Abstract
Post-infarction arrhythmias are most often confined to scar tissue. Scar can be detected by delayed enhanced magnetic resonance imaging (DE-MRI). The purpose of this study was to assess the feasibility of pre-procedural scar identification, and intra-procedural real-time image registration with an electroanatomical map (EAM) in 23 patients with prior infarction and ventricular arrhythmias (VA). Registration accuracy and MRI/EAM correlations were assessed and critical areas for VA were correlated with the presence of scar. With a positional registration error of 3.8±0.8 mm, 86% of low voltage points of the EAM projected onto the registered scar. The DE-MRI defined scar correlated with the area of low voltage (R=0.82, p<0.001). All sites critical to VAs projected on the registered scar. Selective identification and extraction of DE-MRI defined scar followed by registration into a real-time mapping system is feasible and helps to identify and display the arrhythmogenic substrate in post-infarction patients with VAs.
Keywords: magnetic resonance imaging, post-infarction, mapping, ablation, ventricular arrhythmias
Most post-infarction ventricular tachycardias originate from scar tissue and therefore identification of scar has been helpful in mapping and ablating these arrhythmias. Delayed-enhanced magnetic resonance imaging (DE-MRI) precisely identifies scar tissue. The purpose of this study was to assess the feasibility of pre-procedure scar identification and segmentation from DE-MRI images and intra-procedural registration of the scar with the electroanatomical maps (EAM) obtained in real-time.
Methods
Patient Characteristics (Table 1)
Table 1.
Baseline Clinical Characteristics of Patients
| Variables | Number (SD) |
|---|---|
| Age | 60 (12) |
| Gender (m/f) | 19/4 |
| Ejection fraction (%) | 40 (13) |
| Infarct age | 8.8(7.7) |
| Infarct Location - inferior - anterior - lateral |
16 6 1 |
| Targeted Arrhythmia - PVCs - VT |
13 10 |
| Antiarrhythmic therapy | 7 |
Abbrreviations: PVCs= premature ventricular complexes. VT= ventricular tachycardia
Twenty-three consecutive patients (19 men, mean age: 60±12 years, ejection fraction: 44±15%) with post-infarction ventricular arrhythmias underwent DE-MRI prior to mapping and ablation of ventricular arrhythmias. All patients had a prior myocardial infarction. Radiofrequency ablation was performed for ventricular tachycardia (VT) in 10 patients and for symptomatic premature ventricular complexes (PVCs) in 13 patients. No complications occurred. The study was approved by the Institutional Review Board of the University of Michigan.
Delayed Enhancement Magnetic Resonance Imaging
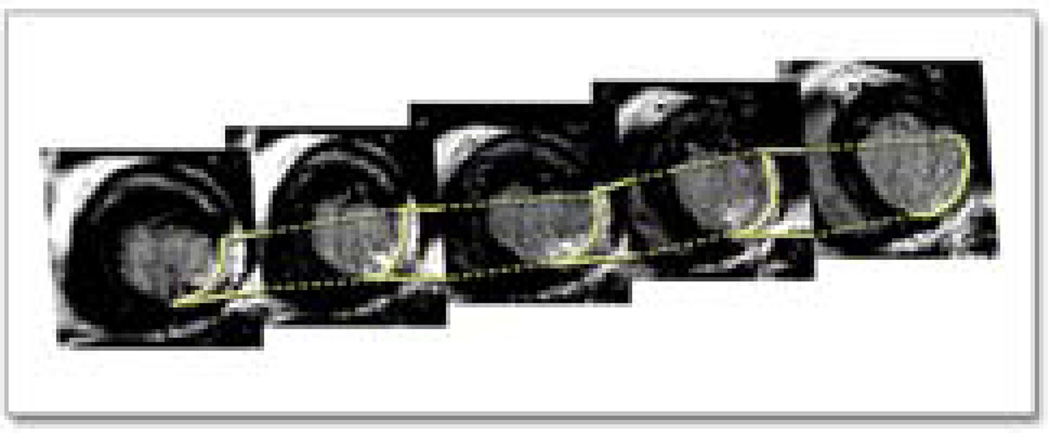
The DE-MRI studies were performed on a 1.5-Tesla magnetic resonance imaging scanner (Signa Excite CV/i, General Electric, Milwaukee, Wis.) with a 4- or 8-element phased array coil placed over the chest of patients in the supine position. Images were acquired with ECG gating during breath-holds. Dynamic short- and long-axis images of the heart were acquired using a segmented, k-space, steady-state, free-precession pulse sequence (repetition time 4.2 ms, echo time 1.8 ms, 1.4×1.4 mm in-plane spatial resolution, 8 mm slice thickness). Fifteen minutes after administration of 0.20 mmol/kg of intravenous gadolinium DTPA (Magnevist, Berlex Pharmaceuticals, Wayne, NJ), 2-D delayed enhancement imaging was performed using an inversion-recovery sequence 1 (repetition time 6.7 ms, echo time 3.2 ms, in-plane spatial resolution 1.4×2.2 mm, slice thickness 8mm) in the short-axis and long-axis of the left ventricle at matching cine-image slice locations. The inversion time (250–350 ms) was optimized by visual inspection for optimal nulling of the normal myocardium. All patients had adequate MRI images as judged by the radiologist. The DE-MRIs were reviewed for the presence or absence of delayed enhancement by 2 observers before the actual mapping/ablation procedure.
Electrophysiologic Study
After informed consent was obtained, a 6 Fr quadripolar electrode catheter was introduced into the right femoral vein and positioned at the right ventricular apex. Programmed right ventricular stimulation with 1–4 extrastimuli was performed in all patients. Sustained VT was defined as VT lasting >30 seconds or requiring termination secondary to hemodynamic compromise.
In 10 patients, a total of 22 distinct, sustained, monomorphic VTs (mean VT cycle length: 325 ms) were targeted.
Mapping and Ablation
A 3-dimensional mapping system (CARTO XP, Biosense Webster, Inc, Diamond Bar, CA) with a 3.5-mm-tip, open-irrigation ablation catheter (Thermocool, Biosense Webster) was used. For the mapping procedure, a bolus of 5000 units of heparin was used initially and heparin was then administered to maintain an activated clotting time of 300 seconds.
The intracardiac electrograms were filtered at 50–500 Hz and displayed along with leads V1, I, II and III on an oscilloscope at a speed of 100 mm/sec. A left ventricular voltage map was generated with 235±108 endocardial points during sinus rhythm.
Low voltage was defined as a bipolar voltage amplitude ≤1.5 mV2. The area of regions with bipolar voltage ≤1.5 and ≤1.0 mV were measured. If a VT was hemodynamically tolerated, entrainment mapping was performed. For PVCs, activation mapping was performed; if PVCs were infrequent or if a targeted VT was not hemodynamically tolerated, pace-mapping was used to identify critical sites.
For PVCs, the site of earliest local activation resulting in PVC elimination with radiofrequency ablation was defined as a critical site. For hemodynamically tolerated VT, a critical site was defined as a site where there was concealed entrainment and where ablation resulted in VT termination. For non-tolerated VTs, an isthmus was defined as a site where there was a pace-map that matched the targeted VT. Radiofrequency energy was delivered to achieve an impedance drop of >10 ohms with a maximal temperature of 45C° at a power of 30–50 watts.
Image Segmentation and Registration
The DE-MRI images (Figure 1) were registered into the EAM (Figure 2, left panel) that was constructed in real-time by the following steps using the vendor integrated segmentation tool of CARTO MERGE: 1. From the DE-MRI images, the endocardial volume was registered as the endocardial shell, 2. Using the same DE-MRI images, the scar on the DE-MRI images was cut out with the cutting tool function leaving only the scar behind. An intensity threshold of 2 standard deviations above the signal of normal myocardium was used to guide the scar segmentation. The scar was then fused together with the endocardial shell using a different color (Figure 2 middle panel), 3. After the DE-MRI images were rendered they were registered real time with the the EAM (Figure 2, right panel). This was achieved with the landmark registration algorithm. In brief, landmark points were identified in the EAM at the aorta, the mitral annulus and the left ventricular apex. Then the landmark registration process with the corresponding MRI landmarks was performed. As the EAM was completed, registration of EAM data and MRI data were refined with surface registration. Using this approach, a 3-D registration of both image modalities was achieved. The inter-and intra-observer variability of the cutting process of the DE-MRI-defined scar was determined by the Pearson correlation coefficient. The Pearson correlation coefficient was 0.986 (p<0.001) for the intra- and 0.987 (p<0.001) for the inter-observer variability.
Figure 1. Short-axis stack of delayed enhanced magnetic resonance images showing a region of scar in the lateral left ventricle.
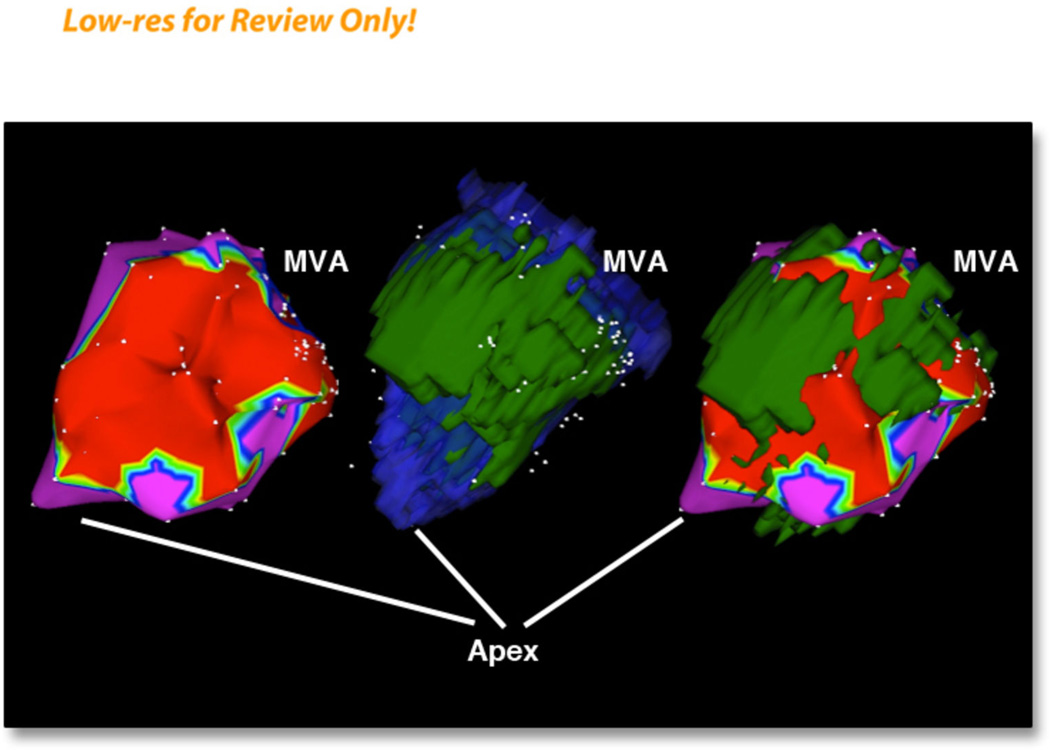
Figure 2. A series of three images of electroanatomical maps and 3 –D reconstruction of scar and left ventricular endocardium.
Left panel: voltage map with a cut-off voltage of 1.5 mV.
Middle panel: merged endocardial MRI shell (blue color) with scar shell (green color)
Right panel: merged MRI scar (green color) with electroanatomical map.
In order to assess accuracy of scar location versus location of low voltage points in a given area of the voltage map, the percentage of points in the low voltage areas of the EAM projecting on the scar was determined. The area of the subendocardial scar was measured after it was registered with the EAM using the CARTO XP software. Only the left ventricular endocardium excluding the papillary muscles was used in the registration process.
Statistics
Continuous data are presented as mean value ± SD. Statistical analysis was performed using SPSS for Windows (Version 16.0, Chicago, Illinois). Comparisons of clinical variables were made using Student’s t test or a paired t-test. Correlations were determined using Pearson’s and Spearman’s correlation coefficient. A p-value < 0.05 was considered significant.
Results
Image Registration
The positional error using surface registration was 3.8±0.8 mm. The registration process took a mean of 26±5 minutes. The subendocardial scar area measured by DE-MRI (median 12.0 cm2, interquartile range 6.4–41.5 cm2) correlated with the area of low voltage (<1.0 mV: median 13.5 cm2; interquartile range 7.0–30.1 cm2, R=0.82, p<0.001; <1.5 mV: median 29.1 cm2, interquartile range 13.8–62.5 cm2, R=0.62, p=0.003).
More than 80% of low-voltage points (88±9% when low voltage was defined as <1.0mV and 86±7% when low voltage was defined as <1.5 mV) projected on the registered scar. The average bipolar voltage of all low-voltage points that projected on the registered scar was 0.70±0.18 mV. Twenty-two patients had a single discrete low voltage area identified on the EAM and a single scar identified by DE-MRI. One patient had two low voltage areas on the EAM and two scars on DE-MRI. In all patients, each low voltage area on the EAM correlated with the scar identified by DE-MRI.
The registration of the DE-MRI-rendered scar and the low-voltage area on the EAM was compared with the registration of the DE-MRI-rendered left ventricle and the left ventricular EAM. This demonstrated that 92.4% of the low voltage points were within 5 mm of the DE-MRI-defined scar and 92.2% of the points of the entire EAM were within 5 mm of the MRI-rendered left ventricular endocardial shell.
Ablation
In the 13 patients with PVCs, the site of origin was identified and effectively ablated in all patients. In patients with ventricular tachycardia a critical isthmus was identified for 19 VTs. All critical sites for PVCs and for VTs were confined to the registered, DE-MRI-based scar.
Follow-Up
The patients in this study were followed for an average of 49 months (range 15–74 months). Amiodarone was continued in 1 patient in whom all VTs were not ablated. In 1 patient, treatment with amiodarone was initiated for atrial fibrillation. One patient had recurrent VT and was treated with sotalol. In patients with frequent PVCs, the PVC burden was reduced from 20.2 ± 15.3% to 0.57±0.94%, representing a reduction of 96% (p=0.001). One of the patients had recurrence of the ablated PVCs. The ejection fraction increased from 47±11% prior to ablation to 54±10% post-ablation in patients in whom PVCs were targeted (p=0.078) and remained unchanged in patients in whom VTs were targeted (43.7±18 vs 41.5±14; p=0.98).
Discussion
The results of this study demonstrate that identification and registration of post-infarction scar with EAMs can be accomplished in real-time with the segmentation tool that is integrated into the CARTO XP software. Because post-infarction arrhythmias originate from scar tissue, this approach may facilitate the mapping process by identifying the areas of scar.
Post-Infarction Arrhythmias
This study confirms the results of prior studies in which different imaging modalities were used to identify scar tissue in post-infarction patients3, 4. The present study is the first study in which previously acquired DE-MRI data were registered in real time during an ablation procedure. Integration of a scar image into the mapping system allows the mapping process to focus on a particular area.
Image Registration Process
Registration of MRI data with electroanatomical mapping data has been accomplished in prior reports4, 5. However, these registrations were performed post hoc without direct benefit to the patient in whom the MRI was obtained. In the present study, registration of the exported scar was helpful with the mapping and ablation procedure in that it allowed to focus the mapping process to a particular area.
A prior study used customized software that is not accessible to others5. In contrast, we demonstrate in this study that the vendor-supplied integrated segmentation tool can be used to identify and selectively extract the 3D structure of the scar that is displayed in the DE-MR images. Both the short- and long-axis images can be used for this purpose. The scar selection and extraction process can be performed with minimal training and minimal time yet it is accurate.
Limitations
The main limitation of the study is the absence of a control group. The study demonstrates the feasibility of real-time registration of electroanatomical data with the scar from a previously obtained MRI. A randomized study will need to be done to show that efficacy or efficiency is improved by scar registration.
Table 2.
Characterization of Infarcts
| Variables | PVCs | VT | p-value |
|---|---|---|---|
| Patients (n) | 13 | 10 | |
| Infarct size - cm3 - (%) of LV mass |
12.44 ± 17.31 7.14 ± 7.59 |
29.75 ± 13.93 17.16 ± 6.42 |
0.03 0.01 |
| Subendocardial extent | 13 | 10 | |
| Degree of transmurality (n) - 1–25% - 25–50% - >50% |
2 7 4 |
0 2 8 |
Abbreviations: LV= left ventricular
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Drs Bogun and Oral have received a grant from the Leducq foundation
References
- 1.Simonetti OP, Kim RJ, Fieno DS, Hillenbrand HB, Wu E, Bundy JM, Finn JP, Judd RM. An improved mr imaging technique for the visualization of myocardial infarction. Radiology. 2001;218:215–223. doi: 10.1148/radiology.218.1.r01ja50215. [DOI] [PubMed] [Google Scholar]
- 2.Marchlinski FE, Callans DJ, Gottlieb CD, Zado E. Linear ablation lesions for control of unmappable ventricular tachycardia in patients with ischemic and nonischemic cardiomyopathy. Circulation. 2000;101:1288–1296. doi: 10.1161/01.cir.101.11.1288. [DOI] [PubMed] [Google Scholar]
- 3.Dickfeld T, Lei P, Dilsizian V, Jeudy J, Dong J, Voudouris A, Peters R, Saba M, Shekhar R, Shorofsky S. Integration of three-dimensional scar maps for ventricular tachycardia ablation with positron emission tomography- computed tomography. J Am Coll Cardiol Img. 2008;1:73–82. doi: 10.1016/j.jcmg.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Desjardins B, Crawford T, Good E, Oral H, Chugh A, Pelosi F, Morady F, Bogun F. Infarct architecture and characteristics on delayed enhanced magnetic resonance imaging and electroanatomic mapping in patients with postinfarction ventricular arrhythmia. Heart Rhythm. 2009;6:644–651. doi: 10.1016/j.hrthm.2009.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Codreanu A, Odille F, Aliot E, Marie PY, Magnin-Poull I, Andronache M, Mandry D, Djaballah W, Regent D, Felblinger J, de Chillou C. Electroanatomic characterization of post-infarct scars comparison with 3-dimensional myocardial scar reconstruction based on magnetic resonance imaging. Journal of the American College of Cardiology. 2008;52:839–842. doi: 10.1016/j.jacc.2008.05.038. [DOI] [PubMed] [Google Scholar]