Abstract
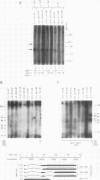
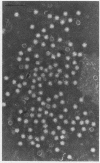
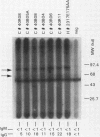
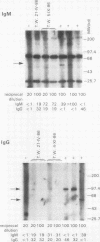
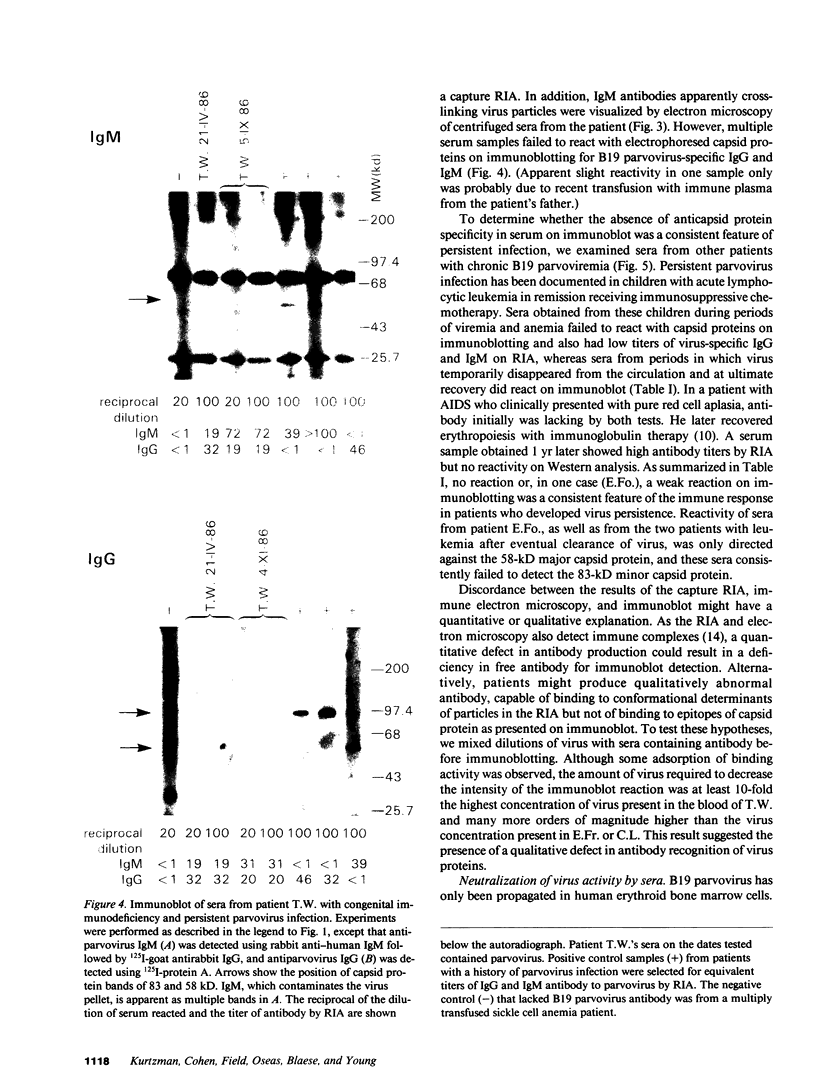
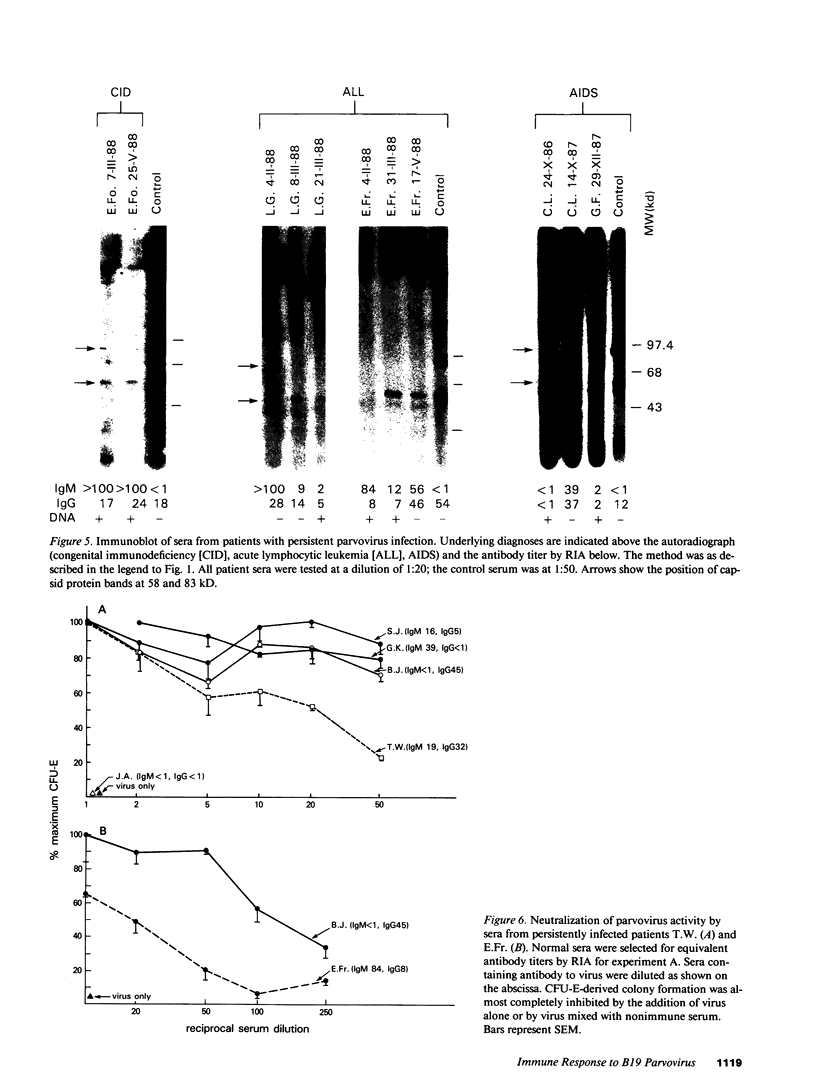
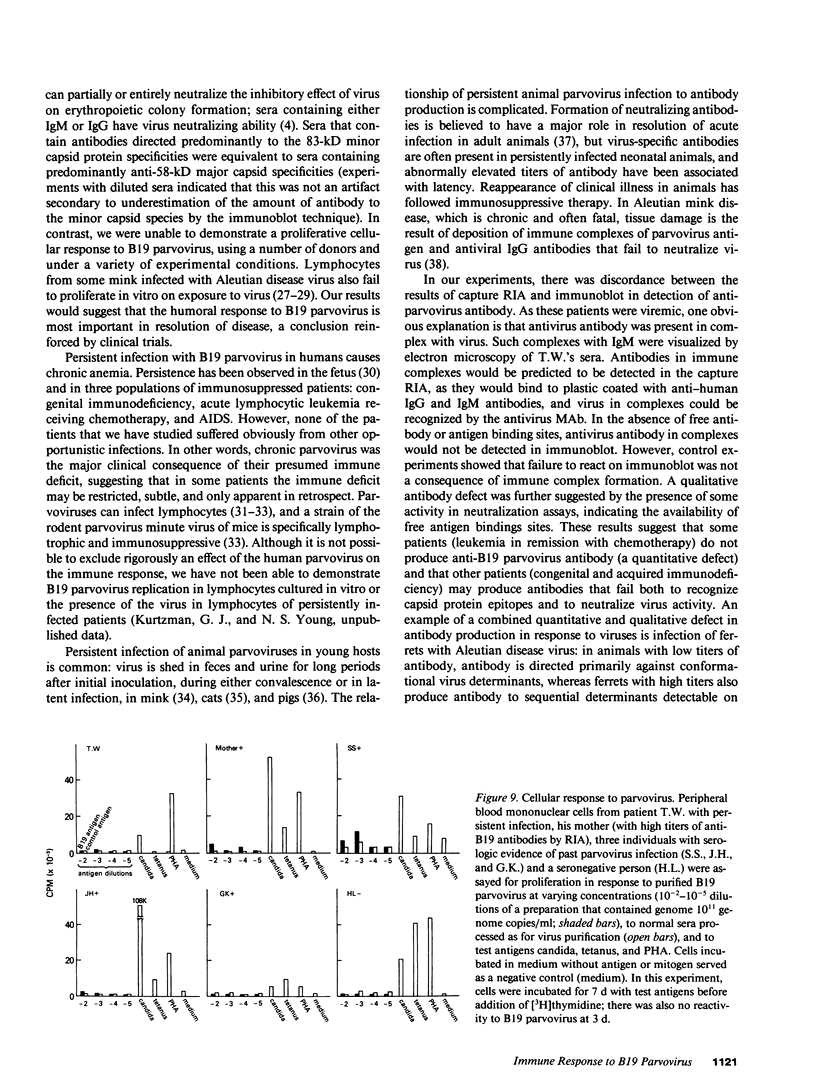
B19 parvovirus has been shown to persist in some immunocompromised patients, and treatment with specific antibodies can lead to decreased quantities of circulating virus and hematologic improvement. A defective immune response to B19 parvovirus in these patients was shown by comparison of results using a capture RIA and immunoblotting. In normal individuals, examination of paired sera showed that the dominant humoral immune response during early convalescence was to the virus major capsid protein (58 kD) and during late convalescence to the minor capsid species (83 kD). In patients with persistent parvovirus infection, variable titers against intact particles were detected by RIA, but the sera from these patients had minimal or no IgG to capsid proteins determined by Western analysis. Competition experiments suggested that this discrepancy was not explicable on the basis of immune complex formation alone and that these patients may have a qualitative abnormality in antibody binding to virus. In neutralization experiments, in which erythroid colony formation in vitro was used as an assay of parvovirus activity, sera from patients with poor reactivity on immunoblotting were also inadequate in inhibiting viral infectivity. A cellular response to purified B19 parvovirus could not be demonstrated using proliferation assays and PBMC from individuals with serologic evidence of exposure to virus. These results suggest that production of neutralizing antibody to capsid protein plays a major role in limiting parvovirus infection in man.
Full text
PDF
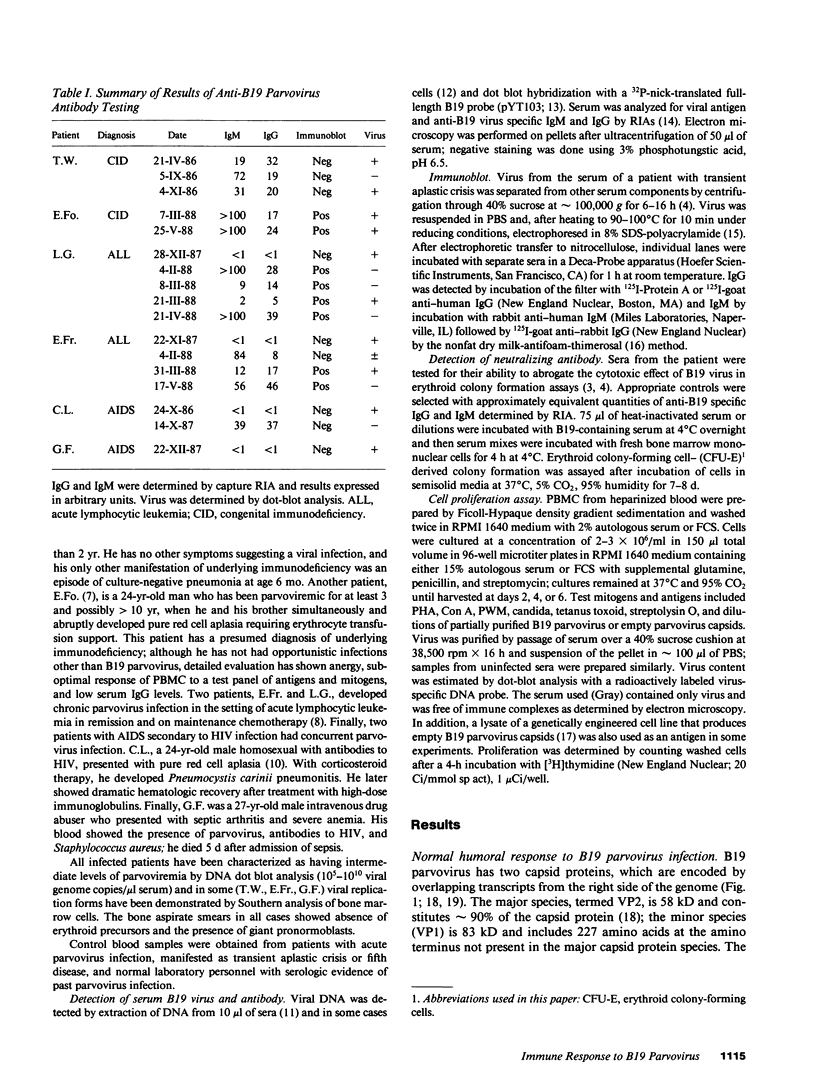
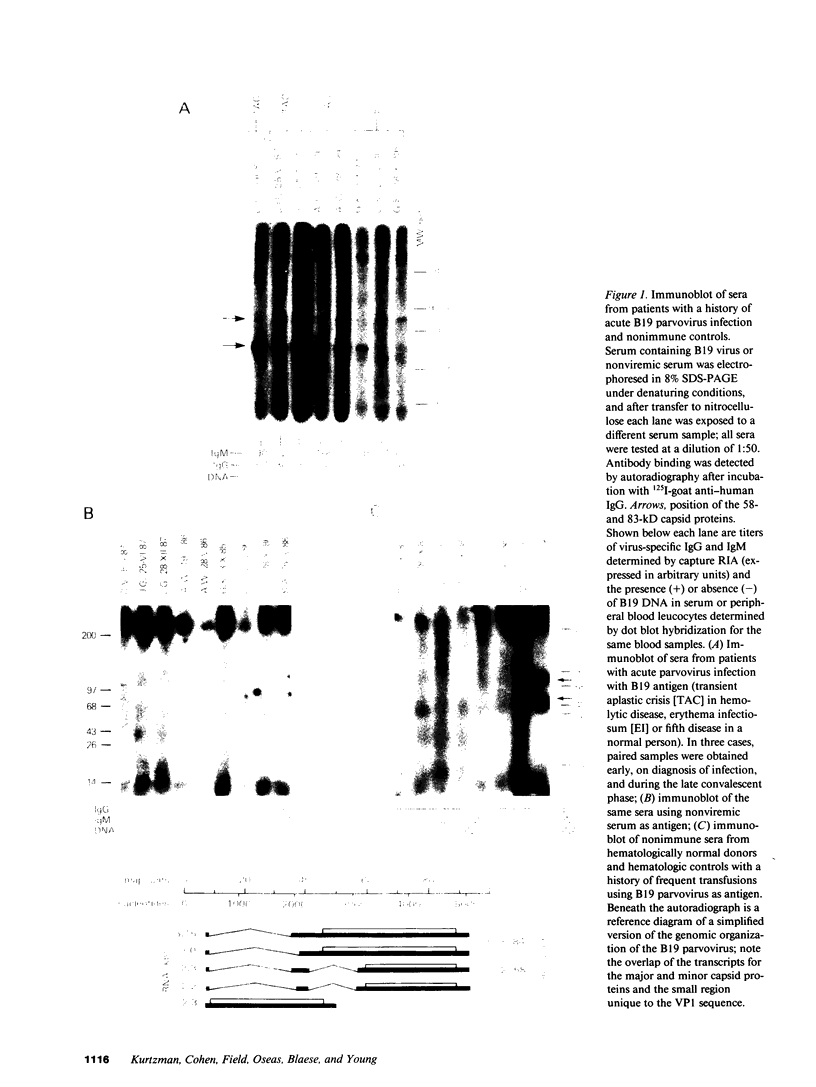
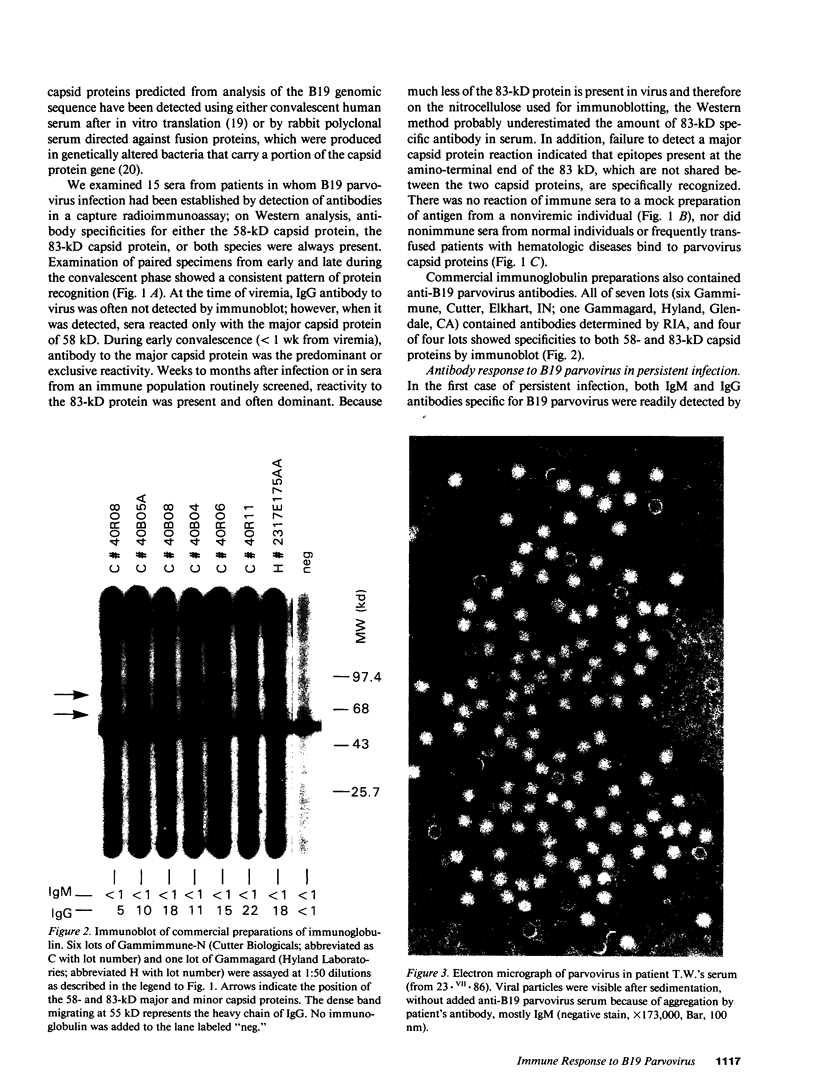



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexandersen S., Bloom M. E., Wolfinbarger J. Evidence of restricted viral replication in adult mink infected with Aleutian disease of mink parvovirus. J Virol. 1988 May;62(5):1495–1507. doi: 10.1128/jvi.62.5.1495-1507.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An S. H., Ingram D. G. Detection of inapparent Aleutian disease virus infection in mink. Am J Vet Res. 1977 Oct;38(10):1619–1624. [PubMed] [Google Scholar]
- An S. H., Wilkie B. N. Mitogen- and viral antigen-induced transformation of lymphocytes from normal mink and from mink with progressive or nonprogressive Aleutian disease. Infect Immun. 1981 Oct;34(1):111–114. doi: 10.1128/iai.34.1.111-114.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand A., Gray E. S., Brown T., Clewley J. P., Cohen B. J. Human parvovirus infection in pregnancy and hydrops fetalis. N Engl J Med. 1987 Jan 22;316(4):183–186. doi: 10.1056/NEJM198701223160403. [DOI] [PubMed] [Google Scholar]
- Anderson M. J., Higgins P. G., Davis L. R., Willman J. S., Jones S. E., Kidd I. M., Pattison J. R., Tyrrell D. A. Experimental parvoviral infection in humans. J Infect Dis. 1985 Aug;152(2):257–265. doi: 10.1093/infdis/152.2.257. [DOI] [PubMed] [Google Scholar]
- Chorba T., Coccia P., Holman R. C., Tattersall P., Anderson L. J., Sudman J., Young N. S., Kurczynski E., Saarinen U. M., Moir R. The role of parvovirus B19 in aplastic crisis and erythema infectiosum (fifth disease). J Infect Dis. 1986 Sep;154(3):383–393. doi: 10.1093/infdis/154.3.383. [DOI] [PubMed] [Google Scholar]
- Clewley J. P. Detection of human parvovirus using a molecularly cloned probe. J Med Virol. 1985 Feb;15(2):173–181. doi: 10.1002/jmv.1890150210. [DOI] [PubMed] [Google Scholar]
- Cohen B. J., Mortimer P. P., Pereira M. S. Diagnostic assays with monoclonal antibodies for the human serum parvovirus-like virus (SPLV). J Hyg (Lond) 1983 Aug;91(1):113–130. doi: 10.1017/s0022172400060095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotmore S. F., McKie V. C., Anderson L. J., Astell C. R., Tattersall P. Identification of the major structural and nonstructural proteins encoded by human parvovirus B19 and mapping of their genes by procaryotic expression of isolated genomic fragments. J Virol. 1986 Nov;60(2):548–557. doi: 10.1128/jvi.60.2.548-557.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csiza C. K., Scott F. W., de Lahunta A., Gillespie J. H. Immune carrier state of feline panleukopenia virus-infected cats. Am J Vet Res. 1971 Mar;32(3):419–426. [PubMed] [Google Scholar]
- Engers H. D., Louis J. A., Zubler R. H., Hirt B. Inhibition of T cell-mediated functions by MVM(i), a parvovirus closely related to minute virus of mice. J Immunol. 1981 Dec;127(6):2280–2285. [PubMed] [Google Scholar]
- Johnson R. H., Collings D. F. Transplacental infection of piglets with a porcine parvovirus. Res Vet Sci. 1971 Nov;12(6):570–572. [PubMed] [Google Scholar]
- Kurtzman G. J., Cohen B., Meyers P., Amunullah A., Young N. S. Persistent B19 parvovirus infection as a cause of severe chronic anaemia in children with acute lymphocytic leukaemia. Lancet. 1988 Nov 19;2(8621):1159–1162. doi: 10.1016/s0140-6736(88)90233-4. [DOI] [PubMed] [Google Scholar]
- Kurtzman G. J., Gascon P., Caras M., Cohen B., Young N. S. B19 parvovirus replicates in circulating cells of acutely infected patients. Blood. 1988 May;71(5):1448–1454. [PubMed] [Google Scholar]
- Kurtzman G. J., Ozawa K., Cohen B., Hanson G., Oseas R., Young N. S. Chronic bone marrow failure due to persistent B19 parvovirus infection. N Engl J Med. 1987 Jul 30;317(5):287–294. doi: 10.1056/NEJM198707303170506. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mortimer P. P., Humphries R. K., Moore J. G., Purcell R. H., Young N. S. A human parvovirus-like virus inhibits haematopoietic colony formation in vitro. 1983 Mar 31-Apr 6Nature. 302(5907):426–429. doi: 10.1038/302426a0. [DOI] [PubMed] [Google Scholar]
- Ozawa K., Ayub J., Young N. Functional mapping of the genome of the B19 (human) parvovirus by in vitro translation after negative hybrid selection. J Virol. 1988 Jul;62(7):2508–2511. doi: 10.1128/jvi.62.7.2508-2511.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa K., Kurtzman G., Young N. Replication of the B19 parvovirus in human bone marrow cell cultures. Science. 1986 Aug 22;233(4766):883–886. doi: 10.1126/science.3738514. [DOI] [PubMed] [Google Scholar]
- Ozawa K., Young N. Characterization of capsid and noncapsid proteins of B19 parvovirus propagated in human erythroid bone marrow cell cultures. J Virol. 1987 Aug;61(8):2627–2630. doi: 10.1128/jvi.61.8.2627-2630.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul P. S., Mengeling W. L., Brown T. T., Jr Replication of porcine parvovirus in peripheral blood lymphocytes, monocytes, and peritoneal macrophages. Infect Immun. 1979 Sep;25(3):1003–1007. doi: 10.1128/iai.25.3.1003-1007.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter D. D. Aleutian disease: a persistent parvovirus infection of mink with a maximal but ineffective host humoral immune response. Prog Med Virol. 1986;33:42–60. [PubMed] [Google Scholar]
- Race R. E., Bloom M. E., Coe J. E. Demonstration of Aleutian disease virus-specific lymphocyte response in mink with progressive Aleutian disease: comparison of sapphire and pastel mink infected with different virus strains. J Immunol. 1983 Sep;131(3):1558–1564. [PubMed] [Google Scholar]
- Saarinen U. M., Chorba T. L., Tattersall P., Young N. S., Anderson L. J., Palmer E., Coccia P. F. Human parvovirus B19-induced epidemic acute red cell aplasia in patients with hereditary hemolytic anemia. Blood. 1986 May;67(5):1411–1417. [PubMed] [Google Scholar]
- Schultz R. D., Mendel H., Scott F. W. Effect of feline panleukopenia virus infection on development of humoral and cellular immunity. Cornell Vet. 1976 Jul;66(3):324–332. [PubMed] [Google Scholar]
- Schwarz T. F., Roggendorf M., Deinhardt F. Human parvovirus B19: ELISA and immunoblot assays. J Virol Methods. 1988 Jun;20(2):155–168. doi: 10.1016/0166-0934(88)90149-8. [DOI] [PubMed] [Google Scholar]
- Shade R. O., Blundell M. C., Cotmore S. F., Tattersall P., Astell C. R. Nucleotide sequence and genome organization of human parvovirus B19 isolated from the serum of a child during aplastic crisis. J Virol. 1986 Jun;58(3):921–936. doi: 10.1128/jvi.58.3.921-936.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner L. A., Eisen H. N. Sequential changes in the relative affinity of antibodies synthesized during the immune response. J Exp Med. 1967 Dec 1;126(6):1161–1183. doi: 10.1084/jem.126.6.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surleraux M., Bodeus M., Burtonboy G. Study of canine parvovirus polypeptides by immunoblot analysis. Arch Virol. 1987;95(3-4):271–281. doi: 10.1007/BF01310785. [DOI] [PubMed] [Google Scholar]
- Young N. S., Mortimer P. P., Moore J. G., Humphries R. K. Characterization of a virus that causes transient aplastic crisis. J Clin Invest. 1984 Jan;73(1):224–230. doi: 10.1172/JCI111195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young N. Hematologic and hematopoietic consequences of B19 parvovirus infection. Semin Hematol. 1988 Apr;25(2):159–172. [PubMed] [Google Scholar]