INTRODUCTION
Epiretinal membranes (ERM)s cause distortion of the retina and vision loss.1,2 Initial classifications of these membranes include the classic work by Gass.2 Ophthalmoscopic grading includes early disease, also termed grade 0 or cellophane maculopathy in which a sheen from the inner retinal surface is observed with no distortion. This is followed by Grade 1, or crinkled cellophane maculopathy, when membrane contraction causes small irregular folding of the inner retina surface. Gass emphasized fine superficial radiating folds that can be seen extending from membrane epicenters. Grade 2 includes an opaque or semi-opaque membrane causing significant vessel dragging. More recently, OCT has allowed visualization of membranes and their relationship to the retinal surface.3,4,5,6 However, the predictive value of optical coherence tomography (OCT) and clinical examination for surgical planning and in determining ease or difficulty of surgical removal remains unclear.4,7
Time domain OCT studies have shown that primary epiretinal membranes are more often adherent ‘globally’ to broad areas of the retina as opposed to membranes that occur after retinal breaks or detachment which are more often focally adherent 8 More recently, spectral domain optical coherence tomography (SD-OCT) analysis of epiretinal membranes has allowed determination of points of adhesion and relationships of the epiretinal membrane to the internal limiting membrane with a higher degree of detail 9,10 Although SD-OCT allows better definition of the geometry of membrane adhesion to the retina and it is suggested that membranes may be globally or focally adherent, ERM structure may not correlate with vision.11,12,13,14 Since the treatment of vision distortion from ERMs is surgical, correlation of ERM-retinal adherence with degree of surgical difficulty may aid in surgical planning.
In this study, we evaluated pre-operative structural characteristics of epiretinal membranes including extent of retinal adhesion and presence of fibrillary changes using SD-OCT. These structural findings were correlated with ease or difficulty of surgical removal by reviewing surgical videography using masked observers.
METHODS
Surgical difficulty and extent of ERM-retinal adhesion was evaluated in a consecutive series of eyes (N=31) undergoing idiopathic macular ERM removal. Inclusion criteria included visual acuity of 20/50 or worse or metamorphosia. All patients underwent a 3-port 25-gauge pars plana vitrectomy with core vitrectomy and posterior hyaloid removal. Epiretinal membranes were removed with or without triamcinolone (TCA) dusting (400 mg/ml) or indocyanine green (1.25 mg/ml) as a negative stain during a period of 8 months performed by a single experienced retinal surgeon. Since the surgeon was masked to pre-operative OCT findings, the quadrant to initiate ERM peeling was randomized and peeling was performed using ILM Forceps Tip (Revolution, Grieshaber). The intended surgical plane was to remove the epiretinal membrane without removal of the internal limiting membrane. Epiretinal membranes with a known etiology of uveitis, diabetic retinopathy, and previous retinal tears or detachments were excluded. Epiretinal membranes with lamellar macular holes were also excluded. Informed consent was obtained from all participants.
Surgical videography was recorded using an AVI adaptor mounted on a surgical microscope (Leica). Images were acquired using a 640 × 480 digital video camera (Sony 3 CCD ExwaveHAD, Tokyo, Japan) mounted onto the surgical microscope. Digital feed was recorded directly onto a computer hard drive at DV resolution (Apple Macbook, Apple Computer, Cupertino, CA). Surgical videos were reviewed by two observers masked to pre-operative SD-OCT imaging. Each masked observer was an experienced retinal surgeon and did not participate in ERM removal in this series of eyes. Degree of difficulty of membrane removal was determined for each quadrant of the macula and foveal area (total 5 areas per eye) following consensus by both reviewers. A grading scale (grade 1–3) was assigned according to surgical difficulty as follows: 1 – Peeling occurs easily with no evidence of retinal traction; 2 – Some evidence of retinal traction but retina retracts quickly following membrane removal; 3 – Appreciable retinal traction is observed with difficulty in ERM separation from the retina and retina remains elevated after ERM removal.
SD-OCT images (Spectralis OCT, Heidelberg Engineering, Vista, CA) of ERM structure along the vertical and horizontal meridians over the fovea (central 1000 microns) and macular quadrants (superior, inferior, nasal, and temporal) were also evaluated in a masked fashion by two observers. Thus, each eye was evaluated in a total of 5 areas (fovea, 4 quadrants). Both vertical and horizontal scanning meridians were obtained for each macular quadrant and foveal area was assessed for extent of ERM-retinal adhesion as well as for the presence of fibrillary changes along both vertical and horizontal scanning meridians. Calipers were used to measure the length of ERM-retinal adherence and divided by the total length of ERM to obtain ERM-retinal adhesion percentage. The extent of ERM-retinal adhesion was then categorized as focal, broad, or complete. Focal adhesion was defined as ERM-retinal adhesion < 50%; broad adhesion between 50-90%; and complete adhesion > 90%.
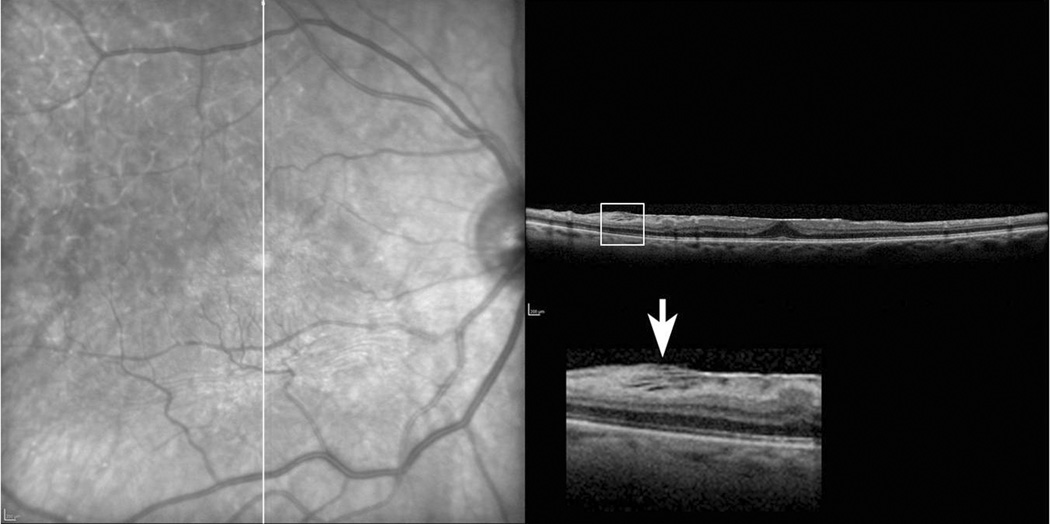
Fibrillary changes were defined as the presence of alternating linear hyper- and hypo-reflective signals observed by SD-OCT extending between the ERM and RNFL that were not parallel to the retinal surface (Figure 1). Fluorescein angiography was performed pre-operatively in all eyes to exclude choroidal neovascularization or other non-ERM pathology.
Figure 1.
Representative grading of epiretinal membrane (ERM)-retinal adherence. Complete ERM-retinal adherence (Grade 3) along the posterior pole with exception inferiorly (broad ERM-retinal adherence, Grade 2). Fibrillary changes (arrow) are seen as alternating linear hyper- and hypo-reflective signals between the ERM and retinal surface (inset).
All analyses used generalized estimating equations to account for intra-eye variations (The SAS system, Generalized Estimated Equations (GEE) analysis).
RESULTS
ERM-retinal adherence pattern resulted in complete concordance (Kappa=1) in each quadrant between the horizontal and vertical meridians for each observer. In addition, assessment of ERM-retinal adherence resulted in an extremely high concordance between masked observers (Kappa=0.9178). Therefore, categorization of ERM-retinal adherence by one observer was used for statistical analysis.
Among 31 consecutive eyes, ERM-retinal adherence pattern was evaluated pre-operatively in a total of 149 areas since no evidence of an ERM was seen in 6 areas. The mean age of our patients in this study was 67.8 ± 11.4 years, (female=14, male=17). Complete ERM-retinal adherence was observed in the majority of areas (86/149) evaluated (Table). The presence of fibrillary changes were similarly distributed among all quadrants in 13 patients.
Table.
Spectral Domain Optical Coherence tomography characteristics of epiretinal membranes in each area evaluated
| Characteristics | Fovea (N) |
Nasal (N) |
Temporal (N) |
Superior (N) |
Inferior (N) |
Total (N) |
|---|---|---|---|---|---|---|
| Focal | 2 | 3 | 1 | 6 | 12 | 24 |
| Broad | 2 | 13 | 9 | 8 | 7 | 39 |
| Complete | 26 | 13 | 20 | 15 | 12 | 86 |
| Fibrillation (+) Fibrillation (−) |
2 28 |
4 25 |
2 28 |
6 23 |
8 23 |
22 127 |
Fibrillation (+) – presence of fibrillary change
Fibrillation (−) – absence of fibrillary change
N – Number of quadrants
Surgical difficulty of ERM removal was also evaluated in the same 149 areas. ERM tissue was easily removed over 59 areas with no evidence of retinal traction (Grade 1) (Figure 5 and Supplement Video 2) (Supplemental material available at AJO.com). Difficult ERM removal (Grade 3) (Figure 2 and Supplement Video 1) (Supplemental material available at AJO.com) was observed in 46 areas with appreciable retinal traction and retinal elevation. Intermediate difficulty in surgical removal (Grade 2) of ERM tissue was observed in 44 areas. A partial thickness retinal defect occurred during ERM removal in 1 case over a single quadrant in which the presence of fibrillary changes were noted pre-operatively. No other complications occurred.
Figure 5.
Easy removal of ERM from the temporal area with no retinal traction seen in a still frame from surgical videography (Supplement, Video 2).
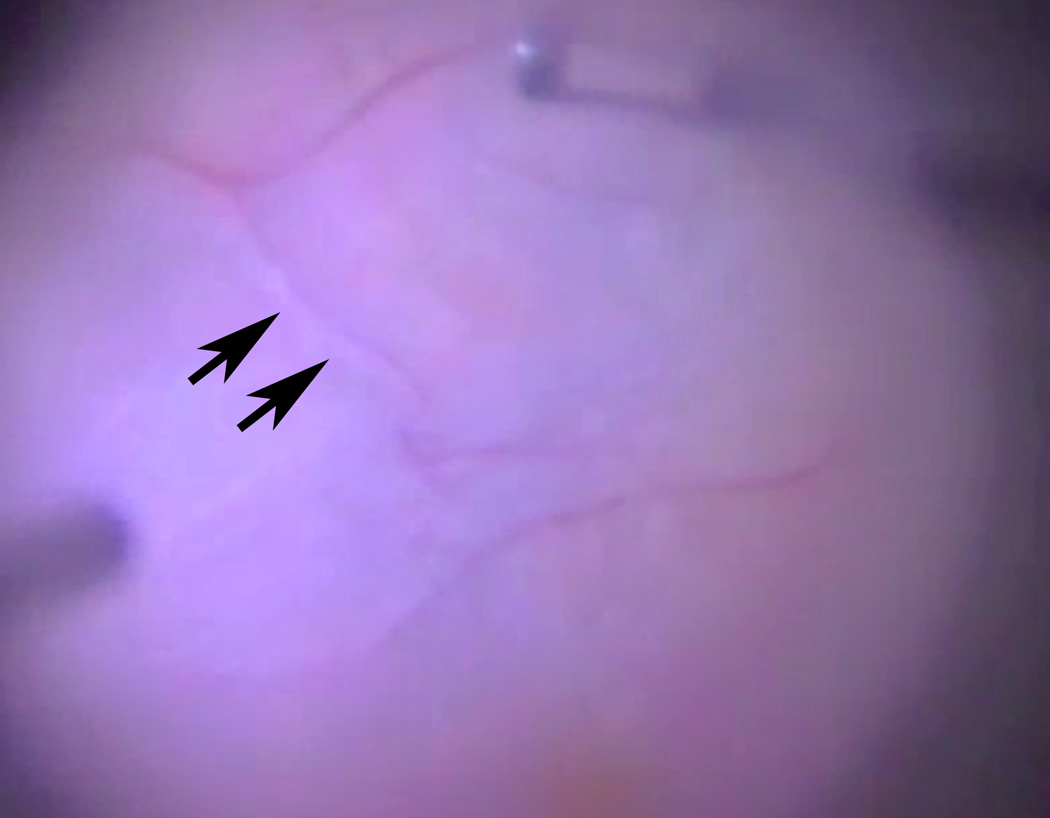
Figure 2.
Representative example of difficult peeling of an epiretinal membrane in which vessel elevation is seen with perivascular white reflection (arrows) by forceps (upper frame) as the ERM is removed. This is a still frame from surgical videography (Supplement, Video 1).
Complete ERM-retinal adhesion correlated with a 3.7- and 8.6-fold increase in surgical removal difficulty compared to broad (p < 0.02) and focal ERM-retinal adhesion (p<0.0001), respectively using generalized estimating equation modeling.
Additionally, irrespective of ERM-retinal adhesion type, the presence of fibrillary changes between the ERM and RNFL observed by SD-OCT correlated with a 25.5-fold increase in difficulty of surgical removal (p<0.0001) compared to the absence of fibrillary changes.
Case 1: Representative Difficult ERM peel (Grade 3)
A 53 year-old man with complete ERM-retinal adherence along the entire posterior pole with exception inferiorly (broad ERM-retinal adherence) and low density ERM-RNFL fibrillation by SD-OCT along the vertical meridian (Figure 1, arrow indicates fibrillation) underwent a difficult ERM peel (Grade 3) (Figure 2, Video Supplement 1) (Supplemental material available at AJO.com). An elevated vessel (arrows) is seen during peeling of the membrane held by forceps in the upper frame (Figure 2). Video (Supplement 1) shows difficulty of membrane removal over area of ERM-RNFL fibrillation.
Case 2: Easy ERM peel (predominantly Grade 1)
Case 2 is an 85 year-old man with focal ERM-retinal adherence superior and inferior to the fovea; broad nasal and temporal; and complete adherence at the fovea area using SD-OCT (Figure 3, 4) underwent an easy ERM peel (predominantly Grade 1) (Figure 5, Video Supplement 2) (Supplemental material available at AJO.com). Video still frame (Figure 5) illustrates the temporal area of the TCA dusted membrane, which was easy to remove with no retinal traction seen.
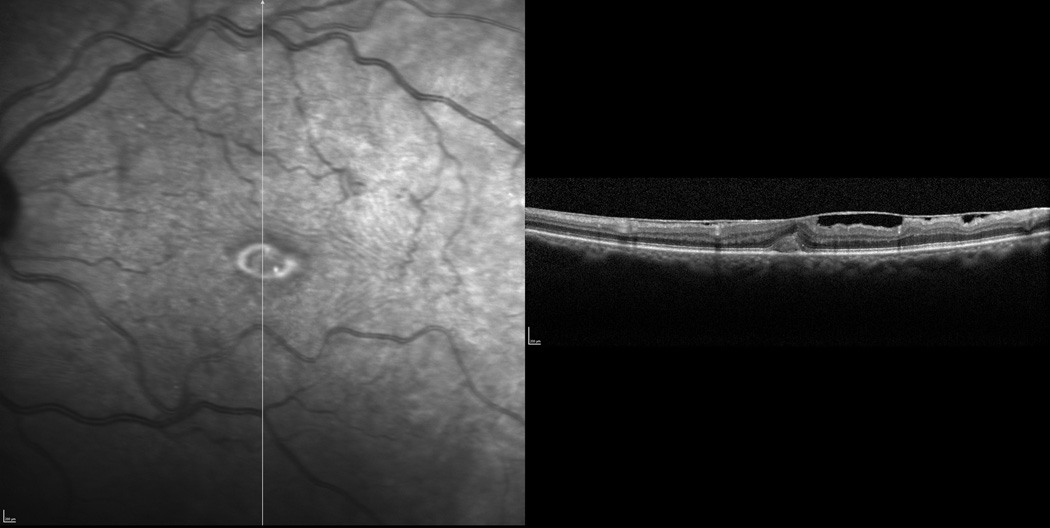
Figure 3.
Focal ERM-retinal adherence is seen both superior and inferior to the fovea along the vertical axis using spectral domain optical coherence tomography (SD-OCT).
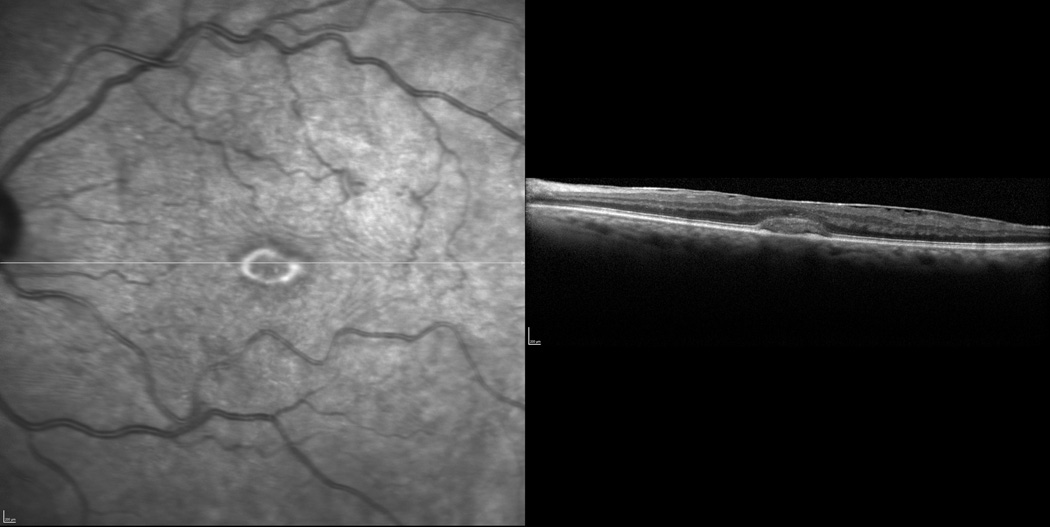
Figure 4.
Broad ERM-retinal adhesion (50–90%) is seen nasally and temporally across the horizontal axis by SD-OCT. Complete ERM-retinal adherence (>90%) is seen at the fovea.
DISCUSSION
Spectral domain optical coherence tomography provides higher resolution imaging of macular disease and in particular, retinal surface pathology.5,9,10,11,13 Our study is the first to characterize ERM-retinal surface details regarding extent of ERM-retinal adhesion and presence of fibrillary changes by SD-OCT. Furthermore, ERM surface characteristics were correlated with difficulty of surgical removal of these membranes.
Although a smaller study suggested that idiopathic epiretinal membrane structure could predict ease of surgical removal, surgical ease of removal was based on operative dictation by the surgeon who was not masked to SD-OCT findings.4 Additionally, surgical ease was determined based on the number of attempts made to remove the ERM, which is more dependent on surgical technique rather than ERM adherence to the retinal surface.
Complete ERM-retinal adhesion resulted in a 3.7- and 8.6-fold increase in surgical difficulty of membrane removal compared to broad (p< 0.02) and focal (p<0.0001) adhesion, respectively. It is not surprising that increased ERM-retinal adherence would increase surgical difficulty of membrane peeling. As the extent of ERM-retinal adhesion increases, the extent of ERM with non-adjacent retinal tissue directly abutting the membrane also decreases. This lamellar space between the ERM and retinal surface likely represents a true tissue-free space between the ERM and retinal surface. Although the presence of fluid cannot be excluded, this tissue-free space may provide a cleavage plane between ERM and retina to facilitate membrane peeling during vitrectomy surgery.
Notably, the presence of fibrillary changes (alternating hyper- and hypo- reflective signals between ERM and retinal surface not parallel to the retinal surface) resulted in a 25.5-fold increase in surgical removal difficulty compared to its absence (p< 0.0001). These fibrillary changes are likely glial in nature and may produce strong adhesional forces between the ERM and the retinal surface which may increase the strength of ERM-retinal adhesion and subsequently increased difficulty in surgical removal (sticky peel). These fibrillary changes are likely a distinct entity from fibrocellular proliferation observed along the posterior surface of the posterior hyaloid in vitreomacular traction described in prior studies given the differences in localization.11, 13 Additonally, ERM associated fibrillary changes described in this study likely contribute adhesion as opposed to tractional forces. In this study, the intended surgical plane was between the ERM and the retinal surface. Future studies with the intended surgical plane at the level of the internal limiting membrane correlating fibrillary changes in ERMs with surgical difficulty of removal may help elucidate the localization of fibrillary ERMs.
Thus, ERM-retinal surface findings by SD-OCT may allow surgeons to anticipate difficulty of surgical removal and facilitate surgical planning. Initiating ERM peeling over an area of focal or broad ERM-retinal adhesion in contrast to areas of complete ERM-retinal adhesion or over areas of fibrillary changes may provide a large enough flap or even peeling momentum to facilitate complete ERM removal more easily. Focal or broad ERM retinal adhesion patterns may allow the underlying retinal tissue to become more plicated. In contrast, complete ERM-retinal adhesion may prohibit the formation of retinal tissue plication. Patients with ERMs of predominantly complete ERM-retinal adherence or fibrillary changes seen by SD-OCT could be advised that surgical removal could potentially be more difficult. Additionally, it would be interesting to assess long term visual outcomes or rates of complications after removing ERMs with differing SD-OCT characteristics in future studies.
Our results note that any particular eye may contain multiple adhesion patterns and varying areas of difficulty for ERM removal. Although characterization of SD-OCT findings in each quadrant along the vertical and horizontal scanning meridians correlated completely, further studies using three-dimensional SD-OCT may provide more extensive topographical understanding of ERM-retinal surface adhesion and may further facilitate surgical approaches to ERM peeling. Additionally, correlating these lamellar spacing and fibrillary surface changes found on SD-OCT with electron microscopy may provide a cellular basis to our understanding of the constituents of ERM-retinal surface adhesion.
In summary, we have shown that SD-OCT may help determine surgical difficulty and thus be an important adjunct for planning ERM surgery. We found that the presence of fibrillary changes may have a stronger association with difficulty in ERM peeling than extent of ERM adhesion to the retinal surface given that the odds ratio was 25.5 compared to complete adhesion with an odds ratio of 8.6. SD-OCT analysis of ERMs provides valuable detailed ERM-retinal surface information to facilitate surgical planning for removal of membranes.
Supplementary Material
Supplement Video 1. Representative example of difficult peeling of an epiretinal membrane in which vessel elevation is seen with perivascular white reflection by forceps (upper frame) as the ERM is removed.
Supplement, Video 2. Easy removal of ERM from the temporal area is seen with no retinal traction.
ACKNOWLEDGEMENTS
This study was supported by The Heed Foundation (CKC), an Unrestricted Research Fund to Jacobs Retina Center at Shiley Eye Center, University of California, San Diego (LC), and NIH EYO 7366 (WRF).
Biographies
JAY CHHABLANI, MD

Jay Chhablani received his medical degree from Pt J N M Medical College, Raipur, India in 2003. He completed his residency at the Kasturba Medical College, Mangalore, India and a vitreoretinal surgery fellowship at Sankara Nethralaya, Chennai, India. Prior to his fellowship at the University of California, San Diego, Dr. Chhablani practiced vitreoretinal surgery at L V Prasad Eye Institute, Hyderabad, India. Dr. Chhablani is interested in macular disorders and advanced imaging techniques.
JAE SUK KIM, MD, PhD

Jae Suk Kim received his MD degree at Inje University School of Medicine, Busan, Korea and completed a residency at the Inje University Seoul Paik Hospital, Seoul, Korea. He received his PhD degree from Chung-Ang University School of Medicine, Seoul, Korea, investigating extracellular matrix remodeling in retinal pigment epithelial cells. Following a research fellowship at the University of California, San Diego, Dr. Kim is currently an Associate Professor at the Inje University Sanggye Paik Hospital, Seoul, Korea.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE
Financial Disclosures: NONE
Contributions to Authors:
Conception and design (JSK, IK, WRF); Analysis and Interpretation (JSK, JC, LC, IK, WRF); Writing the article (JC, WRF); Critical revision of the article (JC, CKC, LC, IK, WRF); Final approval of the article (JSK, CKC, LC, IK, WRF); Data collection (JSK, JC, KH); Provision of materials, patients, or resources (JSK, JC, CKC, WRF); Statistical expertise (LC); Obtaining funding (CKC, LC, WRF); Literature search (JSK, IK); Administrative, technical, or logistic support (JSK, JC, CKC, LC, IK, WRF)
Statement about Conformity with Author Information:
All procedures conformed to the Declaration of Helsinki for research involving human subjects and were approved by the Institutional Review Board of the University of California, San Diego.
REFERENCES
- 1.Watanabe A, Arimoto S, Nishi O. Correlation between metamorphopsia and epiretinal membrane optical coherence tomography findings. Ophthalmology. 2009;116(9):1788–1793. doi: 10.1016/j.ophtha.2009.04.046. [DOI] [PubMed] [Google Scholar]
- 2.Gass JDM. Stereoscopic atlas of macular disease. Vol 2. St. Louis: Mosby; 1987. pp. 693–695. [Google Scholar]
- 3.Wilkins JR, Puliafito CA, Hee MR, et al. Characterization of epiretinal membranes using optical coherence tomography. Ophthalmology. 1996;103(12):2142–2151. doi: 10.1016/s0161-6420(96)30377-1. [DOI] [PubMed] [Google Scholar]
- 4.Hattenbach LO, Höhn F, Fulle G, et al. Preoperative Assessment of Topographic Features in Macular Pucker using High-Definition Optical Coherence Tomography. Klin Monbl Augenheilkd. 2009;226(8):649–653. doi: 10.1055/s-0028-1109490. [DOI] [PubMed] [Google Scholar]
- 5.Georgopoulos M, Geitzenauer W, Ahlers C, et al. High-resolution optical coherence tomography to evaluate vitreomacular traction before and after membrane peeling. Ophthalmologe. 2008;105(8):753–760. doi: 10.1007/s00347-007-1664-0. [DOI] [PubMed] [Google Scholar]
- 6.Falkner-Radler CI, Glittenberg C, Hagen S, et al. Spectral-domain optical coherence tomography for monitoring epiretinal membrane surgery. Ophthalmology. 2010;117(4):798–805. doi: 10.1016/j.ophtha.2009.08.034. [DOI] [PubMed] [Google Scholar]
- 7.Mackenzie SE, Gandorfer A, Rohleder M, et al. Ultrastructure and retinal imaging of epiretinal membrane: a clinicopathologic correlation of trypan blue staining in epiretinal membrane surgery. Retina. 2010;30(4):648–654. doi: 10.1097/IAE.0b013e3181bceda9. [DOI] [PubMed] [Google Scholar]
- 8.Mori K, Gehlbach PL, Sano A, et al. Comparison of epiretinal membranes of differing pathogenesis using optical coherence tomography. Retina. 2004;24(1):57–62. doi: 10.1097/00006982-200402000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Legarreta JE, Gregori G, Knighton RW, et al. Three-dimensional spectral-domain optical coherence tomography images of the retina in the presence of epiretinal membranes. Am J Ophthalmol. 2008;145(6):1023–1030. doi: 10.1016/j.ajo.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 10.Michalewski J, Michalewska Z, Cisiecki S, Nawrocki J. Morphologically functional correlations of macular pathology connected with epiretinal membrane formation in spectral optical coherence tomography. Graefes Arch Clin Exp Ophthalmol. 2007;245(11):1623–1631. doi: 10.1007/s00417-007-0579-4. [DOI] [PubMed] [Google Scholar]
- 11.Chang LK, Fine HF, Spaide RF, et al. Ultrastructural correlation of spectral-domain optical coherence tomographic findings in vitreomacular traction syndrome. Am J Ophthalmol. 2008;146(1):121–127. doi: 10.1016/j.ajo.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wojtkowski M, Bajraszewski T, Gorczyńska I, et al. Ophthalmic imaging by spectral optical coherence tomography. Am J Ophthalmol. 2004;138(3):412–419. doi: 10.1016/j.ajo.2004.04.049. [DOI] [PubMed] [Google Scholar]
- 13.Koizumi H, Spaide RF, Fisher YL, et al. Three-dimensional evaluation of vitreomacular traction and epiretinal membrane using spectral-domain optical coherence tomography. Am J Ophthalmol. 2008;145(3):509–517. doi: 10.1016/j.ajo.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 14.Gupta P, Sadun AA, Sebag J. Multifocal retinal contraction in macular pucker analyzed by combined optical coherence tomography/scanning laser ophthalmoscopy. Retina. 2008;28(3):447–452. doi: 10.1097/IAE.0b013e318160a754. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement Video 1. Representative example of difficult peeling of an epiretinal membrane in which vessel elevation is seen with perivascular white reflection by forceps (upper frame) as the ERM is removed.
Supplement, Video 2. Easy removal of ERM from the temporal area is seen with no retinal traction.