Abstract
Background
Ethanol consumption during pregnancy can lead to fetal growth retardation, mental retardation and neurodevelopmental delay. The fetal brain initiates neurogenesis and vasculogenesis during the second trimester, and depends on maternal-fetal circulation for nutrition and growth signals. We used high resolution in vivo ultrasound imaging to test the hypothesis that ethanol interferes with fetal brain-directed blood flow during this critical developmental period.
Methods
Pregnant mice were lightly anesthetized on gestational day 12 with an isoflurane/oxygen mixture. We assessed the effect of single and repeated binge-like maternal ethanol exposures at 3 g/kg, administered by intragastric gavage or intraperitoneal injection, on maternal circulation and fetal umbilical, aortic, internal carotid and middle cerebral arterial circulation.
Results
Binge maternal ethanol exposure, regardless of exposure route, significantly reduced fetal arterial blood acceleration and velocity time integral (VTI), from umbilical to cerebral arteries, without a change in fetal heart rate and resistivity indices. Importantly a single maternal binge ethanol exposure induced persistent suppression of fetal arterial VTI for at least 24 hours. Repeated binge episodes resulted in a continuing and persistent suppression of fetal VTI. Qualitative assessments showed that maternal ethanol exposure induced oscillatory, non-directional blood flow in fetal cerebral arteries. Maternal cardiac and other physiological parameters remained unaltered.
Conclusion
These data show that binge-type maternal ethanol exposure results in rapid and persistent loss of blood flow from the umbilical artery to the fetal brain, potentially compromising nutrition and the maternal/fetal endocrine environment during a critical period for neuron formation and angiogenesis in the maturing brain.
Keywords: Second trimester, fetal ethanol exposure, high-resolution ultrasound, cerebral blood flow, fetal arterial blood flow
Ethanol consumption during pregnancy can lead to brain, craniofacial, cardiovascular and limb defects that are collectively termed Fetal Alcohol Spectrum Disorders (FASD) (Abel, 1984; Jones et al., 1973). FASD is a leading non-genetic cause of mental retardation (Abel, 1995). Significant numbers of pregnant women continue to consume ethanol into the second trimester (SAMHSA, 2009), a critical period for fetal neurogenesis and brain angiogenesis. FASD continues to be a significant public health problem, with an estimated incidence of 2–5% of the US population (May et al., 2009).
Significant research has focused on the fetal neural basis for the emergence of behavioral deficits associated with maternal ethanol exposure. These studies showed that ethanol directly interferes with several aspects of neural development, including the biology of neural stem cells (Camarillo and Miranda, 2008; Santillano et al., 2005; Vangipuram et al., 2008), neuronal migration (reviewed in (Bearer, 2001)), and the survival and maturation of differentiating neurons (reviewed in (Goodlett and Horn, 2001)). By promoting death (West et al., 2001) and disrupting synaptic connectivity (Carta et al., 2006) of cerebellar neurons for example, ethanol may promote the appearance behavioral deficits like gait disturbances. However, ethanol’s neural effects could additionally be mediated by disturbances within non-neural tissue including developing brain vasculature (Parnell et al., 2007).
During the second trimester period, a network of blood vessels within the sub-arachnoid space give rise to microvessels that invade the fetal brain (Norman and O’Kusky, 1986) during the same time period that neural stem cells generate most of the neurons of the brain. This emergent vasculature supports nutrition needs and endocrine control of fetal growth (Fowden and Forhead, 2009) and promotes neural development (Tam and Watts, 2010). Previous research showed that ethanol exposure during the murine first trimester-equivalent period produced a persistent alteration in cardiac physiology (Serrano et al., 2010), which persisted into the critical second trimester window for brain development. In ovine models, chronic fetal ethanol exposure can increase cerebral blood flow under acidaemic and hypercapnic conditions (Parnell et al., 2007) and second trimester exposure alters subsequent cerebral vascular responses to hypoxia and to vasodilatory hormones like VIP (Mayock et al., 2007; Ngai et al., 2008). However, until recently it has been technically difficult to visualize fetal blood flow, particularly in smaller arteries. Modern high-resolution ultrasonography makes possible repeated, non-invasive and real-time analysis of blood flow in sub-millimeter sized vessels like the murine fetal middle cerebral arteries, making it feasible to ask questions about the direct impact of adverse environmental factors like maternal ethanol consumption on fetal cerebral blood flow. In this study, we used this technology to test the hypothesis that single as well as repeated binge-like episodes of maternal ethanol exposure, which are particularly damaging to the fetus (Maier and West, 2001), both immediately and persistently alter cranially-directed fetal blood flow during a period which encompasses blood vessel and neuron formation in the developing brain.
Methods
All procedures were performed in accordance with institutional animal care committee guidelines and approval. Timed-pregnant C57Bl6 female mice (Harlan laboratories, Houston, TX) were anesthetized using isoflurane (anesthesia was initiated with 3–4%, and maintained with 1% isoflurane), and maintained supine on a temperature-controlled mouse platform (with sensors for monitoring of maternal electrocardiogram, respiration and core body temperature, Visualsonics, Toronto, Canada). Maternal temperature was maintained at 34–37°C and maternal heart rate at ~425 beats/minute by adjusting the level of anesthesia. The abdomen was shaved and depilated (using Nair) to improve contact with the transducer. Ultrasound gel (Ecogel, CA), pre-warmed to 37°C, was applied to the dam’s abdomen prior to positioning the transducer. An initial scan was performed on both uterine horns to verify the number and location of all fetuses. For each pregnant dam, a single fetus in the middle position of a uterine horn was selected for both pre- and post-ethanol treatment scans. Both color and pulse wave Doppler measurements for umbilical arteries, ascending aorta, internal carotid artery (ICA) and middle cerebral arteries (MCA) were obtained using a high-frequency VEVO2100 ultrasound imaging machine coupled to a MS550D Microscan™ transducer with a center frequency of 40MHz (Visualsonics, Canada). The mean (±Standard Error of the Mean, SEM) Doppler angle of insonation for fetal blood vessels was as follows: for the UA (27.6°±2.5), aorta (23.8°±1.96), ICA (29.1°±2.4), and MCA (25.7°±2.4). Importantly, there were no statistically significant differences in angle of insonation between pre- and post-ethanol exposure conditions, indicating the absence of systematic error in estimates of blood flow.
Acute and repeated binge ethanol exposure paradigms
Acute Binge Exposure model
Ethanol, at 3g/kg (prepared from 95% Ethanol, ACS grade, Acros Organics, NJ # 61511, delivered in a final volume of 220ul) was administered either by intraperitoneal injection as a bolus, or by intragastric gavage using polyethylene tubing connected to mini pump (Harvard Apparatus) set to a flow rate of 100 ul/min. Fetal blood vessels and the maternal common carotid artery were subjected to ultrasonography for an average period of 18 minutes before ethanol exposure and data obtained were averaged across that time period. For some experiments, following the pre-exposure period, pregnant dams were administered saline (220ul) intraperitoneally as a bolus, or by intragastric gavage and additional recordings were obtained at time points used for ethanol exposure, as outline below. For other experiments, following the initial baseline scan, pregnant animals were administered ethanol. Starting at 20 minutes after intraperitoneal injection, or at 50 minutes following gavage to account for the effects of route of administration on the temporal course of blood alcohol levels (Figure 1), flow parameters in maternal and fetal blood vessels were assessed for an additional average period of 21 minutes and the data averaged across the recording period. One caveat pertinent to adopting an experimental design wherein blood flow assessment was tied to the peak blood ethanol content (BEC), is that the post-ethanol exposure assessment period in the case of the intragastric gavage condition was delayed by 30 minutes relative to assessment initiation following intraperitoneal ethanol delivery. However, the adopted paradigm does permit direct comparison between two exposure methods at peak BEC. Vascular and cardiac waveforms were analyzed quantitatively using Vevo 2100 software (see Figures 2 & 3 for sample maternal and fetal ultrasound waveforms). Blood (20 ul) was collected from the tail vein using heparinized capillary tubes for determination of BEC by gas chromatography according to our previous publications (Camarillo and Miranda, 2008; Prock and Miranda, 2007; Santillano et al., 2005).
Figure 1.
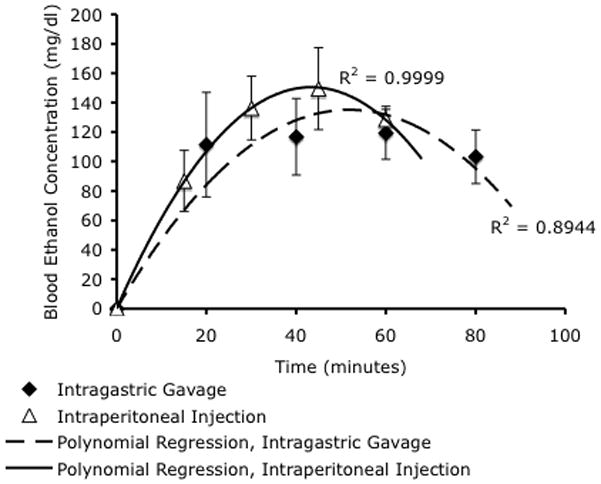
Blood ethanol concentrations were measured by gas chromatography analysis of mouse tail-vein blood. Intragastric gavage and intraperitoneal ethanol injections resulted in peak blood ethanol concentrations (BEC) of 117mg/dL and 150mg/dL respectively. Data were best fit by second-order polynomial regressions (R2=0.89 and 0.99 respectively), indicating that intragastric gavage resulted in a slightly delayed peak blood concentration of ethanol compared to intraperitoneal injection. There was no statistically significant difference in peak BEC between either routes of administration.
Figure 2.
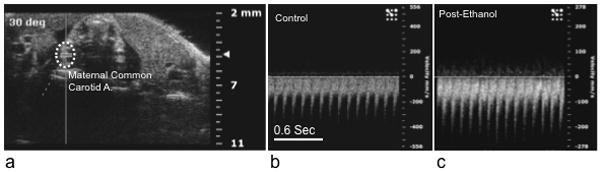
Maternal ethanol exposure (by either intragastric gavage or intraperitoneal injection did not result in a significant change in maternal heart rate or other hemodynamic measures (see results). (a) Sample Doppler image showing angle of insonation and placement of the data acquisition cursor over the maternal common carotid artery. (b and c) show sample sonograms before and after ethanol exposure. Note that the scaling of the ‘Y’ axis (velocity in mm/sec) is different for images b and c, however, there was not a significant difference in maternal carotid artery peak velocity following ethanol exposure. Abbreviations, A; artery. Scale bar (for b and c), 0.6 sec.
Figure 3.
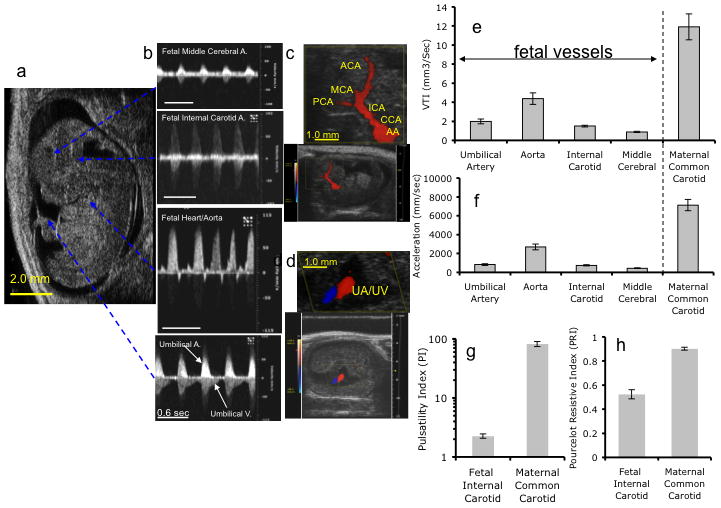
Representative fetal color Doppler Images and pulse waveform recordings obtained from control gestational day (GD) 12.5 mice. a–b Image of GD12.5 fetus (a) and corresponding Doppler waveforms obtained from (b, listed from top to bottom) MCA, ICA, Aorta, and UA (Scale bar, 0.6 sec). (c) Color Doppler image and magnified inset (scale bar, 1mm) depict directional cerebro-fugal blood flow (red) from the aortic arch (AA) through the common carotid artery (CCA), the internal carotid artery (ICA), and the anterior (ACA), middle (MCA) and posterior (PCA) cerebral arteries. The gap between the PCA and the ICA represents the probable location of the anastomotic posterior communicating artery. (d) Color Doppler image and magnified inset (scale bar, 1mm) show blood flow through adjacent umbilical artery (UA) and vein (UV). (e,f) Quantitative analysis of velocity time integral (VTI, e) and acceleration (f) measurements made in respective blood vessels in control GD12.5 fetuses and in maternal common carotid artery, prior to maternal ethanol exposure, demonstrating that in the fetus, the greatest flow was observed in the ascending Aorta for both acceleration and VTI. However, fetal Acc, VTI, PI (g) and PRI (h) are all lower than maternal values.
Multiple binge exposure model
Pregnant dams were administered ethanol at 3 g/kg once daily, by intragastric gavage, on GD12.5, 13.5 and 14.5, corresponding to approximately half of the second trimester-equivalent period of gestation. On each day, ultrasound recordings were obtained immediately before and 50 minutes following maternal ethanol gavage. Therefore, on GD13.5 and GD14.5, the pre-ethanol recordings represented measures of the persistent effects of maternal ethanol exposure 24 hours earlier, and the post-ethanol recordings represented the cumulative effects of multiple episodes of maternal ethanol exposure. As with the single binge exposure paradigm, a single mouse fetus in the mid-position of one uterine horn was assessed in each pregnant dam to prevent litter and uterine position biases, and the same fetus was re-assessed over all three gestational ages.
Assessment of the effects of color doppler imaging on cell and tissue damage
Briefly, a single fetus in each dam was exposed to color doppler imaging of the heart and the brain for 3 consecutive days (GD12.5, 13.5 and 14.5). Ultrasound-exposed fetuses and un-exposed (non-imaged controls were used to minimize variability from extraneous inter-litter variables including variations in litter developmental stage and anesthetic exposure levels among others) littermates from the same pregnant dam were dissected out of uterus on GD14.5 and fixed with 4 % paraformaldehyde. Saggital sections of embryos at 30μm thickness were processed for Nissl and Fluoro-Jade staining. In the case of Fluoro-Jade staining to assess cell death, fetal sections were treated sequentially with 100% ethanol for 3 min, 70 % ethanol for 1 minute, followed by deionized water for 1 minute and finally, 0.004% Fluoro-Jade B (Sigma-Aldrich, St-Louis, MO) for 30 min with gentle shaking. Sections were subsequently washed in deionized water 3 times, then dried at 50°C for 15 min, and finally cleared in xylene and coverslipped with permount. We used Fluoro-Jade staining of coronal tissue sections from an endothelin-1 induced stroke model as a positive control for downstream brain damage resulting from damage to the vascular system (tissue sections were a gift from Dr. Farida Sohrabji, Texas A&M HSC, for protocol details, see (Selvamani and Sohrabji, 2008)). Our expectation was that endothelin-1 vasoconstriction-mediated damage would possess model equivalency to exposing fetal blood vessels to high-frequency ultrasonography to assess associated tissue damage. In this rat stroke model, stereotaxic injections of ET-1 resulted in a significant death of vascular cells, downstream cortical and striatal infarct and substantial neuronal damage in both brain regions that are specifically localized to the injected hemisphere.
Data analysis and statistics
Data from pulse wave Doppler imaging experiments were analyzed using the VEVO2100 measurement and analysis software (Visualsonics, Ca) to assess heart rate (HR), Acceleration (Acc, in mm/sec2) and Velocity-Time Integral (VTI, in mm3/sec), the pulsatility index (PI) and the Pourcelot resistive index (PRI). Acc is defined as the change in blood flow velocity over time from onset of systolic forward flow to peak Velocity (Phoon and Turnbull, 2003). Acc can be used as a measure of cardiac output in peripheral vessels (Chang et al., 2000) in human patients and in embryonic mice studies (Phoon et al., 2000) and correlates well with arterial resistance. Velocity-Time Integral (VTI, in mm3/sec), the area under the velocity envelope (Phoon and Turnbull, 2003) is a surrogate measure of cardiac stroke volume through a specific blood vessel. The fetal and maternal PRI, a measure of vascular resistance, was defined as the velocity difference between peak-systolic to end-diastolic velocity normalized to the peak systolic velocity. PI, also a measure of vascular resistance, is defined as the difference between peak systolic velocity and end-diastolic velocity normalized to the average maximum velocity over one cardiac cycle. In the case of the fetus, f(fetal)Acc and fVTI measurements were obtained from UA, fetal ascending aorta (AA), fetal ICA and fetal MCA, while fPI and fPRI were constant across arteries and therefore assessed in the fetal ICA. In the case of maternal measurements, m(maternal)Acc, mVTI, mPI and mPRI measurements were obtained from the maternal common carotid artery. Each data point represents measurements from one fetus in the middle position of a uterine horn, in one pregnant dam (to eliminate litter and uterine position effects). Results presented are mean ± SEM for each group (n=7–10 pregnant dams), normalized to the average baseline value for the control, pre-ethanol exposed group.
Data were analyzed using a standard General Linear Models (GSM) multivariate (MANOVA)/univariate analysis of variance (Pillai’s Trace Statistic followed by univariate ANOVAs), or when appropriate, a doubly multivariate, repeated measures design to test for both “between-subjects” (route of ethanol exposure) and “within-subjects” (measures of cardiovascular function before and following ethanol exposure). For the latter mixed multivariate/repeated measures design, multivariate statistics were calculated as indicated above, for between-subjects, within-subjects, as well as for the interaction of “between-” and “within-subjects” measures. This was followed by post hoc univariate analyses (Greenhouse-Geisser-corrected Analysis of Variance, gANOVA) for both the main “within-subjects” effects as well as the interaction between “within-subjects” and “between-subjects” effects. Finally, when appropriate, post-hoc or planned comparisons were conducted using Fisher’s least significant difference (LSD) tests (SPSS v18, IBM). Group differences were considered significant at p<0.05.
Results
Intraperitoneal and intra-gastric administration of ethanol results in equivalent peak blood ethanol levels in maternal circulation
In the acute binge-exposure paradigm, we compared the effects for two commonly used methods of ethanol exposure, intragastric gavage and intraperitoneal injection. Maternal ethanol administration by intragastric gavage or intraperitoneal injection, at 3 gm/Kg resulted in a peak blood ethanol concentration of 117 mg/dl and 150 mg/dl respectively. These values were not statistically significantly different from each other (t(10)=1.155, p<0.2, Figure 1) and represent levels attainable in human populations.
Intra-gastric and intraperitoneal ethanol exposure does not alter maternal vascular and respiratory physiology
Multivariate analyses of variance (MANOVA) indicated that ethanol exposure did not significantly alter any maternal physiological and cardiovascular parameters (HR, respiration rate, mACC, mVTI, mPI and mPRI, Pillai’s trace statistic, F(6,7)=1.003, p<0.49, for sample sonograms, see Figure 2), nor was there an effect of route of administration (i.e., intra-peritoneal vs. intragastric gavage ethanol exposure (Pillai’s trace statistic, F(6,7)=1.1, p<0.44). Average maternal parameters, before and after ethanol exposure, are shown in Table 1.
Table 1.
Maternal physiological indices (combined data for intra-gastric and intra-peritoneal ethanol exposure paradigms). Data are presented as Mean ± SEM
| Maternal Indices | Pre-ethanol | Post-ethanol |
|---|---|---|
| Heart Rate (Beats/Minute) | 426.5±13.95 | 442.15±13.78 |
| Respiration (Breaths/minute) | 60.81±8.74 | 66.26±5.59 |
| mVTIICA (mm3/sec) | 11.92±1.37 | 14.89±1.21 |
| mACCICA (mm/sec2) | 7134.92±597.12 | 7075.58±759.01 |
| PI | 89.17±9.96 | 73.86±11.01 |
| PRI | 0.84±0.056 | 0.86±0.025 |
Fetal vascular dynamics
Figure 3 shows sample ultrasonography traces from GD12.5 fetal UA, AA, ICA and MCA, along with baseline fetal arterial physiological measures. As shown in Figure 3e and f, the GD12.5 fetal aorta exhibited the highest acceleration fAcc and Velocity fVTI and the MCA exhibited the lowest indices of blood flow. Control fetal aortic fAcc and fVTI values were 38% and 37% of maternal aortic blood flow values. Other measures, i.e., fPI and fPRI were not altered across different arteries and therefore, the fPI and fPRI, calculated from the fetal ICA, were compared to maternal mPI and mPRI calculated from measurements made in the maternal common carotid artery. As shown in Figures 3g and h, fPI was 2.8% and fPRI 55% that of the maternal values. At GD12.5, control fetal heart rates were 150±11.18 beats/minute (these observed heart rates in GD12.5 mice are slightly lower than previously published values of 174 beats/minute for GD15.5 C57Bl/6 fetal mice (Serrano et al., 2010) and may represent developmental stage or experimental condition variations).
Impact of a single episode of maternal ethanol exposure on fetal vascular dynamics
In a mixed multivariate/repeated measures analysis, the multivariate Pillai’s trace statistic indicated that irrespective of the specific exposure paradigm, there was a statistically significant, and overall suppressive effect of ethanol exposure on vascular function (a significant “within-subjects” effect) in the fetus (F(9,4)=22.26, p< 0.005). Several lines of evidence indicate that the above effects were specifically due to ethanol exposure. Firstly, there was not a statistically significant main effect of route of ethanol exposure (intraperitoneal vs. gavage, F(9,4)=0.62, p<0.75), nor was there a significant interaction between the route of ethanol exposure and the effects of ethanol exposure itself (F(9,4)=0.36, p<0.9), suggesting that intragastric ethanol gavage did not lead to effects that were different from those obtained following intraperitoneal injection, i.e., that the two models were equivalent. Secondly, in control animals, there was no statistically significant alteration in any physiologic measure, over repeated measurements conducted over the span of one hour, separated by an intragastric gavage of saline (Multivariate Pillai’s Trace Statistic, F(3,8)=1.723, p<0.239), indicating that these measurements represent stable indices of cardiovascular function. Finally, repeated Doppler ultrasound measurement in both color and power mode, made over a period of three days did not induce fetal damage as assessed by histological analysis with either nissl (data not shown) or Fluoro-Jade stains in either heart (data not shown) or brain (Figure 4a,b), compared to non ultrasound screened, littermate controls. In contrast, endothelin-1, which causes vasoconstriction and is used as a model for ischemic stroke (Selvamani and Sohrabji, 2008; Selvamani and Sohrabji, 2010), leads to extensive cell death (Figure 4c) not only in neuronal cells (Figure 4e), but also in cells associated with the vasculature (Figure 4d).
Figure 4.
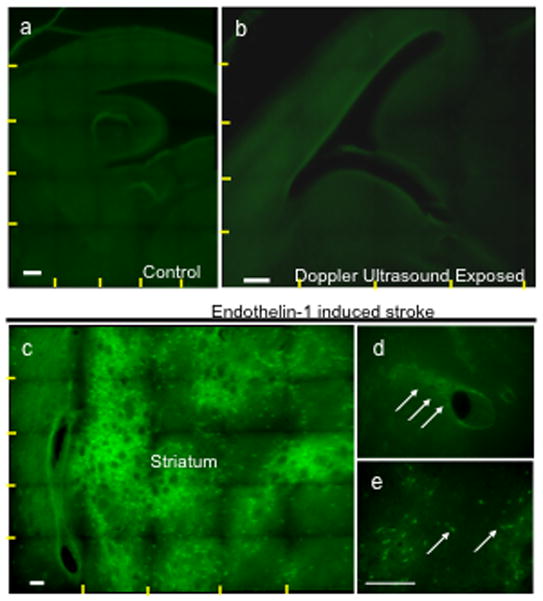
Fluoro-Jade stained sections of the fetal mouse brain show that repeated color and pulse wave Doppler ultrasound scans between GD12.5 and 14.5 do not induce cell death. (a,b) Composite photomicrographs (yellow lines show boundaries of individual images stitched together into a composite) showing saggital brain sections at the level of the lateral ventricle from control littermates (a, unexposed to ultrasound) and ultrasound (b, both color and pulse wave Doppler)-exposed fetuses showed no evidence for cell death. (c–e) Endothelin-1 induced stroke in contrast resulted in significant cell death in the striatum (c, green cellular staining) that is localized both to cells in the vicinity of blood vessels (d) and to neurons (e). Scale bar, 100um.
Univariate analyses indicated that, across both ethanol exposure methods, there was a statistically significant suppressive effect of ethanol exposure on fACC through the UA (49% reduction, F(1,12)= 8.92, p<0.011, Figure 5c), to the fetal aorta (47% reduction, F(1,12)= 10.89, p<0.006, Figure 6c), ICA (40% reduction, F(1,12)= 64.17, p<3.71E-6, Figure 7c) and MCA (40% reduction, F(1,12)= 9.37, p<0.01 Figure 8c). Similarly, there was a statistically significant reduction in fVTI through the UA (53% reduction, F(1,12)=9.22, p<0.01, Figure 5d), fetal ICA (42% reduction, F(1,24)=12.48, p<0.004, Figure 7d) and MCA (45% reduction, F(1,12)=21.58, p<0.001, Figure 8d). The reduction in fVTI through the aorta (Figure 6d) was not statistically significant. Finally, ethanol exposure did not statistically alter fPIICA, fPRIICA or fetal heart rate.
Figure 5.
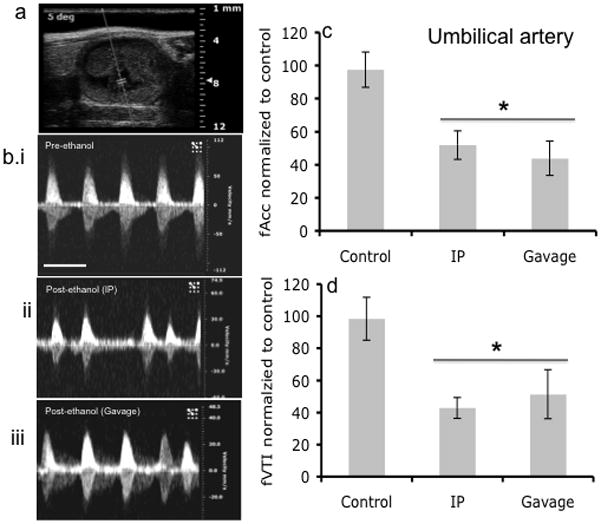
Fetal UA Doppler ultrasound measurements. (a) Doppler ultrasound image of area where waveform analysis was acquired. (b) Representative Doppler ultrasound image of fetal umbilical artery waveform before alcohol administration and after alcohol treatment via intraperitoneal (IP) injection and Gavage routes. (c,d) Maternal ethanol exposure by gavage or IP resulted in a significant decrease in acceleration and VTI (asterisk indicates statistically significant difference from baseline control). No difference was observed based on route of administration. Scale bar, 0.6 sec.
Figure 6.
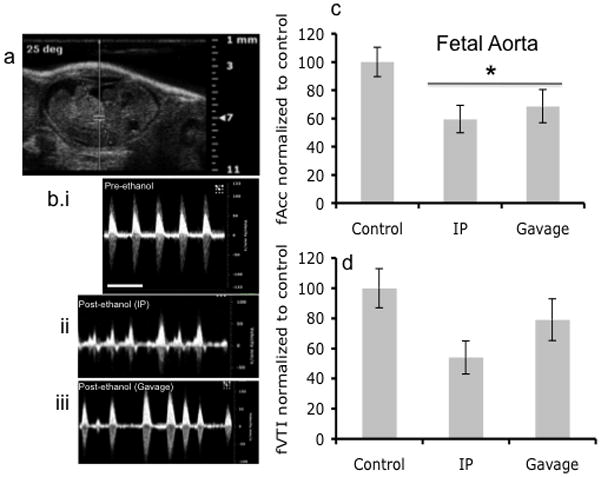
Doppler ultrasound measurement of fetal Aorta. (a) Doppler ultrasound image of fetal Aorta indicating area of analysis. (b) Doppler ultrasound waveform of fetal Aorta before ethanol treatment and after both IP and Gavage routes of administration. (c,d) Blood flow as measured by Acceleration was significantly reduced following maternal ethanol exposure (asterisk indicates statistically significant difference from baseline control), however, VTI, though exhibiting a declining trend, was not significantly decreased. No difference was observed between routes of administration. Scale bar, 0.6 sec.
Figure 7.
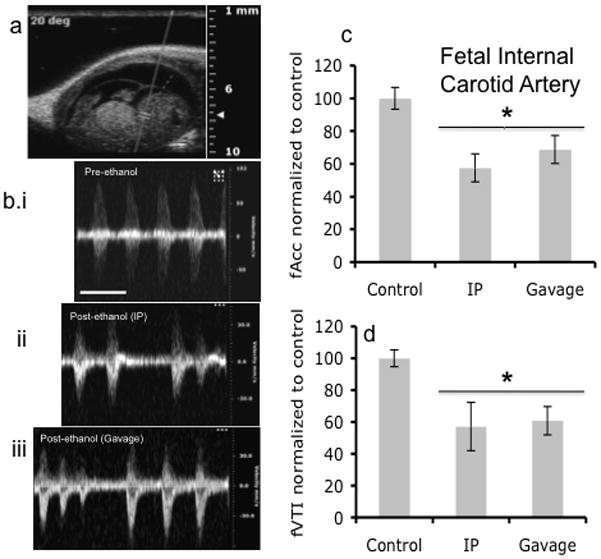
Doppler ultrasound measurements of fetal Internal Carotid Artery. (a) Fetal Doppler ultrasound image showing position marked for analysis of Internal Carotid Artery. (b) Representative Doppler ultrasound waveforms of Internal Carotid Artery before ethanol treatment and after ethanol treatment via IP and gavage routes of administration. (c,d) Maternal ethanol exposure induced a significant decline in both acceleration and VTI, with no difference observed between routes of administration (asterisk indicates statistically significant difference from baseline control). Scale bar, 0.6 sec.
Figure 8.
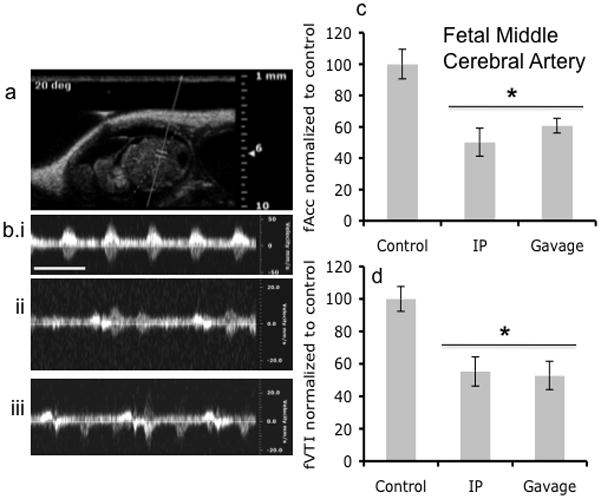
Doppler ultrasound measurements of fetal Middle Cerebral Artery. (a) Fetal Doppler ultrasound image of middle cerebral artery. (b) Doppler ultrasound recordings of the middle cerebral artery before ethanol treatment and after via intraperitoneal and Gavage routes of treatment. (c,d) Doppler ultrasound measurements showed that maternal ethanol exposure induced a significant reduction in acceleration and VTI in the middle cerebral artery, irrespective of the route of administration (asterisk indicates statistically significant difference from baseline control). Scale bar, 0.6 sec.
Qualitative assessment of ultrasonographic traces also revealed evidence for a deleterious impact of maternal ethanol exposure on fetal circulation. Following maternal exposure, all the examined fetal arteries exhibited evidence of arrhythmias with variable systole amplitude, and the appearance of secondary inter-systole contractions (Figures 5b, 6b, 7b, and 8b). While fPIICA was not altered, the pattern of pulsatile blood flow through the downstream MCA (Figure 8b) was particularly disrupted. Moreover, changes in cerebro-vascular flow could also be observed in non-carotid artery-derived vascular projections to the brain. For example, the posterior cerebral artery (PCA), also exhibited a significant loss of directional blood flow. In Figure 9 for example, a sequential series of color Doppler images of the fetal PCA following maternal ethanol exposure provides evidence for oscillatory blood flow towards (red) and away (blue) from the ultrasound transducer suggesting that ethanol exposure induced near cessation of directional blood flow through the vertebral artery and its tributaries. At a higher magnification of the fetal PCA, (Figure 9c) adjacent red and blue pixels can be observed simultaneously within the same image frame, suggesting that maternal ethanol exposure induces local vascular turbulence within the fetal PCA.
Figure 9.
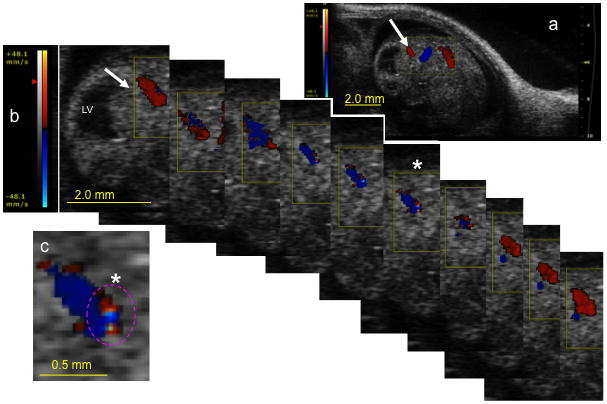
Color Doppler image series document a loss of directional blood flow in the fetal posterior cerebral artery following maternal ethanol exposure. Red color overlay indicates blood flow towards the ultrasonic transducer, whereas a blue overlay indicates flow directed away from the transducer. (a) Low magnification image showing location of the active color Doppler acquisition window (yellow box). White arrow points to the location of the posterior cerebral artery and corresponds to the image sequence in ‘b’. (b) Sequential images acquired over a single cardiac cycle show alternating blood flow towards (red) and away (blue) from the ultrasonic transducer within the same arterial cross-section. Color scale bar (left) indicates velocity (mm/sec). (c) Magnified frame from (b, asterisk) shows the simultaneous presence of adjacent red and blue pixels within the same luminal cross-section, suggesting locally turbulent blood flow. Oscillatory blood flow within the posterior cerebral artery suggests that maternal ethanol exposure can lead to fetal distress. Scale bars; a and b = 2.0mm, c = 0.5mm. Abbreviations, LV, lateral ventricle.
Persistent effects of multiple episodes of maternal ethanol exposure on fetal vascular parameters
In this next series of experiments, fetal vascular parameters (fACC, fVTI and fPIICA) were calculated for a cohort of fetuses (one fetus in the mid-position of the uterine horn of a pregnant dam) at GD12.5. The cohort was divided into two groups, one, a control group, and the second, an ethanol treated group, administered ethanol by intragastric gavage. The same fetus was re-assessed on GD13.5 and 14.5 both before and after a single bolus of ethanol on each of those days. The pre-ethanol screen on GD13.5 and 14.5 provided an assessment of the cumulative, persistent effects of previous-day ethanol exposure on vascular circulation, while the post-ethanol screen provided an assessment of the additive immediate effect of a single binge-like ethanol exposure. A repeated-measures ANOVA showed that there was no statistically significant difference between the acute and persistent effects of ethanol. Therefore, for purposes of further analysis, the data for the acute ethanol effect and the persistent effect 24 hours later were averaged for each sample, and the data subject to a mixed repeated-measures/multivariate analysis. Overall, we observed no significant persistent changes in fAcc or fPI. However, fVTI was significantly altered as a function of repeated ethanol exposure. Multivariate analysis indicated a significant main effect of ethanol exposure on fVTI across all measured fetal blood vessels (Pillai’s Trace Statistic, F(4,6)=5.92, p<0.028). There was not a significant effect of gestation age, nor was there an interaction between gestational age and ethanol exposure. Post-hoc ANOVA for the “between-subjects” effects indicated that fVTI was altered in individual components of the fetal vascular system as well (UA, F(1,9)=19.67, p<0.002; ascending aorta, F(1,9)=24.35, p<0.001; ICA, F(1,9)=11.24, p<0.008; MCA, F(1,9)=8.89, p<0.015). Therefore, ethanol exposure resulted in a statistically significant acute and persistent decrease in fVTI compared to controls (Figure 10), but acute maternal ethanol exposure at GD14.5 and 15.5 did not lead to an additional decline compared to the persistent effects of ethanol administered 24 hours previously. In contrast, a second cohort of pregnant dams that were tested repeatedly, on GD12.5, 13.5 and 14.5, before and after a single bolus of saline exhibited no significant main effect of saline exposure on fVTI following saline exposure (Pillai’s Trace Statistic, F(4,19)=1.16, p<0.36, data not significant (NS)), nor was there an interaction between gestational age and saline exposure (Pillai’s trace statistic, F(8,40)=0.39, p<0.92, NS). Interestingly, in the saline control experiments, there was a significant effect of gestational age on fVTI (Pillai’s trace statistic, F(8,40)=2.79, p<0.015) due principally to a gestational age-related and significant increase in fVTI in the umbilical artery (F(2,22)=16.74, p<0.000038). In contrast, in our ethanol- exposure experiments, the main effect of gestational age on umbilical artery fVTI did not rise to the level of statistical significance because of the magnitude of the ethanol-related decrease in fVTI, though a visual inspection of the control group in that experiment series (Figure 10) also shows a general increase in umbilical artery fVTI with gestational age.
Figure 10.
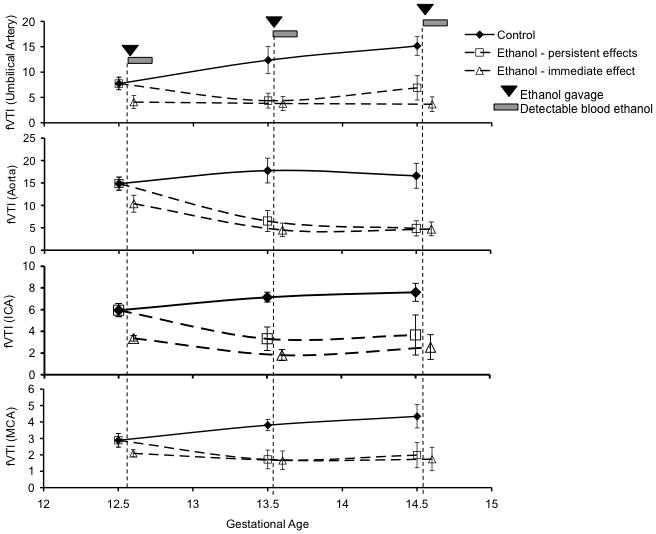
Repeated maternal ethanol exposure results in persistent suppression of fVTI in fetal blood vessels. Pregnant mice were exposed to ethanol (3g/kg) once a day by intragastric gavage for three consecutive days (GD12.5–14.5) and were imaged daily before and following maternal ethanol exposure to detect changes in fetal blood flow. Shown from top to bottom are graphical representations of changes in fVTI in fetal umbilical artery, aorta, internal carotid artery and middle cerebral artery. A persistent fetal effect is defined as one that can be observed 24 hours after an episode of maternal ethanol exposure (i.e., an effect observed at GD13.5 and 14.5 due to exposures on GD12.5 and 13.5), while the acute effect is defined as the additional effect of a maternal binge-like exposure episode on that day, in the period immediately preceding the ultrasound scan. In all vessels, we observed a main effect of ethanol exposure, but no interaction between gestational age and ethanol exposure on fVTI. Visual inspection of the data show that maternal ethanol exposure on GD12.5 induced a persistent decrease in fVTI 24 hours later, and this decrease in fVTI persisted with repeated maternal ethanol exposure, though the magnitude of the decline did not increase with subsequent exposures.
Evidence for protective adaptation of cerebrovascular circulation to repeated ethanol exposure
Since fVTI was a sensitive measure of the persistent effects of ethanol exposure, we measured the drop in fVTI from the aorta and UA to the MCA as an index of cerebrovascular adaptation. At GD12.5, the fVTI at the MCA is 19.45±2.9% of the fVTI measured at the aorta, representing an ~80% drop in fVTI from the heart to the brain. We calculated the ratio of the differences in fVTIMCI and fVTIAA (ΔfVTIMCA-AA) between fetal aorta and MCA measured after ethanol exposure and in control fetuses (ΔΔfVTIMCA-AA = ΔfVTIMCA-AAEthanol exposure/ΔfVTIMCA-AAControl). A ratio of ΔΔfVTIMCA-AA < 1 indicates the presence of compensatory mechanisms that prevent cerebral vascular VTI from declining at a rate comparable to aortic VTI. Repeated daily binge-like maternal ethanol exposure by intragastric gavage significantly decreased ΔΔfVTIMCA-AA (F(2,44)=12.25, p<0.00006). We observed a 33% decline in the ΔΔfVTIMCA-AA ratio following the initial exposure to ethanol, and a drop of up to 76% following repeated ethanol exposure (Figure 11). A similarly calculated ratio of the drop in fVTI from the UA to the MCA (ΔΔfVTIMCA-UA) also exhibited a statistically significant decline (F(2,44)=9.82, p<0.0003), providing additional supporting evidence for a fetal brain sparing adaptation to maternal ethanol exposure, in this case, relative to placental- directed circulation.
Figure 11.
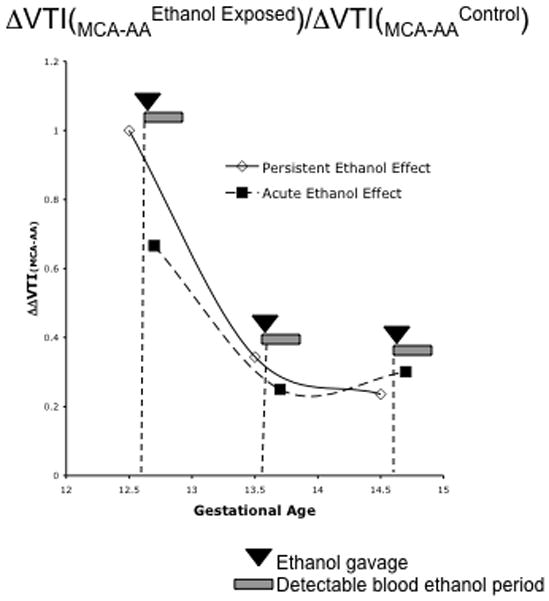
Analysis of the ratio of the decrease in fVTI from the fetal aorta to the fetal middle cerebral artery before and following maternal ethanol exposure (ΔΔfVTI(MCA-AA)) reveals evidence for an adaptive fetal ‘brain-sparing’ response to maternal ethanol exposure. Repeated ethanol exposure resulted in both an acute and a persistent (i.e., an effect observed at GD13.5 and 14.5 due to exposures on GD12.5 and 13.5) drop in the ΔΔfVTI(MCA-AA) ratio suggesting that the decline in fVTI at the MCA is less that predicted from the drop in fVTI at the aorta.
Discussion
The second trimester is a critical period for neuron (Bayer et al., 1993) and blood vessel (Norman and O’Kusky, 1986) formation in the fetal brain. Data from the present study demonstrate that a single second trimester-equivalent maternal binge-like episode of ethanol exposure on GD12.5, significantly and rapidly reduced fetal brain-directed arterial blood flow, irrespective of route of exposure. Following ethanol exposure, fAcc declined significantly in every fetal artery studied, and fVTI declined significantly in every artery except the aorta, while fPIICA and fPRIICA remained un-changed. Importantly, declines in fAcc and fVTI were not accompanied by any change in fetal heart rate, suggesting that maternal ethanol does not disrupt fetal neuromuscular control of the timing of the cardiac contraction cycle. In contrast to our observations of second trimester-equivalent ethanol exposure, a recent study of fetal ethanol exposure during the first trimester-equivalent period (GD6.75) showed that there was a persistent increase in murine fPI and fetal heart rate that could be observed into the mid-second trimester-equivalent period (Serrano et al., 2010). It is likely that developmental stage is an important determinant of fetal responses to ethanol. The first trimester-equivalent period for example, characterized by the initial appearance of a heartbeat at GD6.75 (Ji et al., 2003), coincides more severe ethanol-induced defects (Daft et al., 1986; Serrano et al., 2010).
The decline in fVTI within the UA, ICA and MCA is potentially due to vasodilation in the microvasculature distal to these arteries, a phenomenon that has been previously described as a consequence of ethanol exposure during both the second (Mayock et al., 2008) and third (Parnell et al., 2007) trimester-equivalent periods. Importantly, fVTI was persistently suppressed for 24 hours following a single binge-like episode of ethanol exposure, and a similar magnitude of suppression was observed after repeated daily binge ethanol exposure episodes between GD12.5 and 14.5. The lack of an additional decline in fVTI following repeated daily binge episodes on GD13.5 and GD14.5 suggests that the second trimester-equivalent fetal vascular system rapidly adapts to a changed maternal-fetal environment. Alternatively, the period encompassing GD12.5 may constitute a specific window of structural vulnerability for the maturing vascular system, where agents like ethanol may permanently disrupt blood vessel formation.
Qualitative analysis of fetal blood flow following maternal ethanol exposure yielded additional evidence for fetal vascular stress. Visual inspection of ultrasonography recordings revealed evidence for variability in the inter-systolic interval and variability in the peak amplitude of the cardiac ejection volume. Furthermore, disruption in cerebro-vascular blood flow was not limited to the cerebral projections from the ICA, but could also be observed in the posterior cerebral artery, which arises from the vertebral arterial system. We observed that maternal ethanol exposure in some cases resulted in near complete loss of pulsatile, directional blood flow in fetal cerebral arteries, replaced instead by oscillatory blood movement with local foci of turbulence. This pattern, suggestive of a phenomenon termed ‘end-diastolic flow reversal’, is associated in the clinical literature with intrauterine growth retardation (IUGR, (Marsal, 2009)). However, the appearance of this phenomenon following a single dose of ethanol, suggests that flow reversal may well be an early component of the etiology of maternal ethanol-induced IUGR.
We also observed that ethanol did not alter maternal brain-directed blood flow while significantly disrupting fetal brain-directed blood flow. While these data perhaps reflect a greater sensitivity of small, immature fetal vessels relative to large, mature maternal vessels, they do suggest that, in the presence of an external stressor, maternal physiology is preserved at the expense of fetal cardiovascular function. It is important to determine the extent to which this preferential maintenance of maternal circulation over fetal circulation in a multiparous, short-gestation animal like the mouse is relevant to human species-specific strategies for survival and reproductive success. However, in another uniparous, long-gestation species, the sheep, second trimester ethanol was shown to alter fetal cerebral vasodilator responses to hypoxia (Mayock et al., 2007) and acidosis (Mayock et al., 2008) without a change in maternal blood flow (Mayock et al., 2007), suggesting that mouse data has relevance to human maternal-fetal physiology.
Our interpretation of data is subject to an important caveat, i.e., that we used isoflurane anesthesia to acquire fetal ultrasound data. While consistent with previous experimental approaches (Yu et al., 2008), changes in fVTI and fAcc could well reflect an interaction between anesthesia and maternal ethanol exposure. Our data indicate that maternal heart rate was maintained within the normal range for the mouse during anesthesia, and that ethanol did not alter maternal heart rate, suggesting that the potential for interaction between anesthesia and ethanol was minimal. Secondly, though we assessed fetal physiology at peak maternal BEC for both intragastric and intraperitoneal ethanol exposure, these data may not be strictly comparable because differences in time to peak BEC under the two exposure conditions. However, the lack of difference in fVTI and fACC between the two ethanol delivery methods would argue for equivalency between routes of exposure.
Ethanol’s effects on fetal vasculature during the second trimester are likely to have enduring consequences for brain development because of temporal convergence between cardiac, brain and neurovascular development. Cardiac outflow valve development and active blood flow in the fetal heart is initiated around GD12.5, while cardiac atrial-ventricular flow does not mature until gestational day 14.5 (Yu et al., 2008), nearly the middle of the second trimester equivalent period. Consequently, blood flow from the placenta to the brain may be particularly susceptible to disturbances in the fetal environment.
The murine brain simultaneously initiates neurogenesis during the period of cardiac outflow maturation. Most murine cerebral cortical neurons are born during an eleven-cycle neurogenic window spanning GD11–GD16 (Caviness et al., 2003). Therefore, a 24-hour suppression of fVTI in the MCA following a single maternal binge ethanol exposure, spans 27% of the neurogenic mitotic cycles in the developing murine cortex. This is important because recent evidence (Simamura et al., 2010) shows that maternal-fetal endocrine signals control normal fetal neurogenesis via the fetal vascular system, which is disrupted by ethanol exposure.
The fetal brain also undergoes extensive vasculogenesis during the second trimester (Norman and O’Kusky, 1986). Differentiating neural stem cells secrete VEGF (Camarillo et al., 2007), which stimulates angiogenesis (Hogan et al., 2004). However, VEGF initially induces the formation of leaky, fenestrated-type capillaries (Bates, 2010). Therefore, decreased fVTI, may result in edema from dilated leaky vessels and subsequent fetal brain damage. Moreover invading vascular-derived macrophages, initiate anastomosis of VEGF-stimulated endothelial cell sprouts (Fantin et al., 2010). Ethanol decreases neural production of macrophage chemotactic factors (Camarillo et al., 2007), and membrane expression of cognate receptors on macrophages (Joshi et al., 2005), and may well delay the initiation of active blood flow within the fetal brain. Finally, maternal choline deficiency also decreases blood vessel formation in the developing fetal hippocampus (Mehedint et al., 2010) and presumably, restriction in delivery of choline to the fetal brain due to alterations in blood flow, may have adverse consequences for brain vasculogenesis.
We observed evidence for an adaptive fetal cerebral vascular response to repeated maternal ethanol exposure, in that the decline in fVTI at the fetal MCA was less than that which would be predicted from the drop at the aorta and umbilical arteries. These data suggest that fetal cerebral blood flow rapidly adapts to favor core developing end organs like the brain. This phenomenon, previously termed the “brain sparing effect” is hypothesized to mitigate the effects of adverse changes in the maternal-fetal environment. However, a recent epidemiology study reported that preferential redistribution of fetal circulation to the brain was associated with increased attention and emotional behavior problems in toddlers (Roza et al., 2008). These data collectively suggest that redistribution of fetal blood flow following maternal ethanol exposure may ultimately be maladaptive.
Collectively, our data show that single and repeated binge episodes of maternal ethanol exposure during the critical second trimester window have both a rapid and persistent impact on fetal cranially-directed circulation at doses that have no effect on maternal brain-directed circulation. While these data imply a need to uncover mechanisms underlying fetal vascular vulnerability, they also suggest that fetal blood flow analyses have diagnostic value. High-resolution fetal ultrasound technology has recently emerged as a promising new diagnostic tool for the early detection of fetal defects including fetal growth restriction and anemias (reviewed in (Degani, 2009)). Fetal ultrasound screening for bone and brain growth recently yielded morphometric evidence for in utero ethanol exposure in human populations (Kfir et al., 2009) and ultrasound measurements in children exposed to ethanol in utero, yielded evidence for persistent increases in arterial stiffness (Morley et al., 2010). Therefore, high-resolution ultrasound measurements of fetal blood flow, particularly cerebro-vascular blood flow, may prove to be an important addition to the arsenal of newly emerging ultrasound diagnostic biomarkers for fetal ethanol exposure.
Acknowledgments
We would like to thank Drs. Wei-Jung Chen and Gerard Toussaint for assistance with anatomical orientation, Dr. Gregg Wells for assistance with fetal cardiovascular physiology, Dr. Farida Sohrabji for the gift of tissues for an endothelin-1 model for stroke, Dr. Timothy Cudd for a critical review of the manuscript, Shruti Sharma, Matthew Schilling and Julia Quintana for assistance with animal care and data management. This research was supported by a NIH grant, NIAAA R01AA013440, and by funds from Texas A&M Health Science Center to RCM.
Abbreviations
- AA
ascending aorta
- ACA
anterior cerebral artery
- ICA
internal carotid artery
- IUGR
intrauterine growth restriction
- LV
lateral ventricle
- MCA
middle cerebral artery
- mAcc/fAcc
maternal/fetal arterial acceleration
- mPI/fPI
maternal/fetal pulsatility index
- mPRI/fPRI
maternal/fetal Pourcelot resistive index
- mVTI/fVTI
maternal/fetal arterial velocity time integral
- PCA
posterior cerebral artery
- UA
umbilical artery
References
- Abel EL. Prenatal effects of alcohol. Drug Alcohol Depend. 1984;14(1):1–10. doi: 10.1016/0376-8716(84)90012-7. [DOI] [PubMed] [Google Scholar]
- Abel EL. An update on incidence of FAS: FAS is not an equal opportunity birth defect. Neurotoxicol Teratol. 1995;17(4):437–43. doi: 10.1016/0892-0362(95)00005-c. [DOI] [PubMed] [Google Scholar]
- Bates DO. Vascular endothelial growth factors and vascular permeability. Cardiovasc Res. 2010;87(2):262–71. doi: 10.1093/cvr/cvq105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer S, Altman J, Russo R, Zhang X. Timetables of neurogenesis in the human brain based on experimentally determined patterns in the rat. Neuro Toxicology. 1993;14(1):83–144. [PubMed] [Google Scholar]
- Bearer CF. L1 cell adhesion molecule signal cascades: targets for ethanol developmental neurotoxicity. Neurotoxicology. 2001;22(5):625–33. doi: 10.1016/s0161-813x(01)00034-1. [DOI] [PubMed] [Google Scholar]
- Camarillo C, Kumar LS, Bake S, Sohrabji F, Miranda RC. Ethanol regulates angiogenic cytokines during neural development: evidence from an in vitro model of mitogen-withdrawal-induced cerebral cortical neuroepithelial differentiation. Alcohol Clin Exp Res. 2007;31(2):324–35. doi: 10.1111/j.1530-0277.2006.00308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camarillo C, Miranda RC. Ethanol exposure during neurogenesis induces persistent effects on neural maturation: evidence from an ex vivo model of fetal cerebral cortical neuroepithelial progenitor maturation. Gene Expr. 2008;14(3):159–71. [PMC free article] [PubMed] [Google Scholar]
- Carta M, Mameli M, Valenzuela CF. Alcohol potently modulates climbing fiber-->Purkinje neuron synapses: role of metabotropic glutamate receptors. J Neurosci. 2006;26(7):1906–12. doi: 10.1523/JNEUROSCI.4430-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caviness VS, Jr, Goto T, Tarui T, Takahashi T, Bhide PG, Nowakowski RS. Cell output, cell cycle duration and neuronal specification: a model of integrated mechanisms of the neocortical proliferative process. Cereb Cortex. 2003;13(6):592–8. doi: 10.1093/cercor/13.6.592. [DOI] [PubMed] [Google Scholar]
- Chang CH, Chang FM, Yu CH, Liang RI, Ko HC, Chen HY. Systemic assessment of fetal hemodynamics by Doppler ultrasound. Ultrasound Med Biol. 2000;26(5):777–85. doi: 10.1016/s0301-5629(00)00207-6. [DOI] [PubMed] [Google Scholar]
- Daft PA, Johnston MC, Sulik KK. Abnormal heart and great vessel development following acute ethanol exposure in mice. Teratology. 1986;33(1):93–104. doi: 10.1002/tera.1420330112. [DOI] [PubMed] [Google Scholar]
- Degani S. Evaluation of fetal cerebrovascular circulation and brain development: the role of ultrasound and Doppler. Semin Perinatol. 2009;33(4):259–69. doi: 10.1053/j.semperi.2009.04.004. [DOI] [PubMed] [Google Scholar]
- Fantin A, Vieira JM, Gestri G, Denti L, Schwarz Q, Prykhozhij S, Peri F, Wilson SW, Ruhrberg C. Tissue macrophages act as cellular chaperones for vascular anastomosis downstream of VEGF-mediated endothelial tip cell induction. Blood. 2010;116(5):829–40. doi: 10.1182/blood-2009-12-257832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowden AL, Forhead AJ. Endocrine regulation of feto-placental growth. Horm Res. 2009;72(5):257–65. doi: 10.1159/000245927. [DOI] [PubMed] [Google Scholar]
- Goodlett CR, Horn KH. Mechanisms of alcohol-induced damage to the developing nervous system. Alcohol Res Health. 2001;25(3):175–84. [PMC free article] [PubMed] [Google Scholar]
- Hogan KA, Ambler CA, Chapman DL, Bautch VL. The neural tube patterns vessels developmentally using the VEGF signaling pathway. Development. 2004;131(7):1503–13. doi: 10.1242/dev.01039. [DOI] [PubMed] [Google Scholar]
- Ji RP, Phoon CK, Aristizabal O, McGrath KE, Palis J, Turnbull DH. Onset of cardiac function during early mouse embryogenesis coincides with entry of primitive erythroblasts into the embryo proper. Circ Res. 2003;92(2):133–5. doi: 10.1161/01.res.0000056532.18710.c0. [DOI] [PubMed] [Google Scholar]
- Jones KL, Smith DW, Ulleland CN, Streissguth P. Pattern of malformation in offspring of chronic alcoholic mothers. Lancet. 1973;1(7815):1267–71. doi: 10.1016/s0140-6736(73)91291-9. [DOI] [PubMed] [Google Scholar]
- Joshi PC, Applewhite L, Ritzenthaler JD, Roman J, Fernandez AL, Eaton DC, Brown LA, Guidot DM. Chronic ethanol ingestion in rats decreases granulocyte-macrophage colony-stimulating factor receptor expression and downstream signaling in the alveolar macrophage. J Immunol. 2005;175(10):6837–45. doi: 10.4049/jimmunol.175.10.6837. [DOI] [PubMed] [Google Scholar]
- Kfir M, Yevtushok L, Onishchenko S, Wertelecki W, Bakhireva L, Chambers CD, Jones KL, Hull AD. Can prenatal ultrasound detect the effects of in-utero alcohol exposure? A pilot study. Ultrasound Obstet Gynecol. 2009;33(6):683–9. doi: 10.1002/uog.6379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier SE, West JR. Drinking patterns and alcohol-related birth defects. Alcohol Res Health. 2001;25(3):168–74. [PMC free article] [PubMed] [Google Scholar]
- Marsal K. Obstetric management of intrauterine growth restriction. Best Pract Res Clin Obstet Gynaecol. 2009;23(6):857–70. doi: 10.1016/j.bpobgyn.2009.08.011. [DOI] [PubMed] [Google Scholar]
- May PA, Gossage JP, Kalberg WO, Robinson LK, Buckley D, Manning M, Hoyme HE. Prevalence and epidemiologic characteristics of FASD from various research methods with an emphasis on recent in-school studies. Dev Disabil Res Rev. 2009;15(3):176–92. doi: 10.1002/ddrr.68. [DOI] [PubMed] [Google Scholar]
- Mayock DE, Ness D, Mondares RL, Gleason CA. Binge alcohol exposure in the second trimester attenuates fetal cerebral blood flow response to hypoxia. J Appl Physiol. 2007;102(3):972–7. doi: 10.1152/japplphysiol.00956.2006. [DOI] [PubMed] [Google Scholar]
- Mayock DE, Ngai AC, Mondares RL, Gleason CA. Effects of binge alcohol exposure in the second trimester on intracerebral arteriolar function in third trimester fetal sheep. Brain Res. 2008;1226:111–5. doi: 10.1016/j.brainres.2008.05.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehedint MG, Craciunescu CN, Zeisel SH. Maternal dietary choline deficiency alters angiogenesis in fetal mouse hippocampus. Proc Natl Acad Sci U S A. 2010;107(29):12834–9. doi: 10.1073/pnas.0914328107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley R, Dwyer T, Hynes KL, Cochrane J, Ponsonby AL, Parkington HC, Carlin JB. Maternal alcohol intake and offspring pulse wave velocity. Neonatology. 2010;97(3):204–11. doi: 10.1159/000252973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngai AC, Mondares RL, Mayock DE, Gleason CA. Fetal alcohol exposure alters cerebrovascular reactivity to vasoactive intestinal peptide in adult sheep. Neonatology. 2008;93(1):45–51. doi: 10.1159/000105524. [DOI] [PubMed] [Google Scholar]
- Norman MG, O’Kusky JR. The growth and development of microvasculature in human cerebral cortex. J Neuropathol Exp Neurol. 1986;45(3):222–32. [PubMed] [Google Scholar]
- Parnell SE, Ramadoss J, Delp MD, Ramsey MW, Chen WJ, West JR, Cudd TA. Chronic ethanol increases fetal cerebral blood flow specific to the ethanol-sensitive cerebellum under normoxaemic, hypercapnic and acidaemic conditions: ovine model. Exp Physiol. 2007;92(5):933–43. doi: 10.1113/expphysiol.2007.038091. [DOI] [PubMed] [Google Scholar]
- Phoon CK, Aristizabal O, Turnbull DH. 40 MHz Doppler characterization of umbilical and dorsal aortic blood flow in the early mouse embryo. Ultrasound Med Biol. 2000;26(8):1275–83. doi: 10.1016/s0301-5629(00)00278-7. [DOI] [PubMed] [Google Scholar]
- Phoon CK, Turnbull DH. Ultrasound biomicroscopy-Doppler in mouse cardiovascular development. Physiol Genomics. 2003;14(1):3–15. doi: 10.1152/physiolgenomics.00008.2003. [DOI] [PubMed] [Google Scholar]
- Prock TL, Miranda RC. Embryonic cerebral cortical progenitors are resistant to apoptosis, but increase expression of suicide receptor DISC-complex genes and suppress autophagy following ethanol exposure. Alcohol Clin Exp Res. 2007;31(4):694–703. doi: 10.1111/j.1530-0277.2007.00354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roza SJ, Steegers EA, Verburg BO, Jaddoe VW, Moll HA, Hofman A, Verhulst FC, Tiemeier H. What is spared by fetal brain-sparing? Fetal circulatory redistribution and behavioral problems in the general population. Am J Epidemiol. 2008;168(10):1145–52. doi: 10.1093/aje/kwn233. [DOI] [PubMed] [Google Scholar]
- SAMHSA. The NSDUH Report: Substance Use among Women During Pregnancy and Following Childbirth, vol NSDUH09-0521. Substance Abuse and Mental Health Services Administration, Office of Applied Studies; Rockville, MD: 2009. [Google Scholar]
- Santillano DR, Kumar LS, Prock TL, Camarillo C, Tingling JD, Miranda RC. Ethanol induces cell-cycle activity and reduces stem cell diversity to alter both regenerative capacity and differentiation potential of cerebral cortical neuroepithelial precursors. BMC Neurosci. 2005;6:59. doi: 10.1186/1471-2202-6-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvamani A, Sohrabji F. Reproductive age modulates the impact of focal ischemia on the forebrain as well as the effects of estrogen treatment in female rats. Neurobiol Aging. 2008;31(9):1618–28. doi: 10.1016/j.neurobiolaging.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvamani A, Sohrabji F. The neurotoxic effects of estrogen on ischemic stroke in older female rats is associated with age-dependent loss of insulin-like growth factor-1. J Neurosci. 2010;30(20):6852–61. doi: 10.1523/JNEUROSCI.0761-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano M, Han M, Brinez P, Linask KK. Fetal alcohol syndrome: cardiac birth defects in mice and prevention with folate. Am J Obstet Gynecol. 2010;203(1):75 e7–75 e15. doi: 10.1016/j.ajog.2010.03.017. [DOI] [PubMed] [Google Scholar]
- Simamura E, Shimada H, Higashi N, Uchishiba M, Otani H, Hatta T. Maternal leukemia inhibitory factor (LIF) promotes fetal neurogenesis via a LIF-ACTH-LIF signaling relay pathway. Endocrinology. 2010;151(4):1853–62. doi: 10.1210/en.2009-0985. [DOI] [PubMed] [Google Scholar]
- Tam SJ, Watts RJ. Connecting vascular and nervous system development: angiogenesis and the blood-brain barrier. Annu Rev Neurosci. 2010;33:379–408. doi: 10.1146/annurev-neuro-060909-152829. [DOI] [PubMed] [Google Scholar]
- Vangipuram SD, Grever WE, Parker GC, Lyman WD. Ethanol increases fetal human neurosphere size and alters adhesion molecule gene expression. Alcohol Clin Exp Res. 2008;32(2):339–47. doi: 10.1111/j.1530-0277.2007.00568.x. [DOI] [PubMed] [Google Scholar]
- West JR, Parnell SE, Chen WJ, Cudd TA. Alcohol-mediated Purkinje cell loss in the absence of hypoxemia during the third trimester in an ovine model system. Alcohol Clin Exp Res. 2001;25(7):1051–7. [PubMed] [Google Scholar]
- Yu Q, Leatherbury L, Tian X, Lo CW. Cardiovascular assessment of fetal mice by in utero echocardiography. Ultrasound Med Biol. 2008;34(5):741–52.4. doi: 10.1016/j.ultrasmedbio.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]


