Abstract
The tuberculin skin test (TST) measures the intensity of antimycobacterial acquired immunity and is used to diagnose latent infection with Mycobacterium tuberculosis. We report evidence for a codominant gene explaining ∼65% of the TST variability. Disregarding the host genetic background may lead to misclassifications of TST-based diagnosis of latent M. tuberculosis infection.
A widely used test for latent Mycobacterium tuberculosis infection is the tuberculin skin test (TST). The test measures induration of the skin after intradermal inoculation of M. tuberculosis purified protein derivative. The TST triggers a classic T-cell–mediated delayed-type hypersensitivity reaction against mycobacterial antigens [1, 2]. Hence, the extent of the TST reaction is a measure of the intensity of the antimycobacterial immune response. However, persons exposed to M. tuberculosis who persistently show zero TST reactivity may be innately resistant to infection by M. tuberculosis.
Several genetic epidemiological studies in tuberculosis-endemic areas have reported high levels of heritability (ie, ≥70%) for TST reactivity [3, 4], and some studies have correlated genetic variants with TST negativity or positivity. A study in Ghana showed that an IL10 haplotype, associated with low interleukin-10 production, was significantly less frequent in TST-negative controls than in TST-positive controls [5]. One single nucleotide polymorphism of this haplotype was also found to be associated with TST negativity and positivity in Brazil [6]. More recently, a South African study found a major locus (TST2) located on chromosomal region 5p15 that controls the extent of the TST [7].
To further investigate the genetic component of TST reactivity, we conducted a genetic epidemiological study in a Colombian familial sample of individuals recently exposed to a tuberculosis case patient in their household. Our objectives were to investigate the presence of familial correlations and to perform a complex segregation analysis to discriminate between the different factors causing familial resemblance.
SUBJECTS AND METHODS
Family Study
Ninety multigenerational families composed of household contacts of smear-positive pulmonary tuberculosis case patients were recruited from Medellin, Colombia. Medellin had a tuberculosis incidence of 33/100 000 at the time of enrollment (2006) (http://www.dssa.gov.co/index.php/salud-publica/vigilancia-epidemiologica/bias). Patients with tuberculosis were recruited at the health centers where they were diagnosed. Bacteriological confirmation of tuberculosis was performed by detection of acid-fast bacilli at the local tuberculosis control program’s laboratories and confirmed by the microbiology laboratory at Corporacion para Investigaciones Biologicas by sputum microscopy and culture. A household member was considered a contact if he or she had spent time regularly (eg, several hours per day) in the same household as the index case patient for ≥1 month before index case diagnosis [8]. Household contacts with a history of tuberculosis disease and index case patients were excluded from the study.
TSTs were performed using the Mantoux method with tuberculin purified protein derivative RT23 (2 tuberculin units; Staten Serum Institute). Transversal diameters of indurations were measured in millimeters 48–72 hours after the TST had been performed using the “ballpoint pen” method and following a preestablished standard operating procedure [8]. The study was approved by the ethics committee of the Faculty of Medicine at the University of Antioquia (Medellin, Colombia) and the research ethics board at the Research Institute of the McGill University Health Centre (Montreal, Quebec, Canada).
Statistical Methods
First, we investigated the effects of 4 covariates on the extent of TST reactivity by linear regression: sex, age, having a Bacillus Calmette-Guerin vaccine scar, and sleeping in the same bed as the tuberculosis index case patient (as a proxy for contact intensity with the index case patient). The best-fitting model for the age effect was determined among a set of fractional polynomial models, as proposed elsewhere [9], and was found to be the square root. The analyses were performed with the GLM procedure of SAS software (SAS Institute).
We then performed a segregation analysis using the regressive model proposed by Bonney [10] and implemented in the software package S.A.G.E (http://darwin.cwru.edu/sage/). The overall idea of segregation analysis is to fit several models to the observed distribution of TST values and to select the one that best explains the observed data. The simplest model includes only the covariates of interest (sporadic model). Next, we add familial correlations to this sporadic model. Finally, the complete model includes covariates, familial correlations, and the presence of a gene. Estimation of parameters is based on the maximum-likelihood method, and nested models are compared by means of likelihood-ratio test (see supplemental materials for methodological details).
RESULTS AND DISCUSSION
Family Sample
A total of 90 families composed of 2–13 members with a TST value were enrolled. The overall sample size was 371 healthy household contacts of persons with confirmed tuberculosis comprising 271 parent-offspring and 244 sibling (sib)–sib pairs. Among the household contacts, the sex ratio (male-female) was 1.06, and the mean age was 27.8 years (range 1–83 years). There were 302 household contacts (81.4%) with a BCG vaccine scar, and 44 (11.9%) reported sleeping in the same bed as the tuberculosis index case patient. The TST values ranged from 0 to 35 mm with a mean (median) value of 11.5 (13.0) mm, and a standard deviation of 7.3 mm.
Analysis of Covariates
Results of multivariate analysis (ie, testing of all 4 covariates simultaneously) showed that age had the strongest impact on TST intensity. In particular, the mean TST values significantly increased with age from 7.9 mm before 5 years of age to 13 mm after 20 years of age (P = 1 × 10−8). The strong age effect was due to an increase of the proportion of subjects with TST values >0 mm (data not shown), as seen in many studies focusing on the proportions of TST-positive responders, and presumably reflects age-dependent cumulative exposure [11]. We also noticed an impact of BCG vaccination on the TST because individuals with a BCG vaccine scar displayed a significantly higher mean TST value (11.6 vs 10.4 mm; P = .04). Likewise, there was a significant effect of sleeping in the same bed as the tuberculosis index case patient (13.4 vs 11.2 mm; P = .02). By contrast, we did not observe a significant sex effect (P > .2). Therefore, only age, BCG vaccine scar, and sleeping in the same bed as the tuberculosis index case patient were included in the segregation analysis. Overall, these 3 covariates explained ∼10% of the observed TST variability.
Segregation Analysis
There was evidence for strong familial correlations because the model including both parent-offspring and sib-sib correlations was 5.7 × 106 times more likely than the sporadic model including only the 3 covariates. The likelihood of observing such a strong effect by chance was very small (P < 2.10−7). In the presence of familial correlations, strong evidence of a codominant major gene was observed because the model including familial correlations and a major gene was 4.6 × 106 more likely than the model including only familial correlations (P < 10−6). Finally, a codominant mode of inheritance for the major gene was 5.4 × 104 (P = 3.10−6) and 5.4 × 102 (P = .002) times more likely than a dominant and a recessive mode of inheritance, respectively (see supplemental materials for details about the testing procedure). Overall, the results of our study show strong evidence of a codominant major gene controlling the extent of TST reactivity in household contacts of tuberculosis case patients, as illustrated by the excellent agreement between the distribution of the TST values predicted under our “codominant gene model” and the observed one (Figure 1).
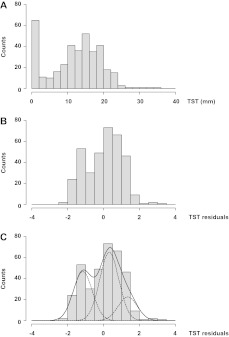
Figure 1.
Observed and predicted distribution of tuberculin skin test (TST) values in 371 healthy household contacts of persons with confirmed tuberculosis. A, Histogram of observed TST values in 371 healthy household contacts of confirmed tuberculosis case patients. B, Histogram of standardized TST residuals after accounting for the effects of age, BCG vaccine scar, and sleeping in the same bed as the index case patient. TST residuals were generated as the difference between observed and predicted TST values, where predicted TST values = mean TST + β√age × (√age − mean √age) + βBCG scar × (BCG scar − mean BCG scar) + βsleeping same bed × (sleeping same bed − mean sleeping same bed) = 11.31 + 0.73 × (√age − 4.85) + 0.29 × (BCG scar − 0.85) + 2.46 × (sleeping same bed − 0.12). Note that “BCG scar” and “sleeping same bed” covariates can only take the value 0 (no) or 1 (yes). Finally, TST residuals were standardized. C, Solid curve represents cumulative distribution of standardized TST residuals as predicted under the codominant gene model with parent-offspring and sibling (sib)–sib familial correlations (model IIIa in Supplemental Table 1) and assuming a normal distribution of TST values for each of the genotypes (dotted curves). The proportions of AA, aA, and aa individuals are 0.35, 0.48, and 0.17, respectively. The means of standardized TST residuals for AA, aA, and aa individuals are −1.15, 0.32, and 1.35, respectively, with a common estimated variance within each genotypic class equal to 0.28.
The proportion of residual TST variance (ie, the variance observed after adjusting on relevant covariates) explained by this major gene (ie, the heritability) is 0.72. This estimate is very consistent with heritability estimates of TST reactivity and related measures of antimycobacterial immunity previously reported in the context of M. tuberculosis community exposure [3, 4, 12]. We estimated the frequency of the A allele predisposing to low values of TST reactivity to be 0.59 (standard deviation, 0.03), indicating that ∼35% of the population carried the AA genotype and were predisposed to low TST reactivity, whereas ∼17% (carriers of aa genotypes) were predisposed to high TST reactivity. As expected from the high heritability, the impact of the major gene on measured TST values was very substantial. For example, we estimated that at 16 years of age, household contacts with a BCG vaccine scar who did not sleep in the same bed as the tuberculosis index case patient had a mean TST value of 2.5, 12.8, or 20 mm if they were AA, Aa, or aa, respectively. Hence, dichotomization of TST reactivity into positive and negative responders at a genotype-independent cutoff would be subject to substantial confounding by host genetic factors.
It remains to be defined if the codominant major gene identified in the present study corresponds to the TST2 locus that we previously identified in a familial sample from South Africa [9]. If the codominant major gene identified in the present study corresponds to the TST2 locus that we previously identified in a familial sample from South Africa [7] remains to be defined. To assess this question, a model-based linkage study of TST reactivity and the TST2 locus, using the genetic model estimated by our segregation analysis, is ongoing in the Colombian sample. Molecular identification of a major gene controlling the extent of TST reactivity will allow a more reliable interpretation of TST reactivity in tuberculosis surveillance by incorporating genotypic information in the threshold definition. Meanwhile, taking into account familial history of TST reactivity could help to better classify individuals as M. tuberculosis infected or uninfected, particularly in BCG-vaccinated populations. Because the TST is the prototypical example of antimycobacterial immunity, identification of major genetic variants that predispose a person to low or high immune reactivity may also be useful for classification of subjects in tuberculosis vaccine trials.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments.
We sincerely thank all members of the community who participated in this study. We thank Lucelly López and Diana Marín for their skillful management of the epidemiological database and Jean-Laurent Casanova (St Giles Laboratory of Human Genetics of Infectious Diseases) for helpful comments on earlier drafts of the manuscript.
Financial support.
This work was supported by Colciencias, Bogotá, Colombia (grants 11150416335 and 34261817270), the Canadian Institutes of Health Research (grant MOP 111172 to E. S.), the Legs Poix (grant to A. A.) the Government of Canada (Banting postdoctoral fellowship BPF 112932 to A. C.), Fondation de la Recherche Médicale (grant to A. C.), and Howard Hughes Medical Institute (International Research Scholarship to E. S.). The results of this paper were obtained by using the program package S.A.G.E., which is supported by a U.S. Public Health Service Resource Grant (RR03655) from the National Center for Research Resources.
Potential conflicts of interest.
All authors: no reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Vukmanovic-Stejic M, Reed JR, Lacy KE, Rustin MH, Akbar AN. Mantoux test as a model for a secondary immune response in humans. Immunol Lett. 2006;107:93–101. doi: 10.1016/j.imlet.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 2.Gallant CJ, Cobat A, Simkin L, et al. Tuberculin skin test and in vitro assays provide complementary measures of antimycobacterial immunity in children and adolescents. Chest. 2010;137:1071–7. doi: 10.1378/chest.09-1852. [DOI] [PubMed] [Google Scholar]
- 3.Sepulveda RL, Heiba IM, King A, Gonzalez B, Elston RC, Sorensen RU. Evaluation of tuberculin reactivity in BCG-immunized siblings. Am J Respir Crit Care Med. 1994;149:620–4. doi: 10.1164/ajrccm.149.3.8118628. [DOI] [PubMed] [Google Scholar]
- 4.Jepson A, Fowler A, Banya W, et al. Genetic regulation of acquired immune responses to antigens of Mycobacterium tuberculosis: a study of twins in West Africa. Infect Immun. 2001;69:3989–94. doi: 10.1128/IAI.69.6.3989-3994.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thye T, Browne EN, Chinbuah MA, et al. IL10 haplotype associated with tuberculin skin test response but not with pulmonary TB. PLoS One. 2009;4:e5420. doi: 10.1371/journal.pone.0005420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zembrzuski VM, Basta PC, Callegari-Jacques SM, et al. Cytokine genes are associated with tuberculin skin test response in a native Brazilian population. Tuberculosis (Edinb) 2010;90:44–9. doi: 10.1016/j.tube.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 7.Cobat A, Gallant CJ, Simkin L, et al. Two loci control tuberculin skin test reactivity in an area hyperendemic for tuberculosis. J Exp Med. 2009;206:2583–91. doi: 10.1084/jem.20090892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.del Corral H, Paris SC, Marin ND, et al. IFNgamma response to Mycobacterium tuberculosis, risk of infection and disease in household contacts of tuberculosis patients in Colombia. PLoS One. 2009;4:e8257. doi: 10.1371/journal.pone.0008257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Royston P, Ambler G, Sauerbrei W. The use of fractional polynomials to model continuous risk variables in epidemiology. Int J Epidemiol. 1999;28:964–74. doi: 10.1093/ije/28.5.964. [DOI] [PubMed] [Google Scholar]
- 10.Bonney GE. On the statistical determination of major gene mechanisms in continuous human traits: regressive models. Am J Med Genet. 1984;18:731–49. doi: 10.1002/ajmg.1320180420. [DOI] [PubMed] [Google Scholar]
- 11.Gallant CJ, Cobat A, Simkin L, et al. Impact of age and sex on mycobacterial immunity in an area of high tuberculosis incidence. Int J Tuberc Lung Dis. 2010;14:952–9. [PubMed] [Google Scholar]
- 12.Cobat A, Gallant CJ, Simkin L, et al. High heritability of antimycobacterial immunity in an area of hyperendemicity for tuberculosis disease. J Infect Dis. 2010;201:15–9. doi: 10.1086/648611. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.