
- 18.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 19.Armas LA, Hollis BW, Heaney RP. Vitamin D2 is much less effective than vitamin D3 in humans. J Clin Endocrinol Metab. 2004;89:5387–91. doi: 10.1210/jc.2004-0360. [DOI] [PubMed] [Google Scholar]
- 20.Institute of Medicine. Dietary reference intakes for calcium and vitamin D. Washington, DC: National Academies Press; 2011. Available at: http://books.nap.edu/openbook.php?record_id=13050. Accessed 21 November 2011. [PubMed] [Google Scholar]
- 21.Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–54. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 22.Cummings SR, Block G, McHenry K, Baron RB. Evaluation of two food frequency methods of measuring dietary calcium intake. Am J Epidemiol. 1987;126:796–802. doi: 10.1093/oxfordjournals.aje.a114716. [DOI] [PubMed] [Google Scholar]
- 23.Fux CA, Christen A, Zgraggen S, et al. Effect of tenofovir on renal glomerular and tubular function. AIDS. 2007;21:1483–5. doi: 10.1097/QAD.0b013e328216f15b. [DOI] [PubMed] [Google Scholar]
- 24.Peacock M. Primary hyperparathyroidism and the kidney: biochemical and clinical spectrum. J Bone Miner Res. 2002;17(Suppl 2):N87–94. [PubMed] [Google Scholar]
- 25.Marks KH, Kilav R, Naveh-Many T, Silver J. Calcium, phosphate, vitamin D, and the parathyroid. Pediatr Nephrol. 1996;10:364–7. doi: 10.1007/BF00866787. [DOI] [PubMed] [Google Scholar]
- 26.Domrongkitchaiporn S, Disthabanchong S, Cheawchanthanakij R, et al. Oral phosphate supplementation corrects hypophosphatemia and normalizes plasma FGF23 and 25-hydroxyvitamin D3 levels in women with chronic metabolic acidosis. Exp Clin Endocrinol Diabetes. 2010;118:105–12. doi: 10.1055/s-0029-1202791. [DOI] [PubMed] [Google Scholar]
- 27.Sims NA, Gooi JH. Bone remodeling: multiple cellular interactions required for coupling of bone formation and resorption. Semin Cell Dev Biol. 2008;19:444–51. doi: 10.1016/j.semcdb.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 28.Suda T, Ueno Y, Fujii K, Shinki T. Vitamin D and bone. J Cell Biochem. 2003;88:259–66. doi: 10.1002/jcb.10331. [DOI] [PubMed] [Google Scholar]
- 29.Childs KE, Fishman SL, Constable C, et al. Short communication: inadequate vitamin D exacerbates parathyroid hormone elevations in tenofovir users. AIDS Res Hum Retroviruses. 2010;26:855–9. doi: 10.1089/aid.2009.0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.von Borstel Smith M, Crofoot K, Rodriguez-Proteau R, Filtz TM. Effects of phenytoin and carbamazepine on calcium transport in Caco-2 cells. Toxicol In Vitro. 2007;21:855–62. doi: 10.1016/j.tiv.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 31.Lee RH, Lyles KW, Colón-Emeric C. A review of the effect of anticonvulsant medications on bone mineral density and fracture risk. Am J Geriatr Pharmacother. 2010;8:34–46. doi: 10.1016/j.amjopharm.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Razzaque MS. The FGF23-Klotho axis: endocrine regulation of phosphate homeostasis. Nat Rev Endocrinol. 2009;5:611–9. doi: 10.1038/nrendo.2009.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grigsby IF, Pham L, Mansky LM, et al. Tenofovir treatment of primary osteoblasts alters gene expression profiles: implications for bone mineral density loss. Biochem Biophys Res Commun. 2010;394:48–53. doi: 10.1016/j.bbrc.2010.02.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grigsby IF, Pham L, Gopalakrishnan R, et al. Downregulation of Gnas, Got2 and Snord32a following tenofovir exposure of primary osteoclasts. Biochem Biophys Res Commun. 2010;391:1324–9. doi: 10.1016/j.bbrc.2009.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grigsby IF, Pham L, Mansky LM, et al. Tenofovir-associated bone density loss. Ther Clin Risk Manag. 2010;6:41–7. [PMC free article] [PubMed] [Google Scholar]
- 36.Nakahama K-I. Cellular communications in bone homeostasis and repair. Cell Mol Life Sci. 2010;67:4001–9. doi: 10.1007/s00018-010-0479-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kiser JJ, Fletcher CV, Flynn PM, et al. Pharmacokinetics of antiretroviral regimens containing tenofovir disoproxil fumarate and atazanavir-ritonavir in adolescents and young adults with human immunodeficiency virus infection. Antimicrob Agents Chemother. 2008;52:631–7. doi: 10.1128/AAC.00761-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heaney RP, Armas LAG, Shary JR, Bell NH, Binkley N, Hollis BW. 25-Hydroxylation of vitamin D3: relation to circulating vitamin D3 under various input conditions. Am J Clin Nutr. 2008;87:1738–42. doi: 10.1093/ajcn/87.6.1738. [DOI] [PubMed] [Google Scholar]
- 39.Arpadi SM, McMahon D, Abrams EJ, et al. Effect of bimonthly supplementation with oral cholecalciferol on serum 25-hydroxyvitamin D concentrations in HIV-infected children and adolescents. Pediatrics. 2009;123:e121–6. doi: 10.1542/peds.2008-0176. [DOI] [PMC free article] [PubMed] [Google Scholar]