Abstract
Background
Studies suggest that cancer patients receiving hematopoietic cell transplant (HCT) are at risk for cognitive deficits. To date, little research has investigated the cumulative effects of clinical risk factors on cognitive function in HCT patients.
Methods
Patients (N=278) scheduled for HCT for hematological disease completed neuropsychological assessments prior to HCT and at 6 and 12 months post-HCT. A time-varying cumulative clinical risk variable was examined as a predictor of total neuropsychological performance (TNP). Cumulative clinical risk was calculated from pre-HCT neuropsychological risk factors (e.g., history of cranial irradiation, intrathecal chemotherapy), HCT-related risk factors (e.g., allogeneic transplant, unrelated donor), and post-HCT complications (e.g., severity of mucositis and enteritis, graft versus host disease).
Results
Patients with greater cumulative clinical risk displayed worse TNP at baseline and 6 months post-HCT and less neuropsychological recovery over time than patients with less risk (ps<.05). Greater cumulative clinical risk predicted worse performance on tasks assessing executive function at baseline and 6 months post-HCT and memory at 6 and 12 months post-HCT (ps<.05). Among risk variables, length of hospital stay was the only significant predictor of neuropsychological function (p<.05).
Conclusions
Findings from this study indicate that clinical risk factors may have a cumulative effect on cognitive function in patients treated with HCT. Patients with a complicated clinical course should be referred for evaluation and management of cognitive deficits.
Keywords: bone marrow transplantation, hematopoietic stem cell transplantation, neurobehavioral manifestations, neoplasms
Advances in hematopoietic cell transplant (HCT) technology over the past two decades have resulted in higher survival rates and an increased number of transplants performed.1, 2 The increased prevalence of transplanted patients is reflected in a growing interest in post-transplant quality of life.3–5 Post-transplant cognitive function is an area of particular concern. Impairment in cognitive function can have important consequences for quality of life, such as the ability to return to work or school, function socially, and attain career or educational goals.6–8 HCT is a physically demanding treatment that may result in neurotoxicities such as delirium, seizures, and significant impairment in cognitive function during and after the transplant process.9, 10 On average, acute cognitive changes improve over time.11–13 Nevertheless, a sizable number of patients display enduring cognitive deficits. It is estimated that 20–28% of HCT survivors experience long-term cognitive deficits that are moderate to severe (i.e., more than two standard deviations below population norms)14–16 and 51% experience mild long-term cognitive deficits (i.e., more than one standard deviation below population norms).17 It is important to be able to determine which patients are at greatest risk for long-term cognitive impairment. Early identification of these patients can enable effective clinical management through close monitoring and timely referrals for intervention and support.
Cognitive function tends to vary over the course of the transplant process. Syrjala and colleagues18 reported that prior to allogeneic transplant, 71% of patients displayed impairment on one or more neuropsychological test. At 80 days post-transplant, this number increased to 94%, then declined to 74% at one year post-transplant and 41% at five years post-transplant.18, 19 The authors noted that pre-transplant impairment was predictive of impairment at one year follow-up; 88% of patients who were impaired at baseline also displayed later impairment, while 54% of patients who were unimpaired at baseline displayed later impairment.18 The cognitive domains most likely to be impaired were grip strength, motor dexterity, verbal fluency and verbal memory.18 A number of studies support Syrjala’s findings suggesting that cognitive impairments are common prior to transplant,13, 15–17, 20 remain stable or become worse during the acute transplant phase (i.e., within 100 days post-transplant),16, 21 then show modest recovery over time.13, 15, 17
Observed cognitive deficits in transplanted patients likely result from multiple disease and treatment factors. Patients with a history of total body irradiation or cranial irradiation,22, 23 intrathecal chemotherapy,24 or multiple standard-dose chemotherapy regimens25 may be at risk for greater cognitive impairment. During the acute transplant phase, length of hospital stay, days until engraftment, and severity of mucositis and enteritis, proxies for transplant-related toxicity, may predict cognitive function. Following transplant, allogeneic patients,21 patients with acute or chronic graft versus host disease (GVHD) at the time of assessment,17, 18, 21, 26 or those treated with steroids or narcotics may be at risk.17, 26, 27 We are aware of only one study examining the cumulative effects of clinical risk factors on cognition in HCT patients.23 The study examined cross-sectional relationships between cognitive function and four clinical variables: history of cranial irradiation, high-dose cytosine arabinoside (ara-C), central nervous system disease with intrathecal chemotherapy, and prophylactic intrathecal chemotherapy. Together, these risk factors accounted for 25% of variance in total neuropsychological performance.
Existing literature regarding risk factors for cognitive deficits in patients treated with HCT is beset by methodological limitations. Most studies have small samples, which limits power to detect effects. Many are also cross-sectional, so it is unclear how clinical risk factors affect cognitive function over time. In addition, few studies take patient attrition into account. This is particularly problematic in longitudinal studies of HCT patients, which tend to experience high rates of attrition due to mortality and morbidity.28 As such, studies may overestimate cognitive function because patients who are more severely compromised may not remain in the study.
The goal of the current study was to longitudinally examine the cumulative effects of clinical risk factors for cognitive deficits in hematologic cancer patients treated with HCT. Clinical risk factors included history of cancer treatment known to affect neuropsychological function (e.g., cranial irradiation, intrathecal chemotherapy), HCT-related risk factors (e.g., allogeneic transplant, unrelated donor), and post-HCT complications (e.g., severity of mucositis and enteritis, graft versus host disease). As described previously,11,12 a sequential longitudinal design was employed to statistically control for practice effects of repeated neuropsychological testing and sample attrition. Participants were randomly assigned to different groups that varied on the number of times they were evaluated. This enabled us to statistically partition changes in neuropsychological performance due to practice from true changes in neuropsychological performance over time.
Methods
Participants
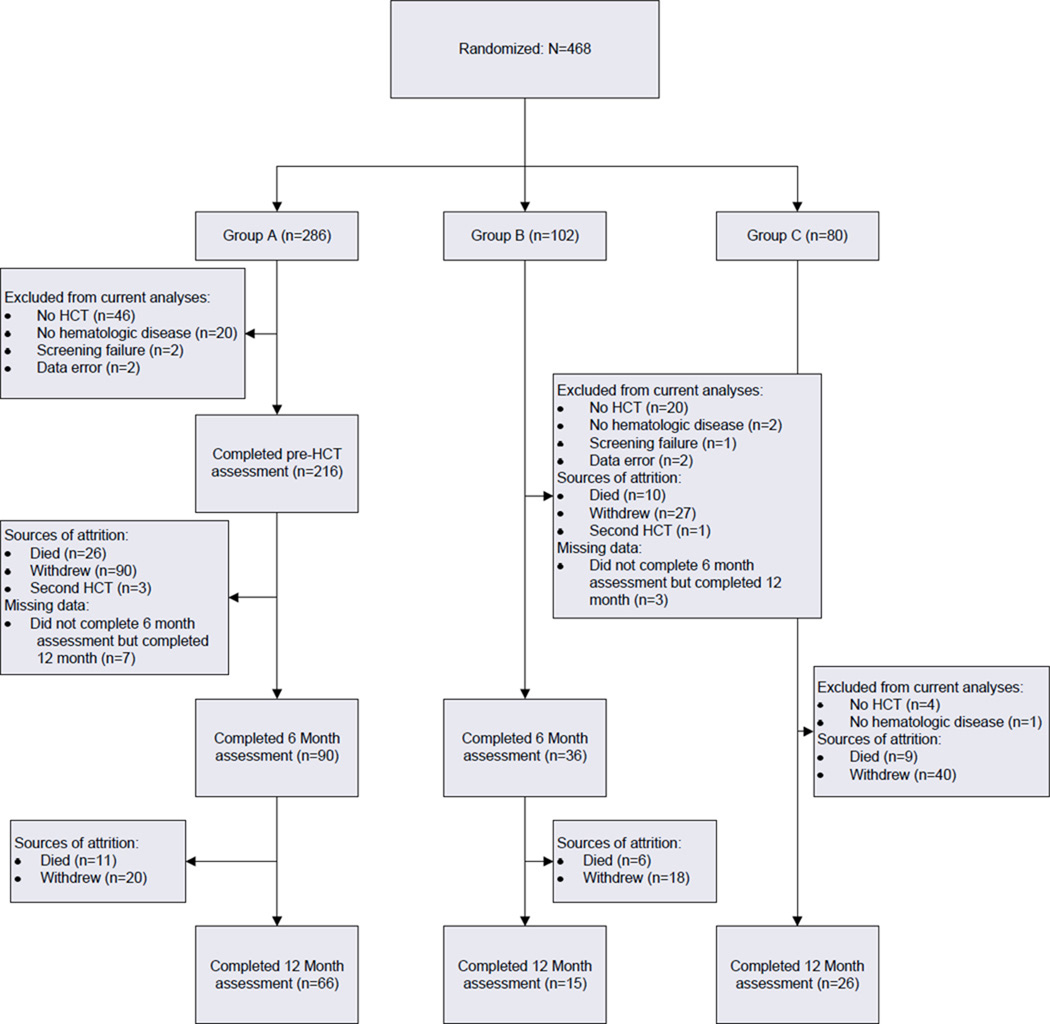
Following IRB approval, individuals identified as HCT candidates were recruited at Moffitt Cancer Center. Additional eligibility criteria were that participants: 1) be between 18 and 75 years of age; 2) have completed at least 8 years of formal education; 3) be able to speak and read standard English; 4) plan to return to Moffitt Cancer Center for follow-up assessments; and 5) provide written informed consent. Patients who had previously received HCT, who were not diagnosed with hematologic disease, or whose HCT was cancelled were excluded from analyses. Sample size and sources of attrition are shown in Figure 1.
Figure 1.
Sample size and sources of attrition. HCT refers to hematopoietic cell transplant.
Procedure
Following Institutional Review Board approval, patients were recruited between February 2001 and September 2004. Patients were approached by study staff at a pre-HCT psychosocial assessment. Patients who signed consent were then randomized to one of three groups in a sequential longitudinal design. Sixty percent of patients were randomized to Group A, which completed neuropsychological testing before hospitalization for HCT, 6 months post-HCT, and 12 months post-HCT. Twenty percent of patients were randomized to Group B, which completed neuropsychological testing at 6 months post-HCT and 12 months post-HCT. Twenty percent of patients were randomized to Group C, which completed neuropsychological testing at 12 months post-HCT only. Participants were paid $25 for each assessment.
Measures
Demographic and Clinical Measures
Gender, date of birth, race/ethnicity, marital status, annual household income, and education were assessed in all participants via self-report. Disease type and date of transplant were assessed through medical chart review. Additional medical data hypothesized to be predictive of cognitive function were also extracted from medical charts, including number of previous distinct chemotherapy regimens, history of total body radiation, history of prophylactic cranial irradiation, history of intrathecal chemotherapy, type of transplant (autologous versus allogeneic), length of hospital stay, days until engraftment [i.e., absolute neutrophil count (ANC) >500], and severity of mucositis and enteritis following transplant [according to Common Terminology Criteria for Adverse Events (CTCAE) 3.0]. At the 6 and 12 month post-HCT assessments, presence of disease relapse, active GVHD, and prescription of narcotics and systemic glucocorticoids were recorded. Medical chart reviews were performed by the cancer registrar and study staff supervised by an HCT physician.
Cognitive Performance
Cognitive performance was assessed using a battery of neuropsychological tests that were selected based on a review of published literature at the time of study design.29–32 Preference was given to tests with demonstrated reliability, validity, and availability of published norms. Alternate test forms were used when available. The battery was designed to assess four major domains of cognitive function: executive function, attention, episodic memory, and motor speed.
Executive function
Executive function refers to the ability to plan, initiate, and carry out goal-directed behavior as well as monitor performance. This domain was assessed using the Digit Symbol subtest of the Wechsler Adult Intelligence Scale -III,34 Trail Making Test,35 Controlled Oral Word Association,36 and Stroop Neuropsychological Screening Test.37
Attention
Attention refers to the ability to focus on incoming stimuli.33 Attention was assessed with the Connors’ Continuous Performance Test.38
Episodic memory
Episodic memory refers to the ability to store and retrieve verbal and visual information. Verbal (California Verbal Learning Test-II; Logical Memory subtest of the Weschler Memory Scales- III)39,40 and non-verbal (Visual Reproduction subtest of the Weschler Memory Scales- III)40 measures of episodic memory were administered.
Motor speed
The Grooved Pegboard41 was used to assess speeded manual dexterity.
Statistical Analysis
Raw neuropsychological test scores were converted into z-scores according to published normative data.42–45 Z-scores were then averaged by domain. A total neuropsychological performance score (TNP) was computed for each participant by averaging performance across the four domains (i.e., executive function, attention, episodic memory, motor speed). An attrition variable was coded for each participant with 0 indicating that the participant completed the study and 1 indicating that the participant left the study before its completion. Because data suggest that practice may give a one-time boost to cognitive performance,46 a practice variable was calculated such that members of Group A and B received a value of 1 and members of Group C received a value of 0.
A time-varying risk variable was calculated to examine the cumulative impact of putative clinical risk factors on TNP. Continuous clinical risk factors were dichotomized using median splits and summed such that a higher score indicated greater risk. Baseline clinical risk factors were history of prophylactic cranial irradiation, history of total body irradiation, history of intrathecal chemotherapy, and number of previous chemotherapy regimens (i.e., less than two versus two or more). Clinical risk factors at 6 months were baseline risk factors plus transplant type (i.e., allogeneic versus autologous), extent of HLA match (i.e., match versus mismatch), donor relationship (i.e., related versus unrelated), length of hospital stay (i.e, less than 20 days versus 20 days or more), days until engraftment (i.e., ANC>500; less than 12 days versus 12 days or more), peak severity of mucositis (i.e., CTCAE grade 1 or 2 versus 3 or 4), peak severity of enteritis (i.e., CTCAE grade 1 or 2 versus 3 or 4), presence of relapsed disease at 6 months (i.e., yes versus no), presence of GVHD at 6 months (i.e., yes versus no), systemic steroid treatment at 6 months (i.e., yes versus no), and narcotic utilization at 6 months (i.e., yes versus no). Clinical risk factors at 12 months were baseline risk factors plus transplant type, extent of HLA match, donor relationship, length of hospital stay, days until engraftment, peak severity of mucositis, peak severity of enteritis, presence of relapsed disease at 12 months, presence of GVHD at 12 months, steroid treatment at 12 months, and narcotic utilization at 12 months.
Mixed model analyses were used to examine the effects of cumulative clinical risk factors on TNP across the three measurement points: pre-HCT baseline, 6 months post-HCT, and 12 months post-HCT. In contrast to repeated measures ANOVA, mixed models analysis allows for the use of all available data at each time point. This is especially important when analyzing longitudinal data from HCT patients, as attrition tends to be high in this population.28 To reduce the possibility of Type 1 error due to multiple statistical tests, analyses were conducted in steps. In the first step, mixed model analyses were planned to examine the main effect of cumulative clinical risk on TNP at each time point and the risk by time interaction. If significant results were found for the risk by time interaction, additional mixed models were planned examining: 1) cumulative clinical risk as a predictor of individual cognitive domains, and 2) individual risk factors as predictors of TNP and statistically-significant domains. Two-sided alpha of .05 was used as the criterion for statistical significance.
Eight effects were estimated in the primary analysis: 1) an intercept representing mean TNP at 6 months post-HCT, 2) the main effect of attrition at 6 months post-HCT, 3) the effect of attrition over time, 4) the main effect of practice at 6 months post-HCT, 5) the effect of practice over time, 6) longitudinal change over time indicating mean change per assessment, 7) the main effect of cumulative clinical risk at 6 months post-HCT, and 8) a cumulative clinical risk by time interaction indicating the effect of cumulative clinical risk on longitudinal change over time. The main effect of risk at pre-HCT baseline was estimated controlling for attrition and attrition by time, while the main effect of risk at 12 months post-HCT was estimated controlling for practice and practice by time, since practice did not vary at baseline and attrition did not vary at 12 months post-HCT.
Results
Demographic and clinical characteristics of the sample are presented in Table 1. Randomization was successful. Of 468 patients randomized, with the exception of a larger proportion of multiple myeloma patients in Group C (61% vs. 46% in Group A and 43% in Group B, p=.04), there were no group differences in sociodemographic (i.e., gender, age, education, race/ethnicity, marital status, annual household income) or clinical variables (i.e., transplant type, donor relationship, donor match, history of prophylactic cranial irradiation, history of total body irradiation, history of intrathecal chemotherapy, number of previous chemotherapy regimens, length of hospital stay, days until engraftment, severity of mucositis, severity of enteritis), although not all patients who were randomized had complete clinical data (see Figure 1). Additional analyses were conducted to examine differences between patients who did and did not complete the study. There were no differences in sociodemographic characteristics (i.e., gender, age, education, race/ethnicity, marital status, annual household income) between the 107 participants who completed a Time 3 assessment and the 361 participants who were randomized but did not complete a Time 3 assessment (ps>.11). Regarding clinical characteristics, patients who completed the study had shorter hospitalizations and were more likely to be diagnosed with multiple myeloma (ps<.01). There were no other significant clinical differences between patients who did and did not complete the study (i.e., transplant type, donor relationship, donor match, history of prophylactic cranial irradiation, history of total body irradiation, history of intrathecal chemotherapy, number of previous chemotherapy regimens, days until engraftment, severity of mucositis, severity of enteritis, ps>.23).
Table 1.
Sociodemographic and Clinical Characteristics of Sample (n=278)
| Group assignment (n, %) | ||
|---|---|---|
| Group A | 216 | 78% |
| Group B | 36 | 13% |
| Group C | 26 | 9% |
| Mean age: years (SD) | 50.50 | 12.52 |
| Mean education: years (SD) | 13.94 | 2.72 |
| Gender (n, % female) | 124 | 45% |
| Race/ethnicity (n, % Caucasian/non-Hispanic) | 225 | 81% |
| Marital status (n, % married) | 211 | 76% |
| Annual household income (n, % > $40k) | 154 | 55% |
| HCT type (n, % allogeneic) | 65 | 23% |
| Unrelated donor (n, %) | 21 | 32% a |
| Mismatched donor (n, %) | 2 | 3% a |
| Matched sibling (n, %) | 42 | 65% a |
| Disease type | ||
| Acute myelogeneous leukemia | 20 | 7% |
| Myelodysplastic syndrome | 8 | 3% |
| Acute lymphoblastic leukemia | 9 | 3% |
| Multiple myeloma | 150 | 54% |
| Chronic lymphocytic leukemia | 2 | 1% |
| Chronic myelogeneous leukemia | 7 | 3% |
| Myeloproliferative disorder | 2 | 1% |
| Non-Hodgkin’s lymphoma | 60 | 22% |
| Aplastic anemia | 3 | 1% |
| Hodgkin’s lymphoma | 17 | 6% |
| History of prophylactic cranial irradiation (n, % yes) | 6 | 2% |
| History of total body irradiation (n, % yes) | 15 | 5% |
| History of intrathecal chemotherapy (n, % yes) | 17 | 6% |
| Median number of previous chemotherapy regimens (range) | 2 | 0–7 |
| Peak severity of post-HCT mucositis (n, % grade 3 or 4) | 200 | 72% |
| Peak severity of post-HCT enteritis (n, % grade 3 or 4) | 106 | 38% |
| Days to engraftment (median, range) | 12 | 9–39 |
| Length of hospital stay (median in days, range) | 19 | 0–96 |
| Disease relapse at 6 months (n, % yes) | 12 | 10% |
| Active GVHD at 6 months (n, % yes) | 10 | 15%a |
| Prescribed steroids at 6 months (n, % yes) | 17 | 16% |
| Prescribed narcotics at 6 months (n, % yes) | 35 | 33% |
| Disease relapse at 12 months (n, % yes) | 15 | 19% |
| Active GVHD at 12 months (n, % yes) | 5 | 8% a |
| Prescribed steroids at 12 months (n, % yes) | 14 | 20% |
| Prescribed narcotics at 12 months (n, % yes) | 17 | 24% |
Denominator is participants with allogeneic transplant.
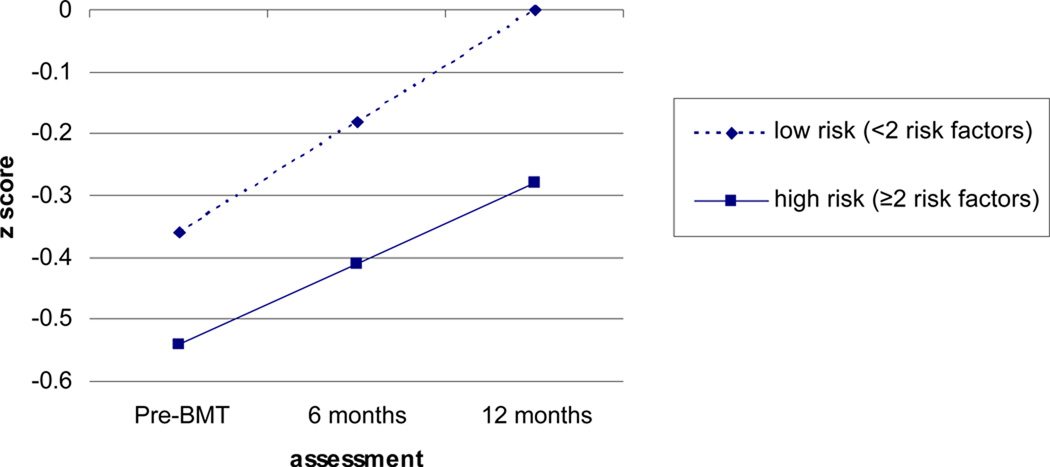
Results of mixed models analyses are shown in Table 2. Regarding TNP, analyses indicated a significant main effect of cumulative clinical risk at pre-HCT baseline and 6 months post-transplant (p<.05) and a significant risk by time interaction. Specifically, patients with a greater number of risk factors displayed worse total neuropsychological performance at pre-HCT baseline and 6 months post-transplant, as well as less neuropsychological recovery over time. For illustrative purposes, cumulative clinical risk at 6 months post-transplant was dichotomized using a median split (i.e., less than two risk factors versus two or more) and estimated mean TNP scores were graphed in Figure 2 controlling for practice, practice by time, attrition, and attrition by time.
Table 2.
Results of Mixed Models Examining Clinical Risk Factors as Predictors of Cognitive Function.
| Outcome | Time | Risk at Baseline |
Risk at 6 months post- HCT |
Risk at 12 months post- HCT |
Risk × Time |
|---|---|---|---|---|---|
| TNP | .28 (.35) | −.07 (.02)** | −.03 (.01)** | −.00 (.02) | .03 (.01)* |
| Memory | −.27 (.53) | −.04 (.03) | −.05 (.02)** | −.05 (.02)* | .00 (.02) |
| Executive Function | .15 (.61) | −.10 (.03)** | −.06 (.03)* | −.02 (.05) | .04 (.04) |
| Attention | .07 (.42) | −.00 (.02) | .01 (.01) | .02 (.02) | .01 (.02) |
| Motor Speed | 1.12 (.95) | −.10 (.05) | −.02 (.04) | .04 (.08) | .06 (.05) |
Note: Non-standardized estimates and standard errors shown. Because mixed models use all available data from each time point, these analyses are based on a sample size of 278 subjects who provided data at one or more time points.
TNP: total neuropsychological performance
p<.05,
p<.01
Figure 2.
Graph of Mean Total Neuropsychological Performance Scores by Cumulative Risk Controlling for Practice and Attrition. Slopes and intercepts were estimated using data from 278 subjects who provided data at one or more time points.
Based on these findings, additional analyses were conducted to examine the effects of cumulative risk over time on domains of cognitive function. As shown in Table 2, main effects of cumulative risk were evident in the domains of memory and executive function, such that patients with higher risk showed lower scores in the executive function domain at pre-HCT baseline and in both domains at 6 months post-transplant. This difference remained significant at 12 months post-transplant in the memory domain (p<.01), but not the executive function domain (p=.48).
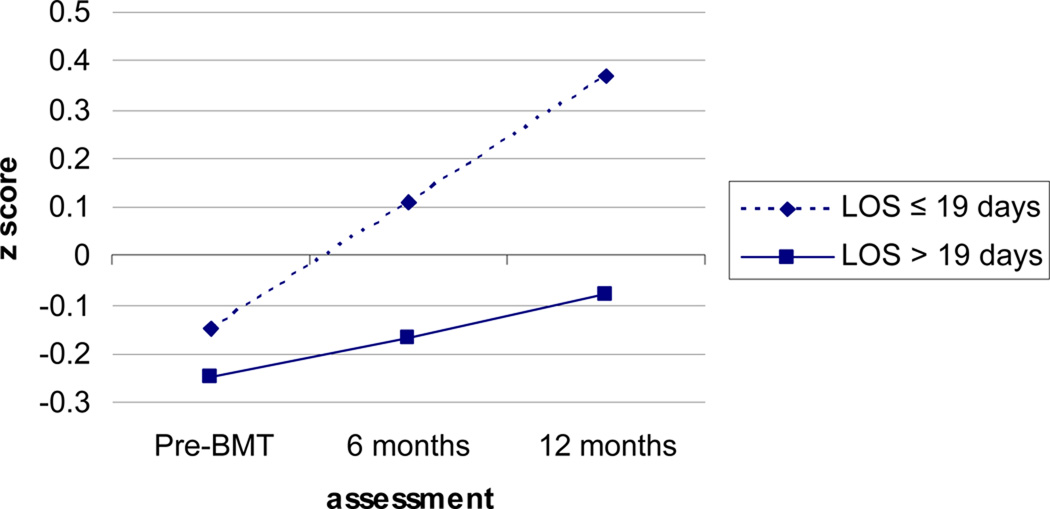
Additional analyses were then conducted examining the effects of individual risk factors on TNP, memory, and executive function. Individual risk factors examined were number of previous chemotherapy cycles, transplant type, days until engraftment, length of hospital stay, severity of mucositis, and severity of enteritis. Other risk factors included in the index of cumulative risk were not examined due to small numbers of patients with these risk factors (see Table 1). A significant interaction was observed between transplant type and time on memory such that patients receiving allogeneic transplant displayed less improvement in memory over time than patients receiving autologous transplant (Est.=−.16, SE=.07, p=.02). A significant interaction was also observed between length of hospital stay and time on memory such that patients with longer hospital stays showed less improvements on memory than patients with shorter hospital stays (Est.=−.02, SE=.00, p<.01). Patients with longer hospital stays also displayed worse memory at 6 months post-HCT (Est.=−.02 SE=.01, p<.01) and 12 months post-HCT (Est.=−.03, SE=.01, p<.01). Because length of hospital stay may be confounded with transplant type, we conducted an additional analysis to examine the independent effects of each to memory. When both variables were entered into the same model, the interaction between transplant type and time became non-significant, while the interaction between length of hospital stay and time remained significant (Est.=−.01, SE=.01, p=.05). Patients with longer hospital stays showed less improvement in memory over time than patients with shorter stays. For illustrative purposes, length of hospital stay was dichotomized using a median split (i.e., 19 days or less versus 20 or more days) and mean memory scores at each time point were graphed controlling for allogeneic transplant, allogeneic transplant by time, practice, practice by time, attrition, and attrition by time (see Figure 3).
Figure 3.
Graph of Mean Memory Scores by Length of Hospital Stay (LOS) Controlling for Practice, Attrition, and Transplant Type. Slopes and intercepts were estimated using data from 278 subjects who provided data at one or more time points.
Discussion
The current study examined clinical predictors of cognitive function in patients treated with HCT. A time-varying index of cumulative clinical risk was created based on previous literature and used to predict cognitive function at pre-HCT baseline, 6 and 12 months post-HCT, as well as recovery of cognitive function over time. Results indicated that patients with higher levels of cumulative clinical risk showed worse total neuropsychological performance at pre-HCT baseline and 6 months post-HCT, as well as worse cognitive recovery over time. The relationship between higher cumulative clinical risk and worse total neuropsychological performance appeared to be driven by worse performance in the domains of memory and executive function. Patients with greater cumulative clinical risk displayed worse performance in executive function at pre-HCT baseline and 6 months post-HCT, as well as worse performance in memory at 6 and 12 months post-HCT. Thus, data from the current study suggest a cumulative cognitive burden of pre-HCT cancer treatment, HCT factors, and HCT-related complications. In addition, the current study suggests that memory and executive function, or the ability to store and retrieve verbal and visual information and to plan and execute higher-order cognitive tasks, are particularly affected.
Analyses examining individual clinical risk factors indicated that allogeneic transplant and longer hospital stay were associated with less improvement in memory over time. When both of these variables were entered into the same model, longer hospital stay significantly predicted less improvement in memory over time, but allogeneic transplant did not. Length of hospital stay can be considered a proxy for a complicated post-transplant recovery, suggesting that clinical course during the acute transplant phase may be important for later cognitive recovery in the memory domain.
These data are consistent with previous findings suggesting that cancer patients treated with HCT display worse performance in memory and executive function.14, 47 Previous research has also suggested that the domains of attention and motor speed are compromised following HCT.14, 47, 48 Taken as a whole, available literature indicates that patients treated with HCT display pervasive cognitive deficits. Patients are characterized by mild diffuse deficits even before HCT.11 Cognitive function improves on average following transplant13, 47 but remains lower than non-cancer controls.47 The current study adds to this literature by suggesting that there are multiple clinical risk factors throughout the transplant process that together predict cognitive deficits.
Our data are consistent with the theory that cumulative physiologic damage secondary to cancer and treatment may overwhelm the brain’s ability to compensate. Functional magnetic resonance imaging (fMRI) studies suggest that cancer patients pre-and post-treatment with standard-dose chemotherapy show activation across larger areas of the brain when completing tasks of attention and working memory compared to individuals without cancer.49, 50 In addition, cancer patients treated with standard-dose chemotherapy display structural changes in white and gray matter.12, 50, 51 These changes are particularly evident in the year after completion of standard-dose chemotherapy.51 White matter abnormalities are also evident after HCT,26 as well as intrathecal chemotherapy for central nervous system lymphoma.8, 52 White matter abnormalities are particularly pronounced in patients with chronic GVHD, as well as patients treated with corticosteroids and cyclosporine.26 Thus, cognitive deficits evident upon neuropsychological testing are consistent with brain abnormalities in patients who experience multiple cognitive insults as a consequence of cancer and its treatment.
The current study has clinical implications for early identification and intervention in HCT patients at risk of cognitive deficits. Before transplant, patients with a history of prophylactic cranial irradiation, total body irradiation, intrathecal chemotherapy, and multiple courses of standard-dose chemotherapy are at risk for reduced cognitive function. If more than one HCT conditioning regimen is under consideration, it is reasonable to select the regimen with the lowest neurotoxicity profile for these patients to limit further neurological insult from cancer treatment.
Following transplant, patients with these baseline risk factors as well as allogeneic transplant, mismatched and unrelated donor, who experience a longer hospital stay, longer time to engraftment, and more severe mucositis and enteritis are less likely to experience cognitive recovery. Patients who have GVHD, relapsed disease, and treatment with narcotics and steroids are also less likely to experience cognitive recovery. These patients may benefit from education by clinical staff to help them set realistic expectations for cognitive function following transplant. In addition, they may benefit from referral to a neuropsychologist for evaluation and management of cognitive deficits.53 Management of cognitive deficits typically involves developing awareness of situations in which cognitive difficulties are likely to arise and rehearsing compensatory strategies.53 These strategies result in moderate to large improvements (i.e., .5 to 1.0 SD) in objective neuropsychological function and self-reported cognition.53
There are several strengths of the current study. It is the first to longitudinally examine the cumulative effects of clinical risk factors on cognitive function in patients treated with HCT. It used a comprehensive, reliable, and valid neuropsychological battery. In addition, the effects of practice and attrition were controlled in analyses, which is important because both practice effects and attrition can cause the false appearance of cognitive improvement over time. Study limitations should also be noted, however. For example, study attrition was high. Although high attrition is not unusual in longitudinal studies of HCT, patients who did not complete the current study had longer hospitalizations and were less likely to be diagnosed with multiple myeloma, suggesting that these patients may have been sicker than patients who completed the study. Because longer hospital stays predicted less improvement in memory over time, patients who did not complete the study may have also had worse cognitive function than patients who completed all assessments. Consequently, attrition was controlled in analyses. A large proportion of the sample consisted of patients receiving autologous HCT for multiple myeloma, so we were unable to adequately investigate the independent effects of risk factors related to allogeneic transplant due to small cell sizes (e.g., donor relationship, GVHD). In addition, despite previous studies suggesting that delirium prospectively predicts worse neuropsychological function,10 we were unable to include post-HCT delirium as a risk factor due to inconsistent documentation in the medical charts. Future research should examine the independent effects of allogeneic HCT-related risk factors on cognitive function over time, as well as adding delirium to our current model of cumulative clinical risk.
In summary, the current study provides evidence that cognitive deficits observed in HCT patients may result from the cumulative effects of multiple courses of treatment, aspects of the transplant itself, and post-transplant complications. Clinical staff should be aware of these risk factors and proactively refer patients at risk for neuropsychological evaluation and management of cognitive deficits. Doing so will help patients achieve the best possible function following transplant.
Acknowledgments
This work was supported by a grant from the American Cancer Society (RSG-01-070-01-PB) to Dr. Booth-Jones. Dr. Jim is supported in part by the National Cancer Institute (K07-CA138499).
References
- 1.Serna DS, Lee SJ, Zhang MJ, Baker S, Eapen M, Horowitz MM, et al. Trends in survival rates after allogeneic hematopoietic stem-cell transplantation for acute and chronic leukemia by ethnicity in the United States and Canada. J Clin Oncol. 2003;21(20):3754–3760. doi: 10.1200/JCO.2003.03.133. [DOI] [PubMed] [Google Scholar]
- 2.Rizzo JD, Wingard JR, Tichelli A, Lee SJ, Van Lint MT, Burns LJ, et al. Recommended screening and preventive practices for long-term survivors after hematopoietic cell transplantation: joint recommendations of the European Group for Blood and Marrow Transplantation, Center for International Blood and Marrow Transplant Research, and the American Society for Blood and Marrow Transplantation (EBMT/CIBMTR/ASBMT) Bone Marrow Transplant. 2006;37(3):249–261. doi: 10.1038/sj.bmt.1705243. [DOI] [PubMed] [Google Scholar]
- 3.Andrykowski MA, Bishop MM, Hahn EA, Cella DF, Beaumont JL, Brady MJ, et al. Long-term health-related quality of life, growth, and spiritual well-being after hematopoietic stem-cell transplantation. J Clin Oncol. 2005;23(3):599–608. doi: 10.1200/JCO.2005.03.189. [DOI] [PubMed] [Google Scholar]
- 4.Bevans MF, Marden S, Leidy NK, Soeken K, Cusack G, Rivera P, et al. Health-related quality of life in patients receiving reduced-intensity conditioning allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2006;38(2):101–109. doi: 10.1038/sj.bmt.1705406. [DOI] [PubMed] [Google Scholar]
- 5.Byar KL, Eilers JE, Nuss SL. Quality of life 5 or more years post-autologous hematopoietic stem cell transplant. Cancer Nurs. 2005;28(2):148–157. doi: 10.1097/00002820-200503000-00010. [DOI] [PubMed] [Google Scholar]
- 6.Ahles TA, Saykin A. Cognitive effects of standard-dose chemotherapy in patients with cancer. Cancer Invest. 2001;19(8):812–820. doi: 10.1081/cnv-100107743. [DOI] [PubMed] [Google Scholar]
- 7.Ahles TA, Saykin AJ, Noll WW, Furstenberg CT, Guerin S, Cole B, et al. The relationship of APOE genotype to neuropsychological performance in long-term cancer survivors treated with standard dose chemotherapy. Psychooncology. 2003;12(6):612–619. doi: 10.1002/pon.742. [DOI] [PubMed] [Google Scholar]
- 8.Fliessbach K, Urbach H, Helmstaedter C, Pels H, Glasmacher A, Kraus JA, et al. Cognitive performance and magnetic resonance imaging findings after high-dose systemic and intraventricular chemotherapy for primary central nervous system lymphoma. Arch Neurol. 2003;60(4):563–568. doi: 10.1001/archneur.60.4.563. [DOI] [PubMed] [Google Scholar]
- 9.de Brabander C, Cornelissen J, Smitt PA, Vecht CJ, van den Bent MJ. Increased incidence of neurological complications in patients receiving an allogenic bone marrow transplantation from alternative donors. J Neurol Neurosurg Psychiatry. 2000;68(1):36–40. doi: 10.1136/jnnp.68.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fann JR, Alfano CM, Roth-Roemer S, Katon WJ, Syrjala KL. Impact of delirium on cognition, distress, and health-related quality of life after hematopoietic stem-cell transplantation. J Clin Oncol. 2007;25(10):1223–1231. doi: 10.1200/JCO.2006.07.9079. [DOI] [PubMed] [Google Scholar]
- 11.Beglinger LJ, Duff K, Van Der Heiden S, Moser DJ, Bayless JD, Paulsen JS, et al. Neuropsychological and psychiatric functioning pre- and posthematopoietic stem cell transplantation in adult cancer patients: a preliminary study. J Int Neuropsychol Soc. 2007;13(1):172–177. doi: 10.1017/S1355617707070208. [DOI] [PubMed] [Google Scholar]
- 12.Deprez S, Amant F, Yigit R, Porke K, Verhoeven J, Van den Stock J, et al. Chemotherapy-induced structural changes in cerebral white matter and its correlation with impaired cognitive functioning in breast cancer patients. Hum Brain Mapp. 2011;32(3):480–493. doi: 10.1002/hbm.21033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacobs SR, Small BJ, Booth-Jones M, Jacobsen PB, Fields KK. Changes in cognitive functioning in the year after hematopoietic stem cell transplantation. Cancer. 2007;110(7):1560–1567. doi: 10.1002/cncr.22962. [DOI] [PubMed] [Google Scholar]
- 14.Booth-Jones M, Jacobsen PB, Ransom S, Soety E. Characteristics and correlates of cognitive functioning following bone marrow transplantation. Bone Marrow Transplant. 2005;36(8):695–702. doi: 10.1038/sj.bmt.1705108. [DOI] [PubMed] [Google Scholar]
- 15.Harder H, Duivenvoorden HJ, van Gool AR, Cornelissen JJ, van den Bent MJ. Neurocognitive functions and quality of life in haematological patients receiving haematopoietic stem cell grafts: a one-year follow-up pilot study. J Clin Exp Neuropsychol. 2006;28(3):283–293. doi: 10.1080/13803390490918147. [DOI] [PubMed] [Google Scholar]
- 16.Meyers CA, Weitzner M, Byrne K, Valentine A, Champlin RE, Przepiorka D. Evaluation of the neurobehavioral functioning of patients before, during, and after bone marrow transplantation. J Clin Oncol. 1994;12(4):820–826. doi: 10.1200/JCO.1994.12.4.820. [DOI] [PubMed] [Google Scholar]
- 17.Sostak P, Padovan CS, Yousry TA, Ledderose G, Kolb HJ, Straube A. Prospective evaluation of neurological complications after allogeneic bone marrow transplantation. Neurology. 2003;60(5):842–848. doi: 10.1212/01.wnl.0000046522.38465.79. [DOI] [PubMed] [Google Scholar]
- 18.Syrjala KL, Dikmen S, Langer SL, Roth-Roemer S, Abrams JR. Neuropsychologic changes from before transplantation to 1 year in patients receiving myeloablative allogeneic hematopoietic cell transplant. Blood. 2004;104(10):3386–3392. doi: 10.1182/blood-2004-03-1155. [DOI] [PubMed] [Google Scholar]
- 19.Syrjala KL, Artherholt SB, Kurland BF, Langer SL, Roth-Roemer S, Elrod JB, et al. Prospective Neurocognitive Function Over 5 Years After Allogeneic Hematopoietic Cell Transplantation for Cancer Survivors Compared With Matched Controls at 5 Years. J Clin Oncol. 2011 doi: 10.1200/JCO.2010.33.9119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harder H, Van Gool AR, Cornelissen JJ, Duivenvoorden HJ, Eijkenboom WM, Barge RM, et al. Assessment of pre-treatment cognitive performance in adult bone marrow or haematopoietic stem cell transplantation patients: a comparative study. Eur J Cancer. 2005;41(7):1007–1016. doi: 10.1016/j.ejca.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 21.Schulz-Kindermann F, Mehnert A, Scherwath A, Schirmer L, Schleimer B, Zander AR, et al. Cognitive function in the acute course of allogeneic hematopoietic stem cell transplantation for hematological malignancies. Bone Marrow Transplant. 2007;39(12):789–799. doi: 10.1038/sj.bmt.1705663. [DOI] [PubMed] [Google Scholar]
- 22.Correa DD, DeAngelis LM, Shi W, Thaler H, Glass A, Abrey LE. Cognitive functions in survivors of primary central nervous system lymphoma. Neurology. 2004;62(4):548–555. doi: 10.1212/01.wnl.0000109673.75316.d8. [DOI] [PubMed] [Google Scholar]
- 23.Andrykowski MA, Schmitt FA, Gregg ME, Brady MJ, Lamb DG, Henslee-Downey PJ. Neuropsychologic impairment in adult bone marrow transplant candidates. Cancer. 1992;70(9):2288–2297. doi: 10.1002/1097-0142(19921101)70:9<2288::aid-cncr2820700913>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 24.Harder H, Holtel H, Bromberg JE, Poortmans P, Haaxma-Reiche H, Kluin-Nelemans HC, et al. Cognitive status and quality of life after treatment for primary CNS lymphoma. Neurology. 2004;62(4):544–547. doi: 10.1212/wnl.62.4.544. [DOI] [PubMed] [Google Scholar]
- 25.Jansen CE, Miaskowski C, Dodd M, Dowling G, Kramer J. A metaanalysis of studies of the effects of cancer chemotherapy on various domains of cognitive function. Cancer. 2005;104(10):2222–2233. doi: 10.1002/cncr.21469. [DOI] [PubMed] [Google Scholar]
- 26.Padovan CS, Yousry TA, Schleuning M, Holler E, Kolb HJ, Straube A. Neurological and neuroradiological findings in long-term survivors of allogeneic bone marrow transplantation. Ann Neurol. 1998;43(5):627–633. doi: 10.1002/ana.410430511. [DOI] [PubMed] [Google Scholar]
- 27.Chapman SL, Byas-Smith MG, Reed BA. Effects of intermediate- and long-term use of opioids on cognition in patients with chronic pain. Clin J Pain. 2002;18(4 Suppl):S83–S90. doi: 10.1097/00002508-200207001-00010. [DOI] [PubMed] [Google Scholar]
- 28.Pidala J, Anasetti C, Jim H. Quality of life after allogeneic hematopoietic cell transplantation. Blood. 2009;114(1):7–19. doi: 10.1182/blood-2008-10-182592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schagen SB, van Dam FSAM, Muller MJ, Boogerd W, Lindeboom J, Bruning PF. Cognitive deficits after postoperative adjuvant chemotherapy for breast carcinoma. Cancer. 1999;85:640–650. doi: 10.1002/(sici)1097-0142(19990201)85:3<640::aid-cncr14>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 30.Wieneke MH, Dienst ER. Neuropsychological assessment of cognitive functioning following chemotherapy for breast cancer. Psychooncology. 1995;4:61–66. [Google Scholar]
- 31.van Dam FSAM, Schagen SB, Muller MJ, Boogerd RM, Wall Evd, Droogleever Fortuyn ME, et al. Impairment of cognitive function in women receiving adjvant treatment for high-risk breast cancer: High-dose versus standard-dose chemotherapy. Journal of the National Cancer Institute. 1998;90:210–218. doi: 10.1093/jnci/90.3.210. [DOI] [PubMed] [Google Scholar]
- 32.Andrykowski MA, Schmitt FA, Gregg ME, Brady MI, Lamb DG, Henslee-Downey J. Neuropsychologic impairment in adjult bone marrow transplant candidates. Cancer. 1992;70:2288–2297. doi: 10.1002/1097-0142(19921101)70:9<2288::aid-cncr2820700913>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 33.Lezak MD, Howieson DB, Loring DW. Neuropsychological Assessment. ed. 4. New York: Oxford University Press; 2004. [Google Scholar]
- 34.Weschler D. WAIS-III administration and scoring manual. San Antonio TX: Psychological Corporation; 1997. [Google Scholar]
- 35.Reitan RM, Wolfson D. The Halstead-Reitan Neuropsychological Test Battery: Theory and clinical interpretation. Tucson, AZ: Neuropsychology Press; 1993. [Google Scholar]
- 36.Benton AL, Hamsher K. Multilingual Aphasia Examination. Iowa City IA: AJA Associates; 1989. [Google Scholar]
- 37.Trenarry MR, Crosson B, DeBoe J, Leber WR. The Stroop Neuropsychological Screening Test. Lutz, FL: Psychological Assessment Resources; 1989. [Google Scholar]
- 38.Connors CK. Connors' Continuous Performance Test 3.0. Toronto, Ontario, Canada: MHS; 2000. [Google Scholar]
- 39.Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test: Adult version. San Antonio TX: Psychological Corporation; 1987. [Google Scholar]
- 40.Weschler D. Weschler Memory Scale - III manual. San Antonio TX: Psychological Corporation; 1997. [Google Scholar]
- 41.Matthews C, Klove H. Instruction Manual for the Adult Neuropsychology Test Battery. Madison, WI: University of Wisconsin Medical School; 1964. [Google Scholar]
- 42.Wechsler D. Wechsler Memory Scale-III Manual. San Antonio, TX: Psychological Corporation; 1997. [Google Scholar]
- 43.Heaton RK. Comprehensive norms for an expanded Halstead-Reitan battery: A supplement to the Wechsler Adult Intellligence Scales-Revised (WAIS-R) Lutz, FL: Psychological Assessment Resources; 1992. [Google Scholar]
- 44.Heaton RK, Grant I, Matthews CG. Comprehensive norms for an expanded Halstead-Reitan battery: Demographic correction, research findings, and clinical application. Lutz, FL: Psychological Assessment Resources; 1991. [Google Scholar]
- 45.Spreen O, Strauss E. A compendium of neuropsychological tests: Administration, norms, and commentary. ed. 2nd. New York: Oxford University Press; 1998. [Google Scholar]
- 46.Hoffman L, Hofer SM, Sliwinski MJ. On the confounds among retest gains and age-cohort differences in the estimation of within-person change in longitudinal studies: A simulation study. Psychol Aging. 2011 doi: 10.1037/a0023910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Syrjala KL, Artherholt SB, Kurland BF, Langer SL, Roth-Roemer S, Elrod JB, et al. Prospective neurocognitive function over 5 years after allogeneic hematopoietic cell transplantation for cancer survivors compared with matched controls at 5 years. J Clin Oncol. 2011;29(17):2397–2404. doi: 10.1200/JCO.2010.33.9119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Friedman MA, Fernandez M, Wefel JS, Myszka KA, Champlin RE, Meyers CA. Course of cognitive decline in hematopoietic stem cell transplantation: a within-subjects design. Arch Clin Neuropsychol. 2009;24(7):689–698. doi: 10.1093/arclin/acp060. [DOI] [PubMed] [Google Scholar]
- 49.Cimprich B, Reuter-Lorenz P, Nelson J, Clark PM, Therrien B, Normolle D, et al. Prechemotherapy alterations in brain function in women with breast cancer. J Clin Exp Neuropsychol. 2010;32(3):324–331. doi: 10.1080/13803390903032537. [DOI] [PubMed] [Google Scholar]
- 50.Ferguson RJ, McDonald BC, Saykin AJ, Ahles TA. Brain structure and function differences in monozygotic twins: possible effects of breast cancer chemotherapy. J Clin Oncol. 2007;25(25):3866–3870. doi: 10.1200/JCO.2007.10.8639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Inagaki M, Yoshikawa E, Matsuoka Y, Sugawara Y, Nakano T, Akechi T, et al. Smaller regional volumes of brain gray and white matter demonstrated in breast cancer survivors exposed to adjuvant chemotherapy. Cancer. 2007;109(1):146–156. doi: 10.1002/cncr.22368. [DOI] [PubMed] [Google Scholar]
- 52.Fliessbach K, Helmstaedter C, Urbach H, Althaus A, Pels H, Linnebank M, et al. Neuropsychological outcome after chemotherapy for primary CNS lymphoma: a prospective study. Neurology. 2005;64(7):1184–1188. doi: 10.1212/01.WNL.0000156350.49336.E2. [DOI] [PubMed] [Google Scholar]
- 53.Ferguson RJ, Ahles TA, Saykin AJ, McDonald BC, Furstenberg CT, Cole BF, et al. Cognitive-behavioral management of chemotherapy-related cognitive change. Psychooncology. 2007;16(8):772–777. doi: 10.1002/pon.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]