Abstract
Transduction of mechanical stimuli by receptor neurons is essential for senses such as hearing, touch, and pain1–4. Ion channels play a role in neuronal mechanotransduction in invertebrates1; however, functional conservation of these ion channels in mammalian mechanotransduction is not observed. For example, NOMPC, a TRP ion channel, acts as a mechanotransducer in Drosophila melanogaster5 and Caenorhabditis elegans6,7; however, it has no orthologues in mammals. DEG/ENaC family members are mechanotransducers in C. elegans8 and potentially in D. melanogaster9; however, a direct role of its mammalian homologues in sensing mechanical force is not shown. Recently, Piezo1 and Piezo2 were identified as components of mechanically activated (MA) channels in mammals10. Piezos represent an evolutionary conserved family of transmembrane proteins. It is unknown whether Piezos function in mechanical sensing in vivo, and if they do, which mechanosensory modalities they mediate. Here, we study the physiological role of the single Piezo member in D. melanogaster (dpiezo). dpiezo expression in human cells induces mechanically activated currents, similar to its mammalian counterparts [Coste et al., accompanying paper11]. Behavioral responses to noxious mechanical stimuli were severely reduced in dpiezo knockout larvae, while responses to another noxious stimulus or touch were not affected. Knocking down dpiezo in sensory neurons that mediate nociception and express the DEG/ENaC ion channel pickpocket (ppk) was sufficient to impair responses to noxious mechanical stimuli. Furthermore, expression of dpiezo in these same neurons rescued the phenotype of the constitutive dpiezo knockout larvae. Accordingly, electrophysiological recordings from ppk-positive neurons revealed a dpiezo dependent, mechanically-activated current. Finally, we found that dpiezo and ppk function in parallel pathways in ppk-positive cells, and that mechanical nociception is abolished in the absence of both channels. These data demonstrate physiological relevance of Piezo family in mechanotransduction in vivo, supporting a role of Piezo proteins in mechanosensory nociception.
D. melanogaster is widely used to study mechanotransduction and genetic studies have identified several ion channels that are essential for mechanosensation5,9,12–14. However, none of these proteins are shown to be activated by mechanical force when expressed in heterologous systems. Since expression of mouse Piezos in a variety of mammalian cells induces large mechanically activated currents10, we set out to test if the fly counterpart is also sufficient to induce mechanosensitivity. Similar to its mammalian counterparts, the Drosophila piezo gene (CG8486) is predicted to consist of a large number of transmembrane domains (39, Supplementary Fig. 1). Albeit fly and mammalian piezos do not exhibit extensive sequence conservation (24% identity), expression of Drosophila piezo in cultured human cells induced large mechanically activated cationic currents, suggesting a role of dpiezo in mechanotransduction11.
To characterize dpiezo expression in flies we used a fusion between the dpiezo enhancer/promoter region and GAL4 (dPiezoP-GAL4). Four independent dPiezoP-GAL4 transgenic insertions were examined to avoid insertional effects on GAL4 expression. We used UAS-GFP for labeling cells except for arborized neurons that were optimally visualized using the membrane-targeted UAS-CD8::GFP. We found fluorescent labeling induced by dpiezo enhancer/promoter region in all types of sensory neurons and several non-neuronal tissues in both adults and larvae (Supplementary Fig. 2). This diverse pattern of dpiezo expression observed in Drosophila is in accord with the expressionof Piezo1 and Piezo2 in mice10.
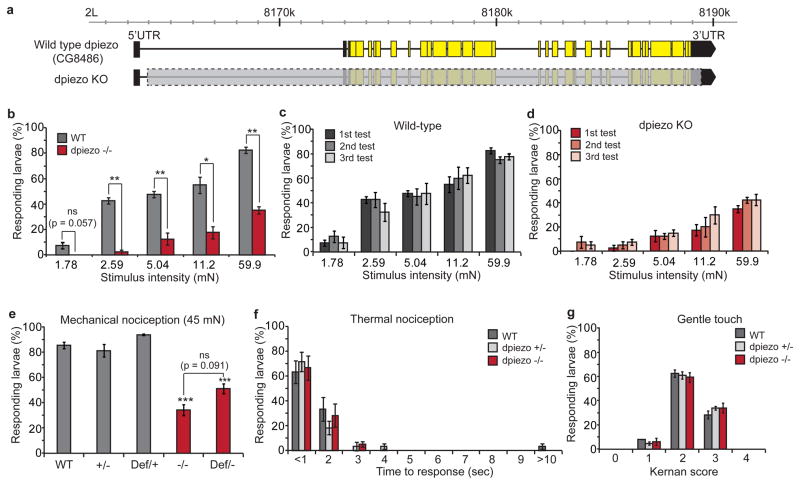
We created dpiezo knockout (KO) flies in which all 31 coding exons were deleted using genomic FLP-FRT recombination15 (Fig. 1a, see Supplementary Fig. 3 for details). The knockout flies were viable, fertile and did not show uncoordination or a defect in bristle mechanoreceptor potential (Supplementary Fig. 4). We studied whether dpiezo KO larvae have mechanical nociception deficits by using a mechanically-induced escape behavior assay9,14,16. Stimulation with von Frey filaments that ranged from 2 to 60 mN demonstrated that dpiezo KO larvae have a severe response deficit over a wide range (Fig. 1b). Repeated stimulations of the same larvae resulted in comparable responsiveness in both wild type and dpiezo KO, indicating that the stimuli did not induce considerable damage to the sensory system (Fig. 1c, d). A 153 ± 11.0 mN filament elicited responses only to the first of three stimulations in wild type larvae, arguing that this amount of force is damaging (not shown). For further experiments, we chose to stimulate the larvae using a 45 mN filament which has been used in a previous study14, and elicits a substantial response in both wild type and dpiezo mutant larvae. 34 ± 4.4 % of dpiezo KO larvae showed a response to 45 mN filament stimulation, compared to over 80 % of wild type or heterozygote larvae (Fig. 1e). As a control for the genetic background, we used larvae that carry the dpiezo KO allele on one chromosome and a deficiency in which the entire dpiezo genomic region is deleted on the homologous chromosome. The defect in the trans-heterozygous larvae was similar to the KO homozygote phenotype (51 ± 3.9 %, p = 0.091). In contrast, dpiezo KO larvae were indistinguishable from wild type in an assay for responses to high temperature, a different noxious stimulus that elicits the same escape response14 (Fig. 1f). Therefore, dpiezo KO larvae retain a normal ability to elicit the escape behavior in response to noxious stimuli, while dpiezo is specifically required for the mechanical modality of nociception. To evaluate the possible role of dpiezo in other modes of larval mechanical sensing, we tested the sensitivity of dpiezo KO to gentle touch which is mediated through ciliated neurons17,18. We observed no defect in the sensitivity of dpiezo KO larvae to innocuous gentle touch (Fig. 1g).
Figure 1. Mechanical nociception defect in dpiezo knockout larvae.
(a) Genomic map showing wild type dpiezo gene (upper) and engineered dpiezo KO (lower). Yellow and black boxes represent coding and non-coding exons, respectively. Deleted genomic segment in dpiezo KO is marked with a gray box. (b) Mechanical nociception assay using a range of stimulus forces in wild type and dpiezo KO larvae. n = 40 from four independent experiments. * p < 0.05, ** p < 0.01 from two tail paired Student t-test. (c, d) Mechanical nociception assay using repeated stimuli of the same larvae. n = 40. (e) Mechanical nociception assay using a 45 mN von Frey filament with wild type (+/+), heterozygous KO (+/−), heterozygous deficiency (Def/+), homozygous KO (−/−) and trans-heterozygous KO (Def/−). n > 85. *** p < 0.001. (f) Thermal nociception assay using heated probe (45 °C). n = 60. (g) Gentle touch assay17. n > 150.
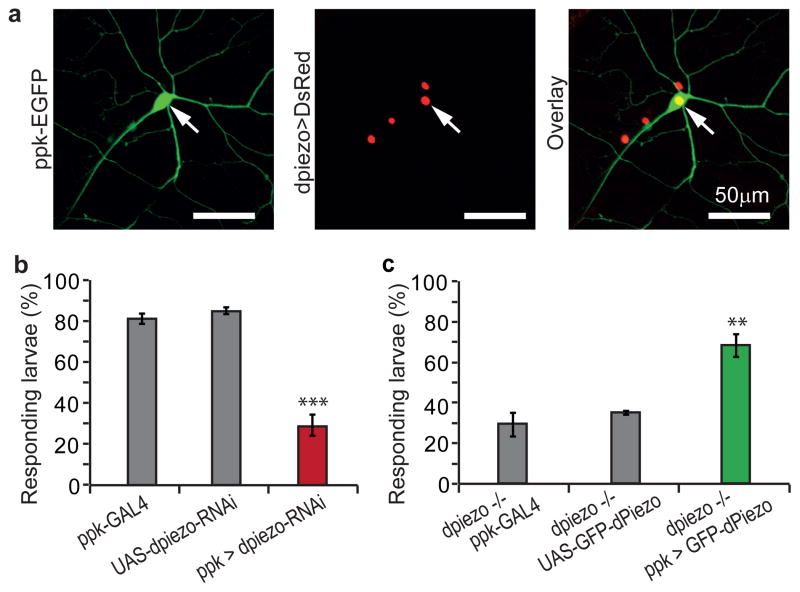
A mechanical nociception phenotype was previously observed in pickpocket (ppk), a DEG/ENaC channel9, and painless (pain), a TRPA ion channel14. The specificity of dpiezo KO to mechanical nociception resembles the phenotype of ppk since pain is also essential for sensing thermal nociception14. We therefore tested the role of dpiezo in ppk-positive cells using ppk-GAL4, which labels subclasses of multidendritic (MD) neurons19,20. The MD neurons are non-ciliated receptor cells that tile the body wall of the larvae and respond to a variety of external stimuli such as mechanical forces, temperature and light9,14,16,21. We used a green fluorescent protein driven directly by the regulatory regions of the ppk gene (ppk-EGFP)22 together with DsRed expression in dpiezo-positive cells to probe dpiezo and ppk co-expression. Indeed we did observe that all ppk-positive cells also expressed dpiezo (Fig. 2a). Next we used ppk-GAL4 to drive the expression of dpiezo RNAi to test whether dpiezo function is specifically required in ppk-expressing cells. The restricted knockdown of dpiezo resulted in a mechanical nociceptive phenotype (Fig. 2b) similar to phenotype observed in dpiezo KO larvae (Fig. 1e). In a complementary approach, we used ppk-GAL4 driven expression of dpiezo cDNA in an attempt to rescue the mechanical nociception phenotype of dpiezo KO. We used a fusion between dPiezo and GFP to monitor expression levels in ppk cells and dPiezo localization within the neurons. GFP-dPiezo fusion protein induces MA currents in human cell lines, similar to untagged dPiezo, confirming functionality (Supplementary Fig. 5a–c). When expressed in the fly, GFP-dPiezo fluorescence was present throughout cell bodies, axons and dendritic arbors of ppk-positive neurons (Supplementary Fig. 5d). Importantly, expression of GFP-dPiezo in ppk-positive neurons alone was sufficient to rescue the mechanical nociception defect of dpiezo KO (Fig. 2c). These data suggests that dpiezo functions in ppk-positive neurons to mediate mechanical nociception.
Figure 2. dpiezo functions in ppk-positive type II sensory neurons.
(a) Double fluorescence labeling using ppk-EGFP (green) and dPiezoP-GAL4 that drives the expression of the nucleus targeted UAS-DsRed-NLS (red). A representative high-magnification image shows one ppk-positive neuron (arrow). All three ppk-positive cells in each hemisegment expressed dpiezo in all segments. (b) Mechanical nociception assay with dpiezo knockdown larvae in ppk-expressing cells by ppk-GAL4 and UAS-dpiezo-RNAi. n > 85, *** p < 0.001. (c) Mechanical nociception assay in rescued dpiezo KO. GFP-dPiezo was expressed in ppk-cells using ppk-GAL4 and UAS-GFP-dPiezo. n > 60. ** p < 0.01.
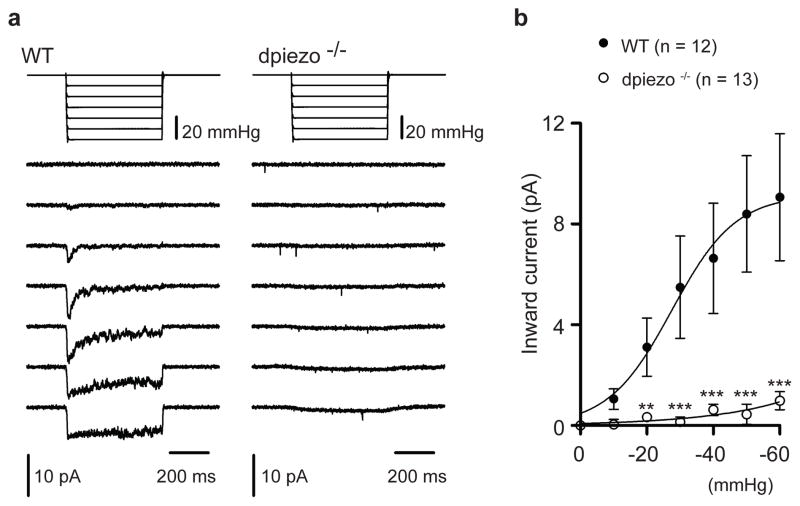
To test if the ppk-positive neurons respond to mechanical stimuli and if dpiezo mediates such responses we performed electrophysiological recordings from isolated cells. Larvae that had GFP labeling in ppk-positive neurons were dissociated using enzymatic digestion and mechanical trituration. Plated fluorescent neurons were then tested using patch clamp recordings in the cell-attached configuration and they were stimulated using a negative pressure through the recording pipette10. Stimulating wild type neurons resulted in a current that was rapidly activated and had a half-maximal activation (P50) of 27.6 ± 7.6 mmHg (Fig. 3). These currents were not observed in the dpiezo KO mutant neurons (Fig. 3). Therefore, ppk-positive neurons which mediate the avoidance response to noxious stimuli display dpiezo-dependent mechanically activated currents.
Figure 3. dpiezo mediates mechanically-activated currents in ppk-positive neurons.
(a) Representative currents elicited by negative pipette pressure (0 to −60 mmHg, Δ 10 mmHg) in cell-attached configuration at −80mV in wild type (left) and dpiezo−/− (right). (b) Average peak current-to pressure relationship of stretch activated currents in wild type (n = 12 cells) and dpiezo−/− (n =13 cells). Data points are mean ± SEM. fitted with a Boltzmann equation. ** P < 0.01, *** P < 0.001, Mann-Whitney test.
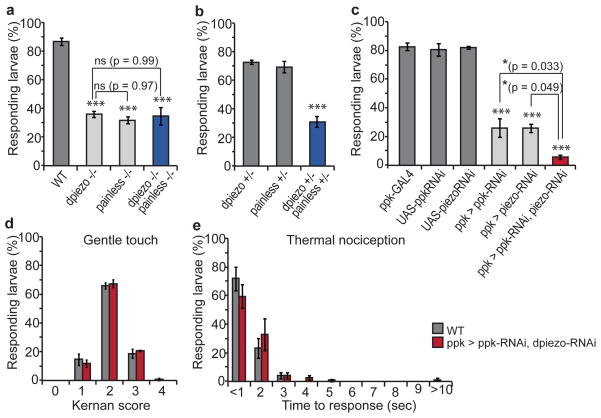
Silencing of ppk cells resulted in a complete abolishment of noxious mechanosensation (Supplementary Fig. 6), in accord with a severe defect observed previously16. In contrast, only a moderate deficit is observed upon eliminating or knocking down ppk in the same cells9, suggesting that there are multiple pathways for mechanical sensing. We tested mechanical nociception in larvae that are deficient in dpiezo and either pain or ppk to gain insight into cellular pathways that involve mechanotransduction in these cells. Once again, we used a 45 mN filament, enabling us to monitor both dpiezo-dependent and -independent mechanisms (Fig. 1b). The dpiezo::pain double mutant had a defect that was comparable to each one of the mutants separately, suggesting that dpiezo and pain might function in the same pathway (Fig. 4a). Larvae that are heterozygous for both dpiezo and pain showed a response deficit while each one of them separately was normal (Fig 4b), further demonstrating their role in a common signaling mechanism. Remarkably, combining both dpiezo and ppk knockdowns resulted in a nearly complete abolishment of responses to noxious mechanical stimuli (Fig. 4c). Importantly, responses to noxious temperatures and touch were normal in larvae with both dpiezo and ppk knocked-down (Fig. 4d, e). These data suggest that dpiezo and ppk function in two parallel pathways in ppk-positive sensory neurons, and that together they constitute the response to noxious mechanical stimuli. There could be many reasons why the mechanically activated currents we observe are entirely dependent on dPiezo (Fig. 3). This can either be because PPK responds to a different modality of mechanical stimulus or due to the specific experimental settings (e.g. level of applied forces, solutions, applied voltage). Future experiments should resolve this issue.
Figure 4. dpiezo and ppk function in parallel pathways.
(a) Mechanical nociception assay using a 45 mN von Frey filament with double null mutant of dpiezo and painless. Single knockout strains were used as controls and the wild type strain is w1118. n > 60. (b) Mechanical nociception assay on heterozygous larvae for dpiezo and/or pain. n (heterozygote dpiezo KO) = 74 from three trials, n (heterozygote painless1) = 169 from five trials, n (trans-heterozygote) = 166 from five trials. (c) Mechanical nociception assay with PPK and Piezo knockdown. ppk and/or dpiezo RNAi were driven by ppk-GAL4. n > 90. * p < 0.5, *** p < 0.001. n (wild type, w1118) = 66, n (UAS-ShiDN/+) = 75 and n (ppk-GAL4/+; UAS-ShiDN/+) = 73 with three trials. (d) Gentle touch sensitivity assay with ppk and dpiezo knockdown. Wild type is w1118. n > 90. (e) Thermal nociception assay using 45 °C probe with ppk and dpiezo knockdown. n > 75.
Using the Drosophila model system we have demonstrated that piezo is essential for sensing noxious mechanical stimulus in vivo. This is the first demonstration that a Piezo family member is essential for mechanotransduction in the whole animal. Indeed, dpiezo is to our knowledge the first eukaryotic excitatory channel component shown to be activated by mechanical force in a heterologous expression system and required for sensory mechanotransduction in vivo. Piezo2 is expressed in mouse DRG neurons that are involved in sensing nociception, and is required for rapidly-adapting mechanically activated currents in such isolated neurons10. This study raises the possibility that mammalian Piezo2 is also required for mechanical pain transduction in vivo. Furthermore, Drosophila genetics can now be utilized to map cellular pathways involved in piezo-dependent mechanotransduction in sensory neurons and beyond.
METHOD SUMMARY
Fly stocks
PiggyBacs (PBac{WH}CG8486-f02291, PBac{RB}CG8486-e00109, Exelixis Collection at the Harvard Medical School), ppk-GAL4 (Bloomington Drosophila Stock Center (BDSC), 32078, 32079), Deficiency (Df(2L)Exel7034/CyO, BDSC, 7807), UAS-dpiezo-RNAi (National Institute of Genetics, Japan, 8486R-3), UAS-ppk-RNAi (Vienna Drosophila RNAi Center, 108683), ppk-EGFP522 (Jan Y.N.), painless1 (BDSC, 27895).
Generating dpiezo KO
The dpiezo knockout fly was generated by FLP-FRT recombination with two PiggyBac lines as described in Parks et al15. The recombined KO fly was confirmed by PCR(Supplementary Fig. 2). The genetic background was cleaned using meiotic recombination with w1118.
Imaging
Fluorescence in adult fly or larva was detected by Nikon C2 Confocal Laser Point Scanning Microscope, Olympus FluoView500 Confocal Microscope or Olympus AX70 microscope.
Cloning
To clone enhancer/promoter of dpiezo gene, the genomic region between 1.0 kb upstream of 5′UTR and the start codon of dpiezo was amplified by PCR and cloned into pPTGAL vector. GFP-dPiezo construct has three Alanines as a linker between N-terminal GFP and C-terminal dPiezo. The construct was cloned in modified pUAST vector to generate transgenic flies and in modified pIRES2-EGFP vector for electrophysiology recordings.
Behavioral assays and statistics
The mechanical nociception was tested as described previously9,14,16 using calibrated von Frey filaments. The thermal nociception was tested as described previously14 using a 45 °C heated metal probe. All error bars represent mean ± SEM.
Isolation of ppk-positive neurons
Third instar larvae that had GFP labeling in ppk-positive neurons were dissected, digested with collagenase and mechanically triturated. The cells were collected by centrifugation and plated on a poly-D-lysine coated glass coverslip. The fluorescent ppk-positive cells were recorded after incubating two hours at room temperature.
Electrophysiology
HEK cells were studied in the whole cell configuration using a polished glass probe for stimulation10 and ppk-positive neurons were stimulated using a negative pressure in the cell attached configuration10.
METHODS
Fly stocks
We used the following stocks: PiggyBacs (PBac{WH}CG8486-f02291, PBac{RB}CG8486-e00109, Exelixis Collection at the Harvard Medical School), ppk-GAL4 (Bloomington Drosophila Stock Center (BDSC), 32078, 32079), Deficiency (Df(2L)Exel7034/CyO, BDSC, 7807), UAS-dpiezo-RNAi (National Institute of Genetics, Japan, 8486R-3), UAS-ppk-RNAi (Vienna Drosophila RNAi Center, 108683), ppk-EGFP522 (Jan Y.N.), painless1 (BDSC, 27895) and UAS-DsRed-NLS (Posakony J.W.). The following stocks were from BDSC: UAS-GFP, UAS-CD8GFP, CyO-GFP, w1118 and Canton-S.
Engineering dpiezo knockout
The dpiezo knockout fly was generated as described in Parks et al15. Two PiggyBac lines which carry FRT sequence were selected for FLP-FRT recombination. PBac{WH}CG8486-f02291 is inserted in the first intron and PBac{RB}CG8486-e00109 in the 3′ UTR of dpiezo gene. After FLP-FRT recombination, 20kb of dpiezo gene, including all 31 coding exons was removed and replaced with 7kb of PiggyBac insertion which contains FRT sequence and white gene. The recombined KO fly was confirmed by PCR reactions (Supplementary Fig. 2). The genetic background was cleaned using meiotic recombination with w1118.
Molecular biology
dpiezo promoter cloning
To clone enhancer/promoter of dpiezo gene, the genomic region between 1.0 kb upstream the beginning of the 5′UTR and the start of the dpiezo coding region was amplified by PCR using forward primer, 5′-atctggcggccgctatctattttttaactagtggaagtct-3′ and reverse primer, 5′-ttactggtaccatggatgcctccggcgccgttctcctccag-3′. The amplified sequence was cloned into pPTGAL vector (Drosophila Genomic Resource Center, 1225) using NotI and KpnI sites and the sequence was verified.
GFP fusion dPiezo (GFP-dPiezo) cloning
For rescue experiments, dpiezo cDNA was amplified from the plasmid reported in Coste et. al. using forward primer 5′-tattagcggccgcagtcttcagctatgcgtgcatggtg-3′ and reverse primer 5′-taattcggtccgttattgcggttgctgtggctgcagttgctccgg -3′ and cloned into a modified pUAST vector using NotI and RsrII. NotI restriction enzyme site was used as a linker by providing three Alanine residues between EGFP and dPiezo. The order of sequences in pUAST vector is as following: UAS-kozak-EGFP-3x(Ala)-dPiezo. To generate transgenic flies, DNA was injected into the isogenized w1118 embryos with Δ2–3. For electrophysiology experiment, EGFP-dPiezo was cloned into mammalian expression vector with CMV promoter.
Behavior assays
The mechanical nociception was tested as described previously9,14,16 using calibrated von Frey filaments. The thermal nociception was tested as described previously14 using calibrated heated metal probe. For both nociception assays, the number of larvae which showed at least one 360 ° rotation was counted for each trial. The gentle touch assay was made as described previously17. Each stimulated larva was scored according to Kernan et al.; 0 = no response, 1 = hesitates, 2 = turns or withdraws anterior, 3 = single reverse contractile wave and 4 = multiple waves. For all behavior assays each third instar larva was stimulated only once. All data were generated from at least three trials.
Calibration of von Frey stimulator
The von Frey filaments for larvae behavior experiments were modified from Touch-Test sensory Evaluator (North Coast Medical) or from monofilament fishing lines. Each monofilament was cut to a length of 18 mm, glued into a pipette tip so that 9 mm of it protruded and mounted on a hand manipulator with a 90° angle. Each von Frey filaments was calibrated as described previously9. The force of each von Frey stimulator was determined by measuring the weight upon filament bending and converting the value into the force: force (mN) = mass (gram) × gravity acceleration constant, g (9.8). Each stimulator was calibrated 15 times and its mean value was used in figures. The calibrated forces (mean ± SEM) of each stimulator are the following (in mN): 1.78 ± 0.15, 2.59 ± 0.15, 5.04 ± 0.19, 11.2 ± 0.66 and 59.9 ± 1.79.
Fluorescence imaging
For identifying tissues or cells expressing fluorescence by dpiezo promoter, both adult flies and third instar larvae, carrying dPiezoP-GAL4 and UAS-GFP or UAS-CD8::GFP were dissected or whole-mounted. For double fluorescent labeling in md neurons, second instar larvae carrying ppk-EGFP, dPiezoP-GAL4 and UAS-DsRed were whole-mounted. For imaging ppk-cells expressing GFP-dPiezo, third instar larvae carrying ppk-GAL4 and UAS-GFP-dPiezo were whole-mounted. Fluorescence images were obtained either by Nikon C2 Confocal Laser Point Scanning Microscope, Olympus FluoView500 Confocal Microscope or Olympus AX70 microscope.
Isolation of larvae ppk-positive neurons
In both wild type and dpiezo KO larvae, ppk-positive neurons were fluorescently labeled by ppk-EGFP which is a direct fusion of ppk genomic regulatory regions with EGFP. Third instar larvae were dissected in M3 media containing 10% heat inactivated FBS. Each larva was cut twice and its internal organs were removed. The cleaned body wall was treated with 5 mg/ml Collagenase type IV at 25 °C for 1 hour in serum free M3 media and washed with serum containing M3 media. The enzyme treated body wall was triturated with fire-polished Pasteur pipettes in M3 media with 2mM EGTA and 10% FBS. The cuticle and debris were removed by centrifugation at 40 g and the small size cells, including neurons were collected by centrifugation at 360 g for 10min. The cell pellet was resuspended with serum co00ntaining M3 media and plated into a poly-D-lysine coated coverslip in a small droplet. After two hours of incubation at room temperature, the coverslips were transferred to the electrophysiology rig for recording.
Electrophysiology
For whole-cell recordings in HEK293T cells, patch pipettes had resistance of 2–3 MΩ when filled with an internal solution consisting of (in mM) 133 CsCl, 10 HEPES, 5 EGTA, 1 CaCl2, 1 MgCl2, 4 MgATP, and 0.4 Na2GTP (pH adjusted to 7.3 with CsOH). The extracellular solution consisted of (in mM) 130 NaCl, 3 KCl, 1 MgCl2, 10 HEPES, 2.5 CaCl2, 10 glucose (pH adjusted to 7.3 with NaOH). Mechanical stimulation was achieved using a fire-polished glass pipette (tip diameter 3–4 μm). The probe had a velocity of 1 μm/ms during the ramp segment of the command for forward motion and the stimulus was applied for 150 ms.
For cell-attached recordings in ppk-positive dissociated neurons, patch pipette had resistance of 3–3.5 MΩ when filled with a solution consisting of (in mM) 130 NaCl, 5 KCl, 10 HEPES, 1 CaCl2, 1 MgCl2, 10 TEA-Cl (pH 7.3 with NaOH). External solution used to zero the membrane potential consisted of (in mM) 140 KCl, 10 HEPES, 1 MgCl2, 10 glucose (pH 7.3 with KOH). Membrane patches were stimulated with brief negative pressure pulses through the recording electrode using a Clampex controlled pressure clamp HSPC-1 device (ALA-scientific). Stretch-activated channels were recorded at a holding potential of −80 mV with pressure steps from 0 to −60 mm Hg (−10 mm Hg increments). Current-pressure relationships were fitted with a Boltzmann equation of the form: I(P) = [1 + exp (−(P – P50)/s)]−1, where I is the peak of stretch-activated current at a given pressure, P is the applied patch pressure (in mm Hg), P50 is the pressure value that evoked a current value which is 50% of Imax, and s reflects the current sensitivity to pressure.
All experiments were done at room temperature. Currents were sampled at 50 or 20 kHz and filtered at 5 or 2 kHz. Voltages were not corrected for a liquid junction potential. Leak currents before mechanical stimulations were subtracted off-line from the current traces.
Supplementary Material
Acknowledgments
We thank Dr. Y.N. Jan of University of California San Francisco for providing ppk-EGFP5. Research was support by the National Institutes of Health and Novartis Research Foundation. S.E.K. and A.C. are supported by Skaggs Institute.
Footnotes
AUTHOR CONTRIBUTIONS
S.E.K. conducted most experiments. B.C. (Coste) performed electrophysiology experiments shown in Fig. 3 and Suplementary Fig 5. A.C. performed fly electrophysiology experiments shown in Supplementary Fig. 4. S.E.K., A.P. and B.C. designed experiments and wrote the manuscript.
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
References
- 1.Chalfie M. Neurosensory mechanotransduction. Nat Rev Mol Cell Biol. 2009;10:44–52. doi: 10.1038/nrm2595. [DOI] [PubMed] [Google Scholar]
- 2.Tsunozaki M, Bautista DM. Mammalian somatosensory mechanotransduction. Curr Opin Neurobiol. 2009;19:362–369. doi: 10.1016/j.conb.2009.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gillespie PG, Muller U. Mechanotransduction by hair cells: models, molecules, and mechanisms. Cell. 2009;139:33–44. doi: 10.1016/j.cell.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Delmas P, Hao J, Rodat-Despoix L. Molecular mechanisms of mechanotransduction in mammalian sensory neurons. Nat Rev Neurosci. 2011;12:139–153. doi: 10.1038/nrn2993. [DOI] [PubMed] [Google Scholar]
- 5.Walker RG, Willingham AT, Zuker CS. A Drosophila Mechanosensory Transduction Channel. Science. 2000;287:2229–2234. doi: 10.1126/science.287.5461.2229. [DOI] [PubMed] [Google Scholar]
- 6.Li W, Feng Z, Sternberg PW, Xu XZSA. C. elegans stretch receptor neuron revealed by a mechanosensitive TRP channel homologue. Nature. 2006;440:684–687. doi: 10.1038/nature04538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kang L, Gao J, Schafer WR, Xie Z, Xu XZS. C elegans TRP family protein TRP-4 is a pore-forming subunit of a native mechanotransduction channel. Neuron. 2010;67:381–391. doi: 10.1016/j.neuron.2010.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Hagan R, Chalfie M, Goodman MB. The MEC-4 DEG/ENaC channel of Caenorhabditis elegans touch receptor neurons transduces mechanical signals. Nat Neurosci. 2005;8:43–50. doi: 10.1038/nn1362. [DOI] [PubMed] [Google Scholar]
- 9.Zhong L, Hwang RY, Tracey WD. Pickpocket is a DEG/ENaC protein required for mechanical nociception in Drosophila larvae. Curr Biol. 2010;20:429–434. doi: 10.1016/j.cub.2009.12.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coste B, et al. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science. 2010;330:55–60. doi: 10.1126/science.1193270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coste B, et al. Piezos are pore-forming subunits of mechanically activated channels. accompanying paper. doi: 10.1038/nature10812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim J, et al. A TRPV family ion channel required for hearing in Drosophila. Nature. 2003;424:81–84. doi: 10.1038/nature01733. [DOI] [PubMed] [Google Scholar]
- 13.Gong Z, et al. Two interdependent TRPV channel subunits, inactive and Nanchung, mediate hearing in Drosophila. J Neurosci. 2004;24:9059–9066. doi: 10.1523/JNEUROSCI.1645-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tracey WD, Jr, Wilson RI, Laurent G, Benzer S. painless, a Drosophila Gene Essential for Nociception. Cell. 2003;113:261–273. doi: 10.1016/s0092-8674(03)00272-1. [DOI] [PubMed] [Google Scholar]
- 15.Parks AL, et al. Systematic generation of high-resolution deletion coverage of the Drosophila melanogaster genome. Nat Genet. 2004;36:288–292. doi: 10.1038/ng1312. [DOI] [PubMed] [Google Scholar]
- 16.Hwang RY, et al. Nociceptive neurons protect Drosophila larvae from parasitoid wasps. Curr Biol. 2007;17:2105–2116. doi: 10.1016/j.cub.2007.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kernan M, Cowan D, Zuker C. Genetic dissection of mechanosensory transduction: mechanoreception-defective mutations of Drosophila. Neuron. 1994;12:1195–1206. doi: 10.1016/0896-6273(94)90437-5. [DOI] [PubMed] [Google Scholar]
- 18.Caldwell JC, Miller MM, Wing S, Soll DR, Eberl DF. Dynamic analysis of larval locomotion in Drosophila chordotonal organ mutants. Proc Natl Acad Sci USA. 2003;100:16053–16058. doi: 10.1073/pnas.2535546100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adams CM, et al. Ripped pocket and pickpocket, novel Drosophila DEG/ENaC subunits expressed in early development and in mechanosensory neurons. J Cell Biol. 1998;140:143–152. doi: 10.1083/jcb.140.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ainsley JA, et al. Enhanced locomotion caused by loss of the Drosophila DEG/ENaC protein Pickpocket1. Curr Biol. 2003;13:1557–1563. doi: 10.1016/s0960-9822(03)00596-7. [DOI] [PubMed] [Google Scholar]
- 21.Xiang Y, et al. Light-avoidance-mediating photoreceptors tile the Drosophila larval body wall. Nature. 2010;468:921–926. doi: 10.1038/nature09576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grueber WB, Ye B, Moore AW, Jan LY, Jan YN. Dendrites of distinct classes of Drosophila sensory neurons show different capacities for homotypic repulsion. Curr Biol. 2003;13:618–626. doi: 10.1016/s0960-9822(03)00207-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.