Abstract
Objective
To determine, in an overweight pediatric population, if an A1C-determined high risk, pre-diabetic state (A1C ≥6.0–6.4%) is associated with decreased insulin sensitivity and β-cell dysfunction, known factors in the pathogenesis of type 2 diabetes.
Study design
We divided 206 healthy overweight Latino adolescents (124 male/82 female; age 13.1±2.0 yrs), into 2 groups: Lower Risk (LR, n=179) had A1C <6.0%; and High Risk (HR, n=27) had A1C 6.0–6.4%. Measures included A1C; OGTT fasting & 2-hr glucose and insulin; insulin sensitivity (SI), acute insulin response (AIR), and disposition index (DI, an index of β-cell function) by frequently sampled FSIVGTT with minimal modeling. Body fat was determined by DEXA.
Results
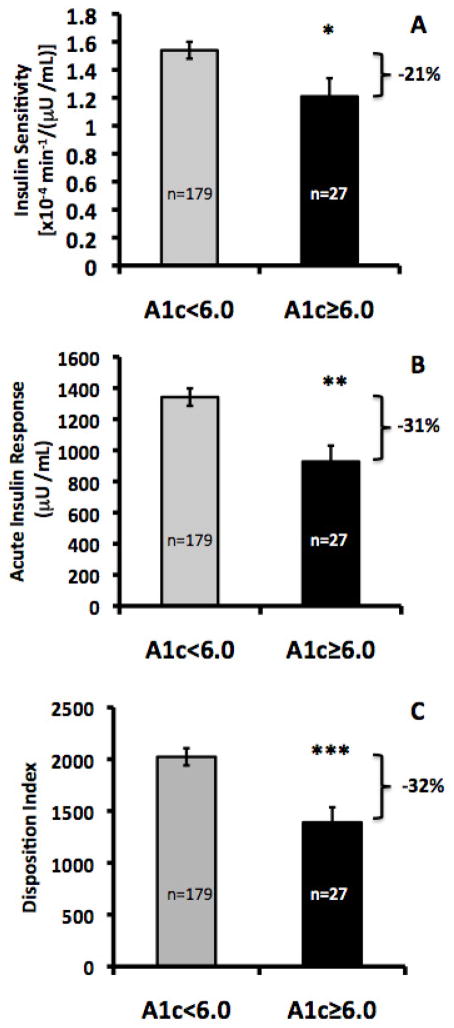
Compared with the LR group, the HR group had 21% lower SI (1.21±0.06 vs. 1.54±0.13, p<0.05), 30% lower AIR (928±102 vs. 1342±56, p<0.01), and 31% lower DI (1390±146 vs. 2023±83, p=0.001) after adjusting for age and total percent body fat.
Conclusion
These data provide clear evidence of greater impairment of β-cell function in those overweight Latino children with A1C 6.0–6.4%, and would thereby support the adoption of the International Expert Committee A1C-determined definition of high risk state for overweight children at risk for type 2 diabetes.
Keywords: Obesity, Prediabetes, A1c, beta cell function, insulin sensitivity
Diabetes and pre-diabetes is present in over 40% of the U.S. population (1). Identification of pre-diabetes allows clinicians to treat and delay its progression to type 2 diabetes (2). Diagnostic criteria for pre-diabetes have been long established using plasma glucose cut-offs based on studies in adults that show associated insulin resistance and other pathophysiologic changes, such as diminished insulin secretion and beta cell function (3–5). In children, those with impaired fasting glucose or impaired glucose tolerance have shown ~15% decline in beta cell function (6, 7).
Until recently, hemoglobin A1C has only been used to monitor people already diagnosed with diabetes, serving as the gold-standard measure of glycemic levels over a three-month period. More recently, as with measures of glucose, A1C levels have been used to describe a continuum of risk for the development of diabetes and associated conditions. The DETECT-2 study (8, 9), determined that the prevalence of non-proliferative diabetic retinopathy begins to rise with A1C levels above 6.5%. Other studies similarly show a relationship of A1C with risk of other diabetic complications and strongly suggest its use as a diagnostic tool (10).
In 2008, the International Expert Committee convened to review current and future methods of diagnosing type 2 diabetes using hemoglobin A1C measures (11). The committee recommended that diabetes should be diagnosed when A1C is ≥ 6.5%, and that diagnosis should be confirmed with a repeat A1C test. In addition, a sub-diabetic “high-risk” state was defined as A1C levels below the threshold for diabetes but ≥6.0%, and a lower risk for type 2 diabetes was defined by A1C level below 6.0%. In 2010, the American Diabetes Association (ADA) released a separate set of recommendations, defining the pre-diabetic state as A1C ≥5.7% and <6.5% (12). Both of these recommendations were intended for adults but they could be extrapolated to pediatric populations if beta cell dysfunction is indeed shown in children to be associated with these newly proposed recommendations.
Current ADA guidelines for the diagnosis of pre-diabetes are based on glucose criteria established in adults, and have generally been adopted for the pediatric population without independent studies to validate their use in children. Our research group has shown that pre-diabetic overweight Latino youth have impaired pancreatic β-cell function. Thus, impaired fasting glucose and impaired glucose tolerance (6, 7) were both associated with ~15% diminished beta cell function in these children. With the new A1C guidelines for diagnosing pre-diabetes, the question is now raised whether these cutoffs similarly identify pediatric patients at risk for type 2 diabetes.
Our hypothesis is that in a Latino pediatric population at high risk for type 2 diabetes, both the International Expert Committee (A1C ≥6.0–6.4%) and ADA (A1C ≥5.7–6.4%) recommendations will be associated with lower insulin sensitivity and diminished beta cell function.
METHODS
The current analysis includes data from two separate studies of diabetes risk in overweight Latino youth: the Study of Overweight Latino Youth At Risk (SOLAR) and Diabetes Risk due to Ectopic Adiposity in Minority youth (DREAM). The DREAM study is an ongoing cross-sectional investigation into relationships between ectopic fat distribution and diabetes risk in overweight minority adolescents. SOLAR is a longitudinal observational cohort study, and data included in this study represent baseline data at entry into the study. Further details of the SOLAR study have been published elsewhere (7, 13).
Two hundred and six overweight (SOLAR=142; DREAM=64), but otherwise healthy Latino boys and girls were included in the present analysis. Participants for both studies were recruited from the greater Los Angeles County through community health clinics, health fairs, and word of mouth, and were required to meet the following inclusion criteria at baseline: 1) Latino ethnicity (all four grandparents of Latino descent); 2) ages 8–17 yr (SOLAR 8–13 yrs; DREAM 8–17 yrs); 3) age and sex BMI ≥85th percentile based on the year 2000 standards of the Centers for Disease Control and Prevention. Participants in the SOLAR study also had to have a first degree relative (parent, grandparent, or sibling) with type 2 diabetes. Although family history of diabetes was not an inclusion criterion for the DREAM study, 86% of participants in fact had a family history of type 2 diabetes by this definition. Children were excluded if they had a previous major illness, including Type 1 or 2 diabetes, took medications, or had a condition known to influence body composition, insulin action, or insulin secretion. Participants and their parents provided written informed consent. Both studies received approval by the Institutional Review Board of the University of Southern California (USC) Health Sciences Campus.
Participants in either the SOLAR or DREAM studies attended two visits to at the USC General Clinical Research Center at the Los Angeles County General Hospital (or Clinical Trials Unit at the USC University Hospital after 2008). On the first visit, participants received a comprehensive medical history, physical examination (including assessment of pubertal maturation stage using Tanner breast development stage for girls and Tanner pubic hair development for boys) by a licensed health care provider (14,15). Clinical staff collected vital signs, blood pressure in triplicate and performed a 2-hour oral glucose tolerance test (OGTT). Within approximately 2 months following the outpatient visit, participants were admitted for an inpatient visit at the USC GCRC for their second visit. Participants were served dinner and a snack before 2000 h, which marked the beginning of an overnight fast. Water alone was permitted during this period. At 0630 h the following morning, a 13-sample insulin-modified frequently sampled intravenous glucose tolerance test (FSIVGTT) was performed as follows. Intravenous catheters were placed in the antecubital fossae of both arms. After two fasting blood samples were taken at −15 and −5 min, glucose (0.3 g/kg body weight) was administered at time 0 over a 1-min period. Subsequent blood samples were collected at 2, 4, 8, and 19 min. Insulin (0.02 U/kg body weight, Humulin R; Eli Lilly, Indianapolis, IN) was administered iv at 20 min, followed by blood sample collection at 22, 30, 40, 50, 70, 100, and 180 min. Plasma was analyzed for glucose and insulin concentration, and results were then entered into MINMOD MILLENNIUM 2003 software (version 5.16; RN Bergman, Los Angeles, CA) for calculation of whole body insulin sensitivity (SI), the acute insulin response to glucose (AIR; the area under the plasma insulin curve between 0 and 10 minutes) and the disposition index (DI; the product of SI and AIR and a measure of the ability of the islet cells to secrete insulin normalized to the degree of insulin resistance).
Body composition measures were performed at either visit based on availability of the participant and staff. Total body composition was completed by dual-energy x-ray absorptiometry using a Hologic QDR 4500 W (Hologic, Bedford, MA). Hemoglobin A1c was measured by HPLC (Tosoh 11c 2.2 HLC-723, Tokyo, Japan), an assay approved by the International Federation of Clinical Chemistry Working Group (IFCC-WG) on A1C standardization (16). The inter-assay coefficients of variations were 0.76% and 0.57% for A1c of 6.2% and 10.3% respectively. Glucose was assayed using a Yellow Springs Instruments analyzer (YSI INC., Yellow Springs, OH) that uses a membrane bound glucose oxidase technique. Insulin for the SOLAR study was assayed using a specific human insulin enzyme-linked immunosorbent assay kit from Linco (St. Charles, MO; intra-assay coefficient of variation 4.7–7.0%, inter-assay coefficient of variation 9.1–11.4%; cross-reaction with human proinsulin 0%). Insulin for the DREAM study was assay using an automated enzyme immunoassay (Tosoh AIA 600 II analyzer, Tosoh Bioscience, Inc., South San Francisco, CA; sensitivity 0.31μU/mL, intra-assay coefficient of variation 2.9%; inter-assay coefficient of variation 5.8%). The two insulin assay methods were validated to show that they did not differ from each other (correlation of two assays, r=0.97, p<0.0001). HOMA-IR was calculated using the following equation: [fasting glucose (in mg/dL) × fasting insulin (in μU/mL)]/405.
Statistical Analysis
Tests of normality (Q-Q plots, Shapiro Wilkes-test) were used to assess the distribution of the outcome variables: SI, AIR and DI. All variables were non-normal and were therefore log-transformed to achieve a normal distribution. Analyses were conducted on log-transformed variables and the results were then back-transformed for figure presentation and ease of interpretation.
The 206 subjects were divided for the primary analysis into 2 groups based on the recommendations of the International Expert Committee. Lower Risk (n=179) had A1C <6.0% and Higher Risk (n=27) had A1C ≥6.0–6.4%. For descriptive purposes, any differences between the two groups in physical and metabolic characteristics were determined using independent t-test and chi-square analyses. Unadjusted means were reported ± standard deviation. Analyses of covariance (ANCOVA) were performed to determine any differences in SI, AIR and DI by Lower or Higher Risk group. Initially, these analyses were adjusted for covariates (age, sex, Tanner pubertal stage, and total % body fat) known from our prior studies to affect these outcomes. However, pubertal stage and sex were not significant in our models, only age and percent body fat were subsequently used as covariates in the final analyses for SI and DI. For the analysis of AIR, the additional covariate of SI was used to assess the independent effects of insulin secretion on pre-diabetes. To assess any potential differences by study type, we also initially adjusted for study (SOLAR or DREAM) and for family history of type 2 diabetes; however all results remained the same so these variables were not included in the final analyses. An additional ANCOVA analysis was employed to determine any differences between 3 risk groups based on the ADA recommended criteria: Subjects with A1C <5.7%; subjects with A1C ≥5.7 and <6.0% and subjects with A1C ≥6.0–6.4%. For this analysis, the group previously identified as “Lower Risk” by IEC criteria (A1C <6.0%) was further divided into 2 groups: A1C <5.7% (n= 130) and A1C ≥5.7–5.9% (n= 49), and the Higher Risk Group (A1C ≥6.0%, n=27) was left intact. A contrast analysis (post-hoc analysis) was used to assess any inter-group differences. Data were analyzed using SPSS for Mac version 18.0 (IBM Inc., Chicago, IL), with an a priori significance level of p<0.05.
RESULTS
The Table shows physical and metabolic characteristics in those subjects at lower risk and higher risk for diabetes. There were no significant differences between groups by sex or Tanner stage. When compared with the Lower Risk Group, the subjects in the Higher Risk Group were significantly older, heavier, taller, and had more total fat, lean tissue mass, and percent body fat (p<0.05). BMI was significantly higher in the Higher Risk Group when compared with the Lower Risk Group (p<0.001) yet the two groups did not differ by BMI percentile (p>0.05). By definition the Higher Risk Group had significantly higher A1C than the Lower Risk Group (p<0.001). Even though fasting & 2-hour glucoses, HOMA-IR, and fasting insulin were higher in the Higher Risk Group, they did not reach significance (p=0.10–0.12). Impaired glucose tolerance was more prevalent in the Higher Risk group than in the Lower risk group (40% vs. 16%, p=0.02) but impaired fasting glucose status did not differ by group. When defining pre-diabetes as having either impaired glucose tolerance or impaired fasting glucose, there more participants with pre-diabetes in the Higher Risk group than the Lower Risk group but the difference was not significant (36% vs. 23%, p=0.09). Assessment of unadjusted insulin dynamics revealed that compared with the Lower Risk group, the Higher Risk group had lower SI (1.31±0.90 vs. 1.91±1.25 x10−4 min−1/(μU/mL), p=0.02) and lower DI (1631±1225 vs. 2338±1149, p<0.01), and AIR did not differ between the two groups (p>0.05).
Table I. Physical and metabolic characteristics of Higher risk vs Lower risk groups (n=206).
Data values are mean ± SD. Independent T-test and chi square were used to compare differences by Lower Risk and Higher Risk Group.
| Lower Risk HbA1c <6.0 (n=179) | Higher Risk HbA1c ≥6.0 (n=27) | |
|---|---|---|
| Age (years) | 12.6 ± 2.2 | 13.6 ± 2.3* |
| Sex (Male/Female) | 106/73 | 18/9 |
| Tanner Stage | ||
| 1 | 39 | 3 |
| 2 | 38 | 5 |
| 3 | 13 | 2 |
| 4 | 40 | 5 |
| 5 | 49 | 12 |
| Height (cm) | 156.8 ± 12.1 | 162.3 ± 10.5* |
| Weight (kg) | 74.1 ± 21.3 | 90.4 ± 24.0*** |
| BMI (kg/m2) | 29.6 ± 5.6 | 33.9 ± 6.9*** |
| BMI z-score | 2.02 ± 0.44 | 2.30 ± 0.44** |
| BMI percentile | 96.3 ± 4.6 | 97.8 ± 3.5 |
| Total Lean Tissue Mass (kg) | 44.2 ± 12.3 | 52.4 ± 13.5** |
| Total Fat Mass (kg) | 26.7 ± 10.3 | 34.6 ± 11.6*** |
| Total Percent Fat (%) | 36.4 ± 6.9 | 39.1 ± 5.5* |
| Fasting Glucose (mg/dL) | 90.4 ± 6.8 | 92.6 ± 7.3 |
| 2-hr Glucose (mg/dL) | 124.1 ± 19.6 | 130.4 ± 27.4 |
| Fasting Insulin (μU/mL) | 16.4 ± 10.8 | 22.3 ± 17.9† |
| 2-hr insulin | 149.2 ± 132.0 | 186.8 ± 181.8 |
| HOMA | 3.9 ± 4.1 | 7.7 ± 11.9 |
| HbA1c (%) | 5.48 ± 0.28 | 6.07 ± 0.10*** |
| Impaired fast glucose status | 16 (9%) | 4 (16%) |
| Impaired glucose tolerance status | 29 (16%) | 10 (40%)* |
| Pre-diabetes (IFG and/or IGT) | 40 (23%) | 10 (36%)† |
| Insulin sensitivity [x10−4/min−1)/(μU/mL)]a | 1.91 ± 1.25 | 1.31 ± 0.90* |
| Acute Insulin Response (μU/mL)a | 1634 ± 1149 | 1554 ± 1428 |
| Disposition indexa | 2338 ± 1149 | 1631 ± 1225** |
p<0.05,
p<0.01,
p<0.001
Identifies variables with non-normal distribution which were log-transformed for analyses. Non-transformed values are displayed for ease of interpretation.
Figure 1 shows the estimated marginal means for SI, AIR and DI by Lower and Higher Risk Groups after adjustment for age and total percent body fat (and SI for AIR). The Higher Risk Group had significantly lower SI (1.21±0.13 vs. 1.54±0.06 x10−4 min−1/(μU/mL), AIR (928±102 vs. 1342±56 μU/mL, p<0.05), and DI (1390±146 vs. 2023±83, p<0.05) when compared with the Lower Risk Group.
Figure 1.
A) Insulin sensitivity, B) Acute Insulin Response, C) Disposition Index. ANCOVAs were performed on log-transformed data and results were then back-transformed for figure presentation. SI and DI estimated marginal means (EMM) are shown ± SE and are adjusted for age and total percent body fat. AIR EMM was also adjusted for SI.
*p<0.05, **p<0.01, ***p<0.001
Repeating this analysis using the ADA recommended cutoffs of Lower Risk Group A1C <5.7% (n=130) and Higher Risk Group with A1C ≥5.7–6.4% (n=76), showed no significant differences in SI, AIR or DI by the 2 groups (p>0.05). This analysis however did not assess whether the children in the intermediary A1C range between 5.7–5.9% have insulin action that would most resemble a lower or higher risk for type 2 diabetes, hence a 3 group analysis was performed next.
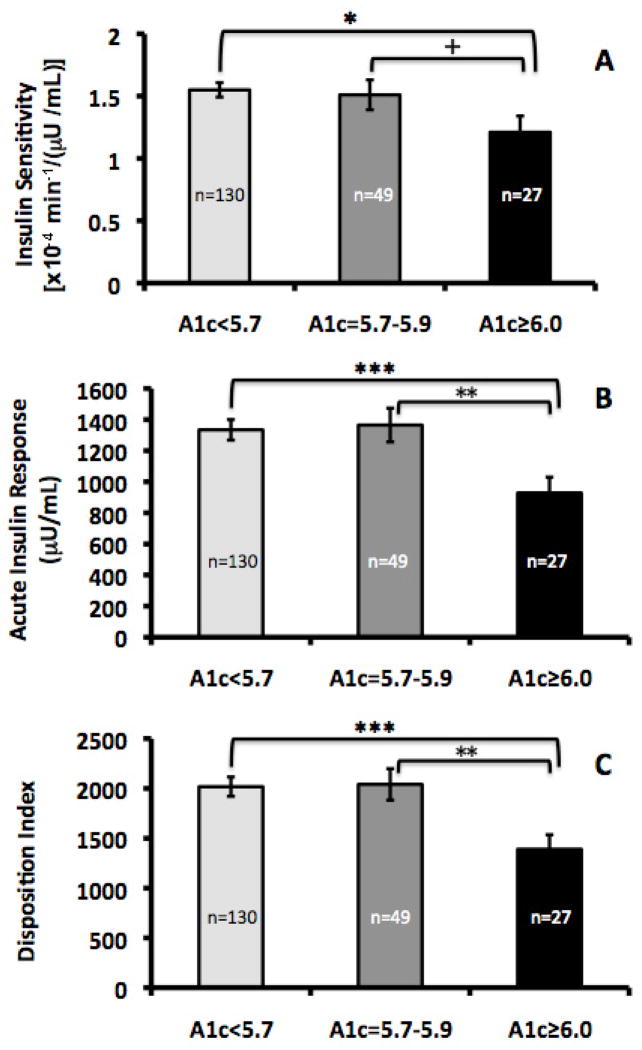
The estimated marginal means for SI, AIR and DI by 3 Risk Groups (subjects with A1C <5.7% (n=130); subjects with A1C ≥5.7–5.9% (n=49) and subjects with A1C ≥6.0–6.4% (n=27) are shown in Figure 2. There was a decrease in SI across the 3 A1C groups from lowest risk to highest risk but it was not statistically significant (p=0.10). Contrast analysis revealed no differences in SI between the two lowest risk groups (p=0.79). The subjects in the Highest Risk Group did have significantly lower SI than those in the subjects with A1c <5.7% (p=0.04) and it was lower than those with A1C ≥5.7–5.9%, but this did not reach significance (p=0.09). With AIR and DI, there were significant decreasing trends across the 3 groups (p<0.01). Contrast analysis revealed no differences in AIR and DI between the A1C <5.7% group and A1C ≥5.7–5.9% group (p=0.89), yet the Highest Risk Group had significantly lower AIR and DI than the subjects in both the A1C<5.7 group and A1C ≥5.7–5.9% group (both p<0.01).
Figure 2.
A) Insulin sensitivity, B) Acute Insulin Response, C) Disposition Index. ANCOVAs were performed on log-transformed data and results were then back-transformed for figure presentation. SI and DI estimated marginal means (EMM) are shown ± SE and are adjusted for age and total percent body fat. AIR EMM was also adjusted for SI.
*p<0.05, **p<0.01, ***p<0.001
DISCUSSION
Our primary result demonstrated that beta cell function (i.e. disposition index) was significantly lower in the Higher Risk groups when compared with the Lower Risk Groups, independent of age and percent body fat. In addition, both insulin sensitivity and insulin secretion (acute insulin response to glucose) were also significantly diminished in the Higher Risk Group. In a secondary objective, we addressed the recent ADA recommendation that used a broader A1c range (≥5.7 to 6.4%). We observed that there were no differences in insulin sensitivity, insulin secretion, or beta cell function when comparing the Lowest Risk Group (A1C<5.7%) with those with A1C between A1C ≥5.7–5.9%. However, there were significant differences in AIR and DI between the 5.7–5.9% group when compared with the Highest Risk Group. This suggests that a distinct drop in beta cell function occurs when A1C reached the 6.0% range in overweight Latino adolescents.
This study addresses two recent recommendations made by the International Expert Committee (High risk ≥6.0%) and the ADA (High risk ≥5.7%). Our post-hoc analysis of three different A1C groups showed no differences between those children with an A1C <5.7% and those with and A1C ≥5.7–5.9%. In contrast, children with an A1C≥6.0–6.4% had significantly lower insulin action than both of the lower risk groups. These results indicate that the intermediate group of children with an A1C ≥5.7–5.9% was unlikely to be at more risk than the Lowest Risk group with A1c<5.7%. Based on these findings, we support the International Expert Committee’s recommendation for the use of A1C≥6.0–6.4% to assess high risk for type 2 diabetes. It is important to caution that we cannot generalize this conclusion beyond our study population of overweight Latino youth. Further study in a multi-ethnic cohort is clearly warranted.
The clinical utility of using A1C as a screen for diabetes and pre-diabetes could greatly improve the options to the current screening recommendations, which include diagnostic criteria for pre-diabetes and diabetes requiring either a fasting glucose or a 2-hour OGTT. Fasting blood draws and OGTT measures may present logistical barriers, such as requiring additional clinic visit to obtain a fasting sample, and the inherent difficulties of a multi-sample stimulation test such as the OGTT. The A1C allows a medical provider to avoid delay in screening a child with risk for asymptomatic type 2 diabetes at the time of a non-fasting visit (17). The test is standardized, is accessible, is easy to use and has low variability from day-to-day testing (18, 19). The disadvantages of using A1C for screening in place of fasting glucose includes higher cost, and potential lack of standardization of A1C assays.
It should be noted that only 36% of the subjects with high-risk A1C would have been identified to have pre-diabetes by glucose criteria alone, and 23% of youth in the low-risk A1C group actually had pre-diabetes by glucose criteria. Therefore, although the specificity of the A1C screen is high (89%), the sensitivity is very low (22%) when it comes to predicting who has prediabetes by glucose criteria. Despite its very low rate of false positives, the A1C test is not as reliable to detect those children who truly have pre-diabetes by glucose criteria. However, it is difficult to say what is the “gold standard” for identifying children at risk, as that would require long-term longitudinal studies as have been done in adults to see which test more accurately predicts progression to type 2 diabetes. For now, our data would suggest that in overweight Latino adolescents, an A1C of 6.0–6.4% is indicative of greater beta cell dysfunction (~30% lower DI) than does a high fasting or 2-hour OGTT glucose (~15% lower DI), and therefore may identify a group at more risk for progression to diabetes than would glucose cutoffs (6, 7). Ultimately, the advantage of screening for pre-diabetes in high-risk youth using an A1C versus a fasting glucose or an oral glucose tolerance test remains to be determined by prospective longitudinal studies demonstrating the sensitivity, specificity, and relative predictive value of A1C versus IFG or IGT in predicting type 2 diabetes progression. Until those studies come to fruition, the A1C test offers the clinician a non-fasting method to detect a population of children with significant beta cell dysfunction, and likely at higher risk to progress.
The strengths of our study include a homogenous group of overweight Latino youth that are typically understudied, the evaluation of both the International Expert Committee and cross-comparison with the ADA recommendations, and the use of rigorous direct measures of insulin action and secretion by FSIVGTT with Minimal Modeling. Limitations include the lack of a lean group of children, for whom ethical considerations by our institutional IRB prevent testing children using FSIVGTT. Also, although we attempted to increase the power of our study by combining subjects from 2 separate studies, the relatively small final sample size in the Higher Risk Group (n=27) still may limit the power to detect some differences between A1C groups. For example, we were unable to achieve an adequate ROC curve to determine an A1C cut-off that can predict a decreased β-cell function, likely due to the low sample size. Lastly, our results cannot be generalized beyond overweight Latino children. Studies in adults have shown that A1C cut-offs have different impacts on different ethnic groups so further research in a multi-ethnic cohort is clearly warranted (20, 21).
Acknowledgments
Supported by NCMHD (grant P60MD002254) and NIDDK (grant R01-DK29511).
Footnotes
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cowie CC, Rust KF, Ford ES, Eberhardt MS, Byrd-Holt DD, Li C, et al. Full accounting of diabetes and pre-diabetes in the U.S. population in 1988–1994 and 2005–2006. Diabetes Care. 2009;32:287–94. doi: 10.2337/dc08-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tripathy D, Carlsson M, Almgren P, Isomaa B, Taskinen MR, Tuomi T, et al. Insulin secretion and insulin sensitivity in relation to glucose tolerance: lessons from the Botnia Study. Diabetes. 2000;49:975–80. doi: 10.2337/diabetes.49.6.975. [DOI] [PubMed] [Google Scholar]
- 4.Kim DJ, Lee MS, Kim KW, Lee MK. Insulin secretory dysfunction and insulin resistance in the pathogenesis of korean type 2 diabetes mellitus. Metabolism. 2001;50:590–3. doi: 10.1053/meta.2001.22558. [DOI] [PubMed] [Google Scholar]
- 5.Festa A, D’Agostino R, Jr, Hanley AJ, Karter AJ, Saad MF, Haffner SM. Differences in insulin resistance in nondiabetic subjects with isolated impaired glucose tolerance or isolated impaired fasting glucose. Diabetes. 2004;53:1549–55. doi: 10.2337/diabetes.53.6.1549. [DOI] [PubMed] [Google Scholar]
- 6.Weigensberg MJ, Ball GD, Shaibi GQ, Cruz ML, Goran MI. Decreased beta-cell function in overweight Latino children with impaired fasting glucose. Diabetes Care. 2005;28:2519–24. doi: 10.2337/diacare.28.10.2519. [DOI] [PubMed] [Google Scholar]
- 7.Goran MI, Bergman RN, Avila Q, Watkins M, Ball GD, Shaibi GQ, et al. Impaired glucose tolerance and reduced beta-cell function in overweight Latino children with a positive family history for type 2 diabetes. J Clin Endocrinol Metab. 2004;89:207–12. doi: 10.1210/jc.2003-031402. [DOI] [PubMed] [Google Scholar]
- 8.Colagiuri S. DETECT-2: early detection of type 2 diabtes and IGT. Diabetes Voice. 2003;48:11–3. [Google Scholar]
- 9.Colagiuri S, Lee CM, Wong TY, Balkau B, Shaw JE, Borch-Johnsen K. Glycemic thresholds for diabetes-specific retinopathy: implications for diagnostic criteria for diabetes. Diabetes Care. 2011;34:145–50. doi: 10.2337/dc10-1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Massin P, Lange C, Tichet J, Vol S, Erginay A, Cailleau M, et al. Hemoglobin A1c and fasting plasma glucose levels as predictors of retinopathy at 10 years: the French DESIR study. Arch Ophthalmol. 2011;129:188–95. doi: 10.1001/archophthalmol.2010.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care. 2009;32:1327–34. doi: 10.2337/dc09-9033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Standards of medical care in diabetes--2010. Diabetes Care. 2010;33 (Suppl 1):S11–61. doi: 10.2337/dc10-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weigensberg MJ, Cruz ML, Goran MI. Association between insulin sensitivity and post-glucose challenge plasma insulin values in overweight Latino youth. Diabetes Care. 2003;26:2094–9. doi: 10.2337/diacare.26.7.2094. [DOI] [PubMed] [Google Scholar]
- 14.Marshall WA, Tanner JM. Variations in pattern of pubertal pattern changes in girls. Arch Dis Child. 1969;44:291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marshall WA, Tanner JM. Variations in pattern of pubertal pattern changes in boys. Arch Dis Child. 1970;45:13–23. doi: 10.1136/adc.45.239.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weykamp C, John WG, Mosca A, Hoshino T, Little R, Jeppsson JO, et al. The IFCC Reference Measurement System for HbA1c: a 6-year progress report. Clin Chem. 2008;54:240. doi: 10.1373/clinchem.2007.097402. [DOI] [PubMed] [Google Scholar]
- 17.Standards of medical care for patients with diabetes mellitus. Diabetes Care. 2000;23 (Suppl 1):S32–42. [PubMed] [Google Scholar]
- 18.Little RR, Rohlfing CL, Wiedmeyer HM, Myers GL, Sacks DB, Goldstein DE. The national glycohemoglobin standardization program: a five-year progress report. Clin Chem. 2001;47:1985–92. [PubMed] [Google Scholar]
- 19.Sacks DB. A1C versus glucose testing: a comparison. Diabetes Care. 2011;34:518–23. doi: 10.2337/dc10-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Christensen DL, Witte DR, Kaduka L, Jorgensen ME, Borch-Johnsen K, Mohan V, et al. Moving to an A1C-based diagnosis of diabetes has a different impact on prevalence in different ethnic groups. Diabetes Care. 2011;33:580–2. doi: 10.2337/dc09-1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kramer CK, Araneta MR, Barrett-Connor E. A1C and diabetes diagnosis: The Rancho Bernardo Study. Diabetes Care. 2011;33:101–3. doi: 10.2337/dc09-1366. [DOI] [PMC free article] [PubMed] [Google Scholar]