Abstract
Multidrug resistance-associated protein 3 (MRP3, ABCC3) plays an important role in protecting hepatocytes and other tissues by excreting an array of toxic organic anion conjugates, including bile salts. MRP3/ABCC3 expression is increased in the liver of some cholestatic patients, but the molecular mechanism of this up-regulation remains elusive. In this report, we assessed liver MRP3/ABCC3 expression in patients (n=22) with obstructive cholestasis due to gallstones blockage of bile ducts and non-cholestatic patient controls (n=22). MRP3/ABCC3 mRNA and protein expression were significantly increased 3.4- and 4.6- fold, respectively in these cholestatic patients where elevated plasma TNFα (4.7-fold, P<0.01) and hepatic SP1 and LRH-1 expression (3.1- and 2.1-fold at mRNA level, 3.5- and 2.5-fold at protein level, respectively) were also observed. The induction of hepatic MRP3/ABCC3 mRNA expression is significantly positively correlated with the level of plasma TNFα in these patients. In HepG2 cells, TNFα treatment induced SP1 and MRP3/ABCC3 expression in a dose- and time-dependent manner, where increased phosphorylation of JNK/SAPK was also detected. These inductions were significantly reduced in the presence of the JNK inhibitor SP600125. TNFα treatment enhanced HepG2 cell nuclear extract binding activity to the MRP3/ABCC3 promoter, but was abolished by SP600125 as demonstrated by EMSA. An increase in nuclear protein binding activity to the MRP3/ABCC3 promoter consisting primarily of SP1 was also seen in liver samples from cholestatic patients as assessed by supershift EMSA assays.
Conclusions
Our findings indicate that up-regulation of hepatic MRP3/ABCC3 expression in human obstructive cholestasis is likely triggered by TNFα, mediated by activations of JNK/SAPK and SP1.
Keywords: MRP3/ABCC3, transcription factor SP1, TNFα, JNK/SAPK signaling pathway, human obstructive cholestasis
Introduction
The multidrug resistance-associated protein 3 (MRP3/Mrp3, ABCC3/Abcc3) is a member of the ATP-binding cassette (ABC) transporter superfamily (1). It is expressed in many tissues, including liver, kidney, bladder, intestine, and adrenal gland in humans and rodents (1, 2). MRP3/Mrp3 is localized on the basolateral membrane of cells and excretes sulfated, glycine and taurine-conjugated bile salts, bilirubin glucuronides, 17α-glucuronosyl estradiol, leukotrienes, and a number of drugs (1–3). The expression of hepatic MRP3/Mrp3 (ABCC3/Abcc3) is low under normal physiological condition, but its expression is significantly up-regulated as an adaptive protective response in cholestasis as demonstrated in rodents following bile duct ligation (BDL) or LPS/CCl4-treatment (4–6). This up-regulation has also been seen in the liver of patients with advanced stages of primary biliary cirrhosis (PBC) or with extrahepatic cholestasis caused by a pancreatic malignancy, but not in patients with early stages of PBC or progressive familial intrahepatic cholestasis (7–10). It is not known if MRP3/ABCC3 expression is altered in cholestasis due to biliary obstruction from bile duct stones. Furthermore, it remains to be determined how MRP3/ABCC3 expression is up-regulated in cholestasis.
The nuclear receptor liver receptor homolog 1 (LRH-1, NR5A2), also called CYP7A promoter-binding factor (CPF) is a positive regulator of MRP3/Mrp3 expression, where TNFα signaling is involved in this up-regulation (5). However, details of this signaling pathway remain to be elucidated, particularly in human cholestatic patients.
We and others have also found that the transcription factor SP1 can directly bind to the MRP3/ABCC3 promoter and stimulate it expression (11–13). Whether TNFα signaling plays a role in SP1 stimulated MRP3/ABCC3 expression is not known.
Recent studies indicate that TNFα-activated JNK/SAPK signaling plays an important role in pseudorabies virus-induced apoptosis in Vero cells and in PKR-deficient mice (14, 15). In addition, inhibition of the JNK/SAPK signaling pathway decreases transcription factor SP1 expression in NK cells and in PC-3 and PC-3N cells (16, 17). Therefore, we hypothesized that up-regulation of hepatic MRP3/ABCC3 expression in cholestatic patients may be mediated by TNFα signaling, and that JNK/SAPK, SP1 and LRH-1 might be involved in this regulation.
To test this hypothesis, we assessed MRP3/ABCC3 expression in the liver of patients with obstructive cholestasis resulting from gallstone blockage of bile ducts. In this report, we describe that increased hepatic MRP3/ABCC3 expression is associated with elevated TNFα levels and enhanced SP1 and LRH-1 expression and binding activity to the MRP3/ABCC3 promoter and speculate that JNK/SAPK signaling may mediate this up-regulation.
Materials and Methods
Patients and liver samples collection
All liver samples were collected from Southwest Hospital, Chongqing, China. This study was approved by the hospital institutional ethics review board, and informed consent was obtained from all participants. Control liver samples were acquired by liver biopsy for exclusion of liver disease or staging of hematologic malignancy (n=7), and also obtained during resections for liver metastases without cholestasis (n=15; 6 colorectal metastases, 7 colonic metastases, 2 rectal metastases). Cholestatic liver samples (n=22) were surgically resected from patients with obstructive cholestasis caused by biliary stones originating from the intrahepatic bile duct and common bile duct, within 3 days of admission due to severe symptoms of biliary obstruction and jaundice. Neither ursodeoxycholic acid (UDCA) nor other preoperative therapy was administered. The isolated liver samples were immediately cut into small pieces and stored in liquid nitrogen. Biochemical characteristics of patients are listed in Table 1.
Table 1.
Clinical features of patientsa
| Controls | Obstructive cholestasis patients | |
|---|---|---|
| Total Samples (males/females) | 22(15/7) | 22(11/11) |
| Age (years) | 49±12 | 48±12 |
| TBIL (μmol/L) | 14.7±5.3 | 196±144* |
| DBIL (μmol/L) | 4.9±1.7 | 104±89.3* |
| IBIL (μmol/L) | 9.8±3.9 | 92.3±68.9* |
| TBA(μmol/L) | 3.9±3.4 | 61.8±61.7* |
| ALT (IU/L) | 47.1±55.1 | 203±237# |
| AST (IU/L) | 45.2±46.9 | 219±269# |
| GGT (IU/L) | 107±85.9 | 359±317* |
| ALP (IU/L) | 83.5 ±42.1 | 407±423* |
TBIL, Total bilirubin; DBIL, Direct bilirubin; IBIL, Indirect bilirubin; TBA, Total bile salts; AST, aspartate aminotransferase; ALT, alanine aminotransferase; GGT, g-glutamyltransferase; ALP, alkaline phosphatase.
Note: values are means ± SD.
p<0.01;
p<0.05 versus controls.
HepG2 cell culture and treatment
Human hepatoma HepG2 cells were cultured as described (5). Before chemical treatment, the cells were starved from serum overnight and then treated with indicated dose of chemicals for designated times. For JNK/SAPK signaling inhibition experiments, HepG2 cells were pretreated with SP600125 (Sigma Chemical Co, St Louis, MO, USA) for 2 h prior to the addition of TNFα.
RNA extraction and quantitative real-time PCR
Total RNA was extracted from the tissues or cultured cells with Trizol reagent (Invitrogen; San Diego, CA, USA). Total RNA was reverse transcribed into cDNA using a RevertAid™ first strand cDNA synthesis kit (MBI Fermentas Inc, Ontario, Canada). Real-time quantitative PCR using a SYBR® premix Ex Taq™ II kit (Takara Biotechnology, Tokyo, Japan) was performed in a Bio-Rad CFX96 real time system machine to determine the mRNA levels of specific genes, whose primers are listed in Table 2. GAPDH and β-actin were used as references for normalizing data and real-time PCR amplification efficiency of target genes were considered, when using CFX manager 2.0 for data analysis.
Table 2.
Sense and antisense primers used for quantitative real-time PCR (Sybr green)
| Gene | Accession No. | Sense primer (5'→3') | Antisense primer (5'→3') |
|---|---|---|---|
| ABCB1 (MDR1) | NM_000927.4 | ctggtgtttggagaaatgacag | aacctgaatgtaagcagcaacc |
| ABCB4 (MDR3) | NM_000443.3 | cttttccttgtcgctgctaaat | agttcagtggtgtcgttgatgt |
| ABCC2 (MRP2) | NM_000392.3 | ctcacttcagcgagaccg | ccagccagttcagggttt |
| ABCC3 (MRP3) | NM_003786.3 | aaaagcagacggcacgaca | gcaggcactgatgaggaagc |
| ABCB11 (BSEP) | NM_003742.2 | cattatccttccagaccagagg | tcttgctccactatcccaatct |
| SLC10A1 (NTCP) | NM_003049.3 | gatgaccacctgctccaccttc | tccctcccttgatgacatagcg |
| CYP7A1 | NM_000780.3 | acactttgtccacctttgatga | tcattgcttctgggttcctaat |
| SP1* | NM_138473.2 | gccgctcccaacttacagaa | cccatcaacggtctggaact |
| LRH-1(NR5A2) | NM_205860.1 | gcgtggaggaaggaataagt | gtcaggtcagagggcatagc |
| Actin | NM_001101.3 | ccacgaaactaccttcaactcc | gtgatctccttctgcatcctgt |
| GAPDH* | NM 002046.3 | ctttggtatcgtggaaggactc | gtagaggcagggatgatgttct |
Note: Primers were also used for semiquantitative RT-PCR as described previously (22).
Western blot analysis
Total protein was extracted using RIPA buffer (Sigma) containing protease and phosphatase inhibitors (Roche, Palo Alto, CA, USA). Membrane protein and nuclear protein were prepared as described previously (18). The dilution of primary antibodies are: MDR1 1:2000, MDR3 1:2000, MRP2 1:1300, MRP3 1:1300, BSEP 1:1000, NTCP 1:1000, CYP7A1 1:2000, SP1 1:1600, JNK 1:2000, LRH-1 1:1600 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), and p-JNK 1:2500 (Cell Signaling, Danvers, MA, USA). GAPDH and SH-PTP1 (Santa Cruz) were used as loading reference for data analysis.
Electrophoretic mobility shift assay (EMSA)
Oligos containing the SP1 and LRH-1 response element in human MRP3/ABCC3 promoter or its mutant forms, as previously reported (5, 11), were labeled with γ-32P -ATP (10mCi/ml). EMSA and Supershift EMSA assay were performed using nuclear extracts as described previously (11).
Determination of plasma proinflammatory cytokines TNFα, IL-1β, and IL-6
Plasma samples from patients were collected prior to biopsy or liver resection (control patients, n=22, and obstructive cholestatic patients, n=22) and stored at −80°C until analysis. Plasma cytokines TNFα, IL-1β, and IL-6 levels were determined using an human TNFα, IL-1β, or IL-6 quantikine ELISA kit (BD Biosciences, San Jose, CA, USA). Bilirubin and bile salt levels encountered in cholestatic plasma samples did not interfere with the quantification of TNFα, IL-1β, and IL-6.
Immunofluorescence and immunohistochemistry analysis
Immunofluorescence microscopy combining the antibodies with MRP3 (1:50), SP1 (1:100), p-JNK (1:400), and JNK (1:200) was performed as described (18). Immunohistochemistry was also performed using p-JNK (1:100) and JNK (1:200) antibodies as described previously (9).
Statistical analysis
All data were analyzed using independent-samples t-test (two-tailed) and are expressed as the mean ± standard deviations. Linear regression analysis was also performed using the SPSS software (PASW Statistics 18, IBM, Chicago IL, USA). A value of P < 0.05 was considered significant.
Results
MRP3/ABCC3 expression is elevated in the livers samples of patients with obstructive cholestasis
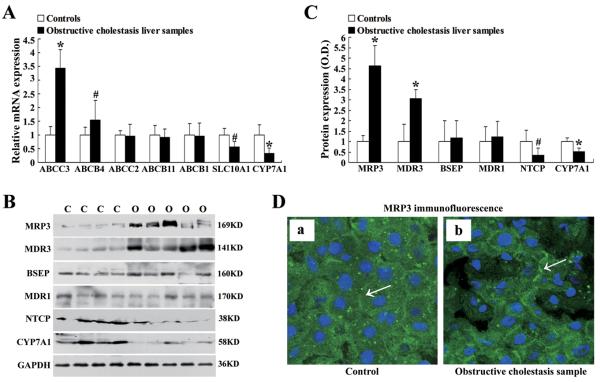
Real-time quantitative PCR revealed that liver MRP3/ABCC3 and MDR3/ABCB4 mRNA expression in obstructive cholestatic patients was significantly increased (3.4-fold and 1.5-fold, respectively) when compared to controls, whereas mRNA expression levels of MRP2/ABCC2, BSEP/ABCB11, and MDR1/ABCB1 were not significantly changed (Fig.1A). In contrast, the mRNA expression of bile salt uptake transporter NTCP/SLC10A1 and bile acid synthetic enzyme CYP7A1 were significantly decreased to 57% and 33% of controls, respectively (Fig.1A). Western blot detected that MRP3 and MDR3 protein expression increased 4.6 and 3.0-fold, respectively (Fig.1B, 1C). The increase in MRP3 protein expression was further confirmed by immunofluorescent labeling of MRP3 in the tissues where MRP3 at the basolateral membrane of cholestatic hepatocytes was more predominant than in control livers (Fig.1D). Reduced expression of NTCP and CYP7A1 protein was also seen in these obstructive cholestatic livers (65% and 50% of controls, respectively), whereas BSEP and MDR1 protein expression was not significantly altered (Fig.1B, 1C).
Figure 1.
The bile acids efflux transporter MRP3/ABCC3 expression increased in the liver samples of patients with obstructive cholestasis. (A) Quantitative RT-PCR analysis of mRNA expression of bile salt transporters MRP3/ABCC3, MDR3/ABCB4, MRP2/ABCC2, BSEP/ABCB11, MDR1/ABCB1, NTCP/SLC10A1 and bile acids synthesis enzyme CYP7A1 (% of control group, n=22 for each group). (B) Representative Western blots for MRP3, MDR3, BSEP, MDR1, NTCP and CYP7A1, and (C) their corresponding densitometry (% of control group, n=22 for each group). (D) Immunofluorescent labeling of MRP3 protein (green) in the liver of patients with obstructive cholestasis (arrow, b) than controls (arrow, a). Nuclei of hepatocytes from patients' liver samples were stained with 4′-6-Diamidino-2-phenylindole = DAPI (blue). C controls, O obstructive cholestasis liver samples. *p < 0.01; #p <0.05 vs. controls.
Plasma TNFα is elevated in patients with obstructive cholestasis
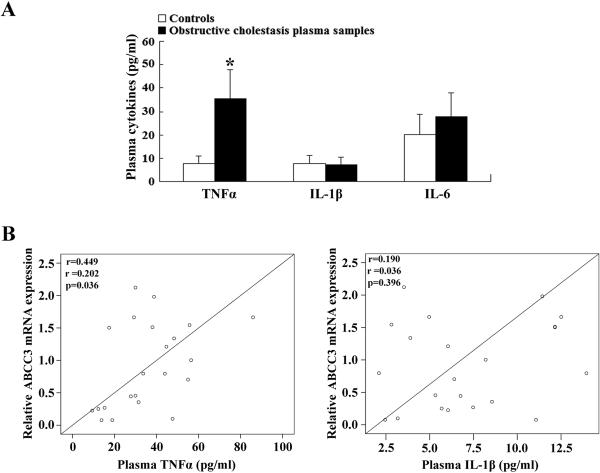
Proinflammatory cytokines TNFα, IL-1β and IL-6 levels are increased in cholestatic rodent models, where elevated hepatic Mrp3/Abcc3 expression is also observed (5, 19). To verify if increases in these cytokines also occurred in our obstructive cholestatic patients, we analyzed the plasma levels of these three cytokines. As shown in Figure 2A, the level of TNFα was significantly increased 4.7-fold in these cholestatic patients, as compared to the control group, whereas the levels of IL-1β and IL-6 were not significantly altered. To examine if there was any correlation between TNFα levels and MRP3/ABCC3 induction, we analyzed the expression of these two genes by linear regression. As shown in Figure 2B, induction of hepatic MRP3/ABCC3 expression positively correlated with the level of plasma TNFα but not IL-1β. Together, these results suggested that the increase in MRP3/ABCC3 expression could be induced by elevated TNFα in these patients as in rodents (5).
Figure 2.
Plasma levels of TNFα, IL-1β and IL-6 in patients (n=22 for each group) (A), where plasma TNFα level is positively correlated to hepatic induction of MRP3/ABCC3 mRNA expression in obstructive cholestatic patients (n=22) by linear regression analysis (B). * p < 0.01 vs. controls.
Transcription factors SP1 and LRH-1 expression and DNA binding activity to the MRP3/ABCC3 promoter are increased in human obstructive cholestasis
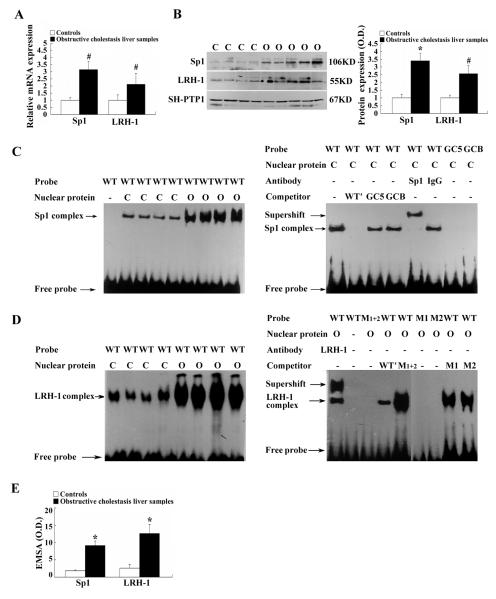
Both SP1 and LRH-1 are positive regulators of MRP3/ABCC3 gene expression (5, 11–13). To determine if these two transcription factors play any role in the up-regulation of MRP3/ABCC3 expression in our bile duct obstructed patients, we first detected the expression levels of these two transcription factors. As shown in Figure 3A, SP1 and LRH-1 mRNA expression significantly increased 3.1 and 2.1-fold, respectively in the cholestatic patients. Western blot detected increases in SP1 and LRH-1 nuclear protein levels ~ 3.5 and 2.5-folds, respectively (Fig.3B). We observed the same results by immunofluorescent labeling of SP1 and LRH-1 in the liver tissues of patients, where SP1 and LRH-1 staining in the nuclei of cholestatic hepatocytes was more predominant than controls (data not shown). The increase in expression of SP1 and LRH-1 prompted us to examine if there were any changes in binding of these transcription activators to the MRP3/ABCC3 promoter. To address this question, we performed EMSA using nuclear protein extract from the liver samples of patients with obstructive cholestasis and controls. As demonstrated in Figure 3C–E, binding of SP1 and LRH-1 to the MRP3/ABCC3 promoter in liver samples from patients with obstructive cholestasis was increased 5.1- and 4.8- fold compared to control livers respectively. This binding to the MRP3/ABCC3 promoter is specific, as confirmed by mutation and supershift EMSA experiments. (Fig. 3C and D).
Figure 3.
Obstructive cholestatic patients liver demonstrated increased expression of transcription factors SP1 and LRH-1 and their binding activity to the MRP3/ABCC3 promoter. (A) mRNA expression of SP1 and LRH-1 (% of control group, n=22 for each group); (B) Representative Western blots and corresponding densitometry of SP1 and LRH-1 (% of control group, n=22 for each group); (C) and (D) Representative EMSA and supershift EMSA of SP1 and LRH-1 response elements in MRP3/ABCC3 promoter using the nuclear extract of patient liver samples, respectively, and (E) corresponding densitometry of EMSA. Competition (WT′), mutation (SP1 mutations GC5 and GCB; LRH-1 mutations WT1, WT2 and WT1+2), and supershift-EMSA were performed to confirm that the induced SP1 or LRH-1 complex was specific and contained SP1 or LRH-1. C controls, O obstructive cholestatic liver samples. * p < 0.01; # p < 0.05 vs. controls.
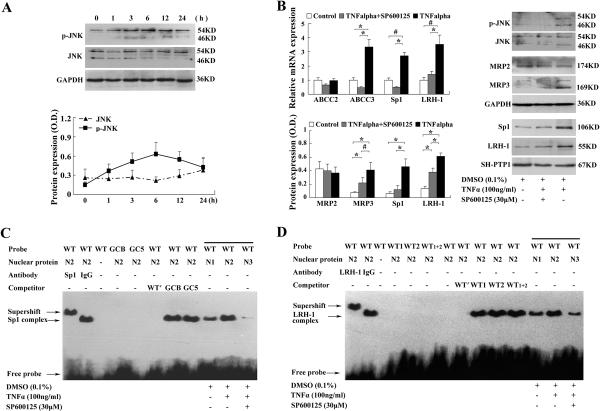
TNFα increased SP1 expression and DNA binding activity to the MRP3/ABCC3 promoter in HepG2 cells
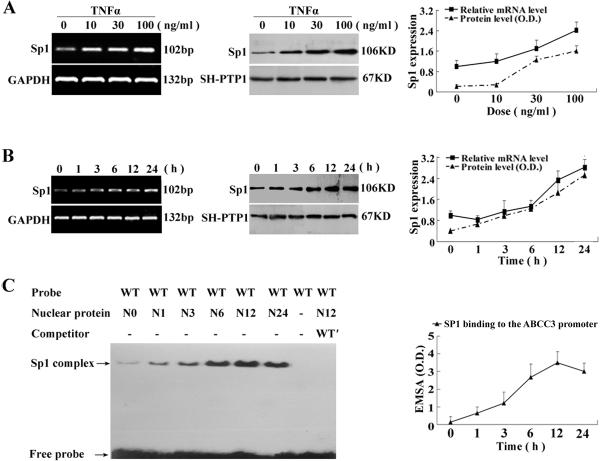
It has been previously observed that TNFα stimulates MRP3/ABCC3 expression in cultured cells (5). To test if TNFα can induce SP1 expression and its nuclear localization, we treated HepG2 cells with TNFα. In this experiment, we found that TNFα increased SP1 mRNA and nuclear protein expression in a dose-dependent and time-dependent manner (Fig.4A, 4B), where MRP3/ABCC3 mRNA expression was also coordinately induced (5). In addition, EMSA experiments, using nuclear extract from TNFα treated HepG2 cells, demonstrated increased binding capacity to the MRP3/ABCC3 promoter when using the SP1 response element (Fig.4C). These results further confirm a role for TNFα mediated SP1 expression and activity in the up-regulation of MRP3/ABCC3 expression.
Figure 4.
TNFα induced SP1 expression in HepG2 cells and binding activity to SP1 response element in MRP3/ABCC3 promoter. TNFα induced SP1 expression in a dose- (A) and time-dependance (B) manner in HepG2 cells by semiquantitative and quantitative (Sybr green) RT-PCR and Westernt blots analysis. (C) EMSA demonstrated increased SP1 binding activity to the SP1 response element in MRP3/ABCC3 promoter in a time-dependent manner after TNFα treatment. HepG2 cells were serum starved for overnight before TNFα treatment.
JNK/SAPK signaling pathway mediated TNFα stimulated SP1 expression and activity in HepG2 cells
Previous studies have shown that the JNK/SAPK signaling pathway can regulate SP1 expression in NK cells, and be activated by TNFα in Vero cells and choroidal neovascularization (CNV) (16, 17, 20). To investigate if the JNK/SAPK signaling pathway can also mediate TNFα induced MRP3/ABCC3 expression, we treated HepG2 cells with TNFα (100ng/ml). As shown in Figure 5A, phosphorylation of JNK (p-JNK) was increased in a time-dependent manner while total JNK was not significantly changed. When HepG2 cells were pretreated with 30μM SP600125 (a JNK specific inhibitor), the TNFα induced phosphorylation of JNK (p-JNK) was abolished in association with reduced inductions of SP1, LRH-1 and MRP3/ABCC3 at mRNA and protein levels in these cells (Fig.5B). In contrast, both MRP2/ABCC2 mRNA and protein levels were not significantly changed (Fig.5B). Further, we assessed SP1 and LRH-1 binding to the MRP3/ABCC3 promoter by TNFα treatment of HepG2 cells in presence or absence of the JNK inhibitor SP600125. Figure 5C and D demonstrate that SP600125 dramatically reduces TNFα stimulated SP1 and LRH-1 binding to the MRP3/ABCC3 promoter. The specificity of SP1 and LRH-1 binding to the MRP3/ABCC3 promoter was further confirmed by competition, mutation and supershift experiments (Fig.5C, 5D). Together, these results indicate that TNFα induced MRP3/ABCC3 expression may be mediated through JNK/SAPK signaling pathway and activation of SP1 and LRH-1.
Figure 5.
JNK/SAPK pathway mediates TNFα induction of MRP3/ABCC3 expression. (A) Western-blot analysis revealed that TNFα induced JNK phosphorylation (p-JNK) in a time-dependent manner in HepG2 cells, where total JNK was not significantly changed. (B) TNFα treatment induced the expression of MRP3/ABCC3, SP1 and LRH-1 at mRNA and protein levels, but not MRP2/ABCC2 in HepG2 cells. These inductions were blocked markedly by SP600125. HepG2 cells were starved from serum for overnight, pretreated with 30 μM SP600125 for 2 h in absence of TNFα, and then treated with 100 ng/ml TNFα for 12 h in presence of SP600125. (C) and (D) EMSA demonstrate that nuclear extract of HepG2 cells treated with TNFα increased SP1 and LRH-1 binding activities to their correspondent response elements. These bindings were substantially inhibited in the presence of 30 μM SP600125. Competition, mutation, and supershift-EMSA confirmed that the induced SP1 or LRH-1 complex was specific and contained SP1 or LRH-1. *p < 0.01,
# p < 0.05.
JNK/SAPK signaling pathway is also activated in liver samples of obstructive cholestasis patients
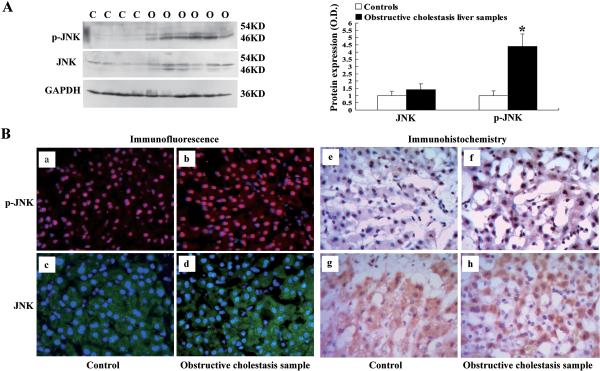
To verify if JNK/SAPK signaling pathway was also activated in the livers from human obstructive cholestasis, we assessed the phosphorylation of JNK (p-JNK) in both obstructive cholestatic patients and control patients. As shown in Figure 6A, p-JNK was markedly induced in liver samples with obstructive cholestasis v/s controls (4.3-fold), while total JNK was not changed significantly. Immunofluorescent analysis also showed that p-JNK staining in the nucleus of patients' cholestatic hepatocytes was more prominent than in controls (Fig.6, a–b). JNK staining was also increased in nucleus of cholestatic hepatocytes v/s controls (Fig.6B, c–d). Immunohistochemisty analysis also demonstrated that p-JNK and JNK expression were induced significantly in nucleus of hepatocytes from cholestatic patients when compared to the controls (Fig.6B, e–h). Altogether, these findings suggest that the JNK/SAPK signaling pathway was also activated in human obstructive cholestasis, where JNK is phosphorylated and translocated into nucleus of hepatocytes under cholestatic conditions.
Figure 6.
Increased phosphorylation of JNK/SAPK was detected in liver samples of patients with obstructive cholestasis. (A) Representative Western-blot analysis of phosphorylation of JNK (p-JNK) and total JNK in liver samples of cholestasis patients and controls (% of control group, n=22 for each group). (B) Phosphorylation of JNK (p-JNK) and total JNK was more prominent in nucleus of hepatocytes from patients with obstructive cholestasis. (a) – (d) Representative immunofluorescent analysis of phosphorylation of JNK (red; a, b) and total JNK (green; c, d) in liver tissue of patients with obstructive cholestasis (b, d) and controls (a, c). Cell nuclei were stained with DAPI (blue). (e) – (h) Representative immunohistochemistry analysis of phosphorylation of JNK (p-JNK) and total JNK in liver tissue of patients with obstructive cholestasis (f, h) and controls (e, g). C controls, O obstructive cholestasis liver samples. *p < 0.01 vs. controls.
Discussion
Elevated expression of MRP3/ABCC3 has been previously reported in cholestatic rodent models and some patients with PBC and obstructive cholestasis due to pancreatic tumors (4–6, 9, 10). The up-regulation of MRP3/ABCC3 is thought to be an adaptive protective response to cholestasis, although the detailed molecular mechanisms remain unclear. In this report, we assessed MRP3/ABCC3 expression in patients with obstructive cholestasis due to gallstones blockage of bile ducts. We demonstrate that MRP3/ABCC3 mRNA and protein expression were significantly increased 3.4- and 4.6- fold, respectively. Interestingly, we found that hepatic MRP3/ABCC3 mRNA expression in these patients significantly and positively correlated with their serum levels of TNF-α but not IL-1β. The correlation of TNF-α levels with hepatic MRP3/ABCC3 mRNA expression in these patients is consistent with our previous observations in cholestatic mice (5), indicating that TNFα signaling modulates hepatic MRP3/Mrp3 expression in both humans and rodents.
SP1 and LRH-1 are two nuclear transcription activators in the human MRP3/ABCC3 promoter (5, 11–13). Recent studies indicate that JNK/SAPK signaling pathway can regulate SP1 expression in human NK cells and in PC-3 and PC-3N cells (16, 17). As mentioned previously, TNFα signaling can also activate JNK/SAPK in pseudorabies virus-induced Vero cells apoptosis and in PKR-deficient mice (14, 15). To elucidate the molecular mechanism of TNFα induced MRP3/ABCC3 expression, we found that: 1) TNFα increased JNK phosphorylation in HepG2 cells (Fig.5A); 2) TNFα also stimulated the expression of SP1 and LRH-1 in HepG2 cells in a time- and dose-dependent manner (Fig.4A, 4B) (5); 3) Increased SP1 and LRH-1 expression was also seen in livers from our cholestatic patients but not from control subjects (Fig.3A, 3B); 4) Enhanced activities of SP1 and LRH-1 binding to the MRP3/ABCC3 promoter was detected, using nuclear extract from both cholestatic human liver and
HepG2 cells treated with TNFα, by EMSA and super-shift assays (Fig.3C–E, Fig.4C) (5); 5) In HepG2 cells, the JNK specific inhibitor, SP600125 blocked TNFα-induced JNK phosphorylation, induction of transcription factors SP1 and LRH-1 expression, as well as MRP3/ABCC3 expression, and binding activities of SP1 and LRH-1 to the MRP3/ABCC3 promoter (Fig.5B–D); 6) Increased JNK phosphorylation was also detected in hepatocytes from our cholestatic patients (Fig.6). Therefore, we speculate that TNFα induces hepatic MRP3/ABCC3 expression through activation of the JNK/SAPK signaling pathway leading to an increase in SP1 and LRH-1 expression and function in human obstructive cholestasis
Increases in TNFα could also explain the increased MRP3/ABCC3 expression that was reported in advanced stages of PBC but not in patients with early stages of PBC (7, 9). In severe cholestasis, liver inflammation may induce TNFα expression and result in up-regulation of MRP3/ABCC3 expression. Whereas TNFα level may be normal in the early stages of PBC so that hepatic MRP3/ABCC3 expression does not change. In addition, induction of MRP3 by TNFα may also contribute to the jaundices/hyperbilirubinemia that is often seen in patients and animals with sepsis (21, 22) and otherwise usually attributed to down regulation of MRP2 (23). Elevated level of plasma TNFα but not IL-1β was also observed in septic cats with hyperbilirubinemia, although it is not known if hepatic Mrp3 expression was increased in these animals (22). However, MRP3/Mrp3 is a bilirubin glucuronide transporter. In particular, Mrp3−/− mice with bile duct obstruction demonstrated lower serum bilirubin glucuronide than its wild-type control (24). Furthermore, up-regulation of Mrp3 was associated with tienilic acid enhanced hyperbilirubinemia in Eisai hyperbilirubinuria rats (25). Therefore, under certain conditions, MRP3/Mrp3 can play a key role in transporting bilirubin out of the hepatocyte back into the blood. During sepsis, serum level of TNFα may increase, which in turn induces hepatic MRP3 expression that further contributes to hyperbilirubinemia associated with sepsis.
Our findings indicate that both SP1 and LRH-1 play roles in TNFα mediated induction of MRP3/ABCC3 expression. However, the relative contribution of each transcription factors remains to be determined. It is conceivable that with the loss of one of these transcription factors, the other may compensate to ensure up-regulation of MRP3/ABCC3 expression when TNFα level is elevated. In addition, activators of nuclear receptors PXR and CAR, and transcription factor Nrf2 induce MRP3/Mrp3 expression in vitro in human cells and in vivo in the liver of rodents (26–28). However, Mrp3/Abcc3 basal expression and induction by Pxr or Car activators is retained in Pxr−/− and Car−/− knockout mice, indicating that Pxr and Car may not play a direct role in Mrp3/Abcc3 expression in the mouse (29, 30). Interestingly, reduced Mrp3/Abcc3 expression was detected in the liver of Nrf2−/− knockout mice, suggesting that Nrf2 play a role in regulating Mrp3/Abcc3 expression, although the detailed mechanism remains elusive (31). Whether TNFα induction of MRP3/Mrp3 may also be mediated through an Nrf2 pathway needs further study.
Although this study focused on the mechanisms of the adaptive response of MRP3/ABCC3 in human cholestatic liver, we also found altered expression of other genes involved in bile salt transport and synthesis in these obstructive cholestatic patients. These, included down regulation of NTCP/SLC10A1 and CYP7A1, increases in expression of MDR3/ABCB4, MRP4/ABCC4 (18) and OSTα/β (data not shown), but no significant change of BSEP/ABCC11, MRP2/ABCC2 and MDR1/ABCB1 (Fig.1). These changes in gene expression are consistent with previous reports and also contribute to the overall adaptive protective responses in cholestatic liver injury (5, 9, 10).
In summary, our current study demonstrates that up-regulation of MRP3/ABCC3 in obstructive cholestasis is positively correlated to serum levels of TNFα. JNK/SAPK activation and increased SP1 and LRH-1 expression and activity may mediate TNFα induction of MRP3/ABCC3 expression in human hepatocytes. These findings also suggest that the proinflammatory cytokine TNFα plays a cytoprotective role in cholestatic liver injury.
Acknowledgments
Financial Support:
Supported by National Natural Science Foundation of China (30570842, 81070320) and Natural Science Foundation of Chongqing (CQ CSTC 2007BA5030), and JLB and S-Y C are supported by NIH grants DK R37 25636 and DK P30 34989.
List of abbreviations
- MRP3
multidrug resistance-associated protein 3
- ABC
ATP–binding cassette
- ABCB
ABC subfamily B
- ABCC
ABC subfamily C
- PBC
primary biliary cirrhosis
- LRH-1
liver receptor homolog 1
- SP1
specificity Protein 1 transcription factor
- TNFα
tumor necrosis factor alpha
- IL-1β
interleukin 1 beta
- IL-6
interleukin 6.
References
- 1.Scheffer GL, Kool M, de Haas M, de Vree JM, Pijnenborg AC, Bosman DK, et al. Tissue distribution and induction of human multidrug resistant protein 3. Lab Invest. 2002;82:193–201. doi: 10.1038/labinvest.3780411. [DOI] [PubMed] [Google Scholar]
- 2.Hirohashi T, Suzuki H, Takikawa H, Sugiyama Y. ATP-dependent transport of bile salts by rat multidrug resistance-associated protein 3 (Mrp3) J Biol Chem. 2000;275:2905–2910. doi: 10.1074/jbc.275.4.2905. [DOI] [PubMed] [Google Scholar]
- 3.Akita H, Suzuki H, Hirohashi T, Takikawa H, Sugiyama Y. Transport activity of human MRP3 expressed in Sf9 cells: comparative studies with rat MRP3. Pharm Res. 2002;19:34–41. doi: 10.1023/a:1013699130991. [DOI] [PubMed] [Google Scholar]
- 4.Soroka CJ, Lee JM, Azzaroli F, Boyer JL. Cellular localization and up-regulation of multidrug resistance-associated protein 3 in hepatocytes and cholangiocytes during obstructive cholestasis in rat liver. Hepatology. 2001;33(4):783–91. doi: 10.1053/jhep.2001.23501. [DOI] [PubMed] [Google Scholar]
- 5.Bohan A, Chen WS, Denson LA, Held MA, Boyer JL. Tumor necrosis factor alpha-dependent up-regulation of Lrh-1 and Mrp3(Abcc3) reduces liver injury in obstructive cholestasis. J Biol Chem. 2003;278(38):36688–98. doi: 10.1074/jbc.M304011200. [DOI] [PubMed] [Google Scholar]
- 6.Donner MG, Warskulat U, Saha N, Haussinger D. Enhanced expression of basolateral multidrug resistance protein isoforms Mrp3 and Mrp5 in rat liver by LPS. Biol Chem. 2004;385(3–4):331–339. doi: 10.1515/BC.2004.029. [DOI] [PubMed] [Google Scholar]
- 7.Zollner G, Fickert P, Zenz R, Fuchsbichler A, Stumptner C, Kenner L, et al. Hepatobiliary transporter expression in percutaneous liver biopsies of patients with cholestatic liver diseases. Hepatology. 2001;33(3):633–646. doi: 10.1053/jhep.2001.22646. [DOI] [PubMed] [Google Scholar]
- 8.Keitel V, Burdelski M, Warskulat U, Kuhlkamp T, Keppler D, Haussinger D, et al. Expression and localization of hepatobiliary transport proteins in progressive familial intrahepatic cholestasis. Hepatology. 2005;41(5):1160–1172. doi: 10.1002/hep.20682. [DOI] [PubMed] [Google Scholar]
- 9.Zollner G, Fickert P, Silbert D, Fuchsbichler A, Marschall HU, Zatloukal K, et al. Adaptive changes in hepatobiliary transporter expression in primary biliary cirrhosis. J Hepatol. 2003;38(6):717–727. doi: 10.1016/s0168-8278(03)00096-5. [DOI] [PubMed] [Google Scholar]
- 10.Schaap FG, van der Gaag NA, Gouma DJ, Jansen PL. High expression of the bile salt-homeostatic hormone fibroblast growth factor 19 in the liver of patients with extrahepatic cholestasis. Hepatology. 2009;49(4):1228–1235. doi: 10.1002/hep.22771. [DOI] [PubMed] [Google Scholar]
- 11.Chen W, Cai SY, Xu S, Denson LA, Soroka CJ, Boyer JL. Nuclear receptors RXRα:RARα are repressors for human MRP3 expression. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1221–G1227. doi: 10.1152/ajpgi.00191.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tzeng SJ, Chang WC, Huang JD. Transcriptional regulation of the rat Mrp3 gene promotor by the specificity protein (Sp) family members and CCAAT/enhancer binding proteins. J Biomed Sci. 2005;12:741–761. doi: 10.1007/s11373-005-9002-5. [DOI] [PubMed] [Google Scholar]
- 13.Takada T, Suzuki H, Sugiyama Y. Characterization of 5'-flanking region of human MRP3. Biochem Biophys Res Commun. 2000;270(3):728–732. doi: 10.1006/bbrc.2000.2507. [DOI] [PubMed] [Google Scholar]
- 14.Yeh CJ, Lin PY, Liao MH, Liu HJ, Lee JW, Chiu SJ, et al. TNF-alpha mediates pseudorabies virus-induced apoptosis via the activation of p38 MAPK and JNK/SAPK signaling. Virology. 2008;381(1):55–66. doi: 10.1016/j.virol.2008.08.023. [DOI] [PubMed] [Google Scholar]
- 15.Takada Y, Ichikawa H, Pataer A, Swisher S, Aggarwal BB. Genetic deletion of PKR abrogates TNF-induced activation of IkappaBalpha kinase, JNK, Akt and cell proliferation but potentiates p44/p42 MAPK and p38 MAPK activation. Oncogene. 2007;26(8):1201–1212. doi: 10.1038/sj.onc.1209906. [DOI] [PubMed] [Google Scholar]
- 16.Kumar D, Hosse J, von Toerne C, Noessner E, Nelson PJ. JNK MAPK pathway regulates constitutive transcription of CCL5 by human NK cells through SP1. J Immunol. 2009;182(2):1011–1020. doi: 10.4049/jimmunol.182.2.1011. [DOI] [PubMed] [Google Scholar]
- 17.Sroka IC, Nagle RB, Bowden GT. Membrane-type 1 matrix metalloproteinase is regulated by sp1 through the differential activation of AKT, JNK, and ERK pathways in human prostate tumor cells. Neoplasia. 2007;9(5):406–417. doi: 10.1593/neo.07193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chai J, Luo D, Wu X, Wang H, He Y, Li Q, et al. Changes of organic anion transporter MRP4 and related nuclear receptors in human obstructive cholestasis. J Gastrointest Surg. 2011;15(6):996–1004. doi: 10.1007/s11605-011-1473-2. [DOI] [PubMed] [Google Scholar]
- 19.Bemelmans MH, Gouma DJ, Greve JW, Buurman WA. Cytokines tumor necrosis factor and interleukin-6 in experimental biliary obstruction in mice. Hepatology. 1992;15(6):1132–1136. doi: 10.1002/hep.1840150626. [DOI] [PubMed] [Google Scholar]
- 20.Xu J, Zhu D, He S, Spee C, Ryan SJ, Hinton DR. Transcriptional regulation of bone morphogenetic protein 4 by tumor necrosis factor and its relationship with age-related macular degeneration. FASEB J. 2011;25(7):2221–2233. doi: 10.1096/fj.10-178350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van den Broek P, Verkade HJ, Hulzebos CV. Hyperbilirubinemia in infants with Gram-negative sepsis does not affect mortality. Early Hum Dev. 2011;87(8):515–519. doi: 10.1016/j.earlhumdev.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 22.Declue AE, Delgado C, Chang CH, Sharp CR. Clinical and immunologic assessment of sepsis and the systemic inflammatory response syndrome in cats. J Am Vet Med Assoc. 2011;238(7):890–897. doi: 10.2460/javma.238.7.890. [DOI] [PubMed] [Google Scholar]
- 23.Trauner M, Arrese M, Soroka CJ, Ananthanarayanan M, Koeppel TA, Schlosser SF, et al. The rat canalicular conjugate export pump (Mrp2) is down-regulated in intrahepatic and obstructive cholestasis. Gastroenterology. 1997;113(1):255–264. doi: 10.1016/s0016-5085(97)70103-3. [DOI] [PubMed] [Google Scholar]
- 24.Zelcer N, van de Wetering K, de Waart R, Scheffer GL, Marschall HU, Wielinga PR, et al. Mice lacking Mrp3 (Abcc3) have normal bile salt transport, but altered hepatic transport of endogenous glucuronides. J Hepatol. 2006;44(4):768–775. doi: 10.1016/j.jhep.2005.07.022. [DOI] [PubMed] [Google Scholar]
- 25.Nishiya T, Kataoka H, Mori K, Goto M, Sugawara T, Furuhama K. Tienilic acid enhances hyperbilirubinemia in Eisai hyperbilirubinuria rats through hepatic multidrug resistance-associated protein 3 and heme oxygenase-1 induction. Toxicol Sci. 2006;91(2):651–659. doi: 10.1093/toxsci/kfj162. [DOI] [PubMed] [Google Scholar]
- 26.Jigorel E, Le Vee M, Boursier-Neyret C, Parmentier Y, Fardel O. Differential regulation of sinusoidal and canalicular hepatic drug transporter expression by xenobiotics activating drug-sensing receptors in primary human hepatocytes. Drug Metab Dispos. 2006;34(10):1756–1763. doi: 10.1124/dmd.106.010033. [DOI] [PubMed] [Google Scholar]
- 27.Teng S, Jekerle V, Piquette-Miller M. Induction of ABCC3 (MRP3) by pregnane X receptor activators. Drug Metab Dispos. 2003;31(11):1296–1299. doi: 10.1124/dmd.31.11.1296. [DOI] [PubMed] [Google Scholar]
- 28.Wagner M, Halilbasic E, Marschall HU, Zollner G, Fickert P, Langner C, et al. CAR and PXR agonists stimulate hepatic bile acid and bilirubin detoxification and elimination pathways in mice. Hepatology. 2005;42(2):420–430. doi: 10.1002/hep.20784. [DOI] [PubMed] [Google Scholar]
- 29.Cherrington NJ, Slitt AL, Maher JM, Zhang XX, Zhang J, Huang W, et al. Induction of multidrug resistance protein 3 (mrp3) in vivo is independent of constitutive androstane receptor. Drug Metab Dispos. 2003;31(11):1315–1319. doi: 10.1124/dmd.31.11.1315. [DOI] [PubMed] [Google Scholar]
- 30.Teng S, Piquette-Miller M. Hepatoprotective role of PXR activation and MRP3 in cholic acid-induced cholestasis. Br J Pharmacol. 2007;151(3):367–376. doi: 10.1038/sj.bjp.0707235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anwar-Mohamed A, Degenhardt OS, El Gendy MA, Seubert JM, Kleeberger SR, El-Kadi AO. The effect of Nrf2 knockout on the constitutive expression of drug metabolizing enzymes and transporters in C57Bl/6 mice livers. Toxicol In Vitro. 2011;25(4):785–795. doi: 10.1016/j.tiv.2011.01.014. [DOI] [PubMed] [Google Scholar]