In this randomized, open-label trial of healthcare personnel previously vaccinated with acellular pertussis vaccine, noninferiority of daily symptom monitoring without postexposure prophylaxis (PEP) compared with antibiotic PEP was not demonstrated for preventing infection following pertussis exposure.
Abstract
Background. Antibiotic postexposure prophylaxis (PEP) following pertussis exposure is recommended but has never been evaluated in healthcare personnel (HCP) vaccinated with acellular pertussis vaccine (Tdap).
Methods. Tdap-vaccinated HCP were randomized to receive azithromycin PEP or no PEP following pertussis exposure. Acute and convalescent nasopharyngeal swabs and sera were obtained for pertussis testing by polymerase chain reaction (PCR) and anti–pertussis toxin (PT) immunoglobulin G, respectively. A nasopharyngeal aspirate was also collected for PCR and culture from subjects who reported respiratory symptoms within 21 days following identification of the exposure. Pertussis infection was defined as a positive culture or PCR, a 2-fold rise in anti-PT titer, or a single anti-PT titer of ≥94 enzyme-linked immunosorbent assay units/mL. Daily symptom monitoring without PEP was considered noninferior to PEP after pertussis exposure if the lower limit of the 1-sided 95% confidence interval (CI) for the reduction in pertussis was greater than −7%.
Results. During 30 months of study, 86 subjects were randomized following a pertussis exposure. Using the predefined definition of infection, pertussis infection did not develop in 41 (97.6%) of 42 subjects who received azithromycin PEP and 38 (86.4%) of 44 subjects who did not receive PEP (absolute risk difference, −11.3%; lower bound of the 1-sided 95% CI, −20.6%; P = .81). However, no subject developed symptomatic pertussis confirmed with culture or a specific PCR assay, and possibly no subject developed subclinical pertussis infection based upon additional serologic testing.
Conclusions. Using the predefined definition of pertussis infection, noninferiority for preventing pertussis following exposure was not demonstrated for daily symptom monitoring of Tdap-vaccinated HCP without PEP when compared with antibiotic PEP. However, the small number of exposed HCP warrants further study of this approach.
Clinical Trial Registration. NCT00469274.
Pertussis, or whooping cough, is an acute respiratory tract infection caused by Bordetella pertussis [1]. The incidence of pertussis has been increasing since the 1980s [1]. Although the increase is not completely understood, factors such as increased transmission, waning immunity from childhood vaccination, and changes in diagnostic testing and reporting are likely contributors. Healthcare personnel (HCP) are at increased risk for pertussis due to regular contact with infected patients [2–4]. They may then transmit infection to susceptible patients and other healthcare personnel, as evidenced by numerous reported outbreaks of healthcare-associated pertussis [5–11].
Vaccination is effective for preventing pertussis in healthy adults and adolescents [12]. In 2005, a tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine (Tdap) was licensed for use in adolescents and adults aged 11–64 years [1]. Then in 2006, the Centers for Disease Control and Prevention (CDC) recommended that all HCP with direct patient contact receive a single dose of Tdap to reduce the risk of pertussis transmission within healthcare institutions. Prior to licensure of Tdap, the only method to reduce transmission after pertussis exposure was antibiotic postexposure prophylaxis (PEP) [13]. The decision to provide PEP to an exposed HCP involves detailed assessments of the infectiousness of the index case, the degree of exposure and risk of pertussis in the HCP, the potential for secondary transmission to high-risk contacts (eg, infants), and the capacity to monitor for symptoms in the exposed HCP. Previously, the CDC recommended that exposed, vaccinated HCP receive either antibiotic PEP or daily symptom monitoring without PEP, with prompt evaluation, treatment, and furlough if symptoms develop [1]. To test the best approach for management of pertussis exposure in previously vaccinated HCP, we conducted a randomized, open-label trial to determine if daily symptom monitoring without PEP was noninferior to antibiotic PEP.
METHODS
Study Population
Between May 2007 and October 2009, all HCP working at a 206-bed, tertiary care, pediatric acute care hospital were recruited for enrollment. Inclusion criteria were aged 18–64 years, self-report of direct patient contact, planning to work at least 1 year from enrollment, and willing to cooperate with surveillance. All subjects were vaccinated with Tdap (ADACEL; sanofi pasteur, Toronto, Ontario, Canada). Each dose contained the following active ingredients: 5 Lf tetanus toxoid, 2 Lf diphtheria toxoid, 2.5 μg detoxified pertussis toxin (PT), 5 μg filamentous hemagglutinin (FHA), 3 μg pertactin, and 5 μg fimbriae types 2 and 3 [14]. Most subjects received Tdap at enrollment, but some had previously received Tdap from the Occupational Health Clinic (OHC) or their personal physician. All previous vaccinations were documented with chart review.
Exclusion criteria for enrollment were a history of allergic or adverse reaction to both azithromycin and trimethoprim-sulfamethoxazole, current prolonged treatment with a macrolide or trimethoprim-sulfamethoxazole, and prelicensure receipt of an acellular pertussis vaccine through participation in a prior clinical trial. Additionally, subjects who required Tdap at enrollment were excluded if they had received a booster of tetanus toxoid and reduced diphtheria toxoid vaccine (ie, Td) in the 2 years prior to screening; if they had a history of allergic or severe adverse reaction to diphtheria, tetanus, or pertussis vaccines, a history of encephalopathy within 7 days of a previous dose of a pertussis-containing vaccine not attributable to another identifiable cause, or a history of progressive neurological disorder, uncontrolled epilepsy, or progressive encephalopathy; or if they were pregnant or attempting to become pregnant.
Exposure Evaluation and Randomization
The Department of Infection Control and Prevention conducted routine surveillance of laboratory-confirmed pertussis among patients. After identification of an infected patient, OHC contacted and evaluated potentially exposed HCP. HCP considered exposed (ie, face-to-face contact within 3 feet of the infected patient during which the subject did not wear a mask) by OHC completed a survey of patient care activities performed during the exposure. Exposed HCP were then randomized to receive daily symptom monitoring either with or without antibiotic PEP. Blocked randomization was performed using a randomly varying block size of 4, 6, or 8 according to a computer-generated random number. Subjects involved in multiple exposures during the study were randomized to a separate postexposure strategy following each exposure.
Subjects were excluded from randomization if they had a previous pertussis exposure within the past 4 weeks, if they had fever (eg, temperature ≥38°C), cough, sore throat, or rhinorrhea, if they received PEP outside of the study, if they had been vaccinated with Tdap <7 days prior to the exposure, or if they were recognized as exposed ≥5 days after pertussis was first detected in the index patient because of the likely inability to reliably detect asymptomatic pertussis infection in the exposed subject. Subjects excluded from randomization were referred to OHC to receive prophylaxis per standard hospital procedures and did not provide clinical specimens for testing.
For all randomized subjects, a nasopharyngeal swab and serum sample were collected at the time of randomization (acute) and again 21 days after identification of the infected patient (convalescent). Subjects randomized to PEP were given azithromycin (500 mg on day 1 and 250 mg daily on days 2–5). Subjects in both study arms were queried daily for symptoms of respiratory infection for the 21-day monitoring period. If a subject developed fever, cough, sore throat, or rhinorrhea, a nasopharyngeal aspirate was collected [15]. Symptomatic HCP were treated with azithromycin empirically, regardless of PEP assignment, and referred to OHC to determine when they could return to work.
Diagnostic Methods
Sera were tested for anti-PT immunoglobulin G (IgG) by enzyme-linked immunosorbent assay (ELISA) using a validated assay with a lower limit of detection of 10 ELISA units/mL (EU) [16]. Nasopharyngeal swabs were tested for pertussis by polymerase chain reaction (PCR) using IS481. After collection, specimens were stored at −70°C until time of testing. DNA was purified using the automated Qiagen EZ1 Robot. Each real time PCR assay contained no template controls, high and low B. pertussis controls (cycle threshold [Ct] 28 and 31, respectively) with DNA extracted from ATCC culture 9797, and a B. parapertussis control containing DNA extracted from ATCC culture 15311. Additional details of the single-target PCR procedure are described in the Appendix. B. pertussis PCR was considered positive if Ct was ≤40 cycles.
Nasopharyngeal aspirates were obtained in symptomatic HCP and divided into 2 equal aliquots. One aliquot was used for PCR testing, and the second aliquot was inoculated onto Regan-Lowe transport medium and sent within 24 hours after collection to the Tennessee Department of Health State Laboratory for culture. Specimens were inoculated onto Regan-Lowe Agar plates and placed in a CO2 incubator at 35°C –37°C. Plates were reviewed daily for growth. Any colonies resembling B. pertussis were transferred to a fresh Regan-Lowe plate for isolation and confirmation. Cultures without growth at 7 days were considered negative.
Outcomes and Statistical Analysis
The primary definition of pertussis infection included a positive nasopharyngeal culture or PCR for B. pertussis at any time point, a 2-fold rise in the anti-PT IgG titer between acute and convalescent sera, or a single acute or convalescent anti-PT IgG titer of ≥94 EU (Table 1) [16]. The primary definition excluded symptoms because antibiotic PEP might prevent symptoms without affecting laboratory markers of infection. Post hoc, a modified definition was devised because of concern that the serologic criteria used in the primary definition might actually represent acquisition of pertussis infection prior to the intervention under study. The modified definition of pertussis excluded an acute anti-PT IgG titer of ≥94 EU and an acute nasopharyngeal swab that was positive for B. pertussis by PCR (Table 1).
Table 1.
Summary of Definitions of Pertussis Infectiona
| Criteria | Primary Definition | Modified Definition |
| Nasopharyngeal swab PCR positive | ||
| Acute specimen | Positive | NA |
| Convalescent specimen | Positive | Positive |
| Nasopharyngeal aspirate PCR or culture positiveb | Positive | Positive |
| Anti-PT immunoglobulin G titer | ||
| Acute specimen | ≥94 EU/mL | NA |
| Convalescent specimen | ≥94 EU/mL | ≥94 EU/mL |
| Acute to convalescent antibody increase | ≥2-fold | ≥2-fold |
Abbreviations: EU, enzyme-linked immunosorbent assay (ELISA) units per milliliter; NA, not applicable; PCR, polymerase chain reaction; PT, pertussis toxin.
The presence of a single criterion within a definition was sufficient to establish the diagnosis of pertussis infection.
Nasopharyngeal aspirates were obtained only from subjects who developed fever, cough, runny nose, or sore throat during the postexposure monitoring period.
We estimated the protection rate of vaccination with ADACEL without antibiotic PEP following a healthcare exposure to be >92% [12]. Although this estimate is based upon the protection rate of a different acellular pertussis vaccine, the 2 vaccines are thought to have similar immunogenicity and efficacy [12]. Although data are lacking on the secondary attack rate in recently vaccinated persons who receive antibiotic PEP following a healthcare exposure, published data suggest that erythromycin PEP decreases the secondary attack rate among unvaccinated household contacts by 31%–68% [17, 18]. Therefore, we assumed the protection rate of azithromycin PEP in vaccinated HCP following a healthcare exposure to be ≥95%. Because of the potential risk of secondary transmission from an infected HCP to vulnerable patients, a minimum clinically important difference of −0.07 was selected. Using the method described by Farrington and Manning [19], we estimated that 150 subjects per arm would be sufficient to reject the null hypothesis that daily symptom monitoring without PEP was inferior to antibiotic PEP with α = .05 and β = .20.
Data were analyzed with Stata (Intercooled, version 9.2; Stata Corporation, College Station, TX). Continuous variables are described in percentiles and compared using the Wilcoxon rank sum or Kruskal–Wallis test, as appropriate. Discrete variables are described in frequencies and percentages and compared using Fisher’s exact test. Noninferiority was assessed using the test statistic z where z = (δ+Δ)/se(δ), where δ represents the difference observed between the treatment arms, Δ represents the pre-specified noninferiority margin of −0.07, and se(δ) is the standard error of the observed difference. This assumes the test statistic, z, converges to the standard normal distribution. Daily symptom monitoring without PEP was considered noninferior to antibiotic PEP if the lower limit of the 1-sided 95% confidence interval (CI) for the reduction in pertussis was greater than −7%.
RESULTS
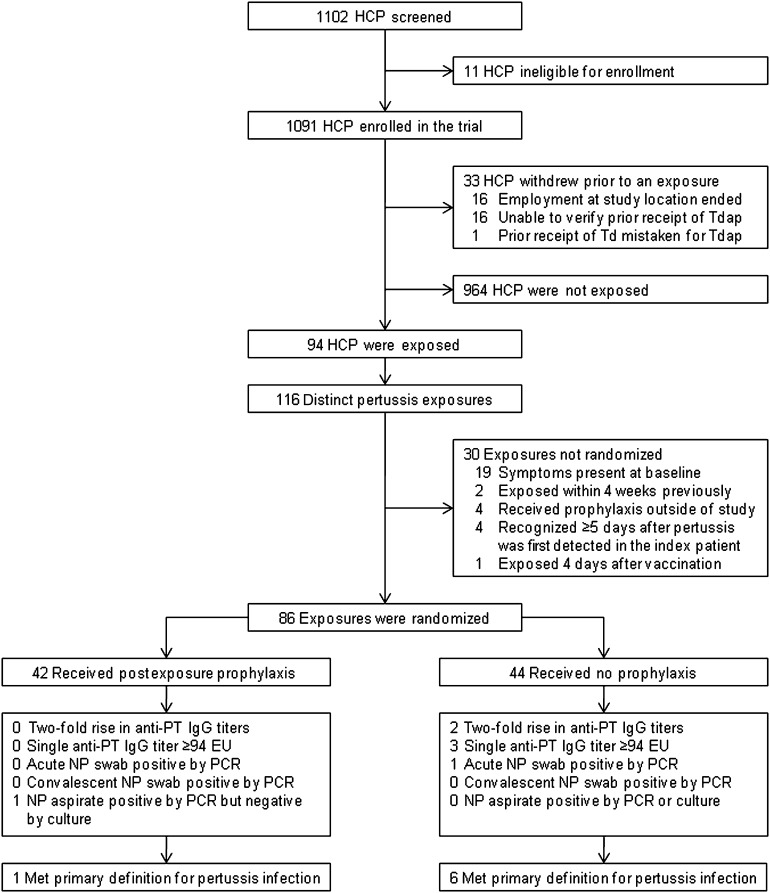
Eleven of the 1102 HCP eligible for enrollment were excluded because of recent vaccination with Td precluded vaccination with Tdap; thus, 1091 HCP were enrolled (Figure 1). During the study period 33 patients with pertussis infection were identified resulting in 116 distinct exposures among 94 different HCP. Thirty of the 116 exposures were not eligible for randomization because of the presence of respiratory symptoms, an earlier pertussis exposure within the previous 4 weeks, receipt of antibiotic prophylaxis outside of the study, exposure only 4 days after vaccination with Tdap, or recognition of the exposure ≥5 days after pertussis was first detected in the index patient (Figure 1). The remaining 86 exposures were randomized to azithromycin PEP (n = 42) or daily symptom monitoring without PEP (n = 44). Demographic characteristics were similar between the 2 groups (Table 2), but subjects randomized to daily symptom monitoring without PEP recalled less total exposure time (Table 3).
Figure 1.
Study flow diagram. Abbreviations: EU, enzyme-linked immunosorbent assay units per milliliter; HCP, healthcare personnel; IgG, immunoglobulin G; NP, nasopharyngeal; PCR, polymerase chain reaction; PT, pertussis toxin; Td, tetanus toxoid and reduced diphtheria toxoid vaccine; Tdap, tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine adsorbed.
Table 2.
Characteristics of Pertussis-Exposed Healthcare Personnela
| Characteristic | Antibiotic Prophylaxis (N = 42) | Daily Symptom Monitoring Without Prophylaxis (N = 44) | Excluded From Randomization (N = 30) | P Value |
| Age at enrollment in years, median (IQR) | 27 (26–31) | 31 (28–39) | 28 (25–42) | .05 |
| Female | 36 (85.7) | 30 (68.2) | 23 (76.7) | .18 |
| Underlying medical conditionb | 1 (2.4) | 1 (2.3) | 1 (3.3) | 1.00 |
| Occupation | .18 | |||
| Registered nurse | 20 (47.6) | 18 (40.9) | 7 (23.3) | |
| Physician | 11 (26.2) | 19 (43.2) | 10 (33.3) | |
| Respiratory therapist | 2 (4.8) | 3 (6.8) | 5 (16.7) | |
| Radiology technician | 5 (11.9) | 3 (6.8) | 6 (20.0) | |
| Nursing aide | 1 (2.4) | 0 (0.0) | 0 (0.0) | |
| Other | 3 (7.1) | 1 (2.3) | 2 (6.7) | |
| Days from vaccination to exposure, median (IQR) | 429 (263–671) | 336 (216–555) | 405 (281–543) | .44 |
| Days from exposure to acute visit, median (IQR) | 11 (7–13) | 10 (7–13) | … | .56 |
Data are No. (%) of subjects, unless otherwise indicated.
Abbreviation: IQR, interquartile range.
Sixteen of 94 healthcare personnel had >1 pertussis exposure and could have contributed data within each column because subjects were randomized following each exposure. Data represents the summary of all exposures.
Underlying medical condition includes diabetes mellitus and chronic lung disease. No subjects reported chronic kidney or liver disease, infection with human immunodeficiency virus, hematologic or solid organ malignancy, or taking oral steroids or other immune suppressants.
Table 3.
Patient Care Activities During Exposures to Patients With Pertussis Infection
| Characteristic | Antibiotic Prophylaxis (N = 42) | Daily Symptom Monitoring Without Prophylaxis (N = 44) | P Value |
| Total exposure time to index patient | .03 | ||
| <10 minutes | 14 (33.3) | 9 (20.5) | |
| 10–29 minutes | 9 (21.4) | 24 (54.6) | |
| 30–59 minutes | 6 (14.3) | 4 (9.1) | |
| ≥1 hour | 9 (21.4) | 4 (9.1) | |
| Cannot recall | 4 (9.5) | 3 (6.8) | |
| Longest single interval of exposure to index patient | .93 | ||
| ≤5 minutes | 10 (23.8) | 9 (20.5) | |
| 6–10 minutes | 10 (23.8) | 11 (25.0) | |
| 11–20 minutes | 10 (23.8) | 14 (31.8) | |
| >20 minutes | 7 (16.7) | 6 (13.6) | |
| Cannot recall | 5 (11.9) | 4 (9.1) | |
| Placed endotracheal, nasogastric or orogastric tube | 1 (2.4) | 3 (6.8) | .62 |
| Suctioned patient, obtained sputum sample, or swabbed patient’s nose or mouth | 11 (26.2) | 6 (13.6) | .18 |
| Changed respiratory tubing or administered aerosolized nebulizer therapy | 1 (2.4) | 2 (4.6) | 1.00 |
| Administered oral med or fed patient by mouth | 5 (11.9) | 2 (4.6) | .26 |
| Taking antibiotics at time of exposure | 1 (2.4) | 1 (2.3) | 1.00 |
Data are No. (%) of subjects.
Seven HCP met the primary definition for pertussis infection (Table 4), but none developed a cough illness. Five subjects fulfilled serologic criteria only. Subjects 1 and 2 had a ≥2-fold increase in anti-PT IgG titer, but the low titers achieved were unlikely associated with acute pertussis infection [20]. Subjects 3–5 had a single acute anti-PT IgG titer ≥94 EU/mL, and all 3 demonstrated a titer decline with the convalescent specimen. For Subject 3, who was vaccinated only 133 days prior to obtaining the acute titer, the elevated titer likely represented a response to Tdap vaccine. Because Subjects 4 and 5 were vaccinated >1 year prior to obtaining the acute titer, their elevated titers likely represented a recent pertussis infection, but the exact timing of the infection could not be determined [21]. To further evaluate whether the observed anti-PT titers were due to pertussis infection, sera from Subjects 1–5 were tested post-hoc for anti-FHA IgG using a previously described validated assay [16]. None of the 5 subjects had a 2-fold rise in the anti-FHA IgG titer or a single acute or convalescent anti-FHA titer ≥358 EU, shown earlier to be associated with an acute pertussis infection [16].
Table 4.
Healthcare Personnel Meeting Primary Definition for Pertussis Infection
| Days From Exposure |
Acute NP Swab PCR Resultsa |
NP Aspirate PCR Resultsc |
Anti-PT IgG (EU) |
Anti-FHA IgG (EU) |
|||||||||
| Subject No. | Prophylaxis | Vaccination | Acute Visit | Convalescent Visit | IS481 | IS481/ptxs1 | Symptoms After the Exposureb | IS481 | IS481/ptxs1 | Acute | Convalescent | Acute | Convalescent |
| 1 | None | −207 | 13 | 34 | − | ND | None | ND | ND | 20 | 42 | 112 | 117 |
| 2 | None | −397 | 20 | 38 | − | ND | None | ND | ND | <10 | 21 | 37 | 40 |
| 3 | None | −125 | 8 | 29 | − | ND | None | ND | ND | 308 | 242 | <10 | <10 |
| 4 | None | −1034 | 21 | 39 | − | ND | None | ND | ND | 125 | 106 | 259 | 256 |
| 5 | None | −382 | 7 | 27 | − | ND | Sore throat | − | − | 111 | 67 | 11 | 12 |
| 6 | None | −110 | 7 | 36 | + | – | None | ND | ND | 81 | 70 | ND | ND |
| 7 | Azithromycin | −773 | 8 | 33 | − | ND | Sore throat | +d | − | <10 | <10 | ND | ND |
Abbreviations: EU, enzyme-linked immunosorbent assay units per milliliter; FHA, filamentous hemagglutinin; IgG, immunoglobulin G; ND, not done; NP, nasopharyngeal; PCR, polymerase chain reaction; PT, pertussis toxin; +, positive result; −, negative result.
NP swabs tested for PCR only. All convalescent swabs were negative for pertussis by PCR.
Subjects were queried daily for 21 days after the exposure was identified for the occurrence of fever, cough, runny nose, and sore throat.
NP aspirate was performed only when symptoms occurred during the postexposure monitoring period.
Culture for Bordetella pertussis was negative.
In addition to the 5 subjects fulfilling serologic criteria, 2 HCP had a single nasopharyngeal specimen positive for B. pertussis by PCR (Subjects 6 and 7; Table 4). Subject 6 had an acute nasopharyngeal swab positive by PCR (Ct, 35.87) but had no respiratory symptoms and only mildly elevated anti-PT IgG titers, likely due to recent vaccination (Table 4). Nasopharyngeal aspirates were collected from 19 subjects who developed respiratory symptoms during the postexposure monitoring period, but only Subject 7 had an aspirate positive by PCR (Ct, 34.51). This subject had sore throat without cough that developed 24 days postexposure, beyond the usual incubation period for pertussis (range, 5–21 days postexposure) [1]. In addition, the aspirate was culture negative. Both specimens that tested positive by PCR did so only when the maximum amount of template DNA was added, suggesting a limited amount of B. pertussis DNA was present. Moreover, a high Ct (ie, >35) may not represent true infection [22]. Alternative explanations for such low bacterial loads include asymptomatic transient colonization because of an effective immune response in a vaccinated person or contamination from amplicon carryover. Because our PCR assay utilized a single-target (IS481) that has been associated with false-positive results in other reports [23], both positive specimens were retested at the CDC using a previously described 2-target PCR assay (IS481 and ptxS1) [24]. With the 2-target assay, neither specimen tested positive for pertussis.
Pertussis infection (according to the primary definition) did not develop postexposure in 41 (97.6%) subjects who received antibiotic PEP and 38 (86.4%) subjects who did not receive PEP (absolute risk difference, −11.3%; lower limit of 1-sided 95% CI, −20.6%; P = .81). Two subjects (Subjects 5 and 6; Table 4) who did not receive PEP did not meet the modified definition for pertussis infection. According to the modified definition, pertussis infection did not develop postexposure in 41 (97.5%) subjects who received antibiotic PEP and 40 (90.9%) subjects who did not receive PEP (absolute risk difference, −6.7%; lower limit of 1-sided 95% CI, −14.8%; P = .54).
DISCUSSION
Our study did not demonstrate noninferiority of daily symptom monitoring of Tdap-vaccinated HCP without PEP compared with antibiotic PEP for preventing pertussis following exposure as defined in the study. However, given the concerns raised in the “Results” section, it is likely that none of the HCP who met the pre-defined serologic or PCR criteria for infection were truly infected with pertussis. None of the 7 subjects who met the primary definition developed a cough illness, and asymptomatic HCP are likely incapable of secondary transmission. Furthermore, the 2 nasopharyngeal specimens in which pertussis DNA was detected on the single-target PCR assay demonstrated high cycle thresholds and were negative when retested at the CDC using the 2-target assay. The 2-target assay included different DNA extraction and amplification methods than the single-target assay. Additionally, the 2-target assay was not performed on the same aliquot of specimen but rather a second aliquot that was divided at the time of collection, stored at −70°C for 12–37 months, and underwent at least 2 freeze-thaw cycles. Lack of concordance by the 2-target assay could also be explained by lack of homogeneity of bacteria number within the paired aliquots, extended storage of the second aliquot prior to testing, or DNA degradation from multiple freeze-thaw cycles. Exhaustion of the recovered DNA from the first aliquot tested prevented further 2-target analysis by the CDC.
Eliminating the need to provide routine antibiotic PEP to vaccinated HCP following pertussis exposure has many advantages. Reducing antibiotic prescriptions will decrease pharmacy costs associated with pertussis exposures and diminish adverse effects secondary to antibiotic use. Additionally, although contact identification to determine the vaccination status of potentially exposed HCP will need to continue, labor costs associated with evaluating the extent of the exposure and need for PEP among vaccinated HCP would likely be reduced. However, a formal cost analysis is necessary to determine whether withholding antibiotic PEP and daily symptom monitoring reduces containment costs of pertussis exposures.
This study has several limitations. The number of pertussis exposures during the study was much less than expected, as the national incidence of pertussis during the study period was substantially lower than the peak in 2004 [25]. In addition, promotion of the study within the hospital may have raised awareness among HCP, resulting in increased recognition of pertussis, prompt institution of respiratory precautions for infected patients, and decreased exposures among HCP. A large proportion of exposed subjects were excluded from randomization, mostly because of the presence of respiratory symptoms at baseline. Most of these symptoms were likely secondary to intercurrent viral infections or seasonal allergies rather than pertussis. The limited number of pertussis-exposed HCP who underwent randomization likely impacted our ability to demonstrate noninferiority.
An additional barrier to demonstrating noninferiority was the small noninferiority margin, which was selected to reduce the potential serious consequence of having an infected HCP expose high-risk patients. Additionally, serology alone may not be appropriate for defining acute pertussis infection in a recently vaccinated asymptomatic HCP, particularly without a baseline post-vaccination titer. In future studies, we would strongly recommend post-vaccination titers be obtained 4 weeks after vaccination. Finally, the duration of immunity following a single dose of Tdap is unknown. All HCP in this study were vaccinated within 4 years prior to their exposure, and these results may not apply to a more extended interval between Tdap vaccination and pertussis exposure. Currently there are no recommendations for revaccination of persons who previously received Tdap.
In conclusion, daily symptom monitoring without PEP in Tdap-vaccinated HCP following pertussis exposure was not noninferior to antibiotic PEP. However, exposed HCP were likely misclassified as having pertussis infection, and no recently vaccinated HCP developed symptomatic pertussis confirmed with a specific PCR assay or culture following pertussis exposure. In light of these data, the Advisory Committee on Immunization Practices recently recommended antibiotic PEP for all exposed HCP likely to secondarily expose high-risk patients (eg, neonates, pregnant women) [26]. Other vaccinated HCP were recommended to receive either antibiotic PEP or daily symptom monitoring for 21 days without PEP after pertussis exposure and antimicrobial treatment if symptoms of pertussis develop.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://www.oxfordjournals.org/our_journals/cid/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments.
We thank Todd Mathis, Carolyn Cooper, Gayle Johnson, Dayna Wyatt, Deborah Hunter, Shanda Adamson, and Robert Sparks for enrolling patients and collecting clinical specimens; Natasha Halasa for her role as safety monitor; Pat Joyce and Tami Skoff for their assistance in conducting the study and examining the results; Kathleen M. Tatti for performing polymerase chain reaction (PCR) testing and her critical review of the manuscript; Andrew Baughman and Brian Plikaytis for their assistance with statistical analysis; Gladys Garrison for performing PCR testing; Tracy Louis and the other infection control practitioners at Vanderbilt University Medical Center for investigating pertussis exposures; members of the Occupational Health Clinic for their assistance in evaluating exposed subjects; and the Tennessee Department of Health State Laboratory for culture of clinical specimens.
Financial support.
This work was supported by the Centers for Disease Control and Prevention (1 U01 IP000095-01); the Vanderbilt Institute for Clinical and Translational Research (1 UL1 RR024975 from the National Center for Research Resources at the National Institutes of Health); and the Vanderbilt–Sanofi Pasteur Healthcare Vaccinology and Epidemiology Training Program Award. Additionally, sanofi pasteur donated vaccine for use in this study.
Potential conflicts of interest.
K. M. E. received research funding from Novartis. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.CDC. Preventing tetanus, diphtheria, and pertussis among adults: use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine recommendations of the Advisory Committee on Immunization Practices (ACIP) and recommendation of ACIP, supported by the Healthcare Infection Control Practices Advisory Committee (HICPAC), for use of Tdap among health-care personnel. MMWR Recomm Rep. 2006;55:1–37. [PubMed] [Google Scholar]
- 2.Deville JG, Cherry JD, Christenson PD, et al. Frequency of unrecognized Bordetella pertussis infections in adults. Clin Infect Dis. 1995;21:639–42. doi: 10.1093/clinids/21.3.639. [DOI] [PubMed] [Google Scholar]
- 3.Wright SW, Decker MD, Edwards KM. Incidence of pertussis infection in healthcare workers. Infect Control Hosp Epidemiol. 1999;20:120–3. doi: 10.1086/501593. [DOI] [PubMed] [Google Scholar]
- 4.De Serres G, Shadmani R, Duval B, et al. Morbidity of pertussis in adolescents and adults. J Infect Dis. 2000;182:174–9. doi: 10.1086/315648. [DOI] [PubMed] [Google Scholar]
- 5.Baggett HC, Duchin JS, Shelton W, et al. Two nosocomial pertussis outbreaks and their associated costs—King County, Washington, 2004. Infect Control Hosp Epidemiol. 2007;28:537–43. doi: 10.1086/513497. [DOI] [PubMed] [Google Scholar]
- 6.Bassinet L, Matrat M, Njamkepo E, Aberrane S, Housset B, Guiso N. Nosocomial pertussis outbreak among adult patients and healthcare workers. Infect Control Hosp Epidemiol. 2004;25:995–7. doi: 10.1086/502332. [DOI] [PubMed] [Google Scholar]
- 7.Boulay BR, Murray CJ, Ptak J, Kirkland KB, Montero J, Talbot EA. An outbreak of pertussis in a hematology-oncology care unit: implications for adult vaccination policy. Infect Control Hosp Epidemiol. 2006;27:92–5. doi: 10.1086/500420. [DOI] [PubMed] [Google Scholar]
- 8.CDC. Outbreaks of pertussis associated with hospitals—Kentucky, Pennsylvania, and Oregon, 2003. MMWR Morb Mortal Wkly Rep. 2005;54:67–71. [PubMed] [Google Scholar]
- 9.Gehanno JF, Pestel-Caron M, Nouvellon M, Caillard JF. Nosocomial pertussis in healthcare workers from a pediatric emergency unit in France. Infect Control Hosp Epidemiol. 1999;20:549–52. doi: 10.1086/501667. [DOI] [PubMed] [Google Scholar]
- 10.Kurt TL, Yeager AS, Guenette S, Dunlop S. Spread of pertussis by hospital staff. JAMA. 1972;221:264–7. [PubMed] [Google Scholar]
- 11.Steketee RW, Wassilak SG, Adkins WN, Jr, et al. Evidence for a high attack rate and efficacy of erythromycin prophylaxis in a pertussis outbreak in a facility for the developmentally disabled. J Infect Dis. 1988;157:434–40. doi: 10.1093/infdis/157.3.434. [DOI] [PubMed] [Google Scholar]
- 12.Ward JI, Cherry JD, Chang SJ, et al. Efficacy of an acellular pertussis vaccine among adolescents and adults. N Engl J Med. 2005;353:1555–63. doi: 10.1056/NEJMoa050824. [DOI] [PubMed] [Google Scholar]
- 13.CDC. Recommended antimicrobial agents for the treatment and postexposure prophylaxis of pertussis: 2005 CDC guidelines. MMWR Recomm Rep. 2005;54:1–16. [PubMed] [Google Scholar]
- 14. Sanofi Pasteur Inc. Tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine adsorbed Adacel package insert. Available at: http://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM142764.pdf. Accessed 23 March 2010.
- 15.Hallander HO, Reizenstein E, Renemar B, Rasmuson G, Mardin L, Olin P. Comparison of nasopharyngeal aspirates with swabs for culture of Bordetella pertussis. J Clin Microbiol. 1993;31:50–2. doi: 10.1128/jcm.31.1.50-52.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baughman AL, Bisgard KM, Edwards KM, et al. Establishment of diagnostic cutoff points for levels of serum antibodies to pertussis toxin, filamentous hemagglutinin, and fimbriae in adolescents and adults in the United States. Clin Diagn Lab Immunol. 2004;11:1045–53. doi: 10.1128/CDLI.11.6.1045-1053.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Serres G, Boulianne N, Duval B. Field effectiveness of erythromycin prophylaxis to prevent pertussis within families. Pediatr Infect Dis J. 1995;14:969–75. doi: 10.1097/00006454-199511000-00009. [DOI] [PubMed] [Google Scholar]
- 18.Halperin SA, Bortolussi R, Langley JM, Eastwood BJ, De Serres G. A randomized, placebo-controlled trial of erythromycin estolate chemoprophylaxis for household contacts of children with culture-positive Bordetella pertussis infection. Pediatrics. 1999;104:e42. doi: 10.1542/peds.104.4.e42. [DOI] [PubMed] [Google Scholar]
- 19.Farrington CP, Manning G. Test statistics and sample size formulae for comparative binomial trials with null hypothesis of non-zero risk difference or non-unity relative risk. Stat Med. 1990;9:1447–54. doi: 10.1002/sim.4780091208. [DOI] [PubMed] [Google Scholar]
- 20.Heininger U, Cherry JD, Stehr K. Serologic response and antibody-titer decay in adults with pertussis. Clin Infect Dis. 2004;38:591–4. doi: 10.1086/381439. [DOI] [PubMed] [Google Scholar]
- 21.Le T, Cherry JD, Chang SJ, et al. Immune responses and antibody decay after immunization of adolescents and adults with an acellular pertussis vaccine: the APERT Study. J Infect Dis. 2004;190:535–44. doi: 10.1086/422035. [DOI] [PubMed] [Google Scholar]
- 22.Guthrie JL, Seah C, Brown S, Tang P, Jamieson F, Drews SJ. Use of Bordetella pertussis BP3385 to establish a cutoff value for an IS481-targeted real-time PCR assay. J Clin Microbiol. 2008;46:3798–9. doi: 10.1128/JCM.01551-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.CDC. Outbreaks of respiratory illness mistakenly attributed to pertussis—New Hampshire, Massachusetts, and Tennessee, 2004–2006. MMWR Morb Mortal Wkly Rep. 2007;56:837–42. [PubMed] [Google Scholar]
- 24.Tatti KM, Wu KH, Tondella ML, et al. Development and evaluation of dual-target real-time polymerase chain reaction assays to detect Bordetella spp. Diagn Microbiol Infect Dis. 2008;61:264–72. doi: 10.1016/j.diagmicrobio.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 25. CDC. Pertussis (whooping cough). Available at: http://www.cdc.gov/pertussis/surv-reporting.html. Accessed 15 December 2010.
- 26. CDC. Immunization of Health-Care Personnel: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2011;60:1--45. [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.