Abstract
Background
Infliximab is used increasingly as maintenance therapy for inflammatory bowel disease (IBD); however the effects of a single maintenance dose of infliximab are unclear with respect to quality of life and hormones related to growth and puberty.
Objective
Determine the time course of inflammatory, hormonal and quality-of-life changes following a single dose of infliximab in the context of on-going therapy, as related to presence of IBD symptoms at time of administration.
Methods
Children and adolescents with IBD receiving on-going therapy with infliximab for clinical indications were recruited. Pediatric Crohn’s Disease Activity Index (PCDAI) was determined at baseline and laboratory measures of hsCRP and hormones of growth and puberty were determined on Days 0, 2 and 14. IBD-related quality of life (IMPACT-III questionnaire) was tested on Days 0 and 14. Subjects who had symptoms of IBD were compared to asymptomatic subjects.
Results
Subjects overall and in the symptomatic group exhibited improved hsCRP by Day 2 following treatment. Symptomatic subjects had higher PCDAI scores and lower quality-of-life scores than asymptomatic subjects on Day 0, while at Day 14 there were no significant differences in quality-of-life scores between the two groups.
Conclusions
Even in the context of on-going treatment, a single dose of infliximab results in decreased hsCRP an improvement that is particularly noted among subjects who are symptomatic at the time of treatment. While randomized trials are needed, these observational data may assist clinicians, patients and families regarding expectations about timing and extent of these changes following a single treatment dose.
Keywords: Inflammatory bowel disease, infliximab, inflammation, quality of life, adolescent
Introduction
Infliximab has become a commonly-used treatment to induce remission among children and adolescents with inflammatory bowel disease (IBD)(1, 2). As a monoclonal antibody that binds TNF-α, infliximab decreases systemic inflammation (1, 3, 4) and lessens disease severity (3, 5, 6). Long-term use of infliximab in children and adolescents results in improved growth velocity (3, 7) and improved quality of life as assessed by IMPACT III, a questionnaire assessing disease-related quality-of-life in multiple domains of care in IBD (5, 8).
Because of its effectiveness in inducing disease remission, infliximab is used increasingly as a maintenance medication for the treatment of pediatric IBD (2, 5). In a recent cohort study, 79% of patients who received infliximab were continued on maintenance therapy thereafter (9). Whereas early studies documented dramatic anti-inflammatory and quality-of-life effects as measured from the time of first treatment (1, 3, 6), the systemic, hormonal and quality-of-life effects of a single dose of infliximab during continued therapy are unclear.
Our goal was to determine the timing and magnitude of anti-inflammatory, hormonal and quality-of-life changes following a single infliximab infusion among a group of subjects in on-going treatment with infliximab, comparing changes seen in those with symptoms (“Symptomatic” group) vs. without symptoms (“Asymptomatic” group) at the time of treatment. We evaluated children and adolescents with IBD for markers of systemic inflammation, hormones related to growth and puberty, and measures of appetite and IBD-related quality of life. We performed these measures just prior to a clinically-indicated treatment (either regularly-scheduled or urgently-scheduled treatment), as well as 2 days and 2 weeks after treatment, with a hypothesis that greater changes would be observed among subjects experiencing symptoms compared to those without symptoms.
Methods
This study was approved by the Institutional Review Board of the University of Virginia. Patients seen in the pediatric gastroenterology clinic at the University of Virginia who were scheduled to have an infusion of infliximab for clinical indications were offered a chance to participate. Interested subjects and at least one parent were required to sign informed consent and assent prior to participation. Inclusion criteria were diagnosed Crohn’s disease or ulcerative colitis, age 6–22 y.o. with medically-indicated need for infliximab and a history of prior infliximab use. Exclusion criteria were prior treatment with infliximab <3 weeks prior to study entry and change in use of other anti-inflammatory medications from 3 days prior to study enrollment to 2 weeks after infliximab treatment. Blood was drawn at the time of IV placement prior to infusion (with the exception of two subjects, whose blood was drawn immediately following infusion, and who were not included in TNF-α calculations). Blood for clinically-indicated laboratory tests (including those needed for pediatric Crohn’s Disease Activity Index (PCDAI)(10) determination) were delivered to the central laboratory, while blood samples for additional tests were brought to the GCRC for centrifugation and storage at −80 degrees. During or just following pre-treatment (diphenhydramine and acetaminophen) and infliximab infusions, subjects answered questions regarding clinical characteristics including presence of diarrhea, abdominal pain or blood in stool over the 4 days prior to the clinic visit. Subjects who had presence of these symptoms in the 4 days prior to clinic visit were classified as being in the “symptomatic” group and those without were classified as being in the “asymptomatic” group. During the infusion subjects filled out the IMPACT-III survey (5, 8). IMPACT III was used by permission (Dr. Anthony Otley, Dalhousie University, Halifax, Nova Scotia, Canada) and scored according to specifications of its creators. Scores for overall IMPACT III (with a range from 35 to 175) and individual domains were then converted to a percent possible, with higher scores associated with better quality of life. We hypothesized that in addition to improved quality of life subjects would experience an increase in appetite following treatment with infliximab. Thus, during the infusion subjects filled out a validated appetite visual-analogue scale (“appetite VAS”). For this measure subjects placed an “X” on individual 100 mm scales to gauge their current degree of appetite, hunger, fullness, intake capacity, nausea and thirst (11). Additional clinical information including original diagnosis of IBD and current height, weight, and disease location was drawn from the chart. PCDAI scores were calculated only for subjects with Crohn’s disease.
On Day 2 following infliximab infusion, subjects had a subsequent blood draw and filled out appetite VAS; on Day 14, subjects had a final blood draw and completed IMPACT questionnaires and the appetite VAS. Following each blood draw, blood was allowed to clot and was spun down before being stored at 4 degrees a maximum of 48 hours prior to being frozen at −80 degrees until time of testing.
Measures of erythrocyte sedimentation rate, albumin and hematorcrit were performed at the University of Virginia Health System central laboratory using standard procedures. Additional measures were batched and performed in the GCRC laboratory at the University of Virginia, using a chemiluminescent enzyme immunoassay (DPC Immulite 2000, Siemens Healthcare Diagnositcs, Inc, Deerfield, IL) to measure serum levels of high sensitivity C-reactive protein (hsCRP), high sensitivity IL-6, high-sensitivity TNF-α, IGF-1, TSH, LH, FSH, estradiol and testosterone. To facilitate inter-group comparison, IGF-1 measurements were converted to age-based z-scores (Esoterix Laboratory Services, Inc, Austin TX). Measures of LH, FSH, estradiol and testosterone were only analyzed for subjects Tanner 3 or more. Given low sample size, we had 80% power to detect changes in the overall group between Day 0 and Days 2 and 14 as low as 2.47 mg/L for hsCRP, 0.93 for hsIL-6 mg/L, 0.90 (change in z-score) for IGF-1, 0.71 uIU/mL for TSH, 0.089 ng/dL for free T4, 1.90 uIU/mL for LH, 1.26 uIU/mL for FSH, 51.6 pg/mL for estradiol and 94.9 ng/mL for testosterone, based on the variance at baseline.
Statistics
Inter-group comparisons between symptomatic and asymptomatic subjects were performed using t-tests. Intra-group comparisons over time were assessed by paired t-tests. All statistical comparisons were performed using Prizm (Graphpad Software, La Jolla, CA). Significance was considered at p<0.05.
Results
Subject characteristics
We recruited a total of 24 subjects, 14 of whom were classified as being in the “symptomatic” group and 10 in the “asymptomatic” group. Subject characteristics by group are shown in the online-only Supplementary Table 1 (http://links.lww.com/MPG/A69). There were no differences in baseline characteristics between the symptomatic and asymptomatic groups, including additional treatment medications, time since diagnosis or time since last infliximab infusion.
Inflammation and disease status
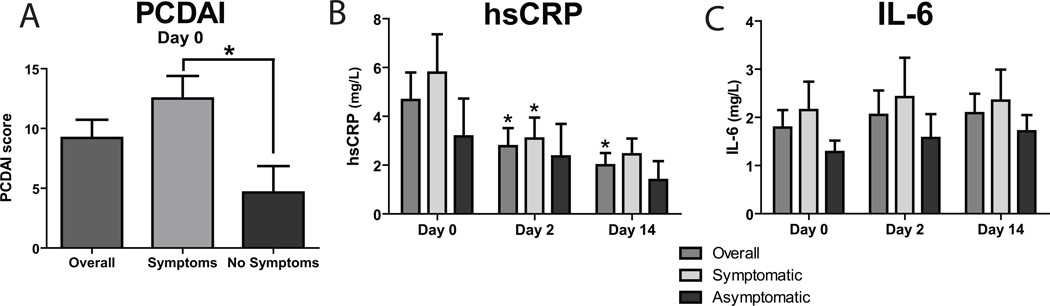
Subjects in the symptomatic group had higher PCDAI scores than the asymptomatic group (Figure 1A). There was a non-significant trend in correlation between PCDAI scores and time-since-last-infliximab for the entire cohort (R2=0.15, p=0.076). There were no differences between groups in hsCRP values at any time point (Figure 1B). Overall subjects exhibited a decrease in hsCRP by Day 2 and Day 14. Subjects in the symptomatic group but not in the asymptomatic group had a decrease in hsCRP at the Day 2 but not the Day 14 time point (Figure 1B). There were not significant differences in IL-6 between groups or significant improvements over time (Figure 1C). Similarly, there was no difference in baseline levels of TNF-α between groups (data not shown). When subjects were analyzed by whether they were on combination therapy with anti-metabolites (irrespective of symptomatic status), this did not yield a significant difference in baseline levels of PCDAI, hsCRP or other markers of inflammation (data not shown).
Figure 1. Disease severity and inflammation.
A. PCDAI pre-infliximab baseline for subjects overall, symptomatic and asymptomatic. B and C. hsCRP and IL-6 for subjects pre-infliximab, on day 2 and day 14 after treatment. Between group comparison: ## p<0.01. Comparison to baseline value within group: * p<0.05.
Hormones of growth and puberty
Levels of hormones by symptomatic classification are shown in the online-only Supplementary Table 2 (http://links.lww.com/MPG/A70). There were no differences in hormone levels between groups, nor were there significant changes in hormone levels following infliximab treatment.
IMPACT III and Appetite scores
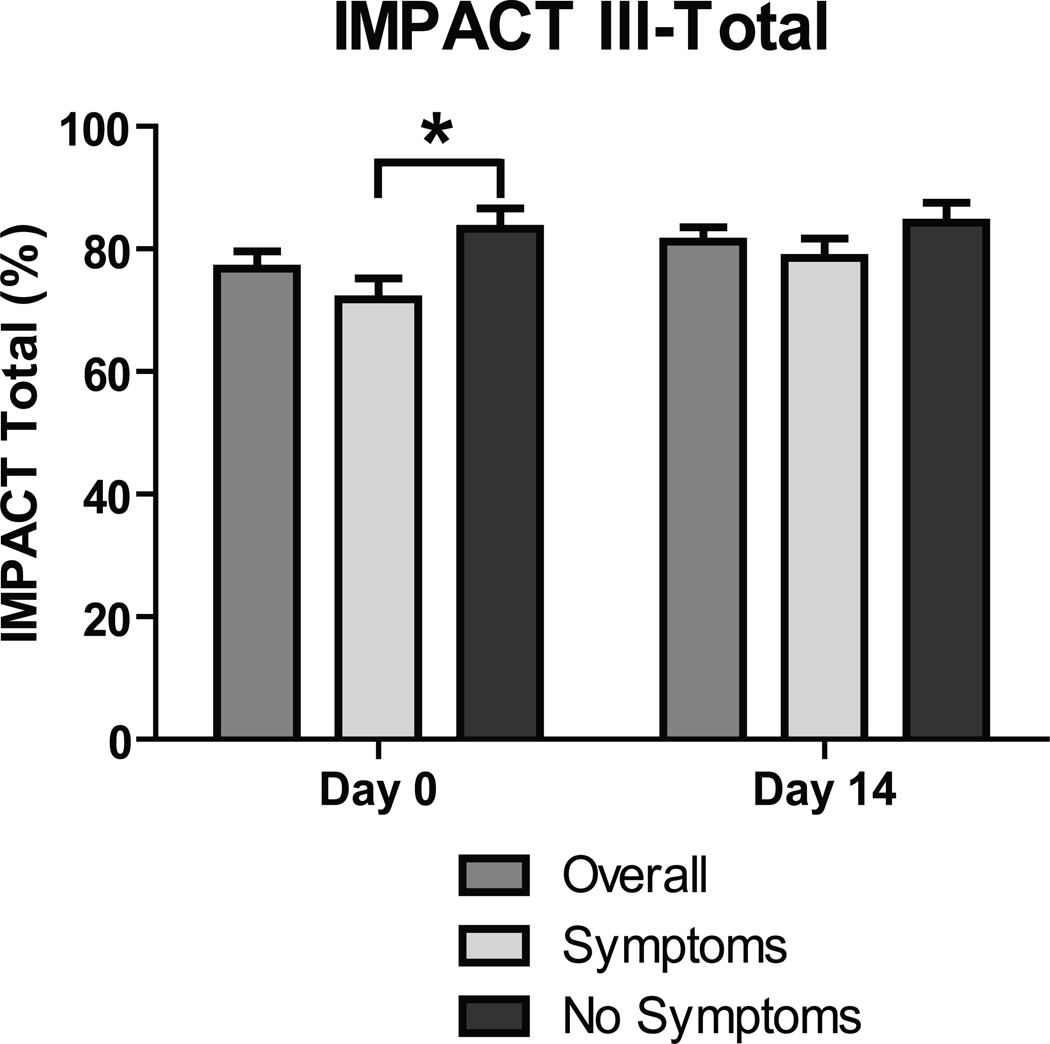
IMPACT III total scores for all subjects 9–17 y.o. are shown in Figure 2 and sub-categories of IMPACT are shown in Table 1. As compared to asymptomatic subjects in this age range, subjects in the symptomatic group at baseline had lower scores for total IMPACT and for sub-categories related to bowel symptoms and emotional symptoms. At Day 14 the symptomatic group had lower scores than the asymptomatic group in sub-categories for bowel symptoms and systemic symptoms. When all subjects (i.e., including those ≥18 y.o.) were included in the analysis there were significant differences in total IMPACT at baseline and bowel subscore at baseline and Day 14 (p<0.05, data not shown).
Figure 2. Quality of life pre-treatment and at 2 weeks.
IMPACT III scores converted as a percent maximum for subjects overall and broken down into those symptomatic and asymptomatic. To obtain raw IMPACT III score, multiply percent maximum by 1.75. Comparison between groups, symptomatic vs. asymptomatic: * p<0.05.
Table 1. IMPACT III sub-scores for subjects overall and by symptomatic group.
Mean and standard deviations for percent score in each domain of IMPACT III on Day 0 and Day 14 for subjects 9–17 years old.
| Overall | Symptomatic | Asymptomatic | |||||
|---|---|---|---|---|---|---|---|
| p value Day 14 vs. Day 0 |
p value Day 14 vs. Day 0 |
p value Day 14 vs. Day 0 |
P value Symptomatic vs. Asymptomatic |
||||
| Total score | 76.8 (11.1) | 71.8 (10.2) | |||||
| Day 0 | 81.2 (9.0) | 0.14 | 78.5 (9.2) | 83.3 (9.0) | <0.05 | ||
| Day 14 | 0.14 | 84.3 (8.4) | 0.69 | 0.22 | |||
| Bowel symptoms | |||||||
| Day 0 | 76.3 (16.3) | 67.9 (14.0) | 86.9 (12.1) | <0.05 | |||
| Day 14 | 79.2 (14.2) | 0.69 | 72.5 (13.0) | 0.69 | 86.9 (12.2) | 1.00 | <0.05 |
| Systemic symptoms | |||||||
| Day 0 | 70.4 (14.8) | 64.4 (12.9) | 78.0 (14.3) | 0.06 | |||
| Day 14 | 74.7 (17.5) | 0.57 | 65.8 (15.7) | 0.79 | 84.8 (14.3) | 0.18 | <0.05 |
| Emotional symptoms | |||||||
| Day 0 | 76.4 (14.2) | 70.2 (15.0) | 84.5 (8.4) | <0.05 | |||
| Day 14 | 81.0 (9.5) | 0.42 | 80.7 (9.6) | <0.05 | 81.2 (10.0) | 1.00 | 0.92 |
| Social functioning | |||||||
| Day 0 | 79.6 (11.8) | 76.1 (9.8) | 83.5 (13.3) | 0.20 | |||
| Day 14 | 85.1 (7.1) | 0.08 | 84.4 (5.6) | 0.1 | 85.8 (8.7) | 0.50 | 0.70 |
| Body image | |||||||
| Day 0 | 70.8 (18.0) | 70.4 (20.3) | 71.4 (16.2) | 0.89 | |||
| Day 14 | 72.4 (18.7) | 0.78 | 71.7 (21.0) | 1.00 | 73.3 (17.2) | 0.67 | 0.87 |
| Treatment/interventions | |||||||
| Day 0 | 77.9 (16.1) | 76.3 (14.6) | 80.0 (18.9) | 0.66 | |||
| Day 14 | 83.1 (13.7) | 0.34 | 81.7 (14.1) | 0.45 | 84.8 (14.3) | 0.60 | 0.68 |
Regarding change in IMPACT scores between Day 0 and Day 14 for subjects 9–17 y.o. there was a significant improvement only in emotional symptoms among symptomatic subjects (Table 1). When all subjects (including those ≥18 y.o.) were included in the analysis there were significant increases in scores of total IMPACT, emotional symptoms and social functioning in the group overall (all p<0.05, data not shown), with a non-significant trend toward increased scores for bowel symptoms (p=0.07). In this evaluation including subjects of all ages, the symptomatic group exhibited increases in scores for total IMPACT, bowel symptoms, emotional symptoms and social functioning (data not shown). In the asymptomatic group there was only a non-significant increase in the systemic symptoms sub-score (p=0.06, data not shown).
The appetite VAS measuring hunger, fullness, intake capacity, nausea and thirst revealed no significant differences between the symptomatic and asymptomatic groups nor any significant changes between Day 0, Day 2 or Day 14 (data not shown).
Discussion
We noted rapid improvements in systemic inflammation and quality-of-life in symptomatic but not asymptomatic subjects following a single dose of infliximab in the context of on-going treatment. Prior reports have focused on magnitude of change between 2 weeks and one year after first beginning treatment with infliximab for moderate- to severely-affected patients with pre-treatment PCDAI scores of 31–56 (1, 4–6) and initial CRP levels of 28–30 mg/L (3, 4). While early changes in CRP in these settings were striking (40–75% decrease), later changes during maintenance treatment reflected no change or even an increase in CRP (3, 4). In our sample of mildly-affected children and adolescents (mean PCDAI of 8.5 overall, 13.1 in the symptomatic group), we noted a sustained decrease in hsCRP levels at both 2 days and 2 weeks, both overall and among symptomatic subjects. These decreases in hsCRP levels below pre-treatment levels at two weeks provide some level of expectation for changes in inflammation following a single dose of infliximab during ongoing treatment. It is not clear why these subjects exhibited symptoms despite receiving on-going infliximab therapy. This may represent a group that does not respond optimally to infliximab from a long-term perspective. In this manner these findings may under-represent the impact of a single infusion of infliximab on the average patient.
Subjects who were receiving on-going therapy with infliximab in the absence of symptoms did not demonstrate significant changes in inflammation. The lack of significant decrease in hsCRP levels in the asymptomatic group is perhaps not surprising, given lower PCDAI scores in this group and slightly (but non-significantly) lower initial hsCRP levels. In addition, our small number of asymptomatic subjects rendered us significantly underpowered to detect changes in hsCRP among the asymptomatic group, though we also cannot exclude the possibility of the presence of human anti-chimeric antibodies as an explanation for the lack of improvement by 14 days. Nevertheless, our observed lack of difference in the asymptomatic group is consistent with prior studies that failed to note further decreases in CRP levels during maintenance treatment (3, 4) and may reflect a subset of children and adolescents with a more stable disease course.
We did not note a difference in time-since-last infliximab treatment or total number of prior infliximab doses between the symptomatic and asymptomatic subjects. A previous study randomizing subjects to regular infusions vs. as-needed infusions of infliximab demonstrated a longer remission and a lower rate of relapse in the group receiving regular infusions (3). While the majority of our subjects were receiving regularly-scheduled infliximab infusions every 2 months, a subset were receiving infusions on a more periodic basis, and it remains possible that these longer intervals may have had some influence on the symptomatic status of these children and adolescents.
Quality of life as assessed by IMPACT III had improved by 2 weeks after infusion among all subjects, demonstrating tangible benefits to a single treatment, even among patients receiving continued therapy. As may have been expected, quality of life was not altered significantly among those who were asymptomatic at the time of treatment but did improve among those who had been symptomatic, and this improvement applied to both the overall IMPACT III scores and to sub-scores related to emotional symptoms and social functioning. Previous studies in children had demonstrated improvements following ongoing infliximab treatment over the course of a year (5, 9), and a study in adults showed improved quality-of-life 4 weeks after treatment of a single dose of infliximab in individuals naïve to therapy and with a high degree of disease activity (12). However, our study is the first that we know of demonstrating improved quality-of-life over a period as short two weeks’ time and the first evaluating for changes in quality-of-life among subjects with mild disease activity.
Systemic inflammation and IBD disease severity contribute to regulation of hormones related to growth and puberty, as evidenced by delayed puberty and decreased growth velocity in the setting of pediatric IBD (13–19). Animal models have demonstrated that the poor growth and delayed puberty are worse than seen in animals that are food restricted, suggesting a role of disease-factors besides poor weight gain (20–23). In animal models, levels of LH and FSH are suppressed during systemic inflammation (24). Similarly, thyroid hormone levels can be notoriously suppressed during significant illness (“sick euthyroid” syndrome), which can improve rapidly after resolution of illness (25, 26). In the setting of IBD an important factor related to these effects is systemic inflammation (27). Levels of IGF-1 have been shown to increase as rapidly as 2 weeks following initiation of enteral feeding in severely-affected children and striking changes in testosterone have also been observed, both coinciding with a dramatic decrease in CRP (19, 28, 29). However, these effects are confounded by the high calorie delivery that may also affect IGF-1 levels in that setting. It is not known how rapidly a decrease in systemic inflammation might affect hormones related to growth and puberty, though it was our hypothesis that we would note changes in hormonal levels over the 2 week period of study. However, we did not note significant changes in levels of these hormones over the time course that we studied here. This study was originally powered to detect differences in hsCRP, and it is likely that we were underpowered to detect changes in levels of hormones such as IGF-1. Additionally, two weeks may not have been an adequate time to observe significant changes in these hormone in this context. Further studies are needed to determine the effect of infliximab treatment on hormones of growth and puberty.
This study had several weaknesses, including its observational nature. Randomized trials will be needed to determine whether quality-of-life improvements were due specifically to treatment with infliximab. We did not perform analysis of serum levels of infliximab—either at baseline or peak concentrations—and were thus not able to determine a correlation between serum levels and outcomes. Similarly, we did not measure levels of HACA, which can be useful in determining cause of a poor response to infliximab (30). We determined “symptomatic” status of our subjects by asking 4 questions pertaining to recent symptoms attributable to IBD. While these questions were rigorously applied to our subjects, it is acknowledged that this was not a validated tool, which may have confounded our results. Finally, many of the subjects filled out their IMPACT and appetite questionnaires while receiving their pre-medication of IV diphenhydramine but did not receive diphenhydramine prior to filling out their follow-up evaluation forms. It is possible that the diphenhydramine affected their initial assessment of their quality-of-life of appetite—though this may be expected to equally affect Symptoms and No symptoms subjects.
In conclusion, among children and adolescents in on-going treatment with infliximab, we found evidence of a rapid decrease in hsCRP levels by 2 days and an increase in quality-of-life scores by 2 weeks after a single dose of infliximab. These changes were present in subjects who had symptoms of disease but not in those without symptoms. While not definitive due to the observational nature of the study, these data may offer treating physicians some guidance regarding clinical changes to expect during on-going therapy using infliximab.
Supplementary Material
Acknowledgement
Funding:
University of Virginia Children’s Hospital Grant-in-Aid
NIH 5K08HD060739-02, 5M01RR000847-37
We would like to acknowledge Anita Vijayagopalan and Shelly Dean for their indispensible help in subject recruitment. This study was funded by University of Virginia Children’s Hospital Grant-in-Aid, NIH 5K08HD060739-02 and the UVa GCRC 5M01RR000847-37.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.jpgn.org).
The authors report no conflicts of interest.
References
- 1.Baldassano R, Braegger CP, Escher JC, DeWoody K, Hendricks DF, Keenan GF, et al. Infliximab (REMICADE) therapy in the treatment of pediatric Crohn's disease. Am J Gastroenterol. 2003;98(4):833–838. doi: 10.1111/j.1572-0241.2003.07343.x. [DOI] [PubMed] [Google Scholar]
- 2.Veres G, Baldassano RN, Mamula P. Infliximab therapy in children and adolescents with inflammatory bowel disease. Drugs. 2007;67(12):1703–1723. doi: 10.2165/00003495-200767120-00005. [DOI] [PubMed] [Google Scholar]
- 3.Ruemmele FM, Lachaux A, Cezard JP, Morali A, Maurage C, Ginies JL, et al. Efficacy of infliximab in pediatric Crohn's disease: a randomized multicenter open-label trial comparing scheduled to on demand maintenance therapy. Inflamm Bowel Dis. 2009;15(3):388–394. doi: 10.1002/ibd.20788. [DOI] [PubMed] [Google Scholar]
- 4.Sinitsky DM, Lemberg DA, Leach ST, Bohane TD, Jackson R, Day AS. Infliximab improves inflammation and anthropometric measures in pediatric Crohn's disease. J Gastroenterol Hepatol. 2010;25(4):810–816. doi: 10.1111/j.1440-1746.2009.06195.x. [DOI] [PubMed] [Google Scholar]
- 5.Hyams J, Crandall W, Kugathasan S, Griffiths A, Olson A, Johanns J, et al. Induction and maintenance infliximab therapy for the treatment of moderate-to-severe Crohn's disease in children. Gastroenterology. 2007;132(3):863–873. doi: 10.1053/j.gastro.2006.12.003. quiz 1165-6. [DOI] [PubMed] [Google Scholar]
- 6.Tiemi J, Komati S, Sdepanian VL. Effectiveness of infliximab in Brazilian children and adolescents with Crohn disease and ulcerative colitis according to clinical manifestations, activity indices of inflammatory bowel disease, and corticosteroid use. J Pediatr Gastroenterol Nutr. 2010;50(6):628–633. doi: 10.1097/MPG.0b013e3181bbf481. [DOI] [PubMed] [Google Scholar]
- 7.Walters TD, Gilman AR, Griffiths AM. Linear growth improves during infliximab therapy in children with chronically active severe Crohn's disease. Inflamm Bowel Dis. 2007;13(4):424–430. doi: 10.1002/ibd.20069. [DOI] [PubMed] [Google Scholar]
- 8.Otley A, Smith C, Nicholas D, Munk M, Avolio J, Sherman PM, et al. The IMPACT questionnaire: a valid measure of health-related quality of life in pediatric inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2002;35(4):557–563. doi: 10.1097/00005176-200210000-00018. [DOI] [PubMed] [Google Scholar]
- 9.Hyams JS, Lerer T, Griffiths A, Pfefferkorn M, Kugathasan S, Evans J, et al. Long-term outcome of maintenance infliximab therapy in children with Crohn's disease. Inflamm Bowel Dis. 2009;15(6):816–822. doi: 10.1002/ibd.20845. [DOI] [PubMed] [Google Scholar]
- 10.Hyams JS, Ferry GD, Mandel FS, Gryboski JD, Kibort PM, Kirschner BS, et al. Development and validation of a pediatric Crohn's disease activity index. J Pediatr Gastroenterol Nutr. 1991;12(4):439–447. [PubMed] [Google Scholar]
- 11.Flint A, Raben A, Blundell JE, Astrup A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int J Obes Relat Metab Disord. 2000;24(1):38–48. doi: 10.1038/sj.ijo.0801083. [DOI] [PubMed] [Google Scholar]
- 12.Lichtenstein GR, Bala M, Han C, DeWoody K, Schaible T. Infliximab improves quality of life in patients with Crohn's disease. Inflamm Bowel Dis. 2002;8(4):237–243. doi: 10.1097/00054725-200207000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Ballinger AB, Camacho-Hubner C, Croft NM. Growth failure and intestinal inflammation. Qjm. 2001;94(3):121–125. doi: 10.1093/qjmed/94.3.121. [DOI] [PubMed] [Google Scholar]
- 14.Ballinger AB, Savage MO, Sanderson IR. Delayed puberty associated with inflammatory bowel disease. Pediatr Res. 2003;53(2):205–210. doi: 10.1203/01.PDR.0000047510.65483.C9. [DOI] [PubMed] [Google Scholar]
- 15.Brain CE, Savage MO. Growth and puberty in chronic inflammatory bowel disease. Baillieres Clin Gastroenterol. 1994;8(1):83–100. doi: 10.1016/s0950-3528(06)80020-5. [DOI] [PubMed] [Google Scholar]
- 16.Pfefferkorn M, Burke G, Griffiths A, Markowitz J, Rosh J, Mack D, et al. Growth abnormalities persist in newly diagnosed children with crohn disease despite current treatment paradigms. J Pediatr Gastroenterol Nutr. 2009;48(2):168–174. doi: 10.1097/MPG.0b013e318175ca7f. [DOI] [PubMed] [Google Scholar]
- 17.Sawczenko A, Ballinger AB, Savage MO, Sanderson IR. Clinical features affecting final adult height in patients with pediatric-onset Crohn's disease. Pediatrics. 2006;118(1):124–129. doi: 10.1542/peds.2005-2931. [DOI] [PubMed] [Google Scholar]
- 18.Thayu M, Denson LA, Shults J, Zemel BS, Burnham JM, Baldassano RN, et al. Determinants of changes in linear growth and body composition in incident pediatric Crohn's disease. Gastroenterology. 2010;139(2):430–438. doi: 10.1053/j.gastro.2010.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fujimura Y, Honda K, Sato I, Takeda M, Murakami M, Kozuka K, et al. Remarkable improvement of growth and developmental retardation in Crohn's disease by parenteral and enteral nutrition therapy. Intern Med. 1992;31(1):39–43. doi: 10.2169/internalmedicine.31.39. [DOI] [PubMed] [Google Scholar]
- 20.Azooz OG, Farthing MJ, Savage MO, Ballinger AB. Delayed puberty and response to testosterone in a rat model of colitis. Am J Physiol Regul Integr Comp Physiol. 2001;281(5):R1483–R1491. doi: 10.1152/ajpregu.2001.281.5.R1483. [DOI] [PubMed] [Google Scholar]
- 21.Ballinger AB, Azooz O, El-Haj T, Poole S, Farthing MJ. Growth failure occurs through a decrease in insulin-like growth factor 1 which is independent of undernutrition in a rat model of colitis. Gut. 2000;46(5):694–700. doi: 10.1136/gut.46.5.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DeBoer MD, Li Y, Cohn S. Colitis causes delay in puberty in female mice out of proportion to changes in leptin and corticosterone. J Gastroenterol. 2010;45(3):277–284. doi: 10.1007/s00535-009-0192-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DeBoer MD, Li Y. Puberty is delayed in male mice with dextran sodium sulfate colitis out of proportion to changes in food intake, body weight, and serum levels of leptin. Pediatr Res. 2011;69(1):34–39. doi: 10.1203/PDR.0b013e3181ffee6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nappi RE, Rivest S. Effect of immune and metabolic challenges on the luteinizing hormone-releasing hormone neuronal system in cycling female rats: an evaluation at the transcriptional level. Endocrinology. 1997;138(4):1374–1384. doi: 10.1210/endo.138.4.5044. [DOI] [PubMed] [Google Scholar]
- 25.DeBoer MD, Lafranchi SH. Pediatric thyroid testing issues. Pediatr Endocrinol Rev. 2007 May; Suppl 1:570–577. [PubMed] [Google Scholar]
- 26.Baloch Z, Carayon P, Conte-Devolx B, Demers LM, Feldt-Rasmussen U, Henry JF, et al. Laboratory medicine practice guidelines. Laboratory support for the diagnosis and monitoring of thyroid disease. Thyroid. 2003;13(1):3–126. doi: 10.1089/105072503321086962. [DOI] [PubMed] [Google Scholar]
- 27.Sawczenko A, Azooz O, Paraszczuk J, Idestrom M, Croft NM, Savage MO, et al. Intestinal inflammation-induced growth retardation acts through IL-6 in rats and depends on the −174 IL-6 G/C polymorphism in children. Proc Natl Acad Sci U S A. 2005;102(37):13260–13265. doi: 10.1073/pnas.0503589102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beattie RM, Camacho-Hubner C, Wacharasindhu S, Cotterill AM, Walker-Smith JA, Savage MO. Responsiveness of IGF-I and IGFBP-3 to therapeutic intervention in children and adolescents with Crohn's disease. Clin Endocrinol (Oxf) 1998;49(4):483–489. doi: 10.1046/j.1365-2265.1998.00562.x. [DOI] [PubMed] [Google Scholar]
- 29.Bannerjee K, Camacho-Hubner C, Babinska K, Dryhurst KM, Edwards R, Savage MO, et al. Anti-inflammatory and growth-stimulating effects precede nutritional restitution during enteral feeding in Crohn disease. J Pediatr Gastroenterol Nutr. 2004;38(3):270–275. doi: 10.1097/00005176-200403000-00007. [DOI] [PubMed] [Google Scholar]
- 30.Afif W, Loftus EV, Jr, Faubion WA, Kane SV, Bruining DH, Hanson KA, et al. Clinical utility of measuring infliximab and human anti-chimeric antibody concentrations in patients with inflammatory bowel disease. Am J Gastroenterol. 2010;105(5):1133–1139. doi: 10.1038/ajg.2010.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.