Abstract
Objective
To investigate whether cartilage degeneration is prevented or minimized in an anterior cruciate ligament (ACL) injury rat model following a single dose-escalated intra-articular injection of lubricin derived from human synoviocytes in culture (HSL).
Methods
Unilateral ACL transection (ACLT) of the right hindlimb was performed in Lewis rats (N = 56). Control animals underwent a capsulotomy alone leaving the ACL intact (N = 11). Intra-articular injections (50μl/injection) of PBS (N = 14) and HSL (N = 14; 1600μg/ml) were performed on day 7 post-surgery. Animals were euthanized on day 70 post-surgery. Histological specimens were immunoprobed for lubricin, and sulfated glycosaminoglycans. Urinary CTX-II (uCTX-II) levels were measured on day 35 and 70 post-surgery. Hindlimb maximum applied force was determined using a variable resistor walkway to monitor quadruped gait asymmetries.
Results
Increased immunostaining for lubricin in the superficial zone and on the surface of cartilage was observed in lubricin-treated and control animals but not the PBS-treated nor the untreated ACLT animals. On post-operative day 35 and 70, uCTXII levels of HSL-treated animals were lower than corresponding untreated and PBS-treated (p=0.005; p<0.001 respectively) animals. ACLT animals treated with HSL and control animals distributed their weight equally between hindlimbs compared to PBS treated or untreated animals (p<0.01).
Conclusion
A single intra-articular injection of concentrated lubricin, following ACLT, reduced collagen type II degradation and improved weight bearing in the affected joint. This study supports the practice of tribosupplementation with lubricin in retarding cartilage degeneration and possibly the development of post-traumatic OA.
Keywords: ACL Injury, Lubricin, PRG-4, SZP, tribonectin, tribosupplementation
INTRODUCTION
Acute anterior cruciate ligament (ACL) injury is a significant risk factor for the development of post-traumatic osteoarthritis (OA) (1, 2). Contributing factors such as joint instability, altered joint loading (3, 4), enzymatic tissue degradation (5, 6), and a lack of lubrication (7) are postulated to play a significant role in the pathogenesis of OA following injury. Synovial fluid (SF) lubricin concentrations in injured joints from patients with ACL injury were significantly lower than those found in uninjured contralateral joints (7). Inhibition of tumor necrosis factor-alpha (TNF-α) with etanercept in a rat ACL transection (ACLT) model was shown to up-regulate lubricin expression, reduced friction and reduced sGAG loss from cartilage (8). However, the translational value of inhibiting TNF-α in preserving chondroprotection may be limited by confounding side-effects related to immune system dysregulation. Thus the direct intra-articular re-introduction of lubricin (i.e. tribosupplementation), during the peri-injury period, may offer an opportunity to preserve cartilage by re-establishing a protective covering of lubricin. This approach appears effective in the rat meniscectomy model using lubricin with a truncated mucin domain (9). Disease modifying effects were noted using 3 injections per week and may have ameliorated pain (10). Lubricin was also effective in the rat ACLT model at post-operative day 35 where human synovial fluid lubricin, human synoviocyte lubricin (HSL) and human recombinant lubricin were tested (11).
ACL rupture is a common injury resulting in over 250,000 operative reconstructions per year in the US. These injuries place the knee at risk for early post-traumatic OA despite surgical treatment (12). The present study was designed to determine if a single lubricin dose escalated treatment, as compared to untreated joints and those treated with PBS as a placebo treatment, could replicate those chondroprotective effects observed previously (11). We also hypothesized that ACLT (13) rat joints treated with lubricin would show less gait asymmetry and fewer degradation products of urinary C-terminal telopeptides of type II collagen (uCTXII) at 10 weeks in the rat ACLT model as compared to untreated and placebo treated counterparts. We also posited that there would be less radiographic evidence of OA, and evidence of glycosaminoglycan and lubricin neosynthesis which was observed earlier following 7 separate intra-articular injections of lubricin (11). The lubricin used, HSL, was purified from synoviocytes in culture, and is a proxy for full-length human recombinant lubricin (11).
METHODS
ACLT Animal Model
ACL transection was performed on the right knee joints of 8–10 weeks old male Lewis rats. A total of 56 animals underwent surgery, and they were randomly assigned to one of four treatment groups with equal sizes: 1) ACLT + HSL 2) ACLT PBS (placebo treatment) 3) ACLT untreated (no injection) 4) capsulotomy (sham, no injection) where the right knee synovium was incised and then closed with PDS suture. Animals were anesthetized with ketamine and medetomidine, the skin was prepped with a topical antiseptic, and an incision was made in the skin laterally to the right knee joint. After the joint capsules were opened, the ACL was transected using a surgical hook and scalpel. In all animals, the right knee joint was the operated joint and the left knee joint was the ACL-intact contra-lateral joint. Prior studies (8, 14) have established that the contralateral joints do not display pathology, appear normal and were thus not included in analyses. A total of 5 animals died during surgery from the untreated ACLT (final N=12) and capsulotomy groups (final N=11). All animals were sacrificed on post-op day 70 and anteroposterior and lateral radiographs were taken of each knee using a high-resolution (14 bit) radiography system (MX-20, Faxitron, Wheeling, Illinois) (15). Afterwards, right knee joints were placed in buffered neutral formalin and paraffin embedded. The study was approved by the Institutional Animal Care and Use Committee. Surgeries and injections were conducted by authors, KM and SA, who had extensive experience in performing and replicating this procedure. Injections were performed by authors KW and KE.
Lubricin
Lyophilized HSL was obtained from SBH Sciences (Natick, MA) and reconstituted in PBS at pH 7.4 at a concentration of 1.6 mg/ml. HSL was purified from conditioned media from human synoviocytes growing confluently. The purification procedure was similar to one reported previously (16). The manufacturer reports that this molecule is anti-adhesive as tested in a cell-based assay (17) and lubricates a cartilage against cartilage bearing assay (18). Sequence confirmation of PRG4 was obtained by liquid chromatography and mass spectrometry of a tryptic digest of purified HSL.
Lubricin Dosing
Seven days following ACLT, animals were anesthetized with inhaled isoflurane and were injected intra-articularly with 80μg HSL in a 50 μl volume (266 μg/kg/animal). Injection was performed through the patellar tendon of the operated knee joint each time while the knee was flexed. Confirmation of intra-articular administration was confirmed by a noticeable and palpable synovial fluid collection.
Quadruped Gait Analysis
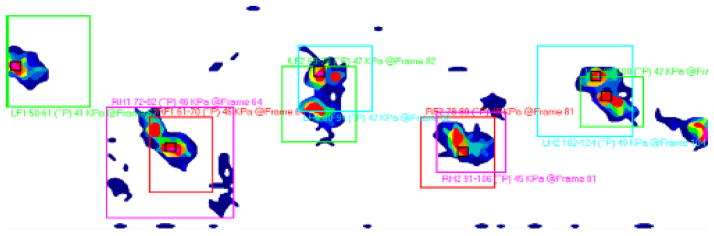
A pressure sensing walkway measuring (5″ W × 14″ L) (Tekscan, Boston, MA) was used to monitor paw pressure and impulse (Newton-secs) over 2 gait cycles with a paw strike resolution of 15.5 sensels/cm2 on post-op day 70 just prior to sacrifice. The walkway was first calibrated with known weights which occupied similar number of sensels as would a rat paw, and the weight of each animal in Newtons was calculated and entered into the Tekscan operating system software (v 7.0) prior to data collection. Data files consisted of consecutive images (frames) sampled at 200 Hz which showed quadruped progression wherein hindlimb strikes superimpose upon forelimb strikes. Paw pressure distribution is illustrated with isobars (Fig 1). Pawstrike maximum force of forelimbs (LF1,2 and RF1,2) and hindlimbs (LH1,2 and RH1,2) and stance time of each gait cycle and within each walkway trial were determined. From this, strike boxes were calculated (Fig 1A) within Tekscan software which calculated the center of symmetry between fore- and hindlimbs and their trajectory. From this parameter the differential weight bearing of the left versus right hind limb was calculated across gait cycles and expressed as a ratio of the average maximum force left divided by the average maximum force right hindlimb. This system has been used in gait studies in previous investigations in small mammals (19, 20, 21).
Figure 1.
Representative rat gait with paw strikes identified over 2 gait cycles. The central maximum pressure within each strike box and its frame number is illustrated. Note that left and right forelimbs (LF and RF) superimpose on left and right hindlimbs (LH, RH) during quadruped gait and are differentiated by frame number.
Determination of urinary CTXII concentrations in urines from untreated, PBS and HSL-treated ACLT animals
On post-operative day 35 and 70 following surgery, animals were housed in metabolic cages and 24 hour urine collections were performed. Urine samples were subsequently centrifuged at 5,000 rpm for 20 min. and stored at −20°C. Urinary CTXII concentrations were determined using the Pre-Clinical Urine Cartilaps ELISA (Immunodiagnostic systems, Scottsdale, AZ). Urinary creatinine was determined using the QuantiChrom creatinine assay (BioAssay Systems, Hayward, CA). Urinary CTXII levels were normalized to urinary creatinine. Due to a limitation in the number of metabolic cages, urine was collected from 12 ACLT untreated, 14 ACLT + HSL, 14 ACLT+PBS and 6 capsulotomized rats.
Histological Analysis and Immuno Staining
Paraffin-embedded coronal sections were taken from weight-bearing areas of the articular cartilage of ACLT joints of each animal as previously described (11). Microtomed coronal sections were collected every 250 μm to find a representative area showing both femoral condyles, tibial plateaus, and the menisci. Two adjacent sections were collected through this region and stained with Safranin O/Methyl green stain for assessment of cartilage sulfated glycosaminoglycan (sGAG) content. Another two adjacent sections were immunoprobed for lubricin and glycosamimoglycans. Specific staining for a glycosylated epitope within the lubricin mucin domain was performed with monoclonal antibody 9G3 (provided by M. Warman) at 1:100 dilution. Biotinylated anti-mouse IgG at 1:200 dilution was utilized and detected using the Vectastain ABC kit (VECTOR Laboratories, Burlingame, CA).
Probing for glycosaminoglycans was performed at 1:100 dilution with monoclonal antibodies 3B3 and 2B6 which are specific for chondroitin-6-sulphate and chondroitin-4-sulfate, respectively (22–24). Biotinylated anti-mouse IgG at 1:200 dilution was utilized for 2B6 antibody and biotinylated anti-mouse IgM at 1:200 dilution for 3B3 antibody. Development was performed using the kit described above. Glycosaminoglycan immunoprobe staining was also counterstained by Methyl green.
Fluorescence In Situ Hydridization (FISH) of Lubricin mRNA
Rat limbs from across treatment groups were decalcified prior to embedding and sectioning. The rat lubricin probe, a 373 bp fragment corresponded to nucleotides 1283 – 1655 of the rat PRG4 NCBI RGD:1308976 gene. The RT-PCR product was created using the forward primer 5′ – GAATGGTGAGTTCAGGCTCCTTAG – 3′ and reverse primer 5′ – CCCAAGACTACAGCGGCAAAAC – 3′ which are complimentary for rat lubricin mucin domain sequence. The PCR product was purifed via agarose gel electrophoresis using QIAquick Gel extraction kit (Qiagen), and quantitated against low DNA mass ladder standard (Invitrogen). In situ hybridization was performed using Random Primed DIG-labeled DNA probe as previously described (25, 26). Hybridized probes were immunodetected with anti-DIG – fluorescein Fab fragments (Roche Applied Science) and viewed via fluorescence microscopy. Two 16 bit grayscale images were acquired with a Nikon E800 microscope (Nikon Inc. Melville, NY) using a 40x PlanApo objective. Camera settings were based on the brightest stained slide and the cameras built-in green filter was used to increase image contrast (Diagnostic Instruments, Sterling Heights, MI). All subsequent images were acquired with the same camera settings. Image processing and analysis was performed using iVision (Biovision, Exton, PA) image analysis software. Positive staining was defined through intensity thresholding and integrated optical density (IOD) was calculated byexamining the thresholded area multiplied by the mean. Images were pseudocolored green in Photoshop CS4.
Caspase 3 Staining
Sections were heated to 60°C for 30 min, deparaffinized and hydrated in Xylene and alcohol. Rabbit polyclonal antibody against active caspase 3 (cat#ab13847, Abcam) at 1: 50 dilution was added to slides at 4°C overnight according to VectaStain procedures. Following the addition of biotinylated secondary antibody solution and DAB, slides were counterstained with 1% Methyl green and coverslip slides fixed with Permount (Fisher).
TUNEL Staining
Decalcified and embedded sections were warmed to 57°C for 5 min, deparaffinized by immersing slides in xylene and ethanol, followed by washing 2 times in 1x PBS, followed by covering sections with proteinase K solution for 20 minutes according to manufacturer’s kit instructions (Trevigen Cat#4812-30-k, Gaithersburg, MD). After washing with deionized water, slides were immersed in 1x TdT labeling buffer, and incubated sections were labeled with reaction mix for 60 minutes at 37°C in a humidity chamber. After using 1x TdT stop buffer to stop the reaction, and 1x PBS wash, slides were incubated with strep-fluor solution for 20 minutes in the dark. After PBS wash, and placement of a glass coverslip using a fluorescent mounting medium, slides were viewed under fluorescence microscopy using a 495nm filter.
Histological Scoring and Image Densitometry
The Osteoarthritis Research Society International (OARSI) modified Mankin score (27) was used to measure tibial cartilage degeneration in each joint compartment and was averaged. A group of at least 3 scorers, blinded to treatment group, independently graded the best appearing Safranin O/Methyl green stained section from each joint and arrived at a consensus score (11).
Knee Joint Radiography and Scoring
Radiographs of the ACLT and contralateral unaffected knee joint were scored using a modification of the Kellgren-Lawrence scoring system. The Kellgren-Lawrence score for knee OA, as originally described, assigns a score from 0 to 4 based on severity of disease in human subjects. Although there are some anatomical differences between rats and humans, the images were scored relative to control radiographs taken of the nonoperative contralateral limb. Radiographs were scored by an orthopaedic surgeon who specializes in adult joint reconstruction and a fifth year orthopaedic surgery resident. Both examiners were blinded to animal identifier and treatment group when scoring. The respective scores of both examiners for each animal were averaged for analysis.
Statistical Analyses
Maximum force of left to right hind limb average ratios during gait analyses and urinary CTX-II concentrations were represented by scatter and box plots respectively. The horizontal line within each scatter plot represents the mean and outer lines are one standard deviation. The horizontal line within each box plot represents the median and outer lines are one standard deviation. Mean and 95% confidence intervals were calculated for all other dependent variables. Analysis of variance using the Kruskall-Wallis test was used to determine differences in average maximum left/right weight ratio, urinary CTXII concentrations and FISH intensity values, OARSI and Kellgren-Lawrence scores across treatment groups. Statistical significance was set at α = 0.05 a priori.
RESULTS
Gait Analysis
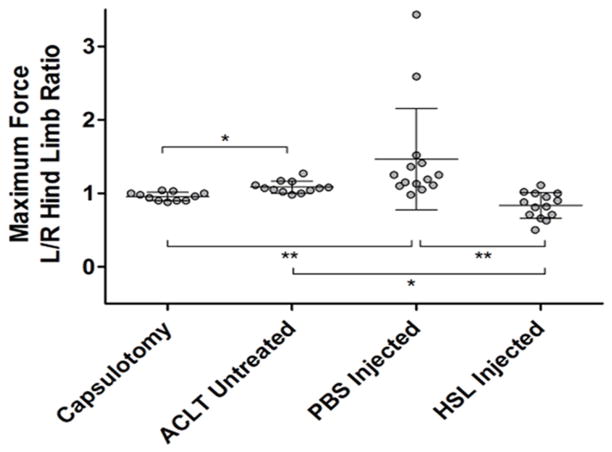
The rats treated with HSL following ACLT demonstrated a left to right hindlimb average maximum force ratio of 0.84 (0.73 – 0.94) which was significantly smaller than rats treated with PBS as placebo, 1.46 (1.11 – 1.83) (p <0.001), and untreated ACLT rats, 1.08 (1.03 – 1.14) (p<0.01) (Fig 2). Capsulotomized rats with intact ACL’s demonstrated a left to right hindlimb average maximum force ratio of 0.95 (0.92 – 1.00). There was no significant difference between the capsulotomized (sham) rats and the ACLT rats which were treated with HSL (p = 0.07).
Figure 2.
Whisker plots of left versus right maximum paw strike force across the 4 groups of capsulotomized controls, untreated, PBS-treated, and human synoviocyte lubricin (HSL)-treated ACL transected rat joints at post-operative day 70. (*p<0.01; **p<0.001)
Urinary CTXII concentrations
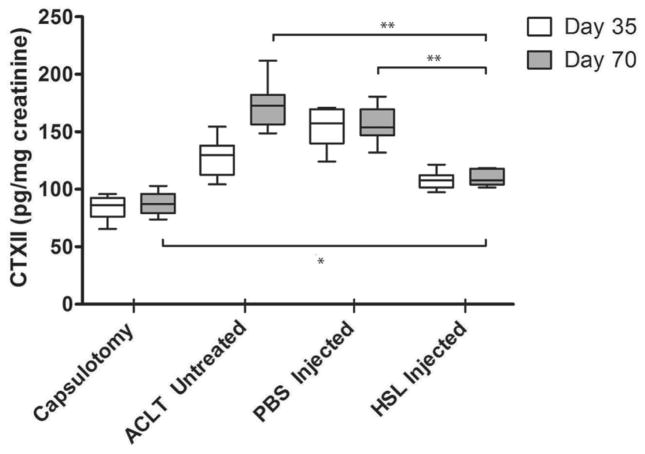
The mean uCTXII levels on post-operative day 35 and 70 were 113.2 (106.9 – 119.5 pg/mg creatinine) and 116.8 (110.2 – 123.4) among HSL-treated animals and were lower than PBS-treated animals 135.4 (123.2 – 149.6) and 157.5 (141.9 – 173.1) at each time (p < 0.001 and p < 0.001 respectively) (Fig 3). uCTXII levels among HSL-treated animals were higher than capsulotomized animals at day 35 (p=0.002) and day 70 (p=0.003). uCTXII levels among HSL-treated animals were lower than for untreated ACLT animals at day 35 (P=0.002) and day 70 (P<0.001). uCTXII levels trended higher over time (p < 0.001) for untreated ACLT animals. There were no significant differences in uCTX-II levels between days in capsulotomized (p = 0.671), HSL-treated animals (p = 0.698) and PBS treated animals (p = 0.622).
Figure 3.
Whisker plots of urinary CTX-II (uCTX-II) levels, normalized to creatinine, from rats with ACLT right hindlimbs randomized to treatment with either HSL or PBS at post-operative day 7. Control animals underwent a sham capsulotomy. Urine was collected for 24hrs from all animals on post-operative days 35 and 70. All uCTXII assays were performed in duplicate. (** p<0.001; * p < 0.01)
OARSI Histological Grading
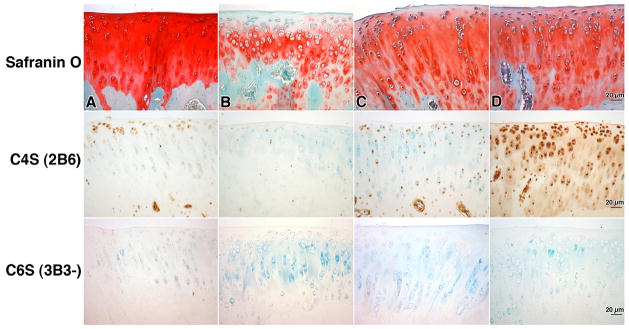
Representative Safranin O/Methyl green stained tibial plateaus from HSL, and PBS-treated animals, untreated ACLT and capsulotomized control animals are shown in Figure 4. Compared to PBS-treated joints, HSL-treated tibial cartilage typically showed more intense staining that spanned the superficial, middle and deep cartilage zones indicating higher sGAG content. HSL-treated joints appeared similar to capsulotomized cartilage in intensity of Safranin O staining. Less chondrocyte cloning was noted in cartilage from HSL-treated joints than in untreated or PBS-treated joints. However, the mean (95%CI) OARSI score of 9.33 (7.91 – 10.74) was not significantly different for HSL-treated joints compared to PBS-treated joints 11.42 (9.36 – 13.49) (p=0.18) and untreated joints 10.75 (6.79 – 14.7) (p = 0.57) (Fig 3). Capsulotomized joints demonstrated an OARSI score 0.5 (−0.17 – 1.17) which was significantly lower than the other three groups.
Figure 4.
Safranin O stained and immunoprobed tibial plateau cartilage from a) capsulotomized, b) untreated, c) PBS-treated, d) human synoviocyte lubricin (HSL)-treated ACLT rat joints. Monoclonal antibodies 3B3(−) and 2B6 probed for chondroitin 6 sulfate (C6S) and chondroitin 4 sulfate(C4S) respectively. Scale bars are 20 μm.
TUNEL Staining and Glycosaminoglycan Immunohistochemistry
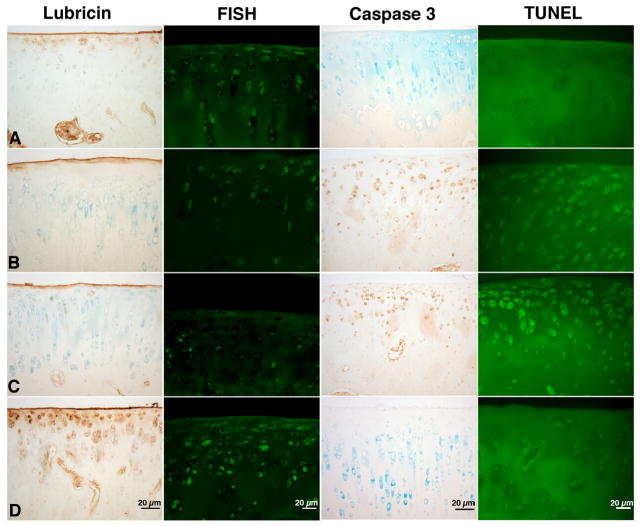
ACLT joints that were untreated or treated with PBS were positive for caspase 3 and were TUNEL positive in the superficial and intermediate zone (Fig 5). Lack of cellular death due to apoptosis, as indicated by absent TUNEL staining, was similar between HSL-treated joints and capsulotomized control cartilage. Apparent greater GAG content in HSL treated joints was supported by greater immunohistochemical staining for 2B6 indicative of C4S. By contrast minimal staining was observed in the untreated or PBS treated ACLT joints (Fig 4) where counterstaining was readily observable in cartilage. There was no detection of nascent C6S in any of the 4 groups (Fig 4).
Figure 5.
Immunostaining of articular cartilage for lubricin probed with mAb 9G3 and in situ hybridization for lubricin mRNA from representative rats at day 70 post-op from a) capsulotomized b) untreated c) PBS-treated d) human synoviocyte lubricin (HSL)-treated ACLT rat joints. Joints which are untreated or PBS-treated display caspase 3 activity and are TUNEL positive. Scale bars are 20 μm.
Lubricin Immuno Staining and FISH
ACL transected joints, treated with single dosing HSL, and probed with 9G3 showed greater surface staining and the presence of superficial zone chondrocytes that stained positive for lubricin intra-cellularly (Fig 5d). Control cartilage from capsulotomized sham joints was similar in appearance (Fig 5a). These findings contrast with a representative PBS-treated (Fig 5c) and untreated ACLT (Fig 5b) joint where lubricin immunostaining of the superficial zone and articular surface was absent or minimal respectively. In situ hybridization indicated that lubricin expression was enhanced following treatment with HSL (Fig 5d). The mean IOD for these specimens 496.8 (330.6 – 662.9) was significantly greater than for ACLT joints treated with PBS 99.6(53.3 – 145.8) (p < 0.001) (Fig 5c), were untreated 215.2(146.8 – 283.7) (p = 0.02) (Fig 4b) or were capsulotomized controls 159.3(95.7 – 222.9) (p < 0.001) (Fig 5a).
Knee Joint Radiography
Kellgren-Lawrence scores showed no significant differences across experimental groups. Mean and 95% CI’s for Kellgren-Lawrence scores for ACLT joints without treatment and those treated with PBS were 2.14 (1.56 – 2.72) and 1.83 (1.49 – 2.17) respectively. Lubricin treated ACLT and capsular incision only joints received scores of 1.89 (1.43 – 2.35) and 1.33 (0.85 – 1.81) respectively.
DISCUSSION
We have shown in this study, and prior ones (11, 15) that some OA degenerative changes following an ACL injury can be mitigated by the intra-articular supplementation of lubricin. Immunohistochemical articular surface staining for lubricin was stronger among the lubricin-treated joints. Untreated and placebo treated ACLT joints displayed less surface and minimal superficial zone lubricin, which in a Prg4 mutant mouse model (28–30) was shown to be critical in the chondroprotection of articular cartilage. In addition, the HSL-treated ACLT joints showed evidence of superficial zone chondrocyte immunopositivity for lubricin which is related to native lubricin synthesis by superficial and intermediate zone chondrocytes. Rationalizing lubricin immunohistology with the findings of in situ hybridization results shown in Fig 5 leads to our conclusion that lubricin expression could be enhanced following injury by the intra-articular delivery of lubricin during the peri-injury period. Without this intervention, loss of lubricin from the cartilage surface occurs due to inflammation and the lack of lubricin expression from the superficial zone chondrocytes (8, 11). Conversely, control cartilage shows a normal amount of lubricin immunostaining (8, 11) and nominal level of expression which may be indicative of homeostasis. Introducing lubricin into the traumatized joint protects the superficial zone, appears to prevent cell death, and also results in greater C4S expression. These observations are similar to our prior lubricin tribosupplementation study (11) conducted with 2 intra-articular injections per week for a total of 7 injections. However, that study also observed immunoreactvity with 3B3(−) which indicates nascent C6S expression and repair, which was not observed in the present, dose escalated single injection study. That previous study also noted an improvement in OARSI scores following lubricin supplementation but overall this metric appeared to be insensitive when compared to other data suggesting anabolic or chondroprotective outcomes in the ACLT joints. The present study was limited to a single dose escalated lubricin intra-articular injection and by contrast no improvement in OARSI scores was observed. This suggests that multiple lubricin injections may be needed to achieve a chondroprotective effect in this animal model that can be readily appreciated histologically by OARSI scoring. The finding that Kellgren-Lawrence scores also showed no significant differences was consistent with these histological results, although we have observed that multiple lubricin injections in the ACLT joint improves radiographic outcomes (15).
The observation that cellular demise of superficial and intermediate zone chondrocytes was prevented by lubricin supplementation is a novel finding. It has been noted previously that blunt trauma induces cell death in articular cartilage (31, 32) but the effects of other offending mechanical stimuli, in this case enhanced friction, were unknown. There is a concern that due to the lack of blunt cartilage trauma, the rat ACLT model is not a true clinical recapitulation of ACL rupture in mammals. However other mechanical sequelae of the ACL deficient joint are resident in this model, such as cellular demise, which appears linked to enhanced friction. In related in vitro work, in bovine osteochondral plugs, we have observed the prevention of apoptosis of superficial zone chondrocytes through the addition of HSL (33).
Chondroprotection following lubricin supplementation may have supported more normal weight bearing in the affected limb. Our analysis of gait was limited to analysis of the maximum force differential between the right and left hind limbs. We observed that lubricin-treated limbs consistently demonstrated a greater maximum force L/R ratio as compared to PBS or untreated ACLT knee joints. The maximum force L/R ratio was almost statistically lower than the control sham operated knees. The lubricin-treated ACLT knee may have supported more force relative to its contralateral counterpart, which may be due to an inflammatory response from a unilateral joint injury with systemic manifestations, affecting the contralateral synovium. Gait analysis in rodents receiving monoarticular IL1β also display a subtle, but reproducibly quantifiable gait disturbance, indicating disease progression (34). In future studies we plan to prospectivelymonitor changes in gait as animals become lame and are then treated with lubricin. It is possible that the ACL transected rats may have transferred more weight bearing to front paws but our analysis was limited to maximum force differential between the right and left hind limbs.
The observation that uCTX-II, a validated marker of collagen type II degradation (35), was less concentrated in the rat urine, normalized for creatinine, among lubricin-treated animals, is an important translational finding. 24 hr urine collections at two differing time points within each animal showed a significant increase in uCTX-II in the ACLT joints. PBS-treated animals reached the higher uCTX-II level on the day 35 urine collection. Treatment with HSL led to statistically significant reductions in uCTX-II compared to ACLT untreated and ACLT + PBS groups,, HSL-treated joints demonstrated similar levels of uCTX-II across the post-op day 35 and 70 urine collections. The magnitude of uCTX-II reduction in the present animals was similar (11) to twice weekly, albeit less concentrated, injections of HSL for 3 weeks. Although uCTX-II levels remained significantly higher than capsulotomized control animals on day 70, the single intra-articular HSL injection also prevented a subsequent increase in uCTX-II across post-op days 35 and 70. Despite the fact that uCTX-II is enzymatically generated, the addition of an anti-adhesive, like lubricin, may prevent the interaction of inflammatory cells and other proteins in SF with the articular surface, in addition to providing boundary lubrication. In the absence of lubricin, biofouling of the articular cartilage with globular proteins is suspected to occur in the lubricin null mouse (28). In inflammatory arthropathies, lubricin may reduce initial collagen type II cleavage by minimizing superficial zone degradation. Preserving collagen type II architecture in articular cartilage is a critical step in ultimately restoring cartilage after a traumatic injury (36). Loss of glycosaminoglycan precedes catabolism of collagen type II. The significant differences in urinary CTX-II in lubricin-treated versus non-lubricin treated groups suggests that exogenous lubricin could play a critical role in a post-traumatic strategy to prevent cartilage degeneration.
Lubricin interacts with collagen (37), hydrophobic (38) and hydrophilic surfaces via its C-terminus (39), and likely forms a loop in its mucin domain allowing the N-terminus to also interact with the same surface (38, 40). Surface bound layers of lubricin appear as both single end-grafted and double-end grafted molecules (41). The O-linked glycosylations which decorate the mucin domain are important in helping to create steric repulsion and hydration shells which manifest as repulsive forces (42) working in an anti-adhesive capacity and keep cartilage asperities from direct contact. Lateral translation of one bearing against its apposed bearing is thus ‘lubricated’ under the conditions that characterize mammalian diarthrodial joints, including very slow sliding speed, high pressures and compliant bearing materials (cartilage) (43). Boundary lubrication becomes the predominant mode of joint lubrication once loaded and pressurized cartilage has stayed in position for up to 10 mins and the coefficient of friction has reached an asymptotic level of 0.14 (44, 45). At this point cartilage has reached zero pore pressure by exuding extracellular matrix fluid. Preventing asperity contact, adhesions and resultant damage is provided by this “carpet” (41) of end-grafted lubricin molecules. This system is critical to chondroprotection and can be re-introduced to weight bearing diarthrodial joints because lubricin is surface active and rapidly adsorbs on surfaces.
The observations of reduced cartilage damage, enhanced chondroprotection and a decrease in inflammation may lead to a mitigation of subsequent OA progression. The chondroprotected joint may thus benefit on a longer term basis from enhanced weight bearing since compressive load and shear together upregulate lubricin expression (46). Recent work on possible mechanisms linking abnormal mechanical stress to chondrocyte degeneration and subsequent OA have focused on the immunostimulatory properties of cartilage breakdown products and subsequent ongoing synovial inflammation as contributors to later OA progression (47). Synovial inflammation has been linked to reduced lubricating capacity (48), more rapid OA progression (49), and increased OA pain symptoms. As discussed by Scanzello et al. in their review comparing OA progression to a chronic wound (47), it is particularly worth noting that synovitis is prominent in OA, and that the early inflammatory responses common in both infection and tissue damage occur via the activation of toll-like receptors (TLR’s). These receptors are activated not only by pathogen surface molecules as occurs in infection, but also by reduced molecular weight hyaluronic acid and fibronectin which may be released into the joint following injury(47, 50). Alternatively, the novel observation that apoptosis is present and responsible for cell death in the ACLT joint, and not present in the lubricin injected joint, suggests that the loss of lubricating ability may possibly initiate apoptosis in of itself (33). Reintroducing lubricin could potentially preserve chondrocytes and the tissue matrix through its regeneration.
In conclusion, this study indicates that lubricin is a disease modifying protein that may prevent the fundamental processes which can lead to post-traumatic OA following ACL injury. A single concentrated injection of lubricin appears generally equivalent to multiple injections (11) with the same final mass, but further work with differing dosing strategies is needed. The strategy of once weekly injections for 3 weeks should be explored. Lubricin is a promising biologic candidate since it is a replacement for a normally occurring mucinous glycoprotein and thus has a low toxicity profile. Patients on average seek medical attention 6.7 days following an acute joint injury (48) which was part of the justification for delayed treatment in this study. Injecting lubricin (tribosupplementation) into the intra-articular space would be an adjunct to viscosupplementation. Human recombinant lubricin will likely have similar chondroprotective effects to the synoviocyte lubricin used in this investigation (11).
Acknowledgments
COI Statement: The first author holds patents which relate to lubricin. This work was funded by NIH/NIAMS R21AR055937, RO1AR050180, R41AR057276 and NCRR COBRE P20 RR024484
The authors would like to thank Dr. Matthew Warman from Boston Children’s Hospital for providing lubricin-specific mAb 9G3, Dr. Bruce Caterson for providing mAb’s 3B3 and 2B6, and Dr. Thomas Bliss for review of data. This research was funded by NIH/NIAMS R21AR055937, RO1AR050180, R41AR057276 and NCRR COBRE P20 RR024484.
References
- 1.Aichroth PM, Patel DV, Zorrilla P. The natural history and treatment of rupture of the anterior cruciate ligament in children and adolescents. A prospective review. J Bone Joint Surg Br. 2002;84(1):38–41. doi: 10.1302/0301-620x.84b1.11773. [DOI] [PubMed] [Google Scholar]
- 2.Fithian DC, Paxton LW, Goltz DH. Fate of the anterior cruciate ligament-injured knee. Orthop Clin North Am. 2002;33(4):621–36. v. doi: 10.1016/s0030-5898(02)00015-9. [DOI] [PubMed] [Google Scholar]
- 3.Li G, Moses JM, Papannagari R, Pathare NP, DeFrate LE, Gill TJ. Anterior cruciate ligament deficiency alters the in vivo motion of the tibiofemoral cartilage contact points in both the anteroposterior and mediolateral directions. J Bone Joint Surg Am. 2006;88(8):1826–34. doi: 10.2106/JBJS.E.00539. [DOI] [PubMed] [Google Scholar]
- 4.Roos H, Adalberth T, Dahlberg L, Lohmander LS. Osteoarthritis of the knee after injury to the anterior cruciate ligament or meniscus: the influence of time and age. Osteoarthritis Cartilage. 1995;3(4):261–7. doi: 10.1016/s1063-4584(05)80017-2. [DOI] [PubMed] [Google Scholar]
- 5.Vasara AI, Jurvelin JS, Peterson L, Kiviranta I. Arthroscopic cartilage indentation and cartilage lesions of anterior cruciate ligament-deficient knees. Am J Sports Med. 2005;33(3):408–14. doi: 10.1177/0363546504268040. [DOI] [PubMed] [Google Scholar]
- 6.Fleming BC, Hulstyn MJ, Oksendahl HL, Fadale PD. Ligament Injury, Reconstruction and Osteoarthritis. Curr Opin Orthop. 2005;16(5):354–362. doi: 10.1097/01.bco.0000176423.07865.d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elsaid KA, Fleming BC, Oksendahl HL, Machan JT, Fadale PD, Hulstyn MJ, et al. Decreased lubricin concentrations and markers of joint inflammation in the synovial fluid of patients with anterior cruciate ligament injury. Arthritis Rheum. 2008;58(6):1707–15. doi: 10.1002/art.23495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elsaid KA, Machan JT, Waller K, Fleming BC, Jay GD. The impact of anterior cruciate ligament injury on lubricin metabolism and the effect of inhibiting tumor necrosis factor alpha on chondroprotection in an animal model. Arthritis Rheum. 2009;60(10):2997–3006. doi: 10.1002/art.24800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flannery CR, Zollner R, Corcoran C, Jones AR, Root A, Rivera-Bermudez MA, et al. Prevention of cartilage degeneration in a rat model of osteoarthritis by intraarticular treatment with recombinant lubricin. Arthritis Rheum. 2009;60(3):840–7. doi: 10.1002/art.24304. [DOI] [PubMed] [Google Scholar]
- 10.Glasson SSR-BT, Mark LS, Whiteside G, Resmini C, et al. Intra-articular lubricin supplementation modifies disease progression and ameliorates pain in a rat model of osteoarthritis. 55th Orthopaedic Research Society; Las Vegas, NV. 2009. [Google Scholar]
- 11.Jay GD, Fleming BC, Watkins BA, McHugh KA, Anderson SC, Zhang LX, et al. Prevention of cartilage degeneration and restoration of chondroprotection by lubricin tribosupplementation in the rat following anterior cruciate ligament transection. Arthritis Rheum. 2010;62(8):2382–91. doi: 10.1002/art.27550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.von Porat A, Roos EM, Roos H. High prevalence of osteoarthritis 14 years after an anterior cruciate ligament tear in male soccer players: a study of radiographic and patient relevant outcomes. Ann Rheum Dis. 2004;63(3):269–73. doi: 10.1136/ard.2003.008136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stoop R, Buma P, van der Kraan PM, Hollander AP, Billinghurst RC, Meijers TH, et al. Type II collagen degradation in articular cartilage fibrillation after anterior cruciate ligament transection in rats. Osteoarthritis Cartilage. 2001;9(4):308–15. doi: 10.1053/joca.2000.0390. [DOI] [PubMed] [Google Scholar]
- 14.Teeple E, Elsaid KA, Fleming BC, Jay GD, Aslani K, Crisco JJ, et al. Coefficients of friction, lubricin, and cartilage damage in the anterior cruciate ligament-deficient guinea pig knee. J Orthop Res. 2008;26(2):231–7. doi: 10.1002/jor.20492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Teeple E, Elsaid KA, Jay GD, Zhang L, Badger GJ, Akelman M, et al. Effects of supplemental intra-articular lubricin and hyaluronic acid on the progression of posttraumatic arthritis in the anterior cruciate ligament-deficient rat knee. Am J Sports Med. 2011;39(1):164–72. doi: 10.1177/0363546510378088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jay GD, Britt DE, Cha CJ. Lubricin is a product of megakaryocyte stimulating factor gene expression by human synovial fibroblasts. J Rheumatol. 2000;27(3):594–600. [PubMed] [Google Scholar]
- 17.Braut-Boucher F, Pichon J, Rat P, Adolphe M, Aubery M, Font J. A non-isotopic, highly sensitive, fluorimetric, cell-cell adhesion microplate assay using calcein AM-labeled lymphocytes. J Immunol Methods. 1995;178(1):41–51. doi: 10.1016/0022-1759(94)00239-s. [DOI] [PubMed] [Google Scholar]
- 18.Schmidt TA, Gastelum NS, Nguyen QT, Schumacher BL, Sah RL. Boundary lubrication of articular cartilage: role of synovial fluid constituents. Arthritis Rheum. 2007;56(3):882–91. doi: 10.1002/art.22446. [DOI] [PubMed] [Google Scholar]
- 19.Lascelles BD, Roe SC, Smith E, Reynolds L, Markham J, Marcellin-Little D, et al. Evaluation of a pressure walkway system for measurement of vertical limb forces in clinically normal dogs. Am J Vet Res. 2006;67(2):277–82. doi: 10.2460/ajvr.67.2.277. [DOI] [PubMed] [Google Scholar]
- 20.Kim J, Kazmierczak KA, Breur GJ. Comparison of temporospatial and kinetic parameters of walking on a pressure sensing walkway in small and large dogs. American Journal of Veterimary Research. 2011;72(7) doi: 10.2460/ajvr.72.9.1171. (in press) [DOI] [PubMed] [Google Scholar]
- 21.Robinson DA, Romans CW, Gordon-Evans WJ, Evans RB, Conzemius MG. Evaluation of short-term limb function following unilateral carbon dioxide laser or scalpel onychectomy in cats. J Am Vet Med Assoc. 2007;230(3):353–8. doi: 10.2460/javma.230.3.353. [DOI] [PubMed] [Google Scholar]
- 22.Richardson JB, Caterson B, Evans EH, Ashton BA, Roberts S. Repair of human articular cartilage after implantation of autologous chondrocytes. J Bone Joint Surg Br. 1999;81(6):1064–8. doi: 10.1302/0301-620x.81b6.9343. [DOI] [PubMed] [Google Scholar]
- 23.Kavanagh E, Osborne AC, Ashhurst DE, Pitsillides AA. Keratan sulfate epitopes exhibit a conserved distribution during joint development that remains undisclosed on the basis of glycosaminoglycan charge density. J Histochem Cytochem. 2002;50(8):1039–47. doi: 10.1177/002215540205000806. [DOI] [PubMed] [Google Scholar]
- 24.Ciombor DM, Lester G, Aaron RK, Neame P, Caterson B. Low frequency EMF regulates chondrocyte differentiation and expression of matrix proteins. J Orthop Res. 2002;20(1):40–50. doi: 10.1016/S0736-0266(01)00071-7. [DOI] [PubMed] [Google Scholar]
- 25.Roche PJ. Preparation of template DNA and labeling techniques. In: Darby IA, editor. In situ Hybridization Protocols. 3. Totowa, NJ: Humana Press; 2006. pp. 9–16. [DOI] [PubMed] [Google Scholar]
- 26.Ge NL, Kocan KM, Murphy GL, Blouin EF. Detection of Anaplasma marginale DNA in bovine erythrocytes by slot-blot and in situ hybridization with a PCR-mediated digoxigenin-labeled DNA probe. J Vet Diagn Invest. 1995;7(4):465–72. doi: 10.1177/104063879500700407. [DOI] [PubMed] [Google Scholar]
- 27.Pritzker KP, Gay S, Jimenez SA, Ostergaard K, Pelletier JP, Revell PA, et al. Osteoarthritis cartilage histopathology: grading and staging. Osteoarthritis Cartilage. 2006;14(1):13–29. doi: 10.1016/j.joca.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 28.Jay GD, Torres JR, Rhee DK, Helminen HJ, Hytinnen MM, Cha CJ, et al. Association between friction and wear in diarthrodial joints lacking lubricin. Arthritis Rheum. 2007;56(11):3662–9. doi: 10.1002/art.22974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rhee DK, Marcelino J, Baker M, Gong Y, Smits P, Lefebvre V, et al. The secreted glycoprotein lubricin protects cartilage surfaces and inhibits synovial cell overgrowth. J Clin Invest. 2005;115(3):622–31. doi: 10.1172/JCI200522263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coles JM, Zhang L, Blum JJ, Warman ML, Jay GD, Guilak F, et al. Loss of cartilage structure, stiffness, and frictional properties in mice lacking Prg4. Arthritis Rheum. 2010 doi: 10.1002/art.27436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Milentijevic D, Rubel IF, Liew AS, Helfet DL, Torzilli PA. An in vivo rabbit model for cartilage trauma: a preliminary study of the influence of impact stress magnitude on chondrocyte death and matrix damage. J Orthop Trauma. 2005;19(7):466–73. doi: 10.1097/01.bot.0000162768.83772.18. [DOI] [PubMed] [Google Scholar]
- 32.D’Lima DD, Hashimoto S, Chen PC, Lotz MK, Colwell CW., Jr In vitro and in vivo models of cartilage injury. J Bone Joint Surg Am. 2001;83-A2(Suppl)(Pt 1):22–4. doi: 10.2106/00004623-200100021-00005. [DOI] [PubMed] [Google Scholar]
- 33.Waller K, Elsaid K, Warman M, Aslani K, Fleming B, Jay G. Lubricin reduces in vitro chondrocyte apoptosis in bovine cartilage bearings. 57th Annual Meeting of Orthopaedic Research Society; 2011. p. 2176. [Google Scholar]
- 34.Allen KD, Adams SB, Jr, Mata BA, Shamji MF, Gouze E, Jing L, et al. Gait and behavior in an IL1beta-mediated model of rat knee arthritis and effects of an IL1 antagonist. J Orthop Res. 2010 doi: 10.1002/jor.21309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garnero P, Landewe R, Boers M, Verhoeven A, Van Der Linden S, Christgau S, et al. Association of baseline levels of markers of bone and cartilage degradation with long-term progression of joint damage in patients with early rheumatoid arthritis: the COBRA study. Arthritis Rheum. 2002;46(11):2847–56. doi: 10.1002/art.10616. [DOI] [PubMed] [Google Scholar]
- 36.Catterall JB, Stabler TV, Flannery CR, Kraus VB. Changes in serum and synovial fluid biomarkers after acute injury ( NCT00332254) Arthritis Res Ther. 2010;12(6):R229. doi: 10.1186/ar3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taguchi M, Sun YL, Zhao C, Zobitz ME, Cha CJ, Jay GD, et al. Lubricin surface modification improves tendon gliding after tendon repair in a canine model in vitro. J Orthop Res. 2009;27(2):257–63. doi: 10.1002/jor.20731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chang DP, Abu-Lail NI, Guilak F, Jay GD, Zauscher S. Conformational Mechanics, Adsorption, and Normal Force Interactions of Lubricin and Hyaluronic Acid on Model Surfaces. Langmuir. 2008;24(4):1183–1193. doi: 10.1021/la702366t. [DOI] [PubMed] [Google Scholar]
- 39.Jones ARC, Gleghorn JP, Hughes CE, Fitz LJ, Zollner R, Wainwright SD, et al. Binding and localization of recombinant lubricin to articular cartilage surfaces. Journal of Orthopaedic Research. 2007;25(3):283–292. doi: 10.1002/jor.20325. [DOI] [PubMed] [Google Scholar]
- 40.Zappone B, Greene GW, Oroudjev E, Jay GD, Israelachvili JN. Molecular Aspects of Boundary Lubrication by Human Lubricin: Effect of Disulfide Bonds and Enzymatic Digestion. Langmuir. 2008;24(4):1495–1508. doi: 10.1021/la702383n. [DOI] [PubMed] [Google Scholar]
- 41.Zappone B, Ruths M, Greene GW, Jay GD, Israelachvili JN. Adsorption, lubrication, and wear of lubricin on model surfaces: polymer brush-like behavior of a glycoprotein. Biophys J. 2007;92(5):1693–708. doi: 10.1529/biophysj.106.088799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jay GD, Harris DA, Cha C-J. Boundary lubrication by lubricin is mediated by O-linked β(1–3)Gal-GalNAc oligosaccharides. Glycoconjugate Journal. 2002;18(10):807–815. doi: 10.1023/a:1021159619373. [DOI] [PubMed] [Google Scholar]
- 43.McCutchen CW. The frictional properties of animal joints. Wear. 1962;5:1–17. [Google Scholar]
- 44.Park S, Costa KD, Ateshian GA. Microscale frictional response of bovine articular cartilage from atomic force microscopy. Journal of Biomechanics. 2004;37(11):1679–1687. doi: 10.1016/j.jbiomech.2004.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Coles JM, Blum JJ, Jay GD, Darling EM, Guilak F, Zauscher S. In situ friction measurement on murine cartilage by atomic force microscopy. J Biomech. 2008;41(3):541–8. doi: 10.1016/j.jbiomech.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nugent GE, Aneloski NM, Schmidt TA, Schumacher BL, Voegtline MS, Sah RL. Dynamic shear stimulation of bovine cartilage biosynthesis of proteoglycan 4. Arthritis Rheum. 2006;54(6):1888–96. doi: 10.1002/art.21831. [DOI] [PubMed] [Google Scholar]
- 47.Scanzello CR, Plaas A, Crow MK. Innate immune system activation in osteoarthritis: is osteoarthritis a chronic wound? Curr Opin Rheumatol. 2008;20(5):565–72. doi: 10.1097/BOR.0b013e32830aba34. [DOI] [PubMed] [Google Scholar]
- 48.Jay GD, Elsaid KA, Zack J, Robinson K, Trespalacios F, Cha CJ, et al. Lubricating ability of aspirated synovial fluid from emergency department patients with knee joint synovitis. J Rheumatol. 2004;31(3):557–64. [PubMed] [Google Scholar]
- 49.Ayral X, Pickering EH, Woodworth TG, Mackillop N, Dougados M. Synovitis: a potential predictive factor of structural progression of medial tibiofemoral knee osteoarthritis --results of a 1 year longitudinal arthroscopic study in 422 patients. Osteoarthritis Cartilage. 2005;13(5):361–7. doi: 10.1016/j.joca.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 50.Jiang D, Liang J, Noble PW. Hyaluronan in tissue injury and repair. Annu Rev Cell Dev Biol. 2007;23:435–61. doi: 10.1146/annurev.cellbio.23.090506.123337. [DOI] [PubMed] [Google Scholar]