Abstract
The neural events that lead to successful or failed detection of suprathreshold sounds are not well established. In this experiment, event‐related potentials (ERPs) and functional magnetic resonance imaging (fMRI) were recorded while participants performed two tasks: a primary difficult duration judgment task on a sequence of tones presented to one ear, and a secondary target detection task on an auditory oddball stream presented to the other ear. The paradigm was designed to elicit competition and variability in detection of auditory targets despite identical input. Successful detection of auditory targets was associated mainly with greater fMRI activity in superior parietal cortex and thalamus. In the ERPs, successful detection was linked with a larger fronto‐central negativity at 200–400 ms, and a later centro‐posterior positivity. Failure to detect targets was associated with greater fMRI signal in the default mode network, a significantly smaller electrical fronto‐central negativity and no late positivity. These findings demonstrate that variability in auditory detection is related to modulation of activity in multimodal parietal and frontal networks active ∼ 200 ms after target onset. Results are consistent with a limited capacity and late selection view of attention. Hum Brain Mapp, 2013. © 2011 Wiley Periodicals, Inc.
Keywords: auditory, attention, capacity, ERP, fMRI
INTRODUCTION
Our senses are constantly flooded with simultaneous information that may or may not reach awareness. The main factor proposed to modulate awareness of multiple sensory events is limits on attentional capacity [Duncan, 1980; Duncan et al., 1997; Navon and Gopher, 1979]. In this account, competition biases allocation of resources toward task‐relevant information. In the case of multiple tasks, a greater share of resources is allocated to the primary task [Desimone and Duncan, 1995]. Furthermore, although the primary and secondary tasks are hypothesized to share a common pool of resources, competition occurs only if the processing load is relatively high [Hill and Miller, 2009; Lavie, 2005; Lavie and Tsal, 1994; Navon and Gopher, 1979; Pashler and Johnston, 1998]. The concept of limited‐capacity is an integral part of early‐ and late‐selection theories of attention [Broadbent, 1958; Deutsch and Deutsch, 1963; Duncan, 1980; Lavie, 2010; Lavie et al., 2004]. In the early selection account, physical features are extracted from incoming stimuli and attention acts as a filter to minimize the sensory encoding of irrelevant information. The information that passed through the filter then enters a limited capacity system responsible for higher‐level processing, such as of semantic properties. In the late selection account, all properties are extracted from incoming stimuli and both relevant and irrelevant information are encoded at a sensory level; however, only the former is selected to enter the limited capacity system and access memory and awareness. Early and late selection has been studied using divided attention paradigms, because this setting allows for competition on resources when participants are engaged in more than one task [Duncan, 1980; Pashler and Johnston, 1998].
Our principal aim in the current study was to investigate the neural events that lead to and are associated with detected or undetected suprathreshold auditory targets of identical input. We selected a paradigm with simultaneous primary and secondary auditory tasks, in which the sensory information is competing to be processed. In the visual modality, Beck et al. [2001] compared blood oxygenation level‐dependent (BOLD) responses to detected and undetected change in face or place images. Participants detected the target letter X in letter strings as a primary task and a change between face or place images as a secondary task. Relative to undetected change, detected change produced larger activations in bilateral superior parietal lobe (SPL) and right dorsolateral prefrontal cortex, and in right fusiform gyrus, regions implicated in the control of attention and category‐specific processing, respectively. Undetected change compared to no change revealed activation in right lingual and fusiform gyri, and in right inferior frontal gyrus (IFG), only for change in faces [Beck et al., 2001]. In an event‐related potential (ERP) study also in the visual modality, Pourtois et al. [2006] employed a simplified version of the paradigm used by Beck et al. [2001]. Participants were engaged in two tasks: identifying a number presented in the central fixation location and detecting a change between images of two faces presented in the periphery. Successful detection of visual change was associated with a larger late positivity, the P3, compared to undetected change. Regions identified by source localization of the P3 included bilateral posterior parietal lobe and lateral occipital lobe [Pourtois et al., 2006]. However, the undetected change and no change conditions generated similar waveforms, suggesting that implicit processes were not distinguished with this method.
In the auditory modality, studies focused primarily on the neural networks implicated in auditory target deviant detection as a single, primary task with various imaging techniques. BOLD activity for target compared to nontarget (standard) trials has been reported in a large and spatially distributed network of regions, including bilateral superior temporal gyrus (STG), inferior, middle and superior frontal gyri (IFG, MFG, SFG), inferior and superior parietal lobules (IPL, SPL), anterior and posterior cingulate, thalamus, caudate, and the amygdala/hippocampal complex [e.g., Kiehl et al., 2001, 2005; Linden, 2005; Linden et al., 1999; Menon et al., 1997; Opitz et al., 1999]. ERP components commonly reported in target detection studies include the N2 (usually a combination of the mismatch negativity, also known as N2a, and the N2b) and P3 [Donchin et al., 1983; Näätänen, 1992; Picton et al., 1992; Sams et al., 1985].
Here, we examined modulations of neural activity associated with variable detection of suprathreshold auditory targets in a divided attention paradigm, using functional magnetic resonance imaging (fMRI) and ERPs in separate sessions. fMRI provides excellent spatial resolution while ERPs provide excellent temporal information related to stages of processing. The primary task was designed to produce high processing load and encourage competition on resources, thereby creating variability in target sensory encoding, perception, and detection [Hill and Miller, 2009; Lavie, 2005; Lavie and Tsal, 1994; Navon and Gopher, 1979; Pashler and Johnston, 1998]. We tested the idea of a limited attentional capacity system and late‐selection, whereby the competition for resources between two tasks would bring differences between detected and undetected targets only at later stages of processing (e.g., perception). In this account, differences would be observed starting 200–300 ms after target occurrence, and would be reflected in the ERP N2 and P3 responses. These ERP responses are thought to originate in STG and SPL [Alho, 1995; Halgren et al., 1995a, b, 1998; Kropotov et al., 1995; Scherg and Von Cramon, 1986; Scherg et al., 1989], and to be associated with attention and working memory. Alternatively, finding reduced sensory processing of undetected targets as reflected in the sensory‐evoked N1 response [Gonsalvez et al., 2007; Picton et al., 1970], thought to originate in primary auditory cortex and STG [Scherg et al., 1989], would be consistent with early selection models of attention.
MATERIALS AND METHODS
Subjects
Participants were 22 healthy adults (10 women, mean age = 27 years, SD = 5.6) with no history of neurological or hearing impairments. All were right‐handed according to the Edinburgh Handedness Inventory [Oldfield, 1971]. ERP data were excluded from nine participants, and fMRI data from five participants, due to one or more of the following reasons: noisy EEG (three participants), excessive movement artifacts during fMRI (two participants), and inadequate behavioral performance (defined as d′ < 0.7 or false alarm rate > 0.2) on the oddball detection task (three participants in the fMRI session and six in the EEG session). There were thus 17 participants in the fMRI and 13 in the ERP group analyses (11 of those contributed to both analyses). Informed consent was obtained from each participant prior to the experiment, in accordance with the Medical College of Wisconsin Institutional Review Board.
Stimuli and Procedure
Participants performed the same tasks in separate fMRI and ERP sessions. In Ear 1, participants performed a demanding duration judgment task, indicating whether a tone was short or long by pressing one of two keys after each tone. Tones were grouped into sequences. Each sequence consisted of seven sinusoidal tones (1,000 Hz; within stream SOA = 1,500 ms; rise‐and‐fall time 5 ms), which could be short (50 ms) or long (60 ms) with equal probability. This task required a motoric response to each tone in the sequence. In Ear 2, participants monitored another sequence for a change in frequency. In this task, participants performed an auditory oddball detection yes/no task, indicating whether a frequency deviance had occurred within a train of 20 repetitive standard tones (1,000 Hz; 55 ms; within stream SOA = 500 ms) by pressing one of two keys at the end of the sequence (see Fig. 1). The magnitude of frequency deviance was determined for each subject individually in a training session, using an adaptive staircase method, and set to a value between 8 and 20 Hz above the frequency of the standard tone. The difficulty of each task was selected to attain ∼ 75–85% accuracy on the duration judgment task and ∼ 50% on the oddball detection task with a low false alarm rate. When presented in isolation in the training phase, accuracy on each single task was >90%. The frequency of the tones in the duration task and of the standard tones in the oddball task was kept identical in order to increase uncertainty and variability in deviant detection. The duration of the tones in the oddball task was set to the midpoint between the short and long stimuli to further promote continuous monitoring of both streams. However, the tones in the left and right ears were presented asynchronously (between streams SOA = 250 ms) and thus never overlapped in time (see Fig. 1). A constant cross‐hair was presented in the center of the screen for the participants to fixate in order to minimize eye movements. The cross‐hair was changed to the text “Change?” at the end of every sequence to prompt participants' response to the oddball detection task. Limiting overt responses in the oddball task to the end of the sequence served to emphasize the secondary nature of this task relative to the ongoing duration judgment task. Participants used the right hand to respond in both tasks.
Figure 1.
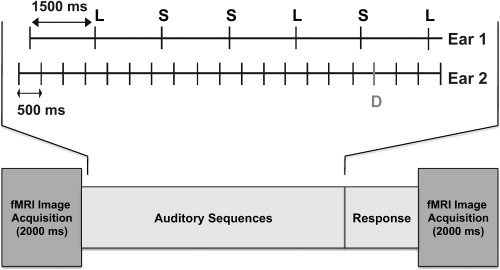
A schematic illustration of stimulus presentation in a single trial: Short (S) and long (L) sequence is shown in Ear 1; within stream SOA = 1,500 ms. An oddball sequence is shown in Ear 2; D = deviant; within stream SOA = 500 ms. Timing between streams sample: A duration tone in Ear 1 was followed by three standard tones in Ear 2 (SOA = 250 ms), which were then followed by a duration tone in Ear 1 (SOA = 250 ms) and a deviant in Ear 2 (SOA = 250 ms). The deviant in the oddball stream could have appeared at 250, 750, or 1,250 ms following a duration stimulus. In the fMRI session, stimuli were presented in the “quiet” time between image acquisitions.
There were 10 ERP and six fMRI runs in two separate sessions. Each run consisted of 40 sequences (blocks) presented in a randomized order, with each sequence 13,500 ms in duration. In the ear presented with the oddball detection task, 30 of the sequences (presented at random) included one frequency deviant (P = 0.14 in a sequence), 25 in positions 11–18 (to maximize their BOLD signal at image acquisition in the fMRI session), and five in positions 3–10 (to introduce uncertainty). Ten of the sequences included only standard tones. Ear of presentation for each task was counterbalanced across the runs within each session. Auditory stimulation was delivered using MRI‐compatible electrostatic headphones (Koss Corp., Milwaukee, WI). Tone presentation was controlled by a personal computer running PsyScope.
fMRI Acquisition and Analysis
Images were acquired on a 3T GE Excite scanner (GE Medical Systems, Milwaukee, WI). Functional data consisted of T2*‐weighted, gradient‐echo, echo‐planar images (TE = 20 ms, flip angle = 85°, NEX = 1), obtained using clustered acquisition (acquisition time = 2,000 ms) at 13,500‐ms intervals to avoid perceptual masking of the sound sequences or contamination of brain activation to task stimuli by the activation to the acoustic noise of the scanner. Volumes were composed of 36 axially oriented 3.50‐mm slices with a 0.5‐mm interslice gap covering the whole brain, with FOV = 220 mm and 64 × 64 matrix, resulting in 3.438 × 3.438 × 3.50 voxel dimensions. High‐resolution anatomical images of the entire brain were obtained using a 3D spoiled gradient‐echo sequence (SPGR) with 0.859 × 0.859 × 1.0 mm voxel dimensions. Head movement was minimized by using a bead pillow molded around the back of the head and neck, and foam padding on the side of the head.
Image analysis was conducted using the AFNI software package [Cox, 1996]. Within‐subject analysis consisted of spatial coregistration to minimize motion artifacts. The first and last images were discarded, leaving a total of 40 images per run. Voxelwise multiple linear regression was applied to analyze individual time series, with two reference functions. One reference function represented the sequences containing detected deviant stimuli compared to standard‐only (baseline) sequences. The second reference function represented the undetected deviant sequences compared to baseline. Trials/images without a button response or false alarm trials (response “yes” on standard sequences) were not included in the analysis. Translation and rotation movement parameters estimated during image registration were included in the regression model to remove residual variance associated with motion‐related changes in BOLD signal. A Gaussian kernel of 6 mm FWHM was used for smoothing prior to the regression analyses. General linear tests were conducted to obtain contrasts of interest between conditions. The individual statistical maps and the anatomical scans were projected into standard stereotaxic space [Talairach and Tournoux, 1988] by linear resampling, and group maps were created using a random‐effects analysis. The group maps were thresholded at a voxelwise P < 0.005, and corrected for multiple comparisons by removing clusters smaller than 704 μl, resulting in a mapwise two‐tailed P < 0.05. The group map comparing directly the two conditions was thresholded at a voxelwise P < 0.05, and corrected for multiple comparisons by removing clusters smaller than 4,639 μl, resulting in a mapwise two‐tailed P < 0.05. The cluster thresholds were determined through Monte‐Carlo simulations that provide the chance probability of spatially contiguous voxels exceeding the threshold.
ERP Acquisition and Analysis
Sixty‐four‐channel EEG activity was acquired using the Maglink system (Neuroscan) in a continuous mode, and the Quik‐Cap electrode positioning system (Neuroscan). Electrode sites conformed to the International 10‐20 System. Vertical eye movements were monitored with bipolar recordings between sites above and below the left eye. Interelectrode resistance was kept below 5 kΩ. Activity was recorded at full bandwidth and digitally sampled at 500 Hz per channel. Potentials at each site were referenced to CPz.
Initial within‐subject analysis consisted of (a) bandpass filtering the data at 1–30 Hz, (b) creating epochs of −100 to +1,000 ms from each tone onset, (c) baseline‐correcting each epoch by removing the mean voltage value of the whole sweep, and (d) rejecting epochs with voltage values exceeding ±100 μV. The remaining epochs were then sorted and averaged according to stimulus type (standard, deviant) and deviant detection response (detected, undetected). As in the fMRI analysis, trials without a button response or false alarm trials were not included in the analysis. Each waveform was baseline‐corrected by subtracting the mean voltage of the prestimulus period from each point in the poststimulus interval. Grand‐average waveforms (across trials in the same condition, and across subjects) were computed for standards, detected deviants, and undetected deviants, and for the difference between detected deviants and standards, undetected deviants and standards, detected and undetected deviants. The resulting waveforms were digitally rereferenced to the mastoids. Pointwise analyses were carried out on the amplitudes of the difference waveforms. Significant differences between waveforms were assessed using pairwise t‐tests applied at each time point. The resulting t‐values were corrected for multiple comparisons using a simulation of α value distribution for filtered data [Guthrie and Buchwald, 1991]. Data were first thresholded at an initial probability of P < 0.05. Points were considered significant if they were members of a contiguous cluster of 25 time points (50 ms) or greater, which corresponds to a corrected P < 0.001.
RESULTS
Behavioral Performance
Oddball detection task
The d′ (z[hit] − z[false alarm]) measure of perceptual sensitivity [Macmillan and Creelman, 1991] was calculated. A “hit” refers to the case in which the observer responded “yes” when the deviant signal was actually presented. The hit rate is P(“yes”|Target). A “false alarm” refers to the case in which the observer responded “yes” when no signal was presented (i.e.,, when the standard stimulus was presented). The false alarm rate is P(“yes”|Standard). These rates are converted to z scores to compute d′.
The group average d′ was 1.97 (0.6 hits) in the fMRI session and 1.24 (0.5 hits) in the EEG session. False alarm rates were low, at 0.07 and 0.1, respectively. Accuracy was greater in the fMRI session probably due to the uniform MRI environment.
Duration judgment task
In the fMRI session, the group average reaction time (RT) was 556 ms and accuracy was 84%. In the EEG session, average RT was 524 ms and accuracy was 80%. There was not a significant difference in RT or in accuracy on the duration task between detected‐ and undetected deviant sequences in the fMRI or EEG sessions, as well as between sessions (all P > 0.05).
In addition, the detection of a deviant did not influence performance on the following duration judgment in either session, whether the duration judgment occurred immediately after the deviant or was separated by standards (P > 0.05). There were no significant correlations (positive or negative) between the performance accuracy on the primary and secondary tasks in either session. RT correlations between the tasks are not informative in this study, because the deviant detection response was required only at the end of each sequence.
fMRI
Overall deviance effects
To examine the main effect of deviance regardless of detection accuracy, fMRI activity for all trials with deviant sequences was contrasted with activity for trials with standard sequences. This contrast, deviant versus standard, is presented in Figure 2A and Table I. Greater activation for the deviants over the standards was observed predominantly in left anterior and middle intraparietal sulcus (IPS), bilateral posterior cingulate gyrus, and bilateral supplementary motor area (SMA) and frontal eye fields (FEF). Smaller foci of activation in auditory cortex included right lateral Heschl's gyrus (HG) and planum polare, bilaterally. Other activations involved the anterior insula and mid thalamus bilaterally, left orbital frontal cortex (OFC), caudate nucleus bilaterally, left lingual gyrus, left MFG, right anterior IPS, and right middle temporal gyrus (MTG).
Figure 2.
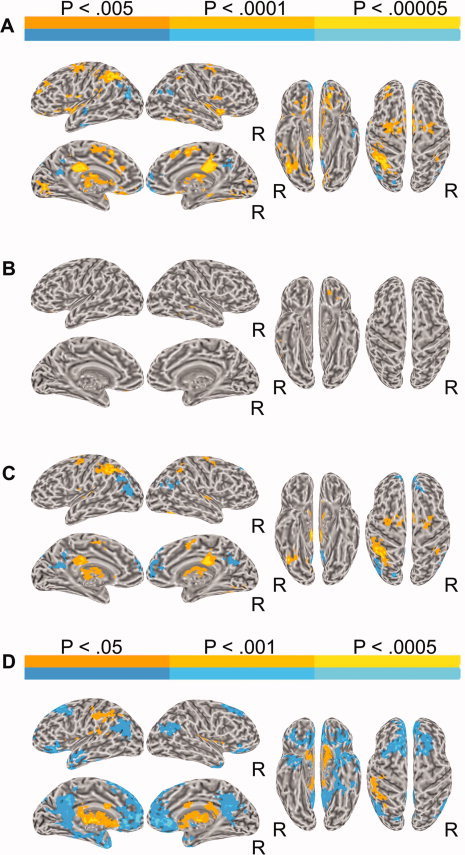
(A) Brain activation for the contrast between deviants and standards. In all figures, left and right lateral and medial views of the inflated brain surface are shown in the left half of the figure, ventral and dorsal views in the right half. The color scale indicates voxelwise probability values. R = right. (B) Brain activation for the contrast between undetected deviants and standards. (C) Brain activation for the contrast between detected deviants and standards. (D) Brain activation for the contrast between detected and undetected deviants.
Table I.
Locations of local extrema
| Contrast | x | y | z | Z‐score | Anatomical location |
|---|---|---|---|---|---|
| Main effect of deviance | 7 | −19 | 24 | 5.44 | R Posterior Cingulate Gyrus |
| 1 | 12 | 47 | 5.40 | L/R MFG | |
| −24 | −53 | 37 | 5.15 | L IPS | |
| −11 | −11 | 6 | 4.94 | L Thalamus | |
| 44 | −36 | −10 | 4.89 | R MTG/ITG | |
| −28 | 36 | −12 | 4.81 | L OFC | |
| 10 | −7 | 54 | 4.71 | R SFG | |
| −10 | 8 | −14 | 4.65 | L OFC | |
| 42 | 3 | −11 | 4.65 | R Planum Polare | |
| −12 | −77 | 6 | 4.61 | L Cuneus | |
| −53 | 9 | 6 | 4.47 | L IFG | |
| −39 | −5 | −4 | 4.46 | L Insula | |
| −26 | 42 | 22 | 4.36 | L MFG | |
| −41 | −36 | 50 | 4.34 | L SPL | |
| −13 | −33 | 18 | 4.12 | L Posterior Cingulate Gyrus | |
| −48 | −42 | 30 | 4.08 | L SMG | |
| 18 | 5 | 18 | 3.46 | R Caudate nucleus | |
| 1 | 53 | −12 | −5.82 | L/R OFC | |
| −47 | −75 | 24 | −4.55 | L Angular Gyrus | |
| 11 | 52 | 11 | −3.60 | R SFG | |
| Detected deviant > standard | −6 | −22 | 31 | 4.81 | L Cingulate Gyrus |
| −32 | −43 | 36 | 4.60 | L SPL | |
| 18 | −7 | 10 | 4.24 | R Thalamus | |
| −46 | −15 | 11 | 4.15 | L HG | |
| −10 | −11 | 6 | 4.11 | L Thalamus | |
| 6 | −8 | 54 | 3.75 | R SFG | |
| 37 | −41 | 32 | 3.70 | R SPL | |
| 26 | −11 | 51 | 3.69 | R MFG | |
| 44 | −15 | −2 | 3.62 | R Planum Polare | |
| −28 | −65 | 43 | 3.43 | L SPL | |
| −47 | −27 | 36 | 3.02 | L Postcentral Gyrus | |
| Undetected deviant > standard | −28 | 35 | −12 | 4.43 | L OFC |
| 47 | −28 | −11 | 4.17 | R MTG | |
| Detected > undetected | −1 | −8 | 8 | 4.3 | L/R Thalamus |
| −8 | −27 | 8 | 4 | L Thalamus | |
| −22 | −11 | 12 | 3.9 | L Putamen | |
| 24 | −5 | 16 | 3.73 | R Putamen | |
| −43 | −24 | 23 | 3.29 | L SMG | |
| −33 | −52 | 42 | 3 | L SPL | |
| −46 | −39 | 50 | 2.88 | L SPL | |
| −37 | −23 | 45 | 2.56 | L Postcentral Gyrus | |
| −3 | −54 | 17 | −2.98 | L Precuneus/Cingulate | |
| −44 | −67 | 21 | −2.46 | L Angular Gyrus | |
| −6 | 39 | 49 | −2.12 | L SFG | |
| 48 | −66 | 21 | −1.99 | R Angular Gyrus | |
| 2 | 43 | 14 | −1.96 | R SFG |
R = right; L = left; IFG = inferior frontal gyrus; IPS = intraparietal sulcus; ITG = inferior temporal gyrus; MFG = middle frontal gyrus; MTG = middle temporal gyrus; OFC = orbital frontal cortex; SFG = superior frontal gyrus; SMG = supramarginal gyrus; SPL = superior parietal lobule; HG = Heschl's gyrus.
Greater activity for the standard over the deviant trials was observed in angular gyrus and posterior cingulate gyrus bilaterally, left anterior temporal lobe, and the medial frontal poles bilaterally.
Detectability effects
We contrasted activity separately for sequences with a behaviorally undetected and detected deviant with that for standard sequences. The contrast, undetected deviant versus standard, revealed small clusters in left OFC and right MTG (Fig. 2B, Table I). The contrast detected deviant versus standard activated similar areas to those observed in the deviant versus standard contrast. These included left anterior and middle IPS, bilateral posterior cingulate, SMA, FEF, thalamus, and caudate nucleus, as well as right anterior IPS, and bilateral HG (Fig. 2C, Table I).
Greater activity for standard sequences was observed over detected deviant sequences (Fig. 2C). This activation involved angular gyrus, posterior cingulate, and medial aspect of the SFG bilaterally.
Detected versus undetected
The contrast detected deviant versus undetected deviant revealed greater activations for detected deviants in left SPL/IPS, and thalamus, putamen, and caudate bilaterally (Fig. 2D). Undetected deviants showed greater activation in ventral‐medial frontal cortex (including OFC, medial SFG, and subgenual cingulate gyrus), posterior cingulate and angular gyrus bilaterally, left anterior temporal pole, and pars orbitalis of the left IFG.
ERPs
Detectability effects
The detected and undetected deviant and standard waveforms at electrodes Fz, Pz, and Cz are superimposed in Figure 3 (Top). The N1 was similar in all conditions, suggesting that deviant sounds were encoded at a sensory level regardless of detection performance. The waveforms separated at about 200 ms with a large negative–positive waveform observed following detected deviants only. Pointwise t‐tests between the detected deviant and standard waveforms, calculated at each electrode, revealed a fronto‐central negativity at ∼ 200 ms, the N2 latency, and a centro‐posterior positivity at ∼ 550 ms, the P3 latency (Fig. 3, Bottom). Pointwise t‐tests between the undetected deviant and standard waveforms, for each electrode, revealed a small fronto‐central negativity in the N2 latency, but no statistically significant positivity in the P3 range (Fig. 3, Bottom).
Figure 3.
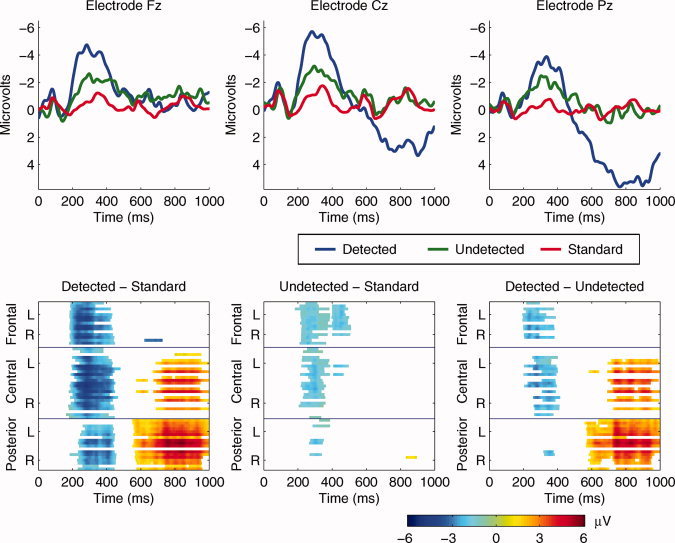
(Top) Group average ERP waveforms superimposed for the standard stimuli, detected deviants, and undetected deviants at frontal (Fz), central (Cz), and parietal (Pz) electrode cites. (Bottom) Spatio‐temporal statistical maps from 60 electrodes: Grand average difference scores of detected deviants minus standards, undetected deviants minus standards, and detected deviants minus undetected deviants at each electrode following pointwise analysis and correction. The y‐axis represents the frontal, central, and posterior electrodes. Each group of electrodes (frontal, central, posterior) is arranged top to bottom according to their lateral position from left (L) to right (R) with the midline electrode in the middle. The color scale represents the amplitude in μV.
Detected versus undetected
Pointwise analysis between the detected deviant and undetected deviant waveforms, for each electrode, is presented in Figure 3 (Bottom). These tests revealed greater fronto‐central negativity from 200 ms, the N2 latency, to 400 ms, and greater centro‐posterior positivity at 450–1,000 ms associated with target detection. There was no statistical difference in the N1 time window.
DISCUSSION
The present ERP and fMRI experiments investigated the neural events leading to and associated with detection of suprathreshold auditory stimuli during performance of two simultaneous tasks. The paradigm was designed specifically to elicit competition and variability in detection of auditory target stimuli despite identical input. The ERP pattern of results suggests that differences underlying successful versus missed deviant detection begin only at ∼ 200 ms poststimulus onset (and not earlier in the time window of the N1). Detected targets elicited a larger N2 component. The P3 component was observed only following detected deviants. The BOLD response distinguishing detected and undetected auditory deviants included left superior parietal cortex (SPL/IPS), bilateral subcortical regions (thalamus, putamen, caudate nucleus), bilateral ventral‐medial frontal cortex, posterior cingulate, and angular gyrus, left anterior temporal pole, and left IFG.
According to the idea of limited‐capacity, general attentional capacity is shared between tasks. Behaviorally, performance is worse when time between targets in two simultaneous tasks is shorter than 500 ms, and with tasks involving a single sensory modality [Duncan et al., 1997]. Capacity limitations have been proposed to take place either early or late in the stream of processing [Broadbent, 1958; Deutsch and Deutsch, 1963; Duncan, 1980; Lavie, 2010; Lavie et al., 2004]. In the current unimodal study, there were no differences in the N1 time range (60–150 ms) depending on detectability, suggesting that all auditory events were encoded at a sensory level even when undetected. N1 is an obligatory potential, that is, it is elicited in response to a sound, regardless of the depth of processing. However, the amplitude of N1 increases with attention [Hillyard et al., 1973], suggesting that this component reflects at least in part the level of auditory sensory analysis [Gonsalvez et al., 2007; Picton et al., 1970]. Our N1 finding is consistent with a late selection view, whereby computations are similar for all sensory input but only relevant information is selected to access a limited capacity system that is required for memory and awareness [Duncan, 1980].
Processes of attention, memory, and awareness have been associated with greater N2 and P3 ERP components [Folstein and Van Petten, 2008; Friedman and Johnson, 2000; Näätänen et al., 2007; Patel and Azzam, 2005; Polich, 2007; Sams et al., 1985]. The greater N2 observed for detected deviants in this study suggests that sufficient attentional resources were allocated for processing the oddball stream. Consistent with this idea, the later P3 potential, an index of memory updating when the stimulus environment is changed and updated [Donchin and Coles, 1988; Polich, 2007], was observed following the N2 only when deviants were reported. Neurons in the right superior temporal plane and middle portion of the STG have been consistently suggested to contribute to the generation of the N2‐P3 frequency deviant response, using a variety of experimental techniques [Alho, 1995; Celsis et al., 1999; Downar et al., 2000; Halgren et al., 1995a, b, 1998; Kropotov et al., 1995; Liebenthal et al., 2003; Opitz et al., 1999, 2002; Sabri et al., 2004; Scherg et al., 1989], in agreement with the greater activity observed in right STG only for detected deviants over standards in the current study. The stringent whole‐brain analysis did not reveal significant activation in STG in the direct contrast between detected and undetected deviants.
Intracranial ERP recordings to auditory targets suggest that additional generators in the SPL contribute to the N2‐P3 responses [Halgren et al., 1995a, b, 1998]. The superior parietal generator is considered to be modality nonspecific in that similar intracerebral potentials have been shown following either auditory or visual deviants. In the current study, the SPL (specifically IPS) was activated more strongly for detected compared to undetected deviants. This result is corroborated by findings of both Beck et al. [2001] and Pourtois et al. [2006], using an analogous paradigm in the visual modality; SPL was involved in successful detection of change between images when also detecting letters in a primary task. Similar tasks involving transition in visual awareness of percepts, for example, during binocular rivalry, also engaged the SPL [e.g., Kleinschmidt et al., 1998; Lumer et al., 1998; Portas et al., 2000]. Importantly, the location in standardized space of IPS clusters reported in visual studies of active target or change detection is very similar to that of the present auditory study ([−32 −43 36, −28 −65 43, 37 −41 32]), despite the modality difference [e.g., Beck et al., 2001[−30 −42 48, 42 −51 51]; Buchel et al., 1998[−30 −54 54, −21 −66 54, −36 −45 60, −39 −36 48, 39 −42 54]; Corbetta et al., 2000[−25 −57 42, −25 −65 48, 33 −51 48]; see also Grosbras et al., 2005 for a review]. The SPL has also been implicated in top–down controlled shifting of auditory or visual spatial attention from attended to unattended stream [Salmi et al., 2007, 2009; Shomstein and Yantis, 2006]. However, in the present study the absence of a significant correlation in performance between the two tasks does not support voluntary switching mechanisms [Miller and Bonnel, 1994; Tombu and Jolicoeur, 2003, 2005] (see below).
The parietal association cortex projects to various neural structures including cortical temporal and frontal cortices and subcortical structures, such as the thalamus [Hyvarinen, 1982]. The thalamus' crucial involvement in attention and awareness is well documented in both human and animal studies [e.g., Frith and Friston, 1996; LaBerge et al., 1992; Newman, 1995; Portas et al., 1998; Posner and Raichle, 1994; Wurtz et al., 1980]. Specifically, the mid thalamus region was shown to be modulated by attention, with increased responses to tones or visual motion when attention was directed toward them [Buchel et al., 1998; Frith and Friston, 1996]. In the present study, the thalamus was activated more strongly for detected than undetected deviants. It is likely that this is due to corticothalamic circuits responsible for synchronizing firing in cortical regions and promoting attention and memory encoding as signified by the N2‐P3. The absence of any difference between detected and undetected deviants in the earlier portions of the ERP suggests that auditory input reached primary auditory cortex regardless of detection performance.
Other regions that showed differential responses to detected and undetected deviants were the ventral‐medial frontal cortex, posterior cingulate, and angular gyrus bilaterally, and left anterior temporal pole and IFG, with activation greater for undetected deviants. These brain regions were previously identified as part of the “default” mode network active in the conscious resting state and deactivated during goal‐directed tasks [Binder et al., 1999; Raichle et al., 2001; Shulman et al., 1997]. This pattern of results suggests lower levels of neural activity in the default system for detected sequences consistent with task goals.
Missing target trials can occur due to dual‐task limitations, which source is of continuous debate [Pashler and Johnston, 1998]. There are two dominant explanations for how divided attention dual‐task paradigms are performed, namely, rapid switching between tasks and capacity sharing models [Miller and Bonnel, 1994; Tombu and Jolicoeur, 2003, 2005]. The switching models commonly predict a negative correlation in performance between tasks that compete on attentional resources [Miller and Bonnel, 1994; Tombu and Jolicoeur, 2003, 2005]. In this study, a correlation in performance between the duration judgment and oddball detection tasks was not observed. This result is in line with capacity sharing rather than attention switching predictions. According to the capacity sharing model, the secondary oddball task does not have full access to attentional resources due to sharing with the primary duration task. It has been demonstrated that accuracy is reduced when a target in one stream is followed within a few hundred milliseconds (e.g., 375 ms) by a target in the other stream, within the same sensory modality [Duncan et al., 1997]. In the current study, there was a trend for higher accuracy in the oddball task when the temporal separation between the duration stimulus and the deviant stimulus was 750 ms. Taken together, the results are more in line with a resource limitation rather than an attention switching explanation.
In summary, these data demonstrate that variability in auditory detection of simple sounds as observed behaviorally is related to modulation of activity in multimodal parietal and frontal networks, and possibly modality specific auditory cortex ∼ 200 ms after target onset. Future investigations will focus on evaluating the different possible explanations for the observed variability in auditory target processing, particularly with regard to limits on attentional capacity and attention switching [Duncan, 1980; Duncan et al., 1997; Miller and Bonnel, 1994; Navon and Gopher, 1979; Tombu and Jolicoeur, 2003, 2005], and task load and type [Lavie, 2005, 2010].
Acknowledgements
The authors thank M. Ellingson and E. Possing for assistance with data collection and/or analyses.
REFERENCES
- Alho K ( 1995): Cerebral generators of mismatch negativity (MMN) and its magnetic counterpart (MMNm) elicited by sound change. Ear Hear 16: 38–51. [DOI] [PubMed] [Google Scholar]
- Beck DM, Rees G, Frith CD, Lavie N ( 2001): Neural correlates of change detection and change blindness. Nat Neurosci 4: 645–650. [DOI] [PubMed] [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Bellgowan PS, Rao SM, Cox RW ( 1999): Conceptual processing during the conscious resting state. A functional MRI study. J Cogn Neurosci 11: 80–95. [DOI] [PubMed] [Google Scholar]
- Broadbent DE ( 1958): Perception and Communication. London: Pergamon Press. [Google Scholar]
- Buchel C, Josephs O, Rees G, Turner R, Frith CD, Friston KJ ( 1998): The functional anatomy of attention to visual motion. A functional MRI study. Brain 121: 1281–1294. [DOI] [PubMed] [Google Scholar]
- Celsis P, Boulanouar K, Doyon B, Ranjeva JP, Berry I, Nespoulous JL, Chollet F ( 1999): Differential fMRI responses in the left posterior superior temporal gyrus and left supramarginal gyrus to habituation and change detection in syllables and tones. Neuroimage 9: 135–144. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Kincade JM, Ollinger JM, McAvoy MP, Shulman GL ( 2000): Voluntary orienting is dissociated from target detection in human posterior parietal cortex. Nat Neurosci 3: 292–297. [DOI] [PubMed] [Google Scholar]
- Cox RW ( 1996): AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 29: 162–173. [DOI] [PubMed] [Google Scholar]
- Desimone R, Duncan J ( 1995): Neural mechanisms of selective visual attention. Annu Rev Neurosci 18: 193–222. [DOI] [PubMed] [Google Scholar]
- Deutsch JA, Deutsch D ( 1963): Attention: Some theoretical considerations. Psychol Rev 70: 80–90. [DOI] [PubMed] [Google Scholar]
- Donchin E, Coles MG ( 1988): Is the P300 component a manifestation of context updating? Behav Brain Sci 11: 357–427. [Google Scholar]
- Donchin E, McCarthy G, Kutas M, Ritter W ( 1983): Event‐related brain potentials in the study of consciousness In: Davidson R, Schwartz G, Shapiro D, editors. Consciousness and Self Regulation. New York: Plenum Press; pp 81–121. [Google Scholar]
- Downar J, Crawley AP, Mikulis DJ, Davis KD ( 2000): A multimodal cortical network for the detection of changes in the sensory environment. Nat Neurosci 3: 277–283. [DOI] [PubMed] [Google Scholar]
- Duncan J ( 1980): The locus of interference in the perception of simultaneous stimuli. Psychol Rev 87: 272–300. [PubMed] [Google Scholar]
- Duncan J, Martens S, Ward R ( 1997): Restricted attentional capacity within but not between sensory modalities. Nature 387: 808–810. [DOI] [PubMed] [Google Scholar]
- Folstein JR, Van Petten C ( 2008): Influence of cognitive control and mismatch on the N2 component of the ERP: A review. Psychophysiology 45: 152–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman D, Johnson R Jr ( 2000): Event‐related potential (ERP) studies of memory encoding and retrieval: A selective review. Microsc Res Tech 51: 6–28. [DOI] [PubMed] [Google Scholar]
- Frith CD, Friston KJ ( 1996): The role of the thalamus in “top down” modulation of attention to sound. Neuroimage 4: 210–215. [DOI] [PubMed] [Google Scholar]
- Gonsalvez CJ, Barry RJ, Rushby JA, Polich J ( 2007): Target‐to‐target interval, intensity, and P300 from an auditory single‐stimulus task. Psychophysiology 44: 245–250. [DOI] [PubMed] [Google Scholar]
- Grosbras MH, Laird AR, Paus T ( 2005): Cortical regions involved in eye movements, shifts of attention, and gaze perception. Hum Brain Mapp 25: 140–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie D, Buchwald JS ( 1991): Significance testing of difference potentials. Psychophysiology 28: 240–244. [DOI] [PubMed] [Google Scholar]
- Halgren E, Baudena P, Clarke JM, Heit G, Liegeois C, Chauvel P, Musolino A ( 1995a): Intracerebral potentials to rare target and distractor auditory and visual stimuli. I. Superior temporal plane and parietal lobe. Electroencephalogr Clin Neurophysiol 94: 191–220. [DOI] [PubMed] [Google Scholar]
- Halgren E, Baudena P, Clarke JM, Heit G, Marinkovic K, Devaux B, Vignal JP, Biraben A ( 1995b): Intracerebral potentials to rare target and distractor auditory and visual stimuli. II. Medial, lateral and posterior temporal lobe. Electroencephalogr Clin Neurophysiol 94: 229–250. [DOI] [PubMed] [Google Scholar]
- Halgren E, Marinkovic K, Chauvel P ( 1998): Generators of the late cognitive potentials in auditory and visual oddball tasks. Electroencephalogr Clin Neurophysiol 106: 156–164. [DOI] [PubMed] [Google Scholar]
- Hill KT, Miller LM ( 2009): Auditory attentional control and selection during cocktail party listening. Cereb Cortex 20: 583–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillyard SA, Hink RF, Schwent VL, Picton TW ( 1973): Electrical signs of selective attention in the human brain. Science 182: 177–180. [DOI] [PubMed] [Google Scholar]
- Hyvarinen J ( 1982): Posterior parietal lobe of the primate brain. Physiol Rev 62: 1060–1129. [DOI] [PubMed] [Google Scholar]
- Kiehl KA, Laurens KR, Duty TL, Forster BB, Liddle PF ( 2001): Neural sources involved in auditory target detection and novelty processing: An event‐related fMRI study. Psychophysiology 38: 133–142. [PubMed] [Google Scholar]
- Kiehl KA, Stevens MC, Laurens KR, Pearlson G, Calhoun VD, Liddle PF ( 2005): An adaptive reflexive processing model of neurocognitive function: Supporting evidence from a large scale (n = 100) fMRI study of an auditory oddball task. Neuroimage 25: 899–915. [DOI] [PubMed] [Google Scholar]
- Kleinschmidt A, Buchel C, Zeki S, Frackowiak RS ( 1998): Human brain activity during spontaneously reversing perception of ambiguous figures. Proc R Soc Lond B Biol Sci 265: 2427–2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kropotov JD, Naatnen R, Sevostianov AV, Alho K, Reinikainen K, Kropotova OV ( 1995): Mismatch negativity to auditory stimulus change recorded directly from the human temporal cortex. Psychophysiology 32: 418–422. [DOI] [PubMed] [Google Scholar]
- LaBerge D, Carter M, Brown V ( 1992): A network simulation of thalamic circuit operations in selective attention. Neural Comput 4: 318–331. [Google Scholar]
- Lavie N ( 2005): Distracted and confused? Selective attention under load. Trends Cogn Sci 9: 75–82. [DOI] [PubMed] [Google Scholar]
- Lavie N ( 2010): Attention, distraction, and cognitive control under load. Curr Dir Psychol Sci 19: 143–148. [Google Scholar]
- Lavie N, Tsal Y ( 1994): Perceptual load as a major determinant of the locus of selection in visual attention. Percept Psychophys 56: 183–197. [DOI] [PubMed] [Google Scholar]
- Lavie N, Hirst A, de Fockert JW, Viding E ( 2004): Load theory of selective attention and cognitive control. J Exp Psychol Gen 133: 339–354. [DOI] [PubMed] [Google Scholar]
- Liebenthal E, Ellingson ML, Spanaki MV, Prieto T, Ropella KM, Binder JR ( 2003): Simultaneous ERP and fMRI of the auditory cortex in a passive oddball paradigm. Neuroimage 19: 1395–1404. [DOI] [PubMed] [Google Scholar]
- Linden D ( 2005): The p300: Where in the brain is it produced and what does it tell us? Neuroscientist 11: 563–576. [DOI] [PubMed] [Google Scholar]
- Linden D, Prvulovic D, Formisano E, Vollinger M, Zanella FE, Goebel R, Dierks T ( 1999): The functional neuroanatomy of target detection: An fMRI study of visual and auditory oddball tasks. Cereb Cortex 9: 815–823. [DOI] [PubMed] [Google Scholar]
- Lumer ED, Friston KJ, Rees G ( 1998): Neural correlates of perceptual rivalry in the human brain. Science 280: 1930–1934. [DOI] [PubMed] [Google Scholar]
- Macmillan NA, Creelman CD ( 1991): Detection Theory: A User's Guide. New York, NY: Cambridge University Press. [Google Scholar]
- Menon VF, Ford JM, Lim KO, Glover GH, Pfefferbaum A ( 1997): Combined event‐related fMRI and EEG evidence for temporal‐parietal cortex activation during target detection. Neuroreport 8: 3029–3037. [DOI] [PubMed] [Google Scholar]
- Miller J, Bonnel AM ( 1994): Switching or sharing in dual‐task line‐length discrimination? Percept Psychophys 56: 431–446. [DOI] [PubMed] [Google Scholar]
- Näätänen R ( 1992): Attention and Brain Function. Hillsdale, NJ: Erlbaum. [Google Scholar]
- Näätänen R, Paavilainen P, Rinne T, Alho K ( 2007): The mismatch negativity (MMN) in basic research of central auditory processing: A review. Clin Neurophysiol 118: 2544–2590. [DOI] [PubMed] [Google Scholar]
- Navon D, Gopher D ( 1979): On the economy of the human‐processing system. Psychol Rev 86: 214–255. [Google Scholar]
- Newman J ( 1995): Thalamic contributions to attention and consciousness. Conscious Cognit 4: 172–193. [DOI] [PubMed] [Google Scholar]
- Oldfield RC ( 1971): The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9: 97–113. [DOI] [PubMed] [Google Scholar]
- Opitz B, Mecklinger A, Von Cramon D, Kruggel F ( 1999): Combining electrophysiological and hemodynamic measures of the auditory oddball. Psychophysiology 36: 142–147. [DOI] [PubMed] [Google Scholar]
- Opitz B, Rinne T, Mecklinger A, Von Cramon D, Schroeger E ( 2002): Differential contribution of frontal and temporal cortices to auditory change detection: fMRI and ERP results. Neuroimage 15: 167–174. [DOI] [PubMed] [Google Scholar]
- Pashler H, Johnston JC ( 1998): Attentional limitations in dual‐task performance In: Pashler H, editor. Attention. Hove, England: Psychology Press; pp 155–189. [Google Scholar]
- Patel SH, Azzam PN ( 2005): Characterization of N200 and P300: Selected studies of the event‐related potential. Int J Med Sci 2: 147–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picton TW, Goodman WS, Bryce DP ( 1970): Amplitude of evoked responses to tones of high intensity. Acta Otolaryngol (Stockh) 70: 77–82. [DOI] [PubMed] [Google Scholar]
- Picton TW, Champagne SC, Kellett AJ ( 1992): Human auditory evoked potentials recorded using maximum length sequences. Electroencephalogr Clin Neurophysiol 84: 90–100. [DOI] [PubMed] [Google Scholar]
- Polich J ( 2007): Updating P300: An integrative theory of P3a and P3b. Clin Neurophysiol 118: 2128–2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portas CM, Rees G, Howseman AM, Josephs O, Turner R, Frith CD ( 1998): A specific role for the thalamus in mediating the interaction of attention and arousal in humans. J Neurosci 18: 8979–8989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portas CM, Krakow K, Allen P, Josephs O, Armony JL, Frith CD ( 2000): Auditory processing across the sleep‐wake cycle: Simultaneous EEG and fMRI monitoring in humans. Neuron 28: 991–999. [DOI] [PubMed] [Google Scholar]
- Posner MI, Raichle ME ( 1994): Images of Mind. New York, NY: Scientific American Library/Scientific American Books. [Google Scholar]
- Pourtois G, De Pretto M, Hauert CA, Vuilleumier P ( 2006): Time course of brain activity during change blindness and change awareness: Performance is predicted by neural events before change onset. J Cogn Neurosci 18: 2108–2129. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL ( 2001): A default mode of brain function. Proc Natl Acad Sci USA 98: 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabri M, Kareken DA, Dzemidzic M, Lowe MJ, Melara RD ( 2004): Neural correlates of auditory sensory memory and automatic change detection. Neuroimage 21: 69–74. [DOI] [PubMed] [Google Scholar]
- Salmi J, Rinne T, Degerman A, Salonen O, Alho K ( 2007): Orienting and maintenance of spatial attention in audition and vision: Multimodal and modality‐specific brain activations. Brain Struct Funct 212: 181–194. [DOI] [PubMed] [Google Scholar]
- Salmi J, Rinne T, Koistinen S, Salonen O, Alho K ( 2009): Brain networks of bottom‐up triggered and top‐down controlled shifting of auditory attention. Brain Res 1286: 155–164. [DOI] [PubMed] [Google Scholar]
- Sams M, Paavilainen P, Alho K, Näätänen R ( 1985): Auditory frequency discrimination and event‐related potentials. Electroencephalogr Clin Neurophysiol 62: 437–448. [DOI] [PubMed] [Google Scholar]
- Scherg M, Von Cramon D ( 1986): Evoked dipole source potentials of the human auditory cortex. Electroencephalogr Clin Neurophysiol 65: 344–360. [DOI] [PubMed] [Google Scholar]
- Scherg M, Vasjar J, Picton TW ( 1989): A source analysis of the late human auditory evoked potentials. J Cogn Neurosci 1: 336–355. [DOI] [PubMed] [Google Scholar]
- Shomstein S, Yantis S ( 2006): Parietal cortex mediates voluntary control of spatial and nonspatial auditory attention. J Neurosci 26: 435–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman GL, Corbetta M, Buckner RL, Raichle ME, Fiez JA, Miezin FM, Petersen SE ( 1997): Top‐down modulation of early sensory cortex. Cereb Cortex 7: 193–206. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P ( 1988): Co‐Planar Stereotaxic Atlas of the Human Brain. New York: Thieme Medical. [Google Scholar]
- Tombu M, Jolicoeur P ( 2003): A central capacity sharing model of dual‐task performance. J Exp Psychol Hum Percept Perform 29: 3–18. [DOI] [PubMed] [Google Scholar]
- Tombu M, Jolicoeur P ( 2005): Testing the predictions of the central capacity sharing model. J Exp Psychol Hum Percept Perform 31: 790–802. [DOI] [PubMed] [Google Scholar]
- Wurtz RH, Goldberg ME, Robinson DL ( 1980): Behavioral modulation of visual responses in the monkey: Stimulus selection for attention and movement. Progr Psychobiol Physiol Psychol 9: 43–83. [Google Scholar]


