Abstract
With 6 agents approved for metastatic renal cell carcinoma (mRCC) within the past 5 years, there has undoubtedly been progress in treating this disease. However, the goal of cure remains elusive, and the agents nearest approval (ie axitinib and tivozanib) abide by the same paradigm as existing drugs (i.e., inhibition of vascular endothelial growth factor, VEGF, or mammalian target of rapamycin, mTOR, signaling). The current review will focus on investigational agents that diverge from this paradigm. Specifically, novel immunotherapeutic strategies will be discussed, including vaccine therapy, cytotoxic T-lymphocyte antigen 4 (CTLA4) blockade, and programmed death-1 (PD-1) inhibition, as well as novel approaches to angiogenesis inhibition, such as abrogation of Ang/Tie-2 signaling. Pharmacologic strategies to block other potentially relevant signaling pathways, such as fibroblast growth factor receptor (FGFR) or MET inhibition, are also in various stages of development. Although VEGF and mTOR inhibition have dramatically improved outcomes for patients with mRCC, a surge above the current plateau with these agents will likely require exploring new avenues.
Keywords: renal cell carcinoma, targeted therapy, XL184, CVX-060, AMG-386, BMS-936558, AV-951
Introduction
Without question, the treatment of metastatic renal cell carcinoma (mRCC) has evolved markedly in recent years. Prior to the introduction of targeted agents more recently, the mainstay of therapy were immune-directed agents such as interleukin-2 (IL-2) or interferon-a (IFN-α). Although IL-2 offers the potential for a durable remission in approximately 5–7% of patients, the vast majority obtain limited clinical benefit.(1) Similarly, the clinical efficacy of IFN-α is quite limited – meta-analytic data from the cytokine era suggests a median progression-free survival (PFS) of 4.7 months and a median overall survival (OS) of 13 months with this therapy.(2) In 2002, it was proposed that these values serve as a benchmark for future therapies for mRCC. Several targeted agents have now surpassed this benchmark. In a phase III study, the vascular endothelial growth factor-tyrosine kinase inhibitor (VEGF-TKI) sunitinib led to an improvement in OS as compared to IFN-α in treatment-naïve patients with clear cell mRCC.(3) In a phase III study enrolling poor-risk patients with mRCC, the mammalian target of rapamycin (mTOR) inhibitor temsirolimus similarly showed a survival advantage over IFN-α.(4) Data from these and other randomized trials have led to the approval of 6 agents in less than 5 years for advanced RCC. A limitation of this milestone, however, is that these agents share common molecular targets. Akin to sunitinib, the monoclonal antibody bevacizumab and the TKIs sorafenib and pazopanib target signaling via the VEGF receptor (VEGFR).(5–8) Also, similar to temsirolimus, everolimus is a small molecule inhibitor of mTOR.(9)
Because of this conundrum, the research community is at a crossroads. Should further research be directed at developing agents that also antagonize VEGF- or mTOR-mediated signaling? Over the past year, the VEGF-TKI axitinib met its primary endpoint in a phase III study, showing an improvement in PFS as compared to sorafenib in patients with mRCC refractory to first-line therapy.(10) Data for other VEGF-TKIs, such as tivozanib (AV-951), are eagerly anticipated.(11) At some point, however, it is possible that a ceiling effect may occur with these therapies. Experiences to date suggest that not all patients will obtain benefit from VEGF- or mTOR-directed treatments and, even amongst those that do, responses are unlikely maintained indefinitely. Thus, parallel efforts are in place to investigate novel signaling axes which may offer unique benefit to patients beyond existing therapies. Herein, these efforts will be described in detail.
Angiogenesis Inhibition: Unique Strategies Beyond VEGFR Targeting
Inhibition of the Ang/Tie-2 signaling axis
The majority of angiogenesis inhibitors used in clinical practice today function via direct inhibition of VEGFR. However, other putative approaches exist to disrupt tumor blood vessel growth and formation. Targeting Tie-2 signaling is one such strategy. The Tie-2 receptor is expressed principally on the vascular endothelium and knockout leads to embryonic lethality in murine models due to vascular disruption.(12) Signaling via Tie-2 is mediated by several key ligands, including angiopoietin-1 (Ang-1) and angiopoiein-2 (Ang-2). Ang-1 was been shown to bind to the Tie-2 receptor and up-regulate survivin, an inhibitor of apoptosis, in endothelial cells.(13) The net effect of this interaction is stabilization of vasculature. In contrast, binding of Ang-2 to Tie-2 leads to increased endothelial cell proliferation. In a rat corneal model of angiogenesis, Ang-2 blockade prevented VEGF-induced neovascularization.(14) Of interest, Ang-2 concentrations appear to be higher in patients with RCC, suggesting the potential role of this moiety as a therapeutic target. Tie-2 gene expression appears to correlate with Ang-2 expression in tumors, suggesting the potential role of both as putative targets. (15) In a correlative study including 34 patients with mRCC treated with standard doses of sunitinib, blood was collected at the start of therapy and during the course of treatment.(16) A total of 20 patients ultimately had progression on sunitinib therapy – in this subgroup, Ang-2 levels decreased in 18 patients (90%) after initiation of sunitinib, but increased in 14 patients (70%) at the time resistance was evoked.
Several clinical strategies have been employed to abrogate signaling through the Ang/Tie-2 signaling axis. The compound AMG-386 is a peptibody that disrupts the interaction of Ang-1 and Ang-2 with Tie-2. In a phase I clinical trial including 32 patients, the most commonly incurred toxicity was fatigue and peripheral edema.(17) Patients received weekly intravenous doses of AMG-386 at up to 30 mg/kg; of note, no maximum tolerated dose (MTD) was reached. Ten patients (32%) were noted to have some degree of radiographic shrinkage, although only one partial response was observed in a patient with refractory ovarian cancer. Four patients (13%) were noted to have stable disease (SD) for greater than 16 weeks. These results culminated in a randomized, phase II study exploring the agent in patients with mRCC.(18) In this study, patients were randomized in a 1:1:1 ratio to receive either sorafenib with AMG-386 at 10 mg/kg intravenous (IV) weekly (Arm A), sorafenib with AMG-386 at 3 mg/kg IV weekly (Arm B) or sorafenib with IV placebo weekly (Arm C). A total of 152 patients were randomized, with a PFS of 9.0, 7.5, and 9.0 months in Arms A, B and C, respectively. For the comparison of Arms A and B combined versus Arm C, the hazard ratio (HR) for PFS was 0.88 (95% CI 0.68–1.14, P=0.523). Although disappointing that the primary endpoint of improved PFS was not met, several items warrant mention. First, the observation of a 9.0 month PFS in association with sorafenib monotherapy is higher than expected based on the phase III experience leading to the approval of the drug, where a PFS of 5.5 months was observed. Second, the combination of sorafenib with AMG-386 did appear to have modest antitumor activity as compared to sorafenib alone. The maximum change in the sum of longest diameters (SLD) from baseline to post-baseline nadir was −34.3%, −29.2%, and −25.2% in Arms A, B, and C, respectively. Similarly, the response rate was higher in treatment arms containing AMG-386 (38%, 37%, and 24% in Arms A, B, and C, respectively). Added toxicity from AMG-386 appeared to be modest, with the safety profile for combination therapy resembling that of sorafenib monotherapy. Specifically, the most frequently incurred adverse events were diarrhea, hand-foot syndrome (HFS), alopecia and hypertension.
A second approach to abrogating Ang/Tie-2 signaling is selective targeting of the ligand. One such agent, CVX-060, is a fusion protein comprised of two Ang-2 binding peptides.(19) Preliminary results from a phase I study including 34 patients have been recently reported. With data available for 30 of these patients, no MTD was reached with doses escalating to 15 mg/kg IV weekly. The toxicity profile of the agent appeared to be relatively mild. Fatigue represented the most common adverse event, occurring in 23% of patients. Proteinuria (primarily grade 1/2) and hemorrhage (grade 1) were observed in a low percentage of patients (17% and 7%, respectively). A total of 24 patients (71%) remained on study therapy for ≥ 8 weeks. A randomized, phase II study will compare axitinib with or without CVX-060.(20) The study is anticipated to open in December of 2011, and will enroll a total of 165 patients. Following the theme of combining Ang-2 inhibition with VEGF inhibition, a distinct compound (CVX-241) is currently under development. Akin to CVX-060, this agent is a peptibody, but has affinity for both VEGF and Ang-2. Data from a phase I study evaluating CVX-241 has been recently reported. In 17 patients with solid tumors to date, no proteinuria or hemorrhage was reported (unlike the experience with CVX-060). The most commonly reported toxicities were fatigue, decreased appetite, back pain, and dyspnea. Of 13 evaluable patients, the best response observed is stable disease (SD) in 7 patients. It remains to be seen how the strategy of selective Ang-2 targeting will compare to the strategy of Tie-2 inhibition. Preclinical data from Coxon et al suggests that Ang-1 activity may be unmasked with use of Ang-2 inhibitors and thus dual inhibition of Ang-1/2 activity may be a preferred approach.(21)
Thalidomide and Lenalidomide
Despite widespread utilization of thalidomide and lenalidomide to treat multiple myeloma and myelodysplastic syndromes with 5q deletion, the mechanisms of these agents remain somewhat poorly understood. It is know that these agents have a complex effect on the tumor microenvironment, enhancing T-cell proliferation and modulating expression of various cytokines, including IL-2, IFN-γ, and IL-12.(22)
Escudier et al reported the activity of thalidomide in 40 patients with mRCC.(23) Patients received thalidomide at a starting dose of 400 mg daily, which was increased to 800 mg daily after 6 weeks if progressive disease (PD) was observed. If disease progression continued after a further 6 or 12 weeks of therapy at 800 mg dosing, the dose was then increased to 1200 mg. Of the 40 patients enrolled, 6 patients (15%) had received no prior therapy, while 8 (20%) had received 3 or more lines of treatment. Two patients (5%) exhibited a partial response (PR), and a median OS of 10 months was reported. The toxicities associated with thalidomide were substantial – for instance, of those patients who had received thalidomide for at least 12 months, 100% had demonstrable neuropathy on electromyography. A total of 9 patients (22.5%) developed thromboembolism, and 3 patients (7.5%) developed pulmonary embolism. Thus, the modest activity associated with thalidomide in mRCC was mitigated by the substantial toxicity profile.
Several other studies aimed to determine if lower doses of thalidomide could be combined with biologics. Hernberg et al assessed thalidomide at up to 300 mg daily with IFN-α dosed at 0.9 million international units (MIU) subcutaneously (SQ) 3 times daily.(24) With a total of 30 patients enrolled, 6 patients (20%) achieved a PR. Median time to treatment failure (TTF) was 7.7 months, and median OS was 14.9 months. When considering historical benchmarks, these results do not clearly suggest a benefit with the addition of thalidomide.(2) With this in mind, a phase III study comparing IFN-α with or without thalidomide may more definitively address this issue.(25) The study has been completed, although results have not yet been published. The combination of thalidomide and IL-2 has also been explored in 31 patients with mRCC. Patients received thalidomide at up to 400 mg daily in combination with IL-2 at 7 MIU/m2 with GM-CSF on days 1–5 from weeks 2 to 5 of therapy. After 7 weeks, patients repeated the same 6-week regimen up to 6 times. Clinical benefit was observed in 17 patients (55%), with 3 patients (10%) attaining a CR and 8 patients (26%) achieving a PR. The applicability of these results is limited by the IL-2 regimen utilized. Presumably, combination of high-dose IL-2 with thalidomide could result in substantially greater toxicity.
Phase II studies of lenalidomide have produced rather similar results. Chouieri et al reported results from a phase II study examining lenalidomide in 28 patients with mRCC who had received no more than 1 prior therapy.(26) Lenalidomide was administered at a dose of 25 mg daily for 3 weeks of a 4 week cycle. Three patients (15%) achieved a PR and remained progression free for longer than 15 months. A further 11 patients (39%) had stable disease lasting longer than 3 months, and median survival had not been achieved at the time of publication. The most frequent toxicities incurred with lenalidomide in this report were neutropenia, fatigue and dermatologic toxicity. A slightly larger experience was reported by Amato et al utilizing a similar schedule of lenalidomide in a total of 40 patients.(27) With 39 evaluable patients in this report, 1 patient achieved a CR and 3 patients (8%) achieved a PR. A further 21 patients (53%) were noted to have SD as a best response. Nine patients (23%) remained progression free after 12 months of therapy and a median OS of 17 months was reported. Akin to the experience reported by Chouieri et al, the most frequently incurred toxicities were neutropenia and fatigue.
Immunotherapy: Beyond IFN-α and IL-2
CTLA4 Inhibition
Pharmacologic blockade of CTLA4 prevents induction of T-cell anergy, which occurs when CTLA4 on the T-cell surface binds B7 on APCs.(28) Ipilimumab, a monoclonal antibody directed at CTLA4, has recently shown a survival benefit over gp100 vaccine in a phase III evaluation in advanced melanoma.(29) A phase II study was conducted in patients with clear cell mRCC utilizing two distinct dosing regimens, either (1) 3 mg/kg IV followed by 1 mg/kg IV every 3 weeks, or (2) 3 mg/kg IV every 3 weeks.(30) Of 21 evaluable patients treated at the lower dose, one patient had a PR. In contrast, 5 of 40 patients (12.5%) treated at the higher dose had a PR. Enteritis/colitis and dermatitis were the most common adverse events associated with therapy. Interestingly, those patients that developed autoimmune toxicities in association with ipilimumab therapy were noted to have a higher response rate (30%). Although there are no other active studies of ipilimumab in mRCC, a phase I study is currently assessing MDX-1106 in association with ipilimumab therapy in patients with stage III or IV melanoma.(31) If well tolerated, the regimen may be of interest in mRCC. It is unknown whether ipilimumab can be combined safely with current approved VEGF- or mTOR-directed therapies. However, a concerning signal has emerged from a phase I study assessing the CTLA4-directed monoclonal tremelimumab in combination with sunitinib in patients with mRCC.(32) Specifically, rapid acute onset renal failure was noted in a subset of 28 patients enrolled on this study. One patient receiving continuous sunitinib at 37.5 mg daily with tremelimumab at 10 mg/kg experienced sudden death, and 3 of 6 patients receiving the same dose of sunitinib in combination with tremelimumab at 15 mg/kg experienced DLTs.
Programmed Death-1 (PD-1) Inhibition
The interaction between PD-1 and its ligands, PD-L1 and PD-L2, play an integral role in regulating T-cell function. PD-1 is a transmembrane receptor on the T-cell surface, whereas its ligands are present on the surface of the antigen presenting cell (APC). The association of PD-1 and either PD-L1 or –L2 leads to induction of T-cell anergy. Thus, disrupting this interaction is a putative strategy to enhance the antitumor immune response. MDX-1106 is a fully human IgG4 antibody blocking PD-1. In a phase I study including 39 patients with either melanoma, colorectal cancer, castration-resistant prostate cancer, non-small cell lung cancer, or RCC, MDX-1106 was administered at doses of up to 10 mg/kg IV.(33) Three patients (7.7%) exhibited responses to therapy, including 1 complete response (CR) in a patient with colorectal cancer and 2 PRs in patients with melanoma and mRCC. Irrespective of dose, a sustained inhibition of PD-1 was observed, with persistent binding in over 70% of circulating T-cells ≥ 2 months following infusion. A separate phase Ib study sought to determine the safety and efficacy of MDX-1106 in a larger cohort of patients with a similar spectrum of malignancies.(34) Of 126 patients treated, 18 patients had mRCC and 16 of these patients had received MDX-1106 at the maximum dose (10 mg/kg). Patients received MDX-1106 for a median of 7.6 months, and 5 of 16 patients (31.2%) treated at the maximum dose achieved a PR. Six patients (37.5%) achieved SD as a best response. The most common toxicities incurred with therapy were fatigue, rash, pruritus and diarrhea, and one patient died of sepsis after developing grade 4 pneumonitis.
A randomized, phase II study is currently underway to further examine MDX-1106 in mRCC.(35) The study will allocate patients to one of three dose levels of the agent – either 0.3 mg/kg IV every 3 weeks, 2 mg/kg IV every 3 weeks, or 10 mg/kg IV every 3 weeks. A total of 150 patients who have progressed on at least 1 prior anti-angiogenic agent will be enrolled, and accrual is anticipated to complete by April of 2013. Further development of the drug may also include exploration of relevant therapeutic combinations, such as MDX-1106 in combination with currently approved VEGF-TKIs or mTOR inhibitors.
Vaccine Therapy
A multitude of vaccine-based approaches have been devised for the treatment of mRCC. The agent IMA901 was derived through a comprehensive analysis of multiple tumor specimens, primarily consisting of RCC. Tumor associated antigens (TAAs) were identified, including 9 HLA-class I and 1 HLA-class II binding peptides. These TAAs were noted to be highly immunogenic. In a phase II study, 68 patients with clear cell mRCC who had failed primary therapy with either cytokines or VEGF-TKIs were randomized to receive up to 17 vaccinations with IMA901 over a 9 month period with or without a single dose of cyclophosphamide at 300 mg/m2.(36) Survival at 12 months and 18 months was 67% and 54%, respectively. Disease control rate (DCR) at 6 months was higher in patients who had failed prior immunotherapy as compared to the post-TKI group (31% v 12%). With respect to the contribution of cytotoxic chemotherapy, it was noted that Treg quantity 3 days following treatment was markedly reduced in patients who received cyclophosphamide as compared to those who did not (P=0.032).(37) Of interest, survival was improved in those patients who generated detectable T-cell responses to IMA901 (P=0.019). Of 31 patients who generated a multipeptide response, survival at 12 and 18 months was 73% and 63%, respectively. Furthermore, in 8 patients who had received prior cyclophosphamide and had a multipeptide response, 100% of patients were alive at these intervals. A potential caveat of this finding is that more debilitated patients may demonstrate a greater degree of anergy, and would be anticipated to have a poorer outcome. Comparison of patient characteristics in groups stratified by T-cell response could be useful.
Given the apparent efficacy and scant toxicity associated with IMA901 (the most common adverse event was mild infusion reactions), a phase III study is underway to evaluate the agent. In this study, 330 patients with treatment-naïve clear cell mRCC will the randomized to receive either sunitinib alone or sunitinib with IMA901 vaccinations over the course of 4 months.(38) Akin to the previously noted phase II experience, patients receiving IMA901 will additionally receive a single dose of cyclophosphamide and adjunctive GM-CSF therapy. The study is anticipated to complete accrual by April of 2014.
Autologous dendritic cell vaccines have recently established a role in prostate cancer therapy, with the approval of sipuleucel-T for asymptomatic or minimally symptomatic castration resistant disease.(39) A slightly distinct approach has been taken in the domain of mRCC. AGS-003 represents an autogolous immunotherapy product derived from matured dendritic cells that have electroporated in the presence of tumor-derived RNA and CD40 ligand (the latter binds to CD40 on APCs and triggers activation). In a phase II study, AGS-003 was administered to 25 subjects with newly-diagnosed mRCC in association with sunitinib therapy. The vaccine was administered every 3 weeks for a total of 5 doses, and then every 3 months until PD was observed. Of note, no good-risk patients were included in the study – in the intention-to-treat (ITT) population (n=21), 15 patients had intermediate-risk disease while 6 patients had poor-risk disease. PFS in this collective group was 12.5 months. Notably, PFS appeared to be correlated with decreased regulatory T-cell function (r2=0.7662). In addition, patients with a prolonged PFS (i.e., exceeding 10 months) were noted to have expansion of CD27+ memory T-cells. A phase III study assessing sunitinib with or without concomitant vaccination with AGS-003 is anticipated.
Allogeneic vaccines are also under study for mRCC, albeit in a more preliminary phase. Fifteen patients were treated in a phase I study assessing administration of irradiated cells derived from a modified RCC-26 cell line.(40) The modified cell line had increased immunogenic potential via IL-2 secretion and expression of CD80 co stimulatory molecules. The vaccine was administered at doses of up to 40 × 106 cells over 22 weeks in patients with at least one metastatic site. Although no PRs were encountered, a median PFS of 5.3 months was observed. Median OS in the study was 15.6 months. Notably, patients with delayed-type hypersensitivity skin reactions to the vaccine demonstrated a longer survival in this initial report. A distinct allogeneic vaccine, MGN1601, has also been assessed in patients with mRCC. The vaccine is generated from human RCC cells that have been modified to express IL-7, GM-CSF, CD80, and CD154.(41) The vaccine also contains the TLR9-agonist dSLIM-30L1.(42) In murine studies, the vaccine greatly enhanced autoimmune responses, increasing infiltration of CD4, CD8, and CD86 cells up to 20-fold. Phase I/II testing of MGN1601 began in November of 2009, and clinical data associated with this agent is eagerly awaited.
Cytotoxic Therapy: A Resurrection?
Cytotoxic agents are still often employed as a salvage approach for patients with mRCC – most frequently, combinations of fluoropyrimidines with the nucleoside analogue gemcitabine are employed. In a phase II study, 41 patients were treated with continuous infusion 5-fluorouracil (5-FU) and gemcitabine.(43) Of these patients, 23 (57%) had received two or more prior regimens (either chemotherapy or immunotherapy). In this heavily pre-treated population, a modest response rate was observed amongst 39 evaluable patients – 7 patients (17%) achieved a PR, while 5 further patients had minor responses. Several permutations of this regimen, including capecitabine with gemcitabine with or without targeted agents, have been reported in multiple subsequent studies.(44–48)
Since the phase II data of 5-FU/gemcitabine was reported in 2000, several other cytotoxic regimens have been attempted. Most recently, the agent S-1 was examined in a phase II clinical study. S-1 represents an oral agent combining three components: (1) tegafur, (2) potassium oxonate, and (3) 5-choloro-2,4-dihydroxypyridine.(49) The benefit of S-1 over other oral fluropyrimidine formulations is derived from the fact that the two additional biologic modifiers may increase the antitumor activity and reduce bowel toxicity associated with tegafur.(50) The phase II experience enrolled 45 patients with mRCC who had received nephrectomy in addition to cytokine therapy (or, alternatively, patients who were cytokine ineligible). Anorexia and neutropenia were the most frequently encountered grade 3/4 adverse events, occurring in 8.9% of patients. A total of 11 patients (24.4%) demonstrated a PR, while an additional 28 patients (62.2%) had SD as a best response. Median PFS for the overall study population was 9.2 months, while the median OS had not been reached with a median follow-up period of 21.7 months. PFS was significantly longer in patients with low thymidylate synthetase (TS) mRNA expression (P=0.006). Furthermore, the TS mRNA levels were noted to be lower in responders to S-1 therapy (P−0.048).
With the difficulties of cross-trial comparisons in mind, at first glance, these results appear to be somewhat comparable to the results achieved with currently available VEGF- directed therapies in the treatment-naïve and cytokine-refractory setting. However, no phase III trials are currently underway to further evaluate S-1 in this setting.
Several studies have also attempted to define the efficacy of ixabepilone, a novel epothilone with activity in breast cancer, in the setting of mRCC.(51–53) In one phase II experience, patients with mRCC with any number of prior therapies were treated with ixabepilone at 40 mg/m2 IV every 21 days.(54) In the first 12 patients enrolled, no objective response were observed and the median time to progression (TTP) was only 2.3 months. The most common grade 3/4 toxicities encountered were lymphopenia, neutropenia, diarrhea and infection. Using a distinct schedule of ixabepilone in a separate phase II study, a far better efficacy profile was achieved, albeit in a less heavily pre-treated population. In this study, a total of 87 patients with mRCC who had not received prior chemotherapy or targeted therapy were treated with ixabepilone at a dose of 6 mg/m2 IV daily for 5 days every 3 weeks. One CR was observed, and 10 patients further demonstrated a PR as a best response, yielding an overall response rate (ORR) of 12.4%. A further 59 patients (67.8%) demonstrated SD as a best response and the median TTP for the overall study population was 4.8 months. To facilitate comparisons to contemporaneous publications of data related to sunitinib and sorafenib, the authors of this study further reported OS data for patients with clear cell histology and Motzer grade 0 or 1 disease. In this cohort of 74 patients, median OS was 19.3 months.
Targeting MET in mRCC
MET has a number of purported roles in the pathogenesis of RCC. Over a decade ago, germ line and somatic mutations were identified in the tyrosine kinase domain of MET in patients with papillary RCC.(55) MET may also play a critical role in clear cell RCC – inactivation of VHL may actually cause constitutive activation of the moiety, and VHL null RCC cell lines appear to be exquisitely sensitive to MET shRNA.(56–57) Tissue microarray (TMA) data incorporating 317 unique RCC specimens suggested higher expression of MET in tumor tissue relative to paired normal tissue across histologic subtypes.(58) Furthermore, increased MET expression was associated with increased tumor grade (P=0.0019), advanced clinical stage (P=0.021), and decreased survival (P=0.017). For these reasons, targeting MET may have relevance across RCC histologies.
The dual VEGFR2/MET targeting agent, XL184, has recently shown unprecedented activity in the setting of metastatic castration resistant prostate cancer (mCRPC), causing regression of metastases visualized on bone scan in 56 of 65 evaluable patients (86%) enrolled in a randomized, phase II study.(59) Early experiences with XL184 also indicate substantial activity in ovarian cancer and medullary thyroid carcinoma.(60–61) Preliminary results from a drug-drug interaction study assessing the combination of XL184 with rosiglitazone (a CYP2C8 substrate) also indicates impressive activity. Patients on the study had either differentiated thyroid cancer or mRCC with a clear cell component. Amongst 9 patients with mRCC, 4 patients (44.4%) demonstrated a PR – 7 of these patients had received ≥ 2 prior therapies. Given these promising preliminary results, the further development plan for XL184 in mRCC is eagerly anticipated.
ARQ197 is a highly-selective small molecule inhibitor of MET. The agent was recently assessed in a phase I study including 51 patients with advanced solid tumors.(62) Uniquely, the study incorporated paired biopsies performed prior to treatment and either at day 2 or 15 of therapy. Only one patient with mRCC was enrolled in this effort. SD lasting ≥ 4 months was the best response observed in the study, although minor tumor regressions were noted in gastric and Merkel cell tumors. With respect to the extensive correlative analyses performed in this study, marked reductions in total c-MET and phosphorylated FAK were observed. A phase II study of ARQ197 in microphthalmia transcription (MiT)-associated tumors offers a slightly larger experience with the agent in RCC. Amongst 28 patients enrolled at the time of a preliminary report were 4 patients with mRCC. Three of these patients (75%) achieved SD as a best response.(63) Tentative plans exist within the Southwest Oncology Group (SWOG) to assess the agent in patients with papillary mRCC (either with or without erlotinib).(64)
MET-driven tumor growth appears to be contingent upon ligand-activation by hepatocyte-growth factor (HGF).(65) In a series of 45 patients with previously untreated clear cell RCC, levels of HGF were higher as compared to non-cancer controls (P<0.0001).(66) Interestingly, in the subset of patients with higher Fuhrman grades and advanced stages, cause-specific survival was superior in those patients with higher levels of HGF. No such association was found with levels of VEGF.
HGF blockade has been examined as an antitumor strategy in mRCC. AMG-102 represents a monoclonal antibody with affinity for HGF.(67) In one phase II study, 61 patients with mRCC of varying histology and degrees of prior therapy were enrolled.(68–69) Although one patient incurred a confirmed PR that was maintained for over 2.5 years, SD was the best response in the majority of subjects (26 patients, or 43%). Grade 3/4 events occurred in 33% of the study population, with edema representing the most frequent adverse event. An assessment of baseline plasma levels of HGF and soluble c-MET was performed, although no correlation with efficacy was observed. Although clinical evaluation of AMG-102 is underway in a variety of other malignancies, it is unclear whether further assessment will proceed in mRCC.(70–71)
Targeting Fibroblast Growth Factor Receptor (FGFR) in mRCC
Emerging evidence suggests that FGFR may play a critical role in RCC pathogenesis. In 38 patients with mRCC, therapy with sunitinib was rendered and serial plasma collections were conducted during therapy.(72) In those patients who progressed, significant rises in bFGF levels were observed (P<0.01) – in contrast, no significant changes were observed in bFGF levels in those patients who exhibited responses or SD. Several other reports similarly suggest increased FGFR signaling as an escape mechanism for VEGF antagonism.(73–74) Dovitinib (TKI-258) represents a small molecule inhibitor with affinity for FGFR1-3.(75) Preliminary phase II results are available from a phase I/II evaluation of dovitinib in patients with mRCC. In 51 patients evaluable for efficacy, 4 patients (8%) demonstrated a PR, while 19 patients (37%) had SD ≥ 4 months as a best response. Notably, 3 patients who obtained a PR had prior therapy with both VEGF- and mTOR-directed therapies. Median PFS and OS in the study population overall was 6.1 and 16 months, respectively. In 59 patients evaluable for safety, the most common adverse events were nausea, diarrhea and vomiting, although grade 3/4 events occurred at a relatively low rate for each of these toxicities (< 10%). Studies of dovitinib are ongoing in a variety of other malignancies, including breast, gastric, and urothelial carcinoma, and a phase III study is underway comparing dovitinib and sorafenib in patients with mRCC who have failed prior therapy with mTOR-and VEGF-directed therapy.(76–79) The study is anticipated to enroll a total of 550 patients by May of 2013, and will assess a primary endpoint of PFS. Investigations of distinct FGFR inhibitors (e.g., E-3810, brivanib, AZD4547, etc.) are also occurring simultaneously.(80–82) Amongst these agents, brivanib (a dual VEGFR/FGFR inhibitor) is being examined in a phase II trial in patients with clear cell mRCC that have progressed on prior VEGF-directed therapy.(83) Tumor assessments via 124I-cG250 PET/CT will be conducted in association with standard radiographic assessments in this study.(81)
Conclusions
Thus far, drug development in mRCC has followed a relatively predictable paradigm, with a steady stream of VEGF- and mTOR-directed therapies. A number of VEGF-antagonists remain in the pipeline, including axitinib and tivozanib.(84–89) Predicated on greater specificity and higher affinity for VEGF receptors, the clinical benefit of these agents over existing drugs appears to incremental at best. Multiple studies assessing the combination of VEGF- and mTOR-directed therapies are also underway.(90–92) Several combinations (i.e., bevacizumab with sunitinib) have been marred by substantial toxicity.(93) Early results from trials examining better tolerated regimens (i.e., bevacizumab with temsirolimus) appear to show little added efficacy.(94–96)
It therefore appears that substantial progress in the treatment of mRCC will be made by expanding beyond the existing paradigm and exploring novel pathways and therapeutic approaches. Several agents under investigation act on distinct moieties along the VEGF-/mTOR-signaling axis. For example, BEZ235 is a dual PI3K-/mTOR-inhibitor currently being examined in solid tumors – the agent appears to have activity in preclinical models of RCC.(97–99) BKM120 is a distinct PI3K inhibitor that is soon to be examined in a phase I study in combination with bevacizumab in patients with mRCC.(100–102) Data from several studies investigating the agent perifosine, a synthetic alkylphospholipid that modulates a number of signal transduction pathways including Akt, have indicated modest activity in patients refractor to both VEGFR and mTOR inhibitors.(103–104) Although clinical data for these agents are eagerly awaited, landmark improvements in clinical outcome for mRCC will more likely come from targeting entirely distinct pathways. Early efforts to do so have been fraught with challenges – ErbB-directed therapies (lapatinib and erlotinib), IL-6 targeting agents (CNTO-328), and thrombospondin-1 agonists (ABT-510) have yielded modest efficacy at best in the setting of mRCC, and have unclear development plans within the disease as a consequence.(105–107) However, many of the agents reviewed herein (vaccine therapies, PD-1 inhibitors, etc.) are based on an evolving understanding of RCC biology. While the goal of cure remains elusive, trials examining these agents represent a critical step forward.
Figure 1.
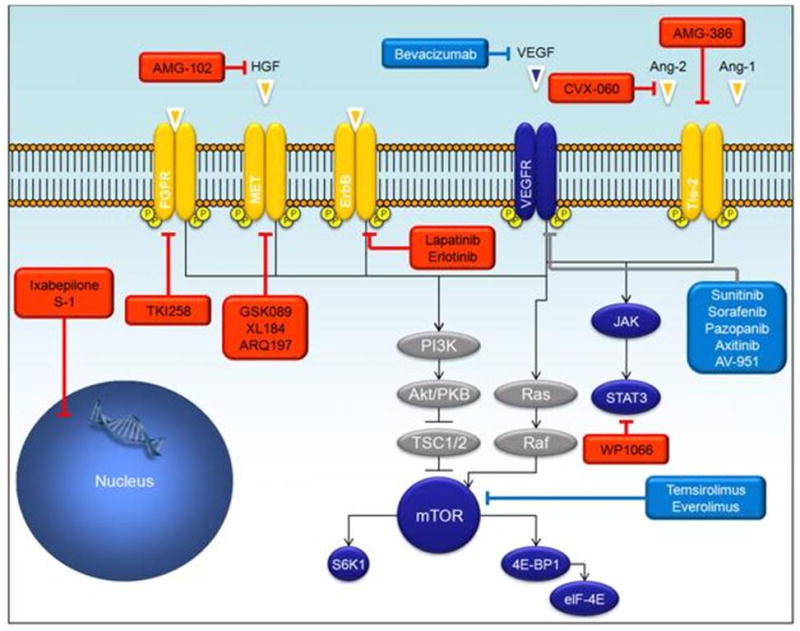
Current and future therapeutic strategies for metastatic renal cell carcinoma (mRCC). Boxes in red highlight strategies currently under investigation.
Figure 2.
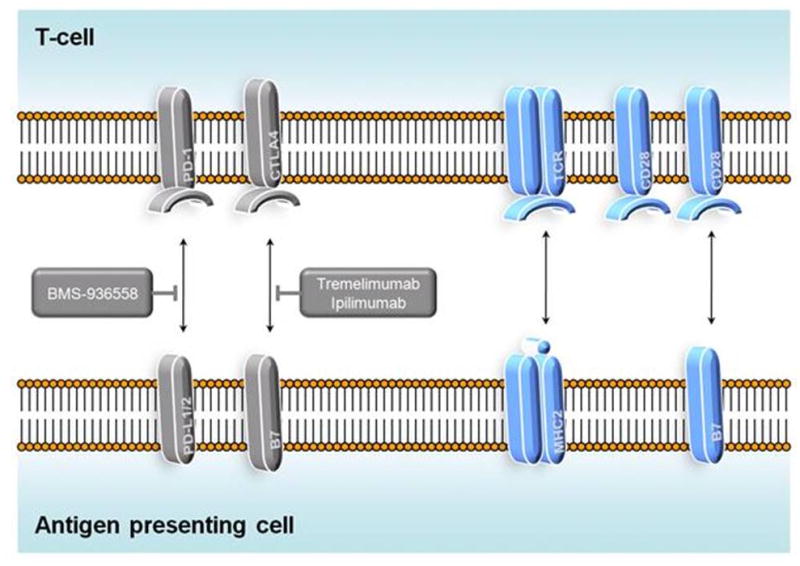
Mechanism of BMS-936558 and tremelimumab/ipilimumab. BMS-936558 binds to PD-1, blocking the interaction between PD-1 and its ligands, PD-L1 and PD-L2. This thereby prevents induction of T-cell anergy and theoretically promotes the antitumor immune response. Tremelimumab and ipilimumab block the interaction between CTLA4 and B7, thereby facilitating T-cell proliferation.
Table 1.
Selected novel therapies for mRCC discussed in the current manuscript.
| Therapeutic Class | Agent | Description |
|---|---|---|
| Angiogenesis Inhibitors | AMG-386 | Peptibody disrupting interaction between Ang-1/2 and Tie-2. |
| CVX-060 | Fusion protein comprised of two Ang-2 binding peptides | |
| CVX-241 | Fusion protein with affinity for both VEGF and Ang-2 | |
| Thalidomide | Immunomodulator; precise mechanism unknown | |
| Lenalidomide | Immunomodulator; precise mechanism unknown | |
| Immunotherapy | BMS-936558 (MDX-1106) | Fully human IgG4 blocking PD-1 |
| IMA901 | Vaccine derived from TAAs, including 9 HLA-class I and 1 HLA- class II binding peptides | |
| AGS-003 | Autologous dendritic cell vaccine generated through electroporation of tumor-derived RNA and CD40L | |
| MGN-1601 | Allogeneic vaccine comprised of human RCC cells modified to express IL-7, GM-CSF, CD80, and CD154 | |
| Ipilimumab | Monoclonal antibody with affinity for CTLA4 | |
| Cytotoxic Therapy | S-1 | Oral formulation comprised of tegafur, potassium oxonate, and 5- choloro-2,4-dihydroxypyridine |
| Ixabepilone | Novel epothilone inhibiting microtubule function | |
| Targeted Agents | XL184 | Small molecule inhibitor of VEGFR2 and MET |
| ARQ197 | Small molecule inhibitor of MET | |
| AMG-102 | Monoclonal antibody with affinity for HGF | |
| Dovitinib (TKI-258) | Small molecule inhibitor of FGFR1-3 | |
| Brivanib | Small molecule inhibitor of VEGFR and FGFR1-3 |
Acknowledgments
Support: Dr. Pal’s efforts are supported by CBCRP 15IB-0140 (California Breast Cancer Research Program Junior IDEA Award) and NIH K12 2K12CA001727-16A1. Dr. Quinn’s efforts are supported by NIH P30CA014089.
Contributor Information
Sumanta Kumar Pal, Email: spal@coh.org, Division of Genitourinary Oncology, Co-Director, Kidney Cancer Program, Department of Medical Oncology & Experimental Therapeutics, City of Hope Comprehensive Cancer Center, Phone: (626) 256-4673, Fax: (626) 301-8233.
Stephen Williams, Email: williams@ocurology.com, Associated Urologists of O.C., 1801 N Broadway, Santa Ana, CA 92706.
David Y. Josephson, Email: josephsond@towerurology.com, Tower Urology, 8635 West 3rd Street Suite 1 West, Los Angeles, CA 90048.
Courtney Carmichael, Email: ccarmichael@coh.org, Division of Genitourinary Malignancies, Department of Medical Oncology & Experimental Therapeutics, City of Hope Comprehensive Cancer Center, Phone: (626) 256-4673.
Nicholas J. Vogelzang, Email: nicholas.vogelzang@usoncology.com, Developmental Therapeutics and Co--chair, GU Committee, US Oncology Research, Comprehensive Cancer Centers NV, 3730 S. Eastern Ave., Las Vegas, NV 89169, Phone: (702) 952-3400.
David I. Quinn, Email: diquinn@usc.edu, Division of Cancer Medicine, Co-Leader, Developmental Therapeutics Program, University of Southern California Norris Comprehensive Cancer Center, 1441 Eastlake Avenue, Los Angeles, CA 90033, Phone: (323) 865-3956, Fax: (323) 8650061.
References
- 1.Fyfe G, Fisher RI, Rosenberg SA, Sznol M, Parkinson DR, Louie AC. Results of treatment of 255 patients with metastatic renal cell carcinoma who received high-dose recombinant interleukin-2 therapy. J Clin Oncol. 1995 Mar;13:688–96. doi: 10.1200/JCO.1995.13.3.688. [DOI] [PubMed] [Google Scholar]
- 2.Motzer RJ, Bacik J, Murphy BA, Russo P, Mazumdar M. Interferon-Alfa as a Comparative Treatment for Clinical Trials of New Therapies Against Advanced Renal Cell Carcinoma. J Clin Oncol. 2002 January 1;20:289–96. doi: 10.1200/JCO.2002.20.1.289. [DOI] [PubMed] [Google Scholar]
- 3.Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Oudard S, et al. Overall Survival and Updated Results for Sunitinib Compared With Interferon Alfa in Patients With Metastatic Renal Cell Carcinoma. J Clin Oncol. 2009 August 1;27:3584–90. doi: 10.1200/JCO.2008.20.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hudes G, Carducci M, Tomczak P, Dutcher J, Figlin R, Kapoor A, et al. Temsirolimus, Interferon Alfa, or Both for Advanced Renal-Cell Carcinoma. N Engl J Med. 2007 May 31;356:2271–81. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 5.Rini BI, Halabi S, Rosenberg JE, Stadler WM, Vaena DA, Archer L, et al. Phase III Trial of Bevacizumab Plus Interferon Alfa Versus Interferon Alfa Monotherapy in Patients With Metastatic Renal Cell Carcinoma: Final Results of CALGB 90206. J Clin Oncol. 2010 May 1;28:2137–43. doi: 10.1200/JCO.2009.26.5561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Escudier B, Bellmunt J, Negrier S, Bajetta E, Melichar B, Bracarda S, et al. Phase III Trial of Bevacizumab Plus Interferon Alfa-2a in Patients With Metastatic Renal Cell Carcinoma (AVOREN): Final Analysis of Overall Survival. J Clin Oncol. 2010 May 1;28:2144–50. doi: 10.1200/JCO.2009.26.7849. [DOI] [PubMed] [Google Scholar]
- 7.Sternberg CN, Davis ID, Mardiak J, Szczylik C, Lee E, Wagstaff J, et al. Pazopanib in Locally Advanced or Metastatic Renal Cell Carcinoma: Results of a Randomized Phase III Trial. J Clin Oncol. 2010 February 20;28:1061–8. doi: 10.1200/JCO.2009.23.9764. [DOI] [PubMed] [Google Scholar]
- 8.Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, et al. Sorafenib in Advanced Clear-Cell Renal-Cell Carcinoma. N Engl J Med. 2007 January 11;356:125–34. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 9.Motzer RJ, Escudier B, Oudard S, Hutson TE, Porta C, Bracarda S, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008 Aug 9;372:449–56. doi: 10.1016/S0140-6736(08)61039-9. [DOI] [PubMed] [Google Scholar]
- 10.Rini BI, Escudier B, Tomczak P, Kaprin A, Hutson TE, Szczylik C, et al. Axitinib versus sorafenib as second-line therapy for metastatic renal cell carcinoma (mRCC): Results of phase III AXIS trial. J Clin Oncol. 2011;29 (suppl; abstr 4503) [Google Scholar]
- 11.Bhargava P, Esteves B, Al-Adhami M, Nosov D, Lipatov ON, Lyulko AA, et al. Activity of tivozanib (AV-951) in patients with renal cell carcinoma (RCC): Subgroup analysis from a phase II randomized discontinuation trial (RDT) J Clin Oncol (Meeting Abstracts) 2010 May 20;28:4599. [Google Scholar]
- 12.Peters KG, Kontos CD, Lin PC, Wong AL, Rao P, Huang L, et al. Functional Significance of Tie2 Signaling in the Adult Vasculature. Recent Progress in Hormone Research. 2004;59:51–71. doi: 10.1210/rp.59.1.51. [DOI] [PubMed] [Google Scholar]
- 13.Papapetropoulos A, Fulton D, Mahboubi K, Kalb RG, O’Connor DS, Li F, et al. Angiopoietin-1 Inhibits Endothelial Cell Apoptosis via the Akt/Survivin Pathway. Journal of Biological Chemistry. 2000 March 31;275:9102–5. doi: 10.1074/jbc.275.13.9102. [DOI] [PubMed] [Google Scholar]
- 14.Oliner J, Min H, Leal J, Yu D, Rao S, You E, et al. Suppression of angiogenesis and tumor growth by selective inhibition of angiopoietin-2. Cancer Cell. 2004;6:507–16. doi: 10.1016/j.ccr.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 15.Currie MJ, Gunningham SP, Turner K, Han C, Scott PAE, Robinson BA, et al. Expression of the angiopoietins and their receptor Tie2 in human renal clear cell carcinomas; regulation by the von Hippel-Lindau gene and hypoxia. The Journal of Pathology. 2002;198:502–10. doi: 10.1002/path.1228. [DOI] [PubMed] [Google Scholar]
- 16.Bullock AJ, Zhang L, O’Neill AM, Percy A, Sukhatme V, Mier JW, et al. Plasma angiopoietin-2 (ANG2) as an angiogenic biomarker in renal cell carcinoma (RCC) J Clin Oncol (Meeting Abstracts) 2010 May 20;28:4630. [Google Scholar]
- 17.Herbst RS, Hong D, Chap L, Kurzrock R, Jackson E, Silverman JM, et al. Safety, Pharmacokinetics, and Antitumor Activity of AMG 386, a Selective Angiopoietin Inhibitor, in Adult Patients With Advanced Solid Tumors. Journal of Clinical Oncology. 2009 July 20;27:3557–65. doi: 10.1200/JCO.2008.19.6683. [DOI] [PubMed] [Google Scholar]
- 18.Rini BI, Szczylik C, Tannir NM, Koralewski P, Tomczak P, Deptala A, et al. AMG 386 in combination with sorafenib in patients (pts) with metastatic renal cell cancer (mRCC): A randomized, double-blind, placebo-controlled, phase II study. Presented at the 2011 Genitourinary Cancers Symposium [Abstr 309]; 2011. [Google Scholar]
- 19.Rosen LS, Mendelson DS, Cohen RB, Gordon MS, Goldman JW, Bear IK, et al. First-in-human dose-escalation safety and PK trial of a novel intravenous humanized monoclonal CovX body inhibiting angiopoietin 2. ASCO Meeting Abstracts. 2010;28:2524. [Google Scholar]
- 20. [last accessed December 12, 2011]; NCT01441414: PF-04856884 (CVX-060) In Combination With Axitinib In Patients With Previously Treated Metastatic Renal Cell Carcinoma. Available at http://www.clinicaltrials.gov.
- 21.Coxon A, Bready J, Min H, Kaufman S, Leal J, Yu D, et al. Context-Dependent Role of Angiopoietin-1 Inhibition in the Suppression of Angiogenesis and Tumor Growth: Implications for AMG 386, an Angiopoietin-1/2–Neutralizing Peptibody. Molecular Cancer Therapeutics. 2010;9:2641–51. doi: 10.1158/1535-7163.MCT-10-0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.LeBlanc R, Hideshima T, Catley LP, Shringarpure R, Burger R, Mitsiades N, et al. Immunomodulatory drug costimulates T cells via the B7-CD28 pathway. Blood. 2004;103:1787–90. doi: 10.1182/blood-2003-02-0361. [DOI] [PubMed] [Google Scholar]
- 23.Escudier B, Lassau N, Couanet D, Angevin E, Mesrati F, Leborgne S, et al. Phase II trial of thalidomide in renal-cell carcinoma. Annals of Oncology. 2002 July 1;13:1029–35. doi: 10.1093/annonc/mdf213. [DOI] [PubMed] [Google Scholar]
- 24.Hernberg M, Virkkunen P, Bono P, Ahtinen H, Mäenpää H, Joensuu H. Interferon Alfa-2b Three Times Daily and Thalidomide in the Treatment of Metastatic Renal Cell Carcinoma. Journal of Clinical Oncology. 2003 October 15;21:3770–6. doi: 10.1200/JCO.2003.01.536. [DOI] [PubMed] [Google Scholar]
- 25. [last accessed August 8, 2011]; NCT00005966: Interferon Alfa-2b With or Without Thalidomide in Treating Patients With Metastatic or Unresectable Kidney Cancer. Available at http://www.clinicaltrials.gov.
- 26.Choueiri TK, Dreicer R, Rini BI, Elson P, Garcia JA, Thakkar SG, et al. Phase II study of lenalidomide in patients with metastatic renal cell carcinoma. Cancer. 2006;107:2609–16. doi: 10.1002/cncr.22290. [DOI] [PubMed] [Google Scholar]
- 27.Amato RJ, Hernandez-McClain J, Saxena S, Khan M. Lenalidomide Therapy for Metastatic Renal Cell Carcinoma. American Journal of Clinical Oncology. 2008;31:244–9. doi: 10.1097/COC.0b013e31815e451f. [DOI] [PubMed] [Google Scholar]
- 28.Weber J. Ipilimumab: controversies in its development, utility and autoimmune adverse events. Cancer Immunol Immunother. 2009 May;58:823–30. doi: 10.1007/s00262-008-0653-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010 Aug 19;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang JC, Hughes M, Kammula U, Royal R, Sherry RM, Topalian SL, et al. Ipilimumab (anti-CTLA4 antibody) causes regression of metastatic renal cell cancer associated with enteritis and hypophysitis. J Immunother. 2007 Nov–Dec;30:825–30. doi: 10.1097/CJI.0b013e318156e47e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. [last accessed August 8, 2011]; NCT01024231: A Phase 1b, Open-label, Multicenter, Multidose, Dose-escalation Study of BMS-936558 (MDX-1106) in Combination With Ipilimumab in Subjects With Unresectable Stage III or Stage IV Malignant Melanoma. Available at http://www.clinicaltrials.gov.
- 32.Rini BI, Stein M, Shannon P, Eddy S, Tyler A, Stephenson JJ, et al. Phase 1 dose-escalation trial of tremelimumab plus sunitinib in patients with metastatic renal cell carcinoma. Cancer. 2011;117:758–67. doi: 10.1002/cncr.25639. [DOI] [PubMed] [Google Scholar]
- 33.Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, et al. Phase I Study of Single-Agent Anti–Programmed Death-1 (MDX-1106) in Refractory Solid Tumors: Safety, Clinical Activity, Pharmacodynamics, and Immunologic Correlates. Journal of Clinical Oncology. 2010 July 1;28:3167–75. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McDermott DF, Drake CG, Sznol M, Sosman JA, Smith DC, Powderly JD, et al. A phase I study to evaluate safety and antitumor activity of biweekly BMS-936558 (Anti-PD-1, MDX-1106/ONO-4538) in patients with RCC and other advanced refractory malignancies. Presented at the 2011 Genitourinary Cancers Symposium [Abstr 331]; 2011. [Google Scholar]
- 35. [last accessed August 8, 2011];NCT0135443: A Randomized, Blinded, Phase 2 Dose-Ranging Study Of BMS-936558 (MDX-1106) In Subjects With Progressive, Advanced/Metastatic Clear-Cell Renal Cell Carcinoma Who Have Received Prior Anti-Angiogenic Therapy. Available at http://www.clinicaltrials.gov.
- 36.Reinhardt C, Zdrojowy R, Szczylik C, Ciuleanu T, Brugger W, Oberneder R, et al. Results of a randomized phase II study investigating multipeptide vaccination with IMA901 in advanced renal cell carcinoma (RCC) J Clin Oncol (Meeting Abstracts) 2010 May 20;28:4529. [Google Scholar]
- 37.Singh H, Hilf N, Mendrzyk R, Maurer D, Weinschenk T, Kirner A, et al. Correlation of immune responses with survival in a randomized phase II study investigating multipeptide vaccination with IMA901 plus or minus low-dose cyclophosphamide in advanced renal cell carcinoma (RCC) ASCO Meeting Abstracts. 2010;28:2587. [Google Scholar]
- 38.Rerksuppaphol S, Rerksuppaphol L. Lactobacillus acidophilus and Bifidobacterium bifidum stored at ambient temperature are effective in the treatment of acute diarrhoea. Ann Trop Paediatr. 2010;30:299–304. doi: 10.1179/146532810X12858955921159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010 Jul 29;363:411–22. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 40.Buchner A, Pohla H, Willimsky G, Frankenberger B, Frank R, Baur-Melnyk A, et al. Phase 1 Trial of Allogeneic Gene-Modified Tumor Cell Vaccine RCC-26/CD80/IL-2 in Patients with Metastatic Renal Cell Carcinoma. Human Gene Therapy. 2010;21:285–97. doi: 10.1089/hum.2008.192. [DOI] [PubMed] [Google Scholar]
- 41.Volz B, Schmidt M, Kapp K, Schroff M, Tschaika M, Wittig B. Preclinical efficacy data of MGN1601, a tumor vaccine comprising 4-fold gene-modified and irradiated allogeneic tumor cells in combination with a DNA-based immunomodulator for the treatment of metastatic renal carcinoma. ASCO Meeting Abstracts. 2010 June 14;28:e15067. [Google Scholar]
- 42.Schmidt M, Volz B, Schroff M, Kapp K, Kleuss C, Tschaika M, et al. Safety data of MGN1601, a tumor vaccine, made of allogeneic, transfected, and irradiated tumor cells in combination with an immunomodulator for the treatment of metastatic renal cell carcinoma. ASCO Meeting Abstracts. 2010 June 14;28:e15104. [Google Scholar]
- 43.Rini BI, Vogelzang NJ, Dumas MC, Wade JL, Taber DA, Stadler WM. Phase II Trial of Weekly Intravenous Gemcitabine With Continuous Infusion Fluorouracil in Patients With Metastatic Renal Cell Cancer. Journal of Clinical Oncology. 2000;18:2419–26. doi: 10.1200/JCO.2000.18.12.2419. [DOI] [PubMed] [Google Scholar]
- 44.Waters JS, Moss C, Pyle L, James M, Hackett S, A’Hern R, et al. Phase II clinical trial of capecitabine and gemcitabine chemotherapy in patients with metastatic renal carcinoma. Br J Cancer. 2004;91:1763–8. doi: 10.1038/sj.bjc.6602209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rini BI, Weinberg V, Small EJ. A phase I trial of fixed dose rate gemcitabine and capecitabine in metastatic renal cell carcinoma. Cancer. 2005 Feb 1;103:553–8. doi: 10.1002/cncr.20795. [DOI] [PubMed] [Google Scholar]
- 46.Bellmunt J, Trigo JM, Calvo E, Carles J, Perez-Gracia JL, Rubio J, et al. Activity of a multitargeted chemo-switch regimen (sorafenib, gemcitabine, and metronomic capecitabine) in metastatic renal-cell carcinoma: a phase 2 study (SOGUG-02-06) Lancet Oncol. 2010 Apr;11:350–7. doi: 10.1016/S1470-2045(09)70383-3. [DOI] [PubMed] [Google Scholar]
- 47.Soga N, Yamada Y, Nishikawa K, Hasegawa Y, Kise H, Arima K, et al. Gemcitabine and capecitabine chemotherapy in Japanese patients with immunotherapy-resistant renal cell carcinoma. Int J Urol. 2009 Jun;16:576–9. doi: 10.1111/j.1442-2042.2009.02308.x. [DOI] [PubMed] [Google Scholar]
- 48.Amato RJ, Khan M. A phase I clinical trial of low-dose interferon-alpha-2A, thalidomide plus gemcitabine and capecitabine for patients with progressive metastatic renal cell carcinoma. Cancer Chemother Pharmacol. 2008 May;61:1069–73. doi: 10.1007/s00280-007-0568-7. [DOI] [PubMed] [Google Scholar]
- 49.Naito S, Eto M, Shinohara N, Tomita Y, Fujisawa M, Namiki M, et al. A phase II study of S-1 for the treatment of cytokine-refractory metastatic renal cell carcinoma. ASCO Meeting Abstracts. 2009 June 8;27:5100. [Google Scholar]
- 50.Macdonald JS. Gastric Cancer: Nagoya Is Not New York. Journal of Clinical Oncology. 2011;29:4348–50. doi: 10.1200/JCO.2011.37.5691. [DOI] [PubMed] [Google Scholar]
- 51.Roche H, Yelle L, Cognetti F, Mauriac L, Bunnell C, Sparano J, et al. Phase II clinical trial of ixabepilone (BMS-247550), an epothilone B analog, as first-line therapy in patients with metastatic breast cancer previously treated with anthracycline chemotherapy. J Clin Oncol. 2007 Aug 10;25:3415–20. doi: 10.1200/JCO.2006.09.7535. [DOI] [PubMed] [Google Scholar]
- 52.Denduluri N, Low JA, Lee JJ, Berman AW, Walshe JM, Vatas U, et al. Phase II trial of ixabepilone, an epothilone B analog, in patients with metastatic breast cancer previously untreated with taxanes. J Clin Oncol. 2007 Aug 10;25:3421–7. doi: 10.1200/JCO.2006.10.0784. [DOI] [PubMed] [Google Scholar]
- 53.Thomas ES. Ixabepilone plus capecitabine for metastatic breast cancer progressing after anthracycline and taxane treatment. J Clin Oncol. 2008 May 1;26:2223. doi: 10.1200/JCO.2008.16.5019. [DOI] [PubMed] [Google Scholar]
- 54.Posadas EM, Undevia S, Manchen E, Wade JL, Colevas AD, Karrison T, et al. A phase II study of ixabepilone (BMS-247550) in metastatic renal-cell carcinoma. Cancer Biol Ther. 2007 Apr;6:490–3. doi: 10.4161/cbt.6.4.3831. [DOI] [PubMed] [Google Scholar]
- 55.Schmidt L, Duh F-M, Chen F, Kishida T, Glenn G, Choyke P, et al. Germline and somatic mutations in the tyrosine kinase domain of the MET proto-oncogene in papillary renal carcinomas. Nat Genet. 1997;16:68–73. doi: 10.1038/ng0597-68. [DOI] [PubMed] [Google Scholar]
- 56.Bommi-Reddy A, Almeciga I, Sawyer J, Geisen C, Li W, Harlow E, et al. Kinase requirements in human cells: III. Altered kinase requirements in VHL−/− cancer cells detected in a pilot synthetic lethal screen. Proceedings of the National Academy of Sciences. 2008;105:16484–9. doi: 10.1073/pnas.0806574105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nakaigawa N, Yao M, Baba M, Kato S, Kishida T, Hattori K, et al. Inactivation of von Hippel-Lindau Gene Induces Constitutive Phosphorylation of MET Protein in Clear Cell Renal Carcinoma. Cancer Research. 2006 April 1;66:3699–705. doi: 10.1158/0008-5472.CAN-05-0617. [DOI] [PubMed] [Google Scholar]
- 58.Gibney G, Conrad P, Aziz SA, Camp RL, Schwartz BE, Chen C, et al. C-met as a therapeutic target using ARQ 197 in renal cell carcinoma. ASCO Meeting Abstracts. 2011;29:360. [Google Scholar]
- 59.Hussain M, Smith MR, Sweeney C, Corn PG, Elfiky A, Gordon MS, et al. Cabozantinib (XL184) in metastatic castration-resistant prostate cancer (mCRPC): Results from a phase II randomized discontinuation trial. ASCO Meeting Abstracts. 2011;29:4516. [Google Scholar]
- 60.Buckanovich RJ, Berger R, Sella A, Sikic BI, Shen X, Ramies DA, et al. Activity of cabozantinib (XL184) in advanced ovarian cancer patients (pts): Results from a phase II randomized discontinuation trial (RDT) ASCO Meeting Abstracts. 2011;29:5008. [Google Scholar]
- 61.Salgia R, Sherman S, Hong DS, Ng CS, Frye J, Janisch L, et al. A phase I study of XL184, a RET, VEGFR2, and MET kinase inhibitor, in patients (pts) with advanced malignancies, including pts with medullary thyroid cancer (MTC) ASCO Meeting Abstracts. 2008;26:3522. [Google Scholar]
- 62.Yap TA, Olmos D, Brunetto AT, Tunariu N, Barriuso J, Riisnaes R, et al. Phase I Trial of a Selective c-MET Inhibitor ARQ 197 Incorporating Proof of Mechanism Pharmacodynamic Studies. Journal of Clinical Oncology. 2011;29:1271–9. doi: 10.1200/JCO.2010.31.0367. [DOI] [PubMed] [Google Scholar]
- 63.Goldberg J, Demetri GD, Choy E, Rosen L, Pappo A, Dubois S, et al. Preliminary results from a phase II study of ARQ 197 in patients with microphthalmia transcription factor family (MiT)-associated tumors. ASCO Meeting Abstracts. 2009 June 8;27:10502. [Google Scholar]
- 64.Twardowski P. Personal Communication. Aug 28, 2011.
- 65.Michieli P, Basilico C, Pennacchietti S, Maffe A, Tamagnone L, Giordano S, et al. Mutant Met-mediated transformation is ligand-dependent and can be inhibited by HGF antagonists. Oncogene. 1999 Sep 16;18:5221–31. doi: 10.1038/sj.onc.1202899. [DOI] [PubMed] [Google Scholar]
- 66.Tanimoto S, Fukumori T, El-Moula G, Shiirevnyamba A, Kinouchi S, Koizumi T, et al. Prognostic significance of serum hepatocyte growth factor in clear cell renal cell carcinoma: comparison with serum vascular endothelial growth factor. The Journal of Medical Investigation. 2008;55:106–11. doi: 10.2152/jmi.55.106. [DOI] [PubMed] [Google Scholar]
- 67.Burgess TL, Sun J, Meyer S, Tsuruda TS, Elliott G, Chen Q, et al. Biochemical characterization of AMG 102: a neutralizing, fully human monoclonal antibody to human and nonhuman primate hepatocyte growth factor. Mol Cancer Ther. 2010 Feb;9:400–9. doi: 10.1158/1535-7163.MCT-09-0824. [DOI] [PubMed] [Google Scholar]
- 68.Schöffski P, Garcia JA, Stadler WM, Gil T, Jonasch E, Tagawa ST, et al. A phase II study of the efficacy and safety of AMG 102 in patients with metastatic renal cell carcinoma. BJU International. 2011 doi: 10.1111/j.1464-410X.2010.09947.x. no-no. [DOI] [PubMed] [Google Scholar]
- 69.Gordon MS, Sweeney CS, Mendelson DS, Eckhardt SG, Anderson A, Beaupre DM, et al. Safety, pharmacokinetics, and pharmacodynamics of AMG 102, a fully human hepatocyte growth factor-neutralizing monoclonal antibody, in a first-in-human study of patients with advanced solid tumors. Clin Cancer Res. 2010 Jan 15;16:699–710. doi: 10.1158/1078-0432.CCR-09-1365. [DOI] [PubMed] [Google Scholar]
- 70.Wen PY, Schiff D, Cloughesy TF, Raizer JJ, Laterra J, Smitt M, et al. A phase II study evaluating the efficacy and safety of AMG 102 (rilotumumab) in patients with recurrent glioblastoma. Neuro Oncol. 2011 Apr;13:437–46. doi: 10.1093/neuonc/noq198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rosen PJ, Sweeney CJ, Park DJ, Beaupre DM, Deng H, Leitch IM, et al. A phase Ib study of AMG 102 in combination with bevacizumab or motesanib in patients with advanced solid tumors. Clin Cancer Res. 2010 May 1;16:2677–87. doi: 10.1158/1078-0432.CCR-09-2862. [DOI] [PubMed] [Google Scholar]
- 72.Tsimafeyeu I, Demidov L, Ta H, Stepanova E, Wynn N. Fibroblast growth factor pathway in renal cell carcinoma. ASCO Meeting Abstracts. 2010;28:4621. doi: 10.3109/00365599.2011.552436. [DOI] [PubMed] [Google Scholar]
- 73.Casanovas O, Hicklin DJ, Bergers G, Hanahan D. Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer Cell. 2005;8:299–309. doi: 10.1016/j.ccr.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 74.Alessi P, Leali D, Camozzi M, Cantelmo A, Albini A, Presta M. Anti-FGF2 approaches as a strategy to compensate resistance to anti-VEGF therapy: long-pentraxin 3 as a novel antiangiogenic FGF2-antagonist. Eur Cytokine Netw. 2009 Dec;20:225–34. doi: 10.1684/ecn.2009.0175. [DOI] [PubMed] [Google Scholar]
- 75.Sarker D, Molife R, Evans TRJ, Hardie M, Marriott C, Butzberger-Zimmerli P, et al. A Phase I Pharmacokinetic and Pharmacodynamic Study of TKI258, an Oral, Multitargeted Receptor Tyrosine Kinase Inhibitor in Patients with Advanced Solid Tumors. Clinical Cancer Research. 2008 April 1;14:2075–81. doi: 10.1158/1078-0432.CCR-07-1466. [DOI] [PubMed] [Google Scholar]
- 76.Andre F, Bachelot TD, Campone M, Dalenc F, Perez-Garcia JM, Hurvitz SA, et al. A multicenter, open-label phase II trial of dovitinib, an FGFR1 inhibitor, in FGFR1 amplified and non-amplified metastatic breast cancer. ASCO Meeting Abstracts. 2011;29:508. [Google Scholar]
- 77.Milowsky MI, Carlson GL, Shi MM, Urbanowitz G, Zhang Y, Sternberg CN. A multicenter, open-label phase II trial of dovitinib (TKI258) in advanced urothelial carcinoma patients with either mutated or wild-type FGFR3. ASCO Meeting Abstracts. 2011;29:TPS186. [Google Scholar]
- 78.Tai WMD, Ooi WS, Ngeow JY, Deng N, Wang H, Tham CK, et al. A phase I study of dovitinib in combination with capecitabine and oxaliplatin in upfront treatment of advanced colorectal and gastric cancer with a dose expansion cohort in advanced gastric cancer. ASCO Meeting Abstracts. 2011;29:TPS174. [Google Scholar]
- 79. [last accessed March 19, 2011]; NCT01223027: An Open-label, Randomized, Multi-center, Phase III Study to Compare the Safety and Efficacy of TKI258 Versus Sorafenib in Patients With Metastatic Renal Cell Carcinoma After Failure of Anti-angiogenic (VEGF-targeted and mTOR Inhibitor) Therapies. Available at http://www.clinicaltrials.gov.
- 80. [last accessed August 27, 2011]; NCT01283945: An Open-Label, Dose-escalation, Phase I Study to Determine the Maximum Tolerated Dose, Recommended Dose, Pharmacokinetics, and Pharmacodynamics of the Dual VEGFR-FGFR Tyrosine Kinase Inhibitor, E-3810, Given Orally as Single Agent to Patients With Advanced Solid Tumours. Available at http://www.clinicaltrials.gov.
- 81. [last accessed March 26, 2011]; NCT01253668: Brivanib (BMS-582664, Brivanib Alaninate) in Treatment of Refractory Metastatic Renal Cell Carcinoma - A Phase II Pharmacodynamic and Baseline Biomarker Study. Available on-line at http://www.clinicaltrials.gov.
- 82. [last accessed August 28, 2011]; NCT01202591: A Randomised Double-blind Phase IIa Study (With Combination Safety Run-in) to Assess the Safety and Efficacy of AZD4547 in Combination With Exemestane vs Exemestane Alone in ER+ Breast Cancer Patients With FGFR1 Polysomy or Gene Amplification Who Have Progressed Following Treatment With One Prior Endocrine Therapy (Adjuvant or First-line Metastatic) (GLOW) Available at http://www.clinicaltrials.gov.
- 83.Bhide RS, Lombardo LJ, Hunt JT, Cai ZW, Barrish JC, Galbraith S, et al. The antiangiogenic activity in xenograft models of brivanib, a dual inhibitor of vascular endothelial growth factor receptor-2 and fibroblast growth factor receptor-1 kinases. Mol Cancer Ther. 2010 Feb;9:369–78. doi: 10.1158/1535-7163.MCT-09-0472. [DOI] [PubMed] [Google Scholar]
- 84.Rini BI, Escudier B, Tomczak P, Kaprin A, Hutson TE, Szczylik C, et al. Axitinib versus sorafenib as second-line therapy for metastatic renal cell carcinoma (mRCC): Results of phase III AXIS trial. ASCO Meeting Abstracts. 2011;29:4503. [Google Scholar]
- 85.Bhargava P, Esteves B, Al-Adhami M, Nosov D, Lipatov ON, Lyulko AA, et al. Activity of tivozanib (AV-951) in patients (Pts) with different histologic subtypes of renal cell carcinoma (RCC) ASCO Meeting Abstracts. 2011;29:327. [Google Scholar]
- 86.Rini BI, Wilding G, Hudes G, Stadler WM, Kim S, Tarazi J, et al. Phase II Study of Axitinib in Sorafenib-Refractory Metastatic Renal Cell Carcinoma. J Clin Oncol. 2009 September 20;27:4462–8. doi: 10.1200/JCO.2008.21.7034. [DOI] [PubMed] [Google Scholar]
- 87.Rixe O, Bukowski RM, Michaelson MD, Wilding G, Hudes GR, Bolte O, et al. Axitinib treatment in patients with cytokine-refractory metastatic renal-cell cancer: a phase II study. Lancet Oncol. 2007 Nov;8:975–84. doi: 10.1016/S1470-2045(07)70285-1. [DOI] [PubMed] [Google Scholar]
- 88.Kabbinavar FF, Srinivas S, Hauke RJ, Amato RJ, Esteves B, Dhillon R, et al. A phase I trial of combined tivozanib (AV-951) and temsirolimus therapy in patients (pts) with renal cell carcinoma (RCC) ASCO Meeting Abstracts. 2011;29:330. [Google Scholar]
- 89.Nosov D, Bhargava P, Esteves WB, Strahs AL, Lipatov ON, Lyulko OO, et al. Final analysis of the phase II randomized discontinuation trial (RDT) of tivozanib (AV-951) versus placebo in patients with renal cell carcinoma (RCC) ASCO Meeting Abstracts. 2011;29:4550. [Google Scholar]
- 90. [Accessed December 22, 2009]; NCT00719264: A Randomized, Open-label, Multi-center Phase II Study to Compare Bevacizumab Plus RAD001 Versus Interferon Alfa-2a Plus Bevacizumab for the First-line Treatment of Patients With Metastatic Clear Cell Carcinoma of the Kidney. Available at: http://www.clinicaltrials.gov.
- 91. [last accessed March 29, 2010]; NCT00631371: Phase 3b, Randomized, Open-Label Study of Bevacizumab + Temsirolimus ) vs. Bevacizumab + Interferon-Alfa as First-Line Treatment in Subjects With Advanced Renal Cell Carcinoma. Available at http://www.clinicaltrials.gov.
- 92. [Accessed December 22, 2009]; NCT00378703: The BeST Trial: A Randomized Phase II Study of VEGF, RAF Kinase, and mTOR Combination Targeted Therapy (CTT) With Bevacizumab, Sorafenib and Temsirolimus in Advanced Renal Cell Carcinoma [BeST] Available at: http://www.clinicaltrials.gov.
- 93.Rini BI, Garcia JA, Cooney MM, Elson P, Tyler A, Beatty K, et al. Toxicity of Sunitinib Plus Bevacizumab in Renal Cell Carcinoma. J Clin Oncol. 2010 June 10;28:e284–5. doi: 10.1200/JCO.2009.27.1759. [DOI] [PubMed] [Google Scholar]
- 94.Escudier BJ, Negrier S, Gravis G, Chevreau C, Delva R, Bay J, et al. Can the combination of temsirolimus and bevacizumab improve the treatment of metastatic renal cell carcinoma (mRCC)? Results of the randomized TORAVA phase II trial. J Clin Oncol (Meeting Abstracts) 2010 May 20;28:4516. [Google Scholar]
- 95.Escudier BJ, Negrier S, Perol D, Gravis G, Delva R, Bay J, et al. Prognostic factors for progression-free survival (PFS) in patients with metastatic renal cell carcinoma (mRCC): Results from the French randomized phase II study TORAVA. ASCO Meeting Abstracts. 2011;29:e15118. [Google Scholar]
- 96.Escudier BJ, Perol D, Ferlay C, Gravis G, Chevreau C, Delva R, et al. TORAVA trial: Lessons from this trial in the two control arms, sunitinib and bevacizumab in combination with interferon. Presented at the 2011 Genitourinary Cancers Symposium; p. Abstr 315. [Google Scholar]
- 97.Cho DC, Cohen MB, Panka DJ, Collins M, Ghebremichael M, Atkins MB, et al. The Efficacy of the Novel Dual PI3-Kinase/mTOR Inhibitor NVP-BEZ235 Compared with Rapamycin in Renal Cell Carcinoma. Clinical Cancer Research. 2010 July 15;16:3628–38. doi: 10.1158/1078-0432.CCR-09-3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bottino D, Tabernero J, Burris HA, Britten C, Chen LC, Bendell JC, et al. Clinical pharmacokinetic-pharmacodynamic (PK/PD) modeling study of the novel dual PI3K/mTOR inhibitor BEZ235. ASCO Meeting Abstracts. 2011;29:3056. [Google Scholar]
- 99.Peyton JD, Rodon Ahnert J, Burris H, Britten C, Chen LC, Tabernero J, et al. A dose-escalation study with the novel formulation of the oral pan-class I PI3K inhibitor BEZ235, solid dispersion system (SDS) sachet, in patients with advanced solid tumors. ASCO Meeting Abstracts. 2011;29:3066. [Google Scholar]
- 100. [last accessed August 29, 2011]; NCT01283048: A Phase I Study of Bevacizumab and Escalation Doses of BKM-120 in Patients With Metastatic Renal Cell Carcinoma Who Failed Prior Systemic Therapies. Available at http://www.clinicaltrials.gov.
- 101.Baselga J, De Jonge MJ, Rodon J, Burris HA, Birle DC, De Buck SS, et al. A first-in-human phase I study of BKM120, an oral pan-class I PI3K inhibitor, in patients (pts) with advanced solid tumors. ASCO Meeting Abstracts. 2010;28:3003. [Google Scholar]
- 102.Grana B, Burris HA, Rodon Ahnert J, Abdul Razak AR, De Jonge MJ, Eskens F, et al. Oral PI3 kinase inhibitor BKM120 monotherapy in patients (pts) with advanced solid tumors: An update on safety and efficacy. ASCO Meeting Abstracts. 2011;29:3043. [Google Scholar]
- 103.Cho DC, Figlin RA, Flaherty KT, Michaelson D, Sosman JA, Ghebremichael M, et al. A phase II trial of perifosine in patients with advanced renal cell carcinoma (RCC) who have failed tyrosine kinase inhibitors (TKI) ASCO Meeting Abstracts. 2009 June 8;27:5101. [Google Scholar]
- 104.Vogelzang NJ, Hutson TE, Samlowski W, Somer B, Richey S, Alemany C, et al. Phase II study of perifosine in metastatic renal cell carcinoma (RCC) progressing after prior therapy (Rx) with a VEGF receptor inhibitor. ASCO Meeting Abstracts. 2009 June 8;27:5034. [Google Scholar]
- 105.Ravaud A, Hawkins R, Gardner JP, von der Maase H, Zantl N, Harper P, et al. Lapatinib Versus Hormone Therapy in Patients With Advanced Renal Cell Carcinoma: A Randomized Phase III Clinical Trial. J Clin Oncol. 2008 May 10;26:2285–91. doi: 10.1200/JCO.2007.14.5029. [DOI] [PubMed] [Google Scholar]
- 106.Ebbinghaus S, Hussain M, Tannir N, Gordon M, Desai AA, Knight RA, et al. Phase 2 Study of ABT-510 in Patients with Previously Untreated Advanced Renal Cell Carcinoma. Clinical Cancer Research. 2007 November 15;13:6689–95. doi: 10.1158/1078-0432.CCR-07-1477. [DOI] [PubMed] [Google Scholar]
- 107.Rossi JF, Negrier S, James ND, Kocak I, Hawkins R, Davis H, et al. A phase I/II study of siltuximab (CNTO 328), an anti-interleukin-6 monoclonal antibody, in metastatic renal cell cancer. Br J Cancer. 2010;103:1154–62. doi: 10.1038/sj.bjc.6605872. [DOI] [PMC free article] [PubMed] [Google Scholar]


