Abstract
Symptoms of anxiety and depression are relatively stable over time. Can this stability be explained by genetic influences, or is it caused by the long-lasting effects of accumulating environmental experiences? To address this question, we analyzed longitudinally assessed symptoms of anxiety and depression in eight samples of monozygotic twins of widely varying ages. These samples were drawn from American and European population-based registries. Using hierarchical linear modeling, we examined individual differences and individual changes in the level of symptoms over time. This method enabled us to decompose the variance into the predictable variance shared by both members of each pair of twins, the differences between individuals within pairs, and the residual variance. We then modeled how these components of individual variation changed over time. Within pairs, the twins’ predicted levels of symptoms increasingly diverged from childhood until late adulthood, at which point the divergence ceased. By middle adulthood, environmental experiences contributed substantially to stable and predictable interindividual differences in levels of anxiety and depression.
Keywords: anxiety, depression, adult development, behavior genetics, emotional development
Individuals’ levels of symptoms of depression and anxiety are relatively stable over time (Foley, Neale, & Kendler, 2001; Lovibond, 1998). What is responsible for this stability? The genetic-set-point hypothesis offers one plausible explanation: Genetic factors determine the stable set points to which an individual will eventually return after the anxiety and depression produced by environmental experiences have subsided (Kandler et al., 2010).
Studies of twins have provided consistent empirical support for this hypothesis. In such studies, researchers have found that across the life span, genetic factors both substantially influence levels of anxiety and depression (Boomsma, van Beijsterveldt, & Hudziak, 2005; Kendler, Gardner, & Lichtenstein, 2008; McGue & Christensen, 1997; Silberg et al., 1990) and are largely responsible for their temporal stability (Gillespie et al., 2004; O’Connor, Neiderhiser, Reiss, Hetherington, & Plomin, 1998).
The genetic-set-point hypothesis can be contrasted with an environmentally-influenced-set-point hypothesis, which assumes that environmental experiences produce enduring effects on an individual’s set points for anxiety and depression. According to this alternative hypothesis, strong negative experiences, whether caused by acute events or by chronic difficulties, could elevate symptoms of anxiety and depression for decades, whereas powerful positive experiences could lessen symptoms. At least two mechanisms might underlie these processes. Life experiences could produce enduring effects on symptoms through biological changes in levels of DNA methylation that result in the altered structure and function of neural systems. Alternatively, changes in the intensity of symptoms might occur via psychological and social routes. For example, early environmental adversity could lead individuals to enter into poor interpersonal relationships (Daley & Hammen, 2002) and other kinds of high-risk environments that further exacerbate symptoms (Kendler, Gardner, & Prescott, 2011).
Several studies have offered empirical support for an environmentally influenced set point for anxiety and depression symptoms. Severe environmental stressors, such as childhood sexual abuse, natural disasters, and combat exposure, produce enduring effects on symptoms of anxiety and depression (Fergusson & Mullen, 1999; Goenjian et al., 2005; Kendler et al., 2000; Koenen et al., 2003). In the German Socio- Economic Panel Study (Headey, 2010), a representative longitudinal study that followed members of German households for 20 years, 14% to 30% of subjects experienced a large and stable change to their set point for well-being, a construct strongly correlated with levels of anxiety and depression (Keyes, 2002, 2005). However, most studies suggest that commonly experienced environmental adversities, particularly stressful life events, have only a temporary impact on depression symptoms, increasing risk for episodes of major depression for only a few months (Brown & Harris, 1978; Kendler, Karkowski, & Prescott, 1998; Surtees et al., 1986).
A cohort of monozygotic twins constitutes an ideal sample for assessing the sources of stability in symptoms of anxiety and depression. Because monozygotic twins are born with identical DNA sequences, differences that arise between monozygotic twins during the life course are mainly the result of nongenetic influences. Some of these differences, however, may reflect epigenetic effects, structural variation (Bruder et al., 2008), or random developmental processes (Molenaar, Boomsma, & Dolan, 1993). In this report, we refer to all such differences as “environmental.”
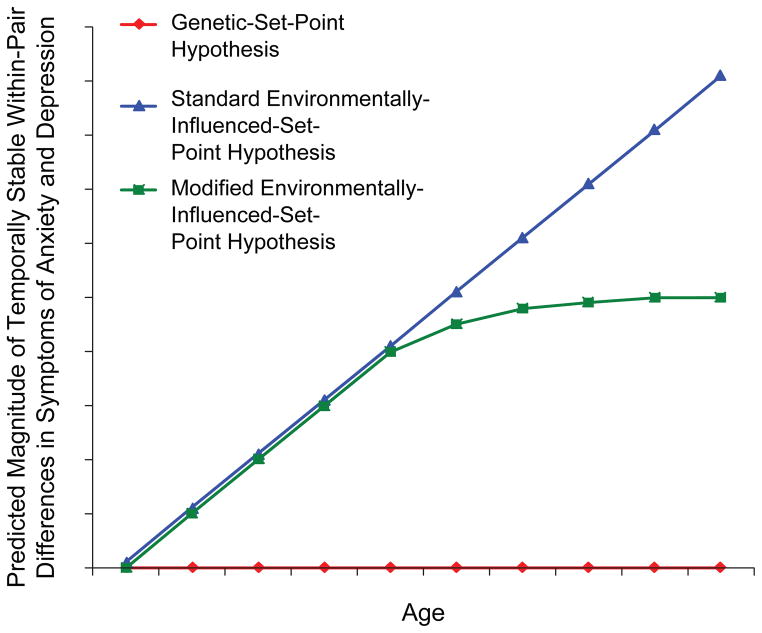
Following the approach suggested by Dickens, Turkheimer, and Beam (2011) for studying intelligence across development, we analyzed symptoms of anxiety and depression in eight samples of longitudinally assessed monozygotic twins derived from population-based registries. Our first goal was to determine whether the data supported a genetically determined or environmentally influenced set point for such symptoms. According to the genetic-set-point hypothesis, the differences in symptoms of anxiety and depression within pairs of monozygotic twins should be stable over the life course. According to the standard environmentally-influenced-set-point hypothesis, levels of anxiety and depression should gradually diverge within monozygotic twin pairs as they age, as a result of the gradual accumulation of different environmental experiences (see Fig. 1).
Fig. 1.
The predicted relationship between age and the magnitude of predictable within-pair difference in symptoms of anxiety and depression in monozygotic twin pairs. Predictions are shown for the genetic-set-point hypothesis, the standard environmentally-influenced-set-point hypothesis, and the modified environmentally-influenced-set-point hypothesis.
Our second goal was to determine whether the differences in symptoms of anxiety and depression within monozygotic twin pairs continue to diverge over the entire life course or stop accumulating at some developmental stage. According to a modified environmentally-influenced-set-point hypothesis (see Fig. 1), differences within twin pairs should stabilize in early adulthood because most severe psychological traumas (e.g., sexual or physical abuse) occur between early childhood and late adolescence. However, changes in emotional processing are especially prominent in older age groups (Mather & Carstensen, 2005). If emotional processing is central to the influence of environmental experiences on symptoms of anxiety and depression, differences in symptoms within monozygotic pairs might accumulate throughout early and middle adulthood and stabilize in late adulthood.
Method
Subjects
We examined seven samples of monozygotic twins with symptoms of depression or anxiety as measured by self-reports on at least three occasions and one sample of monozygotic twins who had reported on such symptoms on two occasions (Table 1). The combined sample contained 12,148 twins, including 8,470 twins in pairs and 3,678 unpaired twins (the data from unpaired twins provided useful information on the temporal stability of symptoms). The mean age of twins in these samples at each evaluation ranged from 10.6 to 66.8 years.
Table 1.
Twin Samples Included in This Study and Instruments Used to Assess Their Symptoms of Depression and Anxiety
| Study, instruments, and wave | Mean age (years) | n |
|---|---|---|
| Virginia Twin Study of Adolescent Behavioral Development (What I Think and Feel Anxiety Scale; Mood and Feeling Depression Scale); RΛ = .78 | ||
| Wave 1 | 10.6 (1.7) | 643 |
| Wave 2 | 12.1 (1.8) | 639 |
| Wave 3 | 15.4 (1.7) | 602 |
| Young Netherlands Twin Register (Youth Self-Report Anxiety and Depression scales); RΛ = .82 | ||
| Wave 1 | 12.0 (0.5) | 464 |
| Wave 2 | 14.6 (0.6) | 490 |
| Wave 3 | 16.8 (0.4) | 555 |
| Wave 4 | 18.5 (0.5) | 270 |
| Swedish Twin Study of Child and Adolescent Developmenta (Child Behavior Checklist Anxiety and Depression scales; Youth Self-Report Anxiety and Depression scales); RΛ = .75 | ||
| Wave 1 | 13–14 | 800 |
| Wave 2 | 16–17 | 830 |
| Wave 3 | 19–20 | 636 |
| Adult Netherlands Twin Register (Youth Self-Report Anxiety and Depression scales); RΛ = .89 | ||
| Wave 1 | 19.7 (1.1) | 399 |
| Wave 2 | 21.6 (2.2) | 796 |
| Wave 3 | 23.5 (2.2) | 533 |
| Wave 4 | 27.3 (2.5) | 727 |
| Virginia Adult Twin Study of Psychiatric and Substance Use Disorders—females (Symptom Checklist-90 Anxiety, Phobic Anxiety, and Depression scales); RΛ = .79 | ||
| Wave 1 | 29.4 (7.4) | 1,086 |
| Wave 2 | 34.5 (7.4) | 1,078 |
| Wave 3 | 37.2 (7.4) | 965 |
| Virginia Adult Twin Study of Psychiatric and Substance Use Disorders—males (Symptom Checklist-90 Anxiety, Phobic Anxiety, and Depression scales); RΛ = .77 | ||
| Wave 1 | 34.5 (9.1) | 1,422 |
| Wave 2 | 36.0 (9.1) | 1,422 |
| Swedish Adoptive Twin Study of Aging (Spielberger State Anxiety Scale); RΛ = .88 | ||
| Wave 1 | 57.0 (13.6) | 454 |
| Wave 2 | 60.0 (13.6) | 483 |
| Wave 3 | 62.1 (13.2) | 457 |
| Wave 4 | 63.4 (13.1) | 409 |
| American Association of Retired Persons twin sample (Symptom Checklist-90 Anxiety, Phobic Anxiety, and Depression scales); RΛ = .91 | ||
| Wave 1 | 63.1 (7.5) | 1,603 |
| Wave 2 | 64.2 (7.5) | 1,623 |
| Wave 3 | 66.8 (7.5) | 1,623 |
Note: Standard deviations are given in parentheses. RΛ represents the reliability of an entire series of longitudinal observations (i.e., how reliably we captured the underlying latent measure by including scores from multiple observations of each individual in our random-coefficients model). Data from the Virginia Twin Study of Adolescent Behavioral Development were drawn from Simonoff et al. (1997). The What I Think and Feel Anxiety Scale was taken from Reynolds and Richmond (1978). The Mood and Feeling Depression Scale was taken from Costello and Angold (1988). Data from the Young Netherlands Twin Register were drawn from Bartels et al. (2007). The Youth Self-Report scales were taken from Achenbach (1991). Data from the Swedish Twin Study of Child and Adolescent Development were drawn from Lichtenstein, Tuvblad, Larsson, and Carlstrom (2007). The Child Behavior Checklist scales were taken from Achenbach and Edelbrock (1983). Data from the Adult Netherlands Twin Register were drawn from Boomsma et al. (2006). Data from the Virginia Adult Twin Study of Psychiatric and Substance Use Disorders for both males and females were drawn from Kendler and Prescott (2006). The Symptom Checklist-90 scales were taken from Derogatis, Lipman, and Covi (1973). Data from the Swedish Adoptive Twin Study of Aging were drawn from Pedersen et al. (1991). The Spielberger State Anxiety Scale was taken from Spielberger, Gorsuch, and Luchene (1970). Data from the American Association of Retired Persons twin sample were drawn from Prescott et al. (1994).
The Child Behavior Checklist scales were used for Waves 1 and 2 of the Swedish Twin Study of Child and Adolescent Development, and the Youth Self-Report scales were used for Wave 3. Both of these instruments included a 12-item Anxiety and Depression scale, which we examined in our analysis. Exact ages were not reported for this study.
Statistical analyses
We analyzed the longitudinal data using a random-coefficients approach involving three steps. In the first step, we used a basic regression model with assessment wave, sex, birth cohort, and age entered as fixed effects. The fixed-effects regression equation provided an estimate for individual subjects’ mean level of anxiety and depression, controlling for sex, birth cohort, and age at interview. Each observation for each individual had a residual representing his or her departure from this mean response. This residual, which is assumed to result from individual characteristics, measurement error, and other random error, was then used to estimate individual growth trajectories. A minimum of three measurements from different waves in a given study was needed to estimate a linear growth trajectory. These individual growth trajectories were characterized by their slopes and intercepts, which were the random coefficients.
Finally, we used the regression equation for the mean symptom levels (the fixed effects) and the individual trajectories to estimate the variance components of interest. Because these trajectories included both intercepts and slopes, the variance included mean between-subjects differences as well as within-subjects change over time. The final model yielded a marginal residual (the deviation of the individual measurement from the value predicted by the fixed-effects regression equation), which was made up of a conditional residual (the deviation of the individual measurement from that predicted by the fixed-effects regression equation plus the individual’s linear growth trajectory) plus the error residual. Thus, the outcome measure was the sum of the mean response, the conditional residual, and the error residual, with a sampling variance equal to the sum of the variance of the conditional residual plus the variance of the error residual.
Because of clustering within monozygotic twin pairs, we were able to further decompose the variance predicted by individual growth trajectories into the variance in symptom levels shared by members of twin pairs and the variance in symptom levels not shared within pairs. We obtained the estimates of the variance components at a particular wave of a particular longitudinal study from the intercept measure by centering the age measure at the individual’s age at that assessment (see Biesanz, Deeb-Sossa, Papadakis, Bollen, & Curran, 2004).
Longitudinal modeling of symptom scores was carried out using the SAS PROC MIXED procedure with a random-coefficients approach. The components of the model were entered as fixed effects, the individual linear growth trajectories were entered as random effects, and individuals were clustered hierarchically into monozygotic twin pairs.
The fixed-effects regression included the following demographic variables: sex, interview wave (to adjust for mean differences across waves), birth year (to adjust for cohort effects), and age centered at an individual’s age in a particular study (in cases in which age was not confounded with birth year). Most of the data sets supplied subjects’ exact age at time of interview. For example, an individual born in 1960 and interviewed at ages 36.36, 39.23, and 40.45 would have a birth year of 1960 at each interview, but the age vectors would be (0.00, 2.87, 4.09), (−2.87, 0.00, 1.22), and (−4.09, −1.22, 0.00) for the purpose of estimating parameters for Waves 1, 2, and 3, respectively. In some cases, however, the dates given for interview waves specified only the year.
All two-way interactions were considered, and those with p values less than .1 were retained. The fixed-effects equation gave the mean score for an individual of a particular sex, birth cohort, centered age, and interview wave. The scales used to measure anxiety and depression were all skewed rightward. Using the square root of the score produced reasonably homoscedastic residuals.
For each wave at which a subject was interviewed, the subject had a residual that represented his or her individual deviation from the mean score. Using these deviations, we constructed individual growth trajectories characterized by their slope and intercept (the value when age = 0, i.e., the age at the specific wave of a particular study for which we were estimating parameters). Individual deviations were then decomposed into two components, one predicted by individual growth trajectories and one due to residual variation (which included measurement error). Because the subjects within twin pairs were hierarchically clustered, we decomposed the variation due to individual growth trajectories into variation shared by both members of a twin pair and variation not shared by both members of the pair. The algorithm alternated between weighted least squares solutions for the fixed-effects regression and residual maximum likelihood estimation of the variance components (intercept, slope, and intercept-slope covariance) attributable to individual growth trajectories. Thus, for each wave of a particular study, we decomposed individual deviation from the mean into three components: a portion due to individual growth trajectories shared by members of a twin pair, a portion due to individual growth trajectories not shared by the members of the pair, and the residual variation.
Our methodology, which followed that used by Laenen, Alonso, and Molenberghs (2007) and Laenen, Alonso, Molenberghs, and Vangeneugden (2009), allowed us to calculate two reliabilities for longitudinal data: R(T), an estimate of cross-sectional reliability (the reliability at a particular wave), and RΛ, an estimate of the reliability of a series of longitudinal observations. For our purposes, “reliability” refers to the proportion of deviation predicted by individual growth trajectories, or the sum of the proportion shared by twins within a twin pair and the proportion not shared by twins within a pair.
Analysis of variance component proportion
For each wave of each study, we decomposed the variance due to deviation from the mean response into the variance due to growth trajectories shared by both members of a monozygotic twin pair (between-pair variance), the variance due to growth trajectories not shared between twins (within-pair variance), and the residual variance (a mixture of measurement error and other variation not explained by the model). We focused on the proportion of the total variance attributable to longitudinal trajectories not shared by twins within a pair (within-pair variance divided by the sum of between-pair variance, within-pair variance, and residual variance). As a statistic, this proportion bears a resemblance to heritability, which also reflects a proportion of variance associated with a variance component. In order to estimate the standard error for this proportion, we used the parameter estimates for the variance components and the variance-covariance matrix of these estimates to generate a population of 10,000 pseudosamples, assuming the parameters are drawn from a multivariate normal distribution. The standard deviation of the proportion in these 10,000 pseudosamples provided an approximate standard error for the proportion. The standard errors calculated by this method were very close to those based on approximate methods (Osborne & Paterson, 1952).
When the ratio of within-pair variance to total variance was plotted against mean age at each sampling point, we observed a curvilinear relationship approaching a maximum divergence asymptotically. Given the observed plots and assuming that monozygotic twins begin life in identical states, we fit several nonlinear growth functions used to describe biological growth and compared these fits with that of a linear growth function using the PROC NLIN procedure in SAS software. All analyses were performed with parameterizations constrained to go through the origin. We tested the following functions: linear (y = b × age), quadratic with a plateau (y = b × age + g × age2), quadratic with a plateau at age = −b ÷ (2 × g), von Bertalanffy (y = c × (1 − exp(−k × age))), and Gompertz (y = c × (exp(−exp(−k × (age − m))))). Both the quadratic function with a plateau and the von Bertalanffy function (two parameters each) are curved convex functions with decreasing slopes that approach an asymptotic limit, whereas the Gompertz function (three parameters) is an asymmetric sigmoidal curve that also approaches an asymptotic limit. At each point, the estimate of the derived ratio was weighted by the inverse of the sampling variance of that estimate (1 ÷ SE2) to account for the fact that different sampling points are estimated with different precision. Model fit was assessed using the root-mean-square error of approximation (RMSEA); RMSEA values under .05 indicate good model fit (Steiger, 1990).
Because statistics derived at different points in a given study applied to the same sample, the linear and quadratic models were fit with a generalized-estimating-equation analysis using PROC GENMOD. This method allowed for the non-independence of parameter estimates from the same study.
Results
We first tested the genetic-set-point hypothesis, according to which monozygotic twins will not differ in their set-point levels of symptoms of anxiety and depression across the life span. Our data fit this model quite poorly, RMSEA = .22. Next, we tested the standard environmentally-influenced-set-point hypothesis, which predicts a linear increase in differences in symptoms between members of monozygotic twin pairs over time. The fit of our data to this model was markedly better, F(1, 24) = 367.5, p < .0001, RMSEA = .056. Finally, we modified the model testing the environmentally-influenced-set-point hypothesis by adding a quadratic growth function with a plateau, so as to capture the idea that the accumulation of differences between monozygotic twins reaches an asymptote at some developmental point. Our data fit this model significantly better than they fit the linear model corresponding to the standard environmentally-influenced-set-point hypothesis, F(1, 23) = 38.2, p < .0001, RMSEA = .035.
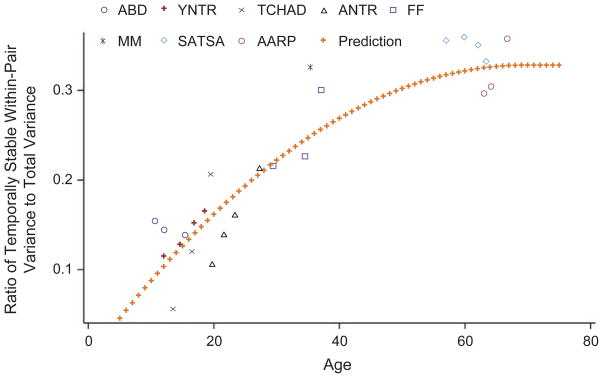
The fit of this best-fitting model is illustrated in Figure 2. As predicted by the modified environmentally-influenced-set-point hypothesis, the data showed a rapid and roughly linear growth in differences within monozygotic pairs in the temporally stable component of their self-reported symptoms through adolescence and into middle adulthood. However, the accumulation of these differences slowed down in late adulthood. The asymptote was estimated as equal to 69.4 years, but this estimate was imprecise (95% confidence interval = [60.1, 89.6]).
Fig. 2.
Actual and predicted ratios of within-pair variance to total variance of the temporally stable component of symptoms of anxiety and depression as a function of the mean age of the tested cohort. The predicted values of the parameter estimates come from the best-fitting model, which had a linear component and a quadratic growth function with a plateau. ABD = Virginia Twin Study of Adolescent Behavioral Development (Simonoff et al., 1997); YNTR = Young Netherlands Twin Register (Bartels et al., 2007); TCHAD = Swedish Twin Study of Child and Adolescent Development (Lichtenstein, Tuvblad, Larsson, & Carlstrom, 2007); ANTR = Adult Netherlands Twin Register (Boomsma et al., 2006); FF = Virginia Adult Twin Study of Psychiatric and Substance Use Disorders—females (Kendler & Prescott, 2006); MM = Virginia Adult Twin Study of Psychiatric and Substance Use Disorders—males (Kendler & Prescott, 2006); SATSA = Swedish Adoptive Twin Study of Aging (Pedersen et al., 1991); AARP = American Association of Retired Persons twin sample (Prescott et al., 1994).
Analysis of our data using generalized estimating equations, and thereby treating estimates at different time points within a study as clustered, also contradicted the standard environmentally-influenced-set-point hypothesis. That is, the fit of the linear-plus-quadratic function was much better than that of the linear term alone (Wald z = 4.49, p < .0001). We tested two other growth functions, the von Bertalanffy function (df = 2) and the Gompertz function (df = 3), neither of which provided a fit superior to that observed with the linear-plus-quadratic model.
We examined the clinical significance of the differences within the monozygotic twin pairs by examining female-female twin pairs from the Virginia Adult Twin Study of Psychiatric and Substance Use Disorders (Kendler & Prescott, 2006). For each month when symptoms of depression and anxiety were assessed, the authors reported whether the twins met the criteria outlined in the fourth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM–IV; American Psychiatric Association, 1994) for major depression (MD) or generalized anxiety disorder (GAD) with a 1-month, rather than 6-month, minimal duration. We generated a score for each subject’s individual trajectory by obtaining the marginal residual (after the removal of the demographic fixed effects) and the conditional residual (based on fixed effects and individual trajectories). The difference between these two residuals was the individual trajectory deviation for that wave. In pairs of twins in which only one twin experienced an episode of MD or GAD at interview, the affected twin was much more likely than the unaffected twin to have the higher trajectory score (odds ratio = 16.0), χ2(1) = 28.4, p < .0001.
Finally, we examined sex differences in the linear and quadratic effects. Using an autoregressive working correlation matrix, we fit four models, comparing the fit using the quasi-likelihood information criterion (QIC), for which lower values indicate better fit (Pan, 2001). For the linear model with no sex interaction, the QIC was 61.69; for the linear model with a sex interaction, the QIC was 60.85; for the quadratic model with no sex interaction, the QIC was 54.35; and for the quadratic model with a sex interaction, the QIC was 58.72. Thus, the best-fitting model included linear and quadratic components but no sex interactions.
Discussion
On the basis of our findings, we reject the genetic-set-point hypothesis, according to which genetic factors alone are responsible for the temporal stability of symptoms of anxiety and depression. Our results are much more consistent with the environmentally-influenced-set-point hypothesis. However, we also reject the standard version of this hypothesis, according to which environmentally driven differences in stable levels of anxiety and depression continue to increase throughout the life cycle. To the contrary, we found that these differences continued to accumulate only until late adulthood. Our results are consistent with studies that have demonstrated an effect of aging on the emotional processing of adverse experiences. For example, Mather and Carstensen (2005) found that individuals show an increasingly selective retention of emotionally positive memories relative to negative memories as they age. It is important to emphasize that in all the analyses presented in this report, the environment, including all influences that contributed to differences between monozygotic twins, was defined as a latent variable.
Our results are consistent with findings obtained in many community studies showing that severe environmental exposures can have enduring effects on levels of depression and anxiety (Fergusson & Mullen, 1999; Goenjian et al., 2005; Kendler et al., 2000; Koenen et al., 2003). The differences in levels of symptoms that developed within the monozygotic twin pairs in the Virginia Adult Twin Study of Psychiatric and Substance Use Disorders (Kendler & Prescott, 2006) were strongly related to differences in risk for DSM–IV MD or GAD. We found no significant differences between males and females in these results.
To our knowledge, there are no other multiwave studies that have used genetically informative designs to measure the stability of environmental influences on symptoms of anxiety and depression and with which our study might be compared. However, a number of twin studies have longitudinally examined personality traits such as neuroticism and negative emotionality, which are closely related to symptoms of anxiety and depression (Jardine, Martin, & Henderson, 1984).
The studies that could most relevantly be compared with ours are those that have calculated within a genetically informative sample the long-term stability of personality traits that results from environmental experiences unique to the individuals. If such environmental experiences produce a cumulative effect on symptoms of anxiety and depression over time, then the environmental correlations for personality traits (i.e., cross-time correlations in personality due to environmental influences) related to anxiety and depression should increase with age. This possibility was first investigated by Viken, Rose, Kaprio, and Koskenvuo (1994) in a study of 15,000 Finnish twins, ages 18 to 53 at baseline, who were tested on two occasions 6 years apart. Across the two waves, environmental correlations for neuroticism, as measured by the short form of the Eysenck Personality Questionnaire (Eysenck, Eysenck, & Barrett, 1985), increased with age. Pedersen and Reynolds (1998) examined neuroticism in a sample of 2,209 twins, ages 26 to over 90, assessed at up to four waves in the Swedish Adoption/Twin Study of Aging. Although Pedersen and Reynolds did not formally calculate environmental correlations across time, their longitudinal model included a stable non-shared environmental component. They found that approximately half of the nonshared environmental variance was transmitted from occasion to occasion, and this transmission contributed substantially to the observed phenotypic stability of neuroticism over time.
More recently, Kandler et al. (2010) used self- and peer-report data from 696 monozygotic and 387 dizygotic twin pairs between the ages of 23 and 55 to investigate stability and change in personality over time. They found a strong trend for increasing environmental correlations for neuroticism with age and concluded that the “phenotypic continuity [of personality] increased as a function of cumulative environmental effects, which became manifest in stable trait variance and decreasing occasion-specific effects with age” (p. 995). Hopwood et al. (2011) studied 626 complete twin pairs, ages 17 to 29, over three waves using the Multidimensional Personality Questionnaire. The researchers found substantially higher environmental correlations for negative emotionality among twins who were 24 to 29 years old than among twins between 17 and 24 years old. Finally, Kupper, Boomsma, de Geus, Denollet, and Willemsen (2011) studied negative affectivity in 3,235 subjects from the Netherlands Twin Register and found that the environmental correlation increased dramatically from ages 17 to 25 to ages 25 to 30. Thus, prior twin studies provide considerable support for an environmentally influenced set point for personality traits, such as neuroticism, that are closely related to symptoms of anxiety and depression.
Two mechanisms might account for the ability of environmental experiences to stably alter levels of reported anxiety and depression. As recently reviewed by McCrory, De Brito, and Viding (2010), the most straightforward mechanism would be a direct effect on biological processes, from gene regulation and the modification of stress-response systems to anatomical changes in key brain structures, all of which could influence levels of depression and anxiety. Alternatively, the process could be psychological, driven by self-perpetuating interactions between symptomatic individuals and their social environment. For example, as shown in one integrated etiological model for depression (Kendler, Gardner, & Prescott, 2002), individuals who experience emotional trauma in childhood are prone to experience early-onset anxiety, which in turn leads to substance misuse, stressful life events, and low levels of social support, all of which predispose individuals to further symptoms. Much as has been described for individuals with normative personality traits (Hopwood et al., 2011), as individuals age, through self-selection they may enter into stability- promoting environments selected on the basis of the individuals’ vulnerabilities. That is, individuals low in depression and anxiety symptoms tend to enter into mental-health-promoting environments, whereas anxious or depressed individuals tend to enter into stressful environments that further exacerbate their symptomatology.
In conclusion, our findings suggest that with respect to symptoms of anxiety and depression, individuals are what they have experienced, to an appreciable degree. Despite recent findings supporting the important influence of genetics on anxiety and depression, our findings contradict the idea that stability of symptoms of depression and anxiety results solely from genetic factors. Although many environmental stressors produce only short-term effects on mood, some aspects of environmental experience have long-term effects, through mechanisms that have not yet been identified, on levels of anxiety and depression symptoms.
Our results should be interpreted in the context of three methodological limitations. First, we combined studies of twins of different ages and countries, and the studies we combined used a range of instruments to assess symptoms. Prior studies have shown that self-reported symptoms of anxiety and self-reported symptoms of depression are related forms of negative affect and are highly correlated in population samples (Clark & Watson, 1991). Second, our results provide no insight into the mechanisms by which environmental experiences alter the affective set point. Third, although we needed to study monozygotic twins to isolate the impact of environmental factors on the predictable component of symptom levels, it is probable that using monozygotic twins produced a downward bias on the magnitude of this effect. Monozygotic twins share much of their rearing environment, including many early environmental adversities. Furthermore, pairs of twins may share some stressful experiences in their adult lives. In addition, given the evidence that genetic factors influence the quality of many social relationships and individuals’ entry into stressful situations (Kendler & Baker, 2007), the environmental exposures of monozygotic twins are likely to be more similar than those of individuals picked at random from a given population. Thus, our results probably underestimate the contribution of environmental exposures to stable individual differences in mood.
Acknowledgments
We thank Linda Corey for assistance with the ascertainment of twins from the Virginia Twin Registry, now part of the Mid-Atlantic Twin Registry.
Funding
The Virginia Adult Twin Study of Psychiatric and Substance Use Disorders is supported in part by National Institutes of Health Grants DA-011287 and MH/AA/DA-49492. The Mid-Atlantic Twin Registry has received support from the National Institutes of Health, the Carman Trust, and the W. M. Keck, John Templeton, and Robert Wood Johnson Foundations. The Young Netherlands Twin Register and Adult Netherlands Twin Register studies are supported by Spinozapremie Grant NWO/SPI 56-464-14192 and Twin-Family Database for Behavior Genetics and Genomics Studies Grant NWO 480-04-004. C. M. M. is supported by a personal grant (NWO-VENI: 916.76.125) and by Genetics of Mental Illness: A Lifespan Approach to the Genetics of Childhood and Adult Neuropsychiatric Disorders and Comorbid Conditions (Grant ERC-230374). The Swedish Twin Study of Child and Adolescent Development and the Swedish Adoptive Twin Study of Aging are supported by the Swedish Council for Working Life and Social Research (Projects 2004-0383 and 2004-1625) and the Swedish Research Council (Projects 2004-1415 and 2007-3205). The Virginia Twin Study of Adolescent Behavioral Development is supported by National Institutes of Health Grants MH-45268 and MH-068521.
Footnotes
Declaration of Conflicting Interests
The authors declared that they had no conflicts of interest with respect to their authorship or the publication of this article.
References
- Achenbach TM. Manual for the Youth Self-Report and 1991 Profile. Burlington: University of Vermont Department of Psychiatry; 1991. [Google Scholar]
- Achenbach TM, Edelbrock C. Manual for the Child Behavior Checklist and revised Child Behavior Profile. Burlington: University of Vermont, Department of Psychiatry; 1983. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: Author; 1994. [Google Scholar]
- Bartels M, van Beijsterveldt CE, Derks EM, Stroet TM, Polderman TJ, Hudziak JJ, Boomsma DI. Young Netherlands Twin Register (Y-NTR): A longitudinal multiple informant study of problem behavior. Twin Research and Human Genetics. 2007;10:3–11. doi: 10.1375/twin.10.1.3. [DOI] [PubMed] [Google Scholar]
- Biesanz JC, Deeb-Sossa N, Papadakis AA, Bollen KA, Curran PJ. The role of coding time in estimating and interpreting growth curve models. Psychological Methods. 2004;9:30–52. doi: 10.1037/1082-989X.9.1.30. [DOI] [PubMed] [Google Scholar]
- Boomsma DI, de Geus EJ, Vink JM, Stubbe JH, Distel MA, Hottenga JJ, Willemsen G. Netherlands Twin Register: From twins to twin families. Twin Research and Human Genetics. 2006;9:849–857. doi: 10.1375/183242706779462426. [DOI] [PubMed] [Google Scholar]
- Boomsma DI, van Beijsterveldt CE, Hudziak JJ. Genetic and environmental influences on Anxious/Depression during childhood: A study from the Netherlands Twin Register. Genes, Brain and Behavior. 2005;4:466–481. doi: 10.1111/j.1601-183X.2005.00141.x. [DOI] [PubMed] [Google Scholar]
- Brown GW, Harris TO. Social origins of depression: A study of psychiatric disorder in women. London, England: Tavistock; 1978. [Google Scholar]
- Bruder CE, Piotrowski A, Gijsbers AA, Andersson R, Erickson S, de Stahl TD, Dumanski JP. Phenotypically concordant and discordant monozygotic twins display different DNA copy-number-variation profiles. American Journal of Human Genetics. 2008;82:763–771. doi: 10.1016/j.ajhg.2007.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark LA, Watson D. Tripartite model of anxiety and depression: Psychometric evidence and taxonomic implications. Journal of Abnormal Psychology. 1991;100:316–336. doi: 10.1037//0021-843x.100.3.316. [DOI] [PubMed] [Google Scholar]
- Costello EJ, Angold A. Scales to assess child and adolescent depression: Checklists, screens, and nets. Journal of the American Academy of Child & Adolescent Psychiatry. 1988;27:726–737. doi: 10.1097/00004583-198811000-00011. [DOI] [PubMed] [Google Scholar]
- Daley SE, Hammen C. Depressive symptoms and close relationships during the transition to adulthood: Perspectives from dysphoric women, their best friends, and their romantic partners. Journal of Consulting and Clinical Psychology. 2002;70:129–141. doi: 10.1037//0022-006x.70.1.129. [DOI] [PubMed] [Google Scholar]
- Derogatis LR, Lipman RS, Covi L. SCL-90: An outpatient psychiatric rating scale—preliminary report. Psychopharmacology Bulletin. 1973;9:13–28. [PubMed] [Google Scholar]
- Dickens WT, Turkheimer E, Beam C. The social dynamics of the expression of genes for cognitive ability. In: Kendler KS, Jaffee SR, Romer D, editors. The dynamic genome and mental health: The role of genes and environments in youth development. Oxford, England: Oxford University Press; 2011. pp. 103–127. [Google Scholar]
- Eysenck SBG, Eysenck HJ, Barrett P. A revised version of the Psychoticism scale. Personal Individual Differences. 1985;6:21–29. [Google Scholar]
- Fergusson DM, Mullen PE. Childhood sexual abuse: An evidence based perspective. Thousand Oaks, CA: Sage; 1999. [Google Scholar]
- Foley DL, Neale MC, Kendler KS. Genetic and environmental risk factors for depression assessed by subject-rated symptom check list versus structured clinical interview. Psychological Medicine. 2001;31:1413–1423. doi: 10.1017/s0033291701004755. [DOI] [PubMed] [Google Scholar]
- Gillespie NA, Kirk KM, Evans DM, Heath AC, Hickie IB, Martin NG. Do the genetic or environmental determinants of anxiety and depression change with age? A longitudinal study of Australian twins. Twin Research. 2004;7:39–53. doi: 10.1375/13690520460741435. [DOI] [PubMed] [Google Scholar]
- Goenjian AK, Walling D, Steinberg AM, Karayan I, Najarian LM, Pynoos R. A prospective study of posttraumatic stress and depressive reactions among treated and untreated adolescents 5 years after a catastrophic disaster. American Journal of Psychiatry. 2005;162:2302–2308. doi: 10.1176/appi.ajp.162.12.2302. [DOI] [PubMed] [Google Scholar]
- Headey B. The set point theory of well-being has serious flaws: On the eve of a scientific revolution? Social Indicators Research. 2010;97:7–21. [Google Scholar]
- Hopwood CJ, Donnellan MB, Blonigen DM, Krueger RF, McGue M, Iacono WG, Alexandra BS. Genetic and environmental influences on personality trait stability and growth during the transition to adulthood: A three-wave longitudinal study. Journal of Personality and Social Psychology. 2011;100:545–556. doi: 10.1037/a0022409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jardine R, Martin NG, Henderson AS. Genetic covariation between neuroticism and the symptoms of anxiety and depression. Genetic Epidemiology. 1984;1:89–107. doi: 10.1002/gepi.1370010202. [DOI] [PubMed] [Google Scholar]
- Kandler C, Bleidorn W, Riemann R, Spinath FM, Thiel W, Angleitner A. Sources of cumulative continuity in personality: A longitudinal multiple-rater twin study. Journal of Personality and Social Psychology. 2010;98:995–1008. doi: 10.1037/a0019558. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Baker JH. Genetic influences on measures of the environment: A systematic review. Psychological Medicine. 2007;37:615–626. doi: 10.1017/S0033291706009524. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Bulik CM, Silberg J, Hettema JM, Myers J, Prescott CA. Childhood sexual abuse and adult psychiatric and substance use disorders in women: An epidemiological and cotwin control analysis. Archives of General Psychiatry. 2000;57:953–959. doi: 10.1001/archpsyc.57.10.953. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Gardner CO, Lichtenstein P. A developmental twin study of symptoms of anxiety and depression: Evidence for genetic innovation and attenuation. Psychological Medicine. 2008;38:1567–1575. doi: 10.1017/S003329170800384X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Gardner CO, Prescott CA. Toward a comprehensive developmental model for major depression in women. American Journal of Psychiatry. 2002;159:1133–1145. doi: 10.1176/appi.ajp.159.7.1133. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Gardner CO, Prescott CA. Toward a comprehensive developmental model for alcohol use disorders in men. Twin Research and Human Genetics. 2011;14:1–15. doi: 10.1375/twin.14.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Karkowski LM, Prescott CA. Stressful life events and major depression: Risk period, long-term contextual threat, and diagnostic specificity. Journal of Nervous and Mental Disease. 1998;186:661–669. doi: 10.1097/00005053-199811000-00001. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA. Genes, environment, and psychopathology: Understanding the causes of psychiatric and substance use disorders. New York, NY: Guilford Press; 2006. [Google Scholar]
- Keyes CLM. The mental health continuum: From languishing to flourishing in life. Journal of Health and Social Behavior. 2002;43:207–222. [PubMed] [Google Scholar]
- Keyes CLM. Mental illness and/or mental health? Investigating axioms of the complete state model of health. Journal of Consulting and Clinical Psychology. 2005;73:539–548. doi: 10.1037/0022-006X.73.3.539. [DOI] [PubMed] [Google Scholar]
- Koenen KC, Lyons MJ, Goldberg J, Simpson J, Williams WM, Toomey R, Tsuang MT. A high risk twin study of combat-related PTSD comorbidity. Twin Research. 2003;6:218–226. doi: 10.1375/136905203765693870. [DOI] [PubMed] [Google Scholar]
- Kupper N, Boomsma DI, de Geus EJ, Denollet J, Willemsen G. Nine-year stability of type D personality: Contributions of genes and environment. Psychosomatic Medicine. 2011;73:75–82. doi: 10.1097/PSY.0b013e3181fdce54. [DOI] [PubMed] [Google Scholar]
- Laenen A, Alonso A, Molenberghs G. A measure for the reliability of a rating scale based on longitudinal clinical trial data. Psychometrika. 2007;72:443–448. [Google Scholar]
- Laenen A, Alonso A, Molenberghs G, Vangeneugden T. Reliability of a longitudinal sequence of scale ratings. Psychometrika. 2009;74:49–64. [Google Scholar]
- Lichtenstein P, Tuvblad C, Larsson H, Carlstrom E. The Swedish twin study of child and adolescent development: The TCHAD-Study. Twin Research and Human Genetics. 2007;10:67–73. doi: 10.1375/twin.10.1.67. [DOI] [PubMed] [Google Scholar]
- Lovibond PF. Long-term stability of depression, anxiety, and stress syndromes. Journal of Abnormal Psychology. 1998;107:520–526. doi: 10.1037//0021-843x.107.3.520. [DOI] [PubMed] [Google Scholar]
- Mather M, Carstensen LL. Aging and motivated cognition: The positivity effect in attention and memory. Trends in Cognitive Sciences. 2005;9:496–502. doi: 10.1016/j.tics.2005.08.005. [DOI] [PubMed] [Google Scholar]
- McCrory E, De Brito SA, Viding E. Research review: The neurobiology and genetics of maltreatment and adversity. Journal of Child Psychology and Psychiatry. 2010;51:1079–1095. doi: 10.1111/j.1469-7610.2010.02271.x. [DOI] [PubMed] [Google Scholar]
- McGue M, Christensen K. Genetic and environmental contributions to depression symptomatology: Evidence from Danish twins 75 years of age and older. Journal of Abnormal Psychology. 1997;106:439–448. doi: 10.1037//0021-843x.106.3.439. [DOI] [PubMed] [Google Scholar]
- Molenaar PC, Boomsma DI, Dolan CV. A third source of developmental differences. Behavior Genetics. 1993;23:519–524. doi: 10.1007/BF01068142. [DOI] [PubMed] [Google Scholar]
- O’Connor TG, Neiderhiser JM, Reiss D, Hetherington EM, Plomin R. Genetic contributions to continuity, change, and co-occurrence of antisocial and depressive symptoms in adolescence. Journal of Child Psychology and Psychiatry. 1998;39:323–336. [PubMed] [Google Scholar]
- Osborne R, Paterson WSB. On the sampling variance of heritability estimates derived from variance analyses. Proceedings of the Royal Society of Edinburgh B. 1952;64:456–471. [Google Scholar]
- Pan W. Akaike’s information criterion in generalized estimating equations. Biometrics. 2001;57:120–125. doi: 10.1111/j.0006-341x.2001.00120.x. [DOI] [PubMed] [Google Scholar]
- Pedersen NL, McClearn GE, Plomin R, Nesselroade JR, Berg S, DeFaire U. The Swedish adoption twin study of aging: An update. Acta Genetica Medica et Gemellologia. 1991;40:7–20. doi: 10.1017/s0001566000006681. [DOI] [PubMed] [Google Scholar]
- Pedersen NL, Reynolds CA. Stability and change in adult personality: Genetic and environmental components. European Journal of Personality. 1998;12:365–386. [Google Scholar]
- Prescott CA, Hewitt JK, Heath AC, Truett KR, Neale MC, Eaves LJ. Environmental and genetic influences on alcohol use in a volunteer sample of older twins. Journal of Studies on Alcohol. 1994;55:18–33. doi: 10.15288/jsa.1994.55.18. [DOI] [PubMed] [Google Scholar]
- Reynolds CR, Richmond BO. What I think and feel: A revised measure of children’s manifest anxiety. Journal of Abnormal Child Psychology. 1978;6:271–280. doi: 10.1007/BF00919131. [DOI] [PubMed] [Google Scholar]
- Silberg JL, Heath AC, Kessler R, Neale MC, Meyer JM, Eaves LJ, Kendler KS. Genetic and environmental effects on self-reported depressive symptoms in a general population twin sample. Journal of Psychiatric Research. 1990;24:197–212. doi: 10.1016/0022-3956(90)90010-n. [DOI] [PubMed] [Google Scholar]
- Simonoff E, Pickles A, Meyer JM, Silberg JL, Maes HH, Loeber R, Eaves LJ. The Virginia Twin Study of Adolescent Behavioral Development: Influences of age, sex, and impairment on rates of disorder. Archives of General Psychiatry. 1997;54:801–808. doi: 10.1001/archpsyc.1997.01830210039004. [DOI] [PubMed] [Google Scholar]
- Spielberger C, Gorsuch R, Luchene R. The State-Trait Anxiety Inventory: Test manual for form X. Palo Alto, CA: Consulting Psychologists Press; 1970. [Google Scholar]
- Steiger JH. Structural model evaluation and modification: An interval estimation approach. Multivariate Behavioral Research. 1990;25:173–180. doi: 10.1207/s15327906mbr2502_4. [DOI] [PubMed] [Google Scholar]
- Surtees PG, Miller PM, Ingham JG, Kreitman NB, Rennie D, Sashidharan SP. Life events and the onset of affective disorder: A longitudinal general population study. Journal of Affective Disorders. 1986;10:37–50. doi: 10.1016/0165-0327(86)90047-9. [DOI] [PubMed] [Google Scholar]
- Viken RJ, Rose RJ, Kaprio J, Koskenvuo M. A developmental genetic analysis of adult personality: Extraversion and neuroticism from 18 to 59 years of age. Journal of Personality and Social Psychology. 1994;66:722–730. doi: 10.1037//0022-3514.66.4.722. [DOI] [PubMed] [Google Scholar]