Abstract
Background
Calcium and vitamin D may be inversely related to breast cancer risk, in part by affecting mammographic density. However, results from previous, mostly cross-sectional studies have been mixed, and there have been few randomized clinical trials of the effect of calcium and vitamin D supplementation on change in mammographic density.
Methods
We assessed the effect of one year of supplementation on mammographic density in 330 postmenopausal women enrolled in the Women’s Health Initiative Hormone Therapy (HT) and Calcium and Vitamin D (CaD) trials. Women were randomized to receive 1000 mg/day of elemental calcium carbonate plus 400 IU/day of vitamin D3 or placebo.
Results
After approximately one year, mammographic density decreased 2% in the CaD supplementation group and increased 1% in the placebo group (ratio of means = 0.97; 95% confidence interval (CI) = 0.81–1.17). Results suggested potential interaction by HT use (P = 0.08). Among women randomized to HT placebo, the ratio of mean density comparing CaD supplementation and placebo groups was 0.82 (95%CI = 0.61–1.11) vs. 1.16 (95%CI = 0.92–1.45) in women randomized to active HT. In sensitivity analyses limited to women taking ≥80% of study supplements, ratios were 0.67 (95%CI = 0.41–1.07) in women not assigned to HT and 1.07 (95%CI = 0.79–1.47) women assigned to HT.
Conclusions
We observed no overall effect of vitamin D and calcium supplementation on mammographic density after one year.
Impact
Potential interaction between these nutrients and estrogen as related to mammographic density warrants further study.
Keywords: breast, calcium, clinical trial, mammography, vitamin D
Introduction
Numerous studies have assessed whether vitamin D and calcium may be related to the risk of breast cancer.(1, 2) Results from prospective studies of dietary intake of these nutrients and/or blood levels of the main circulating vitamin D metabolite, 25-hydroxyvitaminD (25OHD), and breast cancer risk have been inconsistent. While some have observed inverse associations,(3-5) others including the Women’s Health Initiative (WHI) Calcium and Vitamin D Supplementation Trial have reported null findings(6-8), or suggest that the association may vary by menopausal status, age, tumor hormone receptor status, and other hormone-related factors.(9, 10) Vitamin D and calcium may affect breast cancer risk in part by reducing mammographic density, a strong predictor of breast cancer risk.(11, 12) Numerous in vitro studies have indicated that 1,25-dihydroxyvitamin D, the biologically active vitamin D metabolite, can inhibit cellular proliferation and promote differentiation in normal breast tissue as well as tumor tissue.(13). Vitamin D status may thus be associated with lower mammographic density and consequently, lower breast cancer risk. In addition, calcium intake may also influence mammographic density by regulating cell differentiation and proliferation independently of vitamin D.(14)
Previous studies evaluating the relation between calcium, vitamin D and mammographic density have been largely cross-sectional and results have been mixed.(15-28) Few prospective studies of these association have been conducted (29, 30) and have not supported strong associations between vitamin D and calcium intake in childhood (30) or adulthood (29, 30) and mammographic density at midlife. Because results from these observational studies may be subject to residual confounding by other dietary and lifestyle factors affecting density that are also correlated with vitamin D intake, it is important to test these associations in randomized clinical trials. We thus examined the effect of 1 year of supplementation with 400 IU/day of vitamin D along with 1000 mg/day of elemental calcium carbonate compared to placebo on breast density in a subset of postmenopausal women enrolled in the WHI Calcium and Vitamin D trial who were concurrently enrolled in the hormone therapy trials.
Methods
The WHI Hormone Therapy Trials
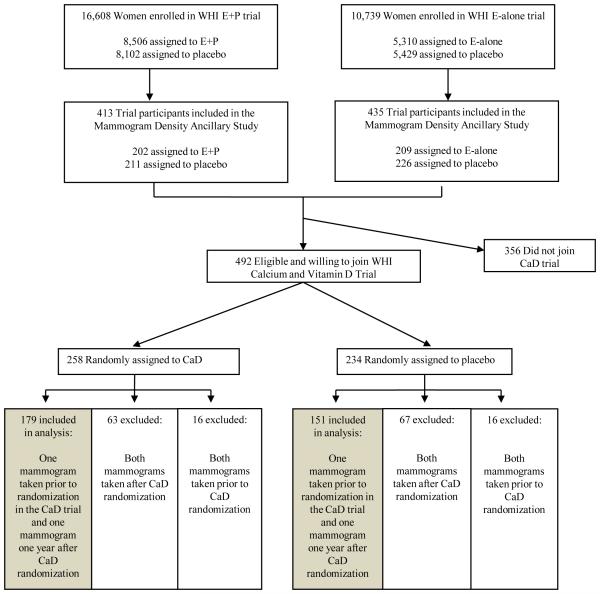
Establishment of the WHI HT trials has been described previously.(31-33) Briefly, between 1993 and 1998, postmenopausal women 50-79 years of age were recruited through direct mailing campaigns and media awareness programs. Recruitment was conducted at 40 clinical centers throughout the US. Major ineligibility criteria included previous history of breast cancer, history of other cancers (other than non-melanoma skin cancer) within the previous 10 years, medical conditions likely to result in death within 3 years, and conditions likely to interfere with retention in the study. Ultimately, 16,608 women who had not had a hysterectomy were randomized to 0.625 mg conjugated equine estrogen plus 2.5 mg medroxyprogesterone acetate per day in a single table or a similar placebo (E+P trial; Figure 1). An additional 10,739 women without a uterus were randomized to 0.625 mg conjugated equine estrogen daily or a similar placebo (E-alone trial; Figure 1). At baseline enrollment visits prior to randomization, participants completed questionnaires that assessed a variety of demographic, reproductive, behavioral and health factors. Participants were required to have had a screening mammogram within 6 months prior to randomization or were referred for a screening mammogram before they were randomized.
Figure 1. Selection of participants for assessment of calcium and vitamin D assessment and mammographic density in the Women’s Health Initiative Hormone Therapy and Calcium and Vitamin D Trials.
E+P = conjugated equine estrogen plus medroxyprogesterone acetate, E-alone = conjugated equine estrogen, HT = hormone therapy, CaD = calcium with vitamin D, and MPD = mammographic percent density.
WHI Calcium and Vitamin D Trial
The WHI Calcium and Vitamin D (CaD) trial included 36,282 women previously enrolled in the HT and/or Dietary Modification (DM) HT trials, and has been described in detail previously.(34) Eligible HT and DM trial participants were invited to join the CaD trial at their first or second annual follow-up clinic visit. More than 95% of HT participants joining the CaD trial did so at their first HT follow-up visit.
As part of the CaD trial, participants were randomized to receive a combined calcium plus vitamin D supplement or an identical-appearing placebo using a permuted block algorithm (Figure 1). Participants were asked to take two pills per day (each containing 500 mg of elemental calcium as calcium carbonate and 200 IU of vitamin D3), for a total daily dose of 1000 mg of elemental calcium and 400 IU of vitamin D3. Women were allowed to continue personal use of calcium and vitamin D supplements, with initial cutoffs of 1000 mg/day for calcium and 600 IU/day for vitamin D (later increased to 1000 IU/day during the trial). Supplementation was terminated if women reported kidney stones, kidney dialysis, hypercalcemia, calcitriol use, or personal use of vitamin D supplements at dosages higher than 600 IU/day (later 1000 IU/day). Participants were contacted 4 weeks post CaD randomization and then twice per year to assess safety, adherence, and clinical outcomes. Adherence in the trials was defined as taking 80% or more of study medication. In the first 3 years of follow-up, adherence ranged from 60-63% and an additional 13-21% of participants took at least half of their medications (1 of 2 pills per day on average).(6) In a sub-study conducted among 448 CaD trial participants, after 2 years of supplementation 25OHD levels among women assigned to calcium and vitamin D were 28% higher than those assigned to placebo.(35) Of note, no biologic correlate of calcium intake is available for similar analyses of calcium status.
The study protocol was approved by institutional review boards at each participating institution and registered at clinicaltrials.gov (NCT00000611). An independent data and safety monitoring board reviewed all clinical outcomes for the study.
Study population
The present analysis includes members of the HT and CaD trials who also enrolled in the Mammogram Density Ancillary Study of the HT trials. This ancillary study was designed to evaluate the effect of postmenopausal hormones on mammographic density and has been described in detail previously. (36, 37) Briefly, women who had a mammogram taken prior to HT randomization and at least one follow-up mammogram 1-2 years after HT randomization were considered eligible and were selected for inclusion in this sub-study using a stratified random sampling protocol.
Among E+P trial participants selected for the random sample, 214 of the 233 women assigned to E+P and 223 of the 240 women assigned to placebo agreed to join in the Mammogram Density Ancillary Study. Complete mammogram data showing no evidence of invasive breast cancer were received from 202 women assigned to E+P and 211 assigned to placebo. Among E-alone trial participants selected for the random sample, 220 of the 234 women assigned to E-alone and 238 of the 264 women assigned to placebo agreed to join the Mammogram Density Ancillary Study. Complete mammogram data showing no evidence of invasive breast cancer were received from 209 women assigned to E-alone and 226 assigned to placebo. Thus, the parent Mammogram Density Ancillary Study included a total of 848 participants (Figure 1). Of these 848 women, 492 were eligible and enrolled in the CaD trial, 258 of whom were randomized to calcium and vitamin D supplementation and 234 of whom were randomized to placebo.
For this analysis, we limited inclusion to women who had a first mammogram prior to CaD randomization (i.e., “CaD baseline”) and a second mammogram approximately one year after randomization (i.e., “CaD follow-up”). This included 179 of the 258 (69.4%) women assigned to CaD supplements and 151 of 234 (64.5%) of women assigned to placebo, for a total of 330 women (Figure 1).
Assessment of mammographic density
After receiving informed consent, mammograms from each participant were requested from their individual mammography provider and then sent to the University of North Carolina for digitizing. Digitizing of films was performed on a Lumisys 85 laser digitizer with a maximum resolution of approximately 50 μm and 12-bit depth, with the digitizer recalibrated between sessions. A standard data-averaging method was used to convert raw image files to bitmap format for display and measurement of mammographic density. For each film, a unique serial number, the date of exam, laterality and view were recorded. The technique used to assess mammographic density has been validated previously(38) and used a computer-assisted interactive thresholding technique with software from the Imaging Research Program (Sunnybrook Health Science Center, Toronto, Ontario, Canada).
Mammograms were sorted separately for 2 trained observers (CM, JP), who both reviewed all films. Inter-observer reliability for measuring percent density was assessed before the study began and found to be very high (i.e., intraclass correlation coefficients >0.92).(37) Observers independently reviewed mammograms from CaD baseline and CaD follow-up, and were blinded to participant identification, randomization status for either trial, the timing of the mammogram (CaD baseline vs. follow-up), result from the other observer, and results of other mammograms from the same woman. The craniocaudal view of the right breast was used if available; otherwise the same view from the left breast was used. Investigators determined the breast edge and noncontinguous areas of mammographic density. The total area of breast and the total combined area of mammographic density were both calculated (pixels), and then the latter was divided by the former to calculate percent density. Each participant’s density was then calculated as the mean of the estimates of percent density from the 2 readers.
Assessment of vitamin D intake and other factors
At their baseline clinic visit, participants completed a semi-quantitative food frequency questionnaire (FFQ) designed for the Women’s Health Initiative and validated in this population. (39) Participants reported their usual intake and portion size of 122 foods or food groups in the 3 previous months. Vitamin D intake from food sources was calculated by multiplying the nutrient content of the specified portion size of each food (University of Minnesota Nutrient Coding Center nutrient database) by its frequency of consumption and summing the contributions of all foods. In a validation study in the WHI, vitamin D and calcium intake measured by FFQ correlated well with intake measured with 4 days of diet recalls + 4 days of food records (de-attenuated r for intake from foods: vitamin D = 0.70, calcium = 0.73; r for foods + supplements: vitamin D = 0.73; calcium = 0.78).(39)
At clinic visits prior to HT randomization and approximately 1 year later, vitamin D intake from supplemental sources was assessed by trained interviewers using a standard questionnaire that ascertained dose, frequency (pills per week), and duration (months and years) of use of multivitamins, multivitamin-mineral, and single supplements. Total vitamin D and calcium intakes were determined by summing intakes from food sources (FFQ) and personal supplements.
Study questionnaires completed during the baseline visits were used to assess breast cancer risk factors, including age, race/ethnicity, previous use of hormone therapy and oral contraceptives, education, alcohol intake, participation in physical activity, history of smoking, age at menarche, and Gail risk score. Weight and height were measured directly and used to calculate body mass index (weight (kg) / height (m) squared). For each of the 40 WHI clinical centers, an estimate of annual level of solar irradiance in Langleys (gm-cal) per cm2 was calculated using measurements from the US Weather Bureau adapted for use in the WHI. (40)
Statistical analysis
Comparisons of mean mammographic density by CaD randomization assignment were made by fitting linear models of log-transformed densities on treatment assignment. Means (95%CI) of the logged densities were then exponentiated to yield geometric means (95%CI). Ratios (95%CI) and p-values, comparing CaD to placebo, were obtained in a similar fashion by fitting linear models on differences between log-transformed densities (CaD follow-up minus CaD baseline) and exponentiating. The log-transformation and differencing ensured an approximate normal distribution for statistical tests. Subgroup analyses were performed to explore whether the effect of CaD was modified by other factors including age, race/ethnicity, total vitamin D intake, HT treatment arm, Gail risk score, BMI, region of residence, and category of mammogram density at CaD baseline. We tested for effect modification by including terms for CaD assignment, the factor (e.g., age), and CaD assignment * the factor in the regression model. Statistical significance was based on the test of interaction where at most one interaction is expected to be significant at the 0.05 level by chance alone. The influence of non-adherence to protocol-assigned treatment was examined by excluding density measures from participants reported consuming <80% of study medications (CaD or HT) or initiating non-study hormone therapy. All analyses were conducted using SAS version 9.1.3 (SAS Institute), and all p-values are two-sided.
Results
Characteristics of the 330 women included in this analysis by CaD randomization group are presented in Table 1. Women randomized to CaD supplements did not differ from those randomized to placebo in terms of mean age (61.8 years (SD=7.5) vs. 62.0 (8.0); P = 0.85) or mean BMI (29.9 kg/m2 (5.7) vs. 29.9 (6.2); P = 0.94). The distribution of other characteristics was similar between randomization groups, including intakes of vitamin and calcium.
Table 1.
Baseline Characteristics of Mammographic Percent Density Subsample that are Participating in the CaD Trial by Randomization Assignment (n=330),a Women’s Health Initiative Calcium and Vitamin D Trial.
| CaD (n = 151) |
Placebo (n = 179) |
||||
|---|---|---|---|---|---|
| Characteristic | N | % | N | % | P-valuea |
| Age (years) | 0.07 | ||||
| 50 to 59 | 73 | 40.8 | 68 | 45.0 | |
| 60 to 69 | 76 | 42.5 | 47 | 31.1 | |
| 70 to 79 | 30 | 16.8 | 36 | 23.8 | |
|
| |||||
| Ethnicity | 0.53 | ||||
| White | 82 | 45.8 | 78 | 51.7 | |
| Black | 65 | 36.3 | 54 | 35.8 | |
| Hispanic | 25 | 14.0 | 14 | 9.3 | |
| Asian/Pacific Islander | 7 | 3.9 | 5 | 3.3 | |
|
| |||||
| U. S. Region | 0.43 | ||||
| Northeast | 13 | 7.3 | 17 | 11.3 | |
| South | 73 | 40.8 | 52 | 34.4 | |
| Midwest | 57 | 31.8 | 54 | 35.8 | |
| West | 36 | 20.1 | 28 | 18.5 | |
|
| |||||
| Smoking status | 0.40 | ||||
| Never | 97 | 55.1 | 78 | 51.7 | |
| Past | 58 | 33.0 | 47 | 31.1 | |
| Current | 21 | 11.9 | 26 | 17.2 | |
|
| |||||
| Body-mass index (kg/m2) | 0.53 | ||||
| < 25 | 35 | 19.6 | 37 | 24.7 | |
| 25 to <30 | 63 | 35.2 | 50 | 33.3 | |
| ≥ 30 | 81 | 45.3 | 63 | 42.0 | |
|
| |||||
| Alcohol consumption (drinks/day) | 0.21 | ||||
| Non drinker | 106 | 59.2 | 83 | 55.0 | |
| ≤ 1 | 65 | 36.3 | 54 | 35.8 | |
| > 1 | 8 | 4.5 | 14 | 9.3 | |
|
| |||||
| Moderate to strenuous physical activity (# episodes ≥ 20 min/wk) | 0.47 | ||||
| No activity | 38 | 23.2 | 33 | 24.1 | |
| Some activity | 71 | 43.3 | 61 | 44.5 | |
| 2 to < 4 | 16 | 9.8 | 19 | 13.9 | |
| ≥4 | 39 | 23.8 | 24 | 17.5 | |
|
| |||||
| Age at menarche (years) | 0.88 | ||||
| ≤ 11 | 35 | 19.7 | 33 | 21.9 | |
| 12 to 13 | 91 | 51.1 | 76 | 50.3 | |
| ≥ 14 | 52 | 29.2 | 42 | 27.8 | |
|
| |||||
| Years since menopause | 0.59 | ||||
| < 5 | 27 | 16.9 | 22 | 16.5 | |
| 5 to < 10 | 32 | 20.0 | 24 | 18.0 | |
| 10 to < 15 | 28 | 17.5 | 17 | 12.8 | |
| ≥ 15 | 73 | 45.6 | 70 | 52.6 | |
|
| |||||
| Parity (number of full term pregnancies) | 0.11 | ||||
| Never pregnant/Never had term pregnancy | 16 | 9.0 | 14 | 9.3 | |
| 1 | 14 | 7.9 | 11 | 7.3 | |
| 2 | 25 | 14.1 | 37 | 24.5 | |
| ≥3 | 122 | 68.9 | 89 | 58.9 | |
|
| |||||
| Age at first birth (years) | 0.36 | ||||
| Never pregnant/No full term pregnancies | 16 | 10.3 | 14 | 10.1 | |
| < 20 | 40 | 25.8 | 36 | 26.1 | |
| 20 - 29 | 89 | 57.4 | 85 | 61.6 | |
| ≥ 30 | 10 | 6.5 | 3 | 2.2 | |
|
| |||||
| Benign breast disease and biopsy history | 0.68 | ||||
| No | 138 | 85.7 | 121 | 89.0 | |
| Yes, 1 biopsy | 16 | 9.9 | 11 | 8.1 | |
| Yes, 2+ biopsies | 7 | 4.3 | 4 | 2.9 | |
|
| |||||
| Family history of female relative w/breast cancer | 0.31 | ||||
| No | 147 | 86.5 | 116 | 82.3 | |
| Yes | 23 | 13.5 | 25 | 17.7 | |
|
| |||||
| Gail Risk Score | 0.86 | ||||
| < 1.25 | 109 | 60.9 | 89 | 58.9 | |
| 1.25 to <1.75 | 42 | 23.5 | 35 | 23.2 | |
| ≥ 1.75 | 28 | 15.6 | 27 | 17.9 | |
|
| |||||
| Duration of HT use at baseline (years) | 0.73 | ||||
| Never-user | 35 | 19.6 | 30 | 19.9 | |
| < 5 | 10 | 5.6 | 7 | 4.6 | |
| 5 to <10 | 125 | 69.8 | 102 | 67.5 | |
| ≥ 10 | 9 | 5.0 | 12 | 7.9 | |
|
| |||||
| Duration of oral contraceptive use (years) | 0.75 | ||||
| Non-user | 55 | 30.7 | 39 | 25.8 | |
| < 5 | 16 | 8.9 | 14 | 9.3 | |
| 5 to < 10 | 92 | 51.4 | 81 | 53.6 | |
| ≥ 10 | 16 | 8.9 | 17 | 11.3 | |
|
| |||||
| Total vitamin D intake (IU/day) | 0.89 | ||||
| < 100 | 39 | 23.1 | 39 | 26.9 | |
| 100 to < 200 | 48 | 28.4 | 38 | 26.2 | |
| 200 to < 400 | 29 | 17.2 | 24 | 16.6 | |
| ≥ 400 | 53 | 31.4 | 44 | 30.3 | |
|
| |||||
| Dietary vitamin D intake (IU/day) | 0.78 | ||||
| < 100 | 55 | 32.5 | 52 | 35.9 | |
| 100 to < 150 | 49 | 29.0 | 37 | 25.5 | |
| 150 to 200 | 26 | 15.4 | 26 | 17.9 | |
| ≥ 200 | 39 | 23.1 | 30 | 20.7 | |
|
| |||||
| Any Supplemental D | 0.82 | ||||
| No | 114 | 63.7 | 98 | 64.9 | |
| Yes | 65 | 36.3 | 53 | 35.1 | |
|
| |||||
| Total calcium intake (mg/day) | 0.67 | ||||
| < 500 | 29 | 17.2 | 33 | 22.8 | |
| 500 to < 750 | 48 | 28.4 | 38 | 26.2 | |
| 750 to < 1000 | 40 | 23.7 | 32 | 22.1 | |
| ≥ 1000 | 52 | 30.8 | 42 | 29.0 | |
|
| |||||
| Dietary calcium intake (mg/day) | 0.43 | ||||
| < 500 | 41 | 24.3 | 46 | 31.7 | |
| 500 to < 650 | 42 | 24.9 | 29 | 20.0 | |
| 650 to < 800 | 25 | 14.8 | 23 | 15.9 | |
| ≥ 800 | 61 | 36.1 | 47 | 32.4 | |
|
| |||||
| Supplemental Calcium (mg/day) | 0.47 | ||||
| None | 42 | 23.5 | 43 | 28.5 | |
| < 500 | 103 | 57.5 | 85 | 56.3 | |
| ≥ 500 | 34 | 19.0 | 23 | 15.2 | |
|
| |||||
| HT trial arm | 0.49 | ||||
| E-alone | 47 | 26.3 | 33 | 21.9 | |
| E-alone placebo | 40 | 22.3 | 42 | 27.8 | |
| E + P | 45 | 25.1 | 32 | 21.2 | |
| E + P placebo | 47 | 26.3 | 44 | 29.1 | |
|
| |||||
| DM trial treatment assignment | 0.49 | ||||
| Not in DM trial | 125 | 69.8 | 99 | 65.6 | |
| Placebo | 32 | 59.3 | 35 | 67.3 | |
| Active | 22 | 40.7 | 17 | 32.7 | |
E+P = conjugated equine estrogen plus medroxyprogesterone acetate; E-alone = conjugated equine estrogen; HT hormone therapy; CaD calcium with vitamin D; DM = dietary modification;
Includes women where mammographic breast density was measured prior to CaD randomization and approximately one year after CaD randomization.
Chi-square test of association.
We did not find study supplementation with calcium and vitamin D to be associated with a significant change in mammographic density between CaD baseline and follow-up, as compared to placebo (Table 2). On average, mammographic density decreased 2% in the CaD supplement group and increased 1% in the CaD placebo group. The ratio of geometric means comparing one-year change in mammographic density in the CaD supplement group to that of the CaD placebo group was 0.97 (95% CI = 0.81 – 1.17; P = 0.77).
Table 2.
Meana Mammographic Percent Density Prior to CaD Randomization (CaD Baseline), Approximately One Year after CaD Randomization (CaD Follow-up), and Ratio of Means by CaD Randomization Assignment.
| Mean % Mammographic Density (95% CI) |
||||||
|---|---|---|---|---|---|---|
| n | At CaD Baseline |
At CaD Follow-up |
Ratio of CaD Follow-up to Baseline (95% CI) |
Ratio in CaD vs. Ratio in Placebo (95% CI) |
P value | |
| CaD | 179 | 3.7 (2.9 – 4.8) | 3.6 (2.9 – 4.6) | 0.98 (0.86 – 1.12) | 0.97 (0.81 - 1.17) | 0.77 |
| Placebo | 151 | 2.8 (2.1 – 3.7) | 2.8 (2.2 – 3.7) | 1.01 (0.88 – 1.15) | ||
CaD calcium with vitamin D
Geometric mean
We observed little evidence of effect modification by age, calcium or vitamin D intake from foods and personal supplements at baseline, Gail risk score, and category of percent density (Table 3). Results stratified by HT treatment arm suggested modest effect modification of supplementation with CaD by hormone therapy (P for interaction = 0.08). Among those in the HT trials assigned to placebo, the ratio of geometric mean mammographic density comparing one year change in density between CaD and placebo groups was 0.82 (95% CI = 0.61 – 1.11). In contrast, among HT users, the ratio of geometric mean density comparing CaD and placebo groups was 1.16 (95% CI = 0.92 – 1.45). Further stratification by E-alone and E+P trial also resulted in a non- significant interaction between CaD supplementation and HT (P = 0.18 for the CaD by E-alone interactin, and P = 0.24 for the CaD by E+P interaction). Among women in the E-alone trial, the ratio of geometric mean mammographic density comparing one year change in density between CaD and placebo groups was 1.29 (95%CI = 0.88 - 1.89) for those randomized to E-alone and 0.86 (95%CI = 0.54 -1.36) randomized to the placebo arm. Similarly, among women in the E+P trial, the ratio of geometric mean mammographic density comparing one year change in density between CaD and placebo groups was 1.03 (95%CI = 0.80 - 1.33) for those randomized to E+P and 0.78 (95%CI = 0.53 - 1.14) randomized to the placebo arm.
Table 3.
Meana Mammographic Percent Density Prior to CaD Randomization (CaD Baseline), Approximately One Year after CaD Randomization (CaD Follow-Up), and Ratio of Means by CaD Randomization Assignment by Subgroup.
| Mean % Mammographic Density (SD) |
||||||
|---|---|---|---|---|---|---|
| n | At CaD Baseline |
At CaD Follow-Up |
Ratio of CaD Baseline to Follow-up (95% CI) |
Ratio in CaD vs. Ratio in Placebo (95% CI) |
P value (int)b |
|
| Age | 0.31 | |||||
| 50 – 57 years | ||||||
| CaD | 59 | 4.6 (3.0 – 6.9) | 4.2 (2.9 – 6.1) | 0.92 (0.74 - 1.15) | 0.95 (0.71 - 1.27) | |
| Placebo | 53 | 3.1 (2.0 – 4.8) | 3.0 (2.0 – 4.6) | 0.97 (0.81 - 1.17) | ||
| 58 – 65 years | ||||||
| CaD | 64 | 3.2 (2.1 – 4.7) | 3.2 (2.1 – 4.9) | 1.02 (0.81 - 1.27) | 0.77 (0.56 - 1.07) | |
| Placebo | 44 | 1.9 (1.1 – 3.5) | 2.5 (1.5 – 4.2) | 1.32 (1.04 - 1.66) | ||
| 66 – 79 years | ||||||
| CaD | 56 | 3.6 (2.2 – 5.9) | 3.6 (2.3 – 5.5) | 1.00 (0.77 - 1.30) | 1.20 (0.83 - 1.72) | |
| Placebo | 54 | 3.6 (2.3 – 5.6) | 3.0 (1.8 – 4.8) | 0.83 (0.65 - 1.08) | ||
|
| ||||||
| Total Vitamin D Intake (IU/day) | 0.75 | |||||
| < 200 | ||||||
| CaD | 87 | 3.6 (2.5 – 5.2) | 3.5 (2.5 – 4.9) | 0.96 (0.79 – 1.18) | 0.97 (0.74- 1.28) | |
| Placebo | 77 | 3.0 (2.1 – 4.5) | 3.0 (2.1 – 4.3) | 0.99 (0.83 – 1.19) | ||
| 200 – < 400 | ||||||
| CaD | 29 | 2.4 (1.1 – 5.3)) | 2.8 (1.4 – 5.6) | 1.13 (0.73 – 1.74) | 0.89 (0.53- 1.47) | |
| Placebo | 24 | 2.5 (1.3 – 5.1) | 3.2 (1.7 – 6.1) | 1.28 (1.03 – 1.59) | ||
| ≥ 400 | ||||||
| CaD | 53 | 4.3 (2.9 – 6.4) | 4.0 (2.6 – 6.0) | 0.93 (0.77 – 1.14) | 1.06 (0.75- 1.50) | |
| Placebo | 44 | 2.7 (1.5 – 4.8) | 2.3 (1.3 – 4.2) | 0.88 (0.65 – 1.19) | ||
|
| ||||||
| Total Calcium Intake (mg/day) | 0.51 | |||||
| <750 | ||||||
| CaD | 77 | 3.6 (2.4 – 5.2) | 3.2 (2.2 – 4.6) | 0.90 (0.71 – 1.14) | 0.99 (0.74-1.34) | |
| Placebo | 71 | 3.1 (2.1 – 4.6) | 2.8 (1.9 – 4.2) | 0.91 (0.75 – 1.09) | ||
| 750-<1000 | ||||||
| CaD | 40 | 3.7 (1.9 – 7.2) | 4.4 (2.6 – 7.5) | 1.19 (0.88 – 1.62) | 1.16 (0.75-1.80) | |
| Placebo | 32 | 2.7 (1.4 – 5.1) | 2.7 (1.6 – 4.7) | 1.03 (0.74 – 1.43) | ||
| ≥ 1000 | ||||||
| CaD | 52 | 3.5 (2.3 – 5.1) | 3.3 (2.2 – 5.1) | 0.96 (0.78 – 1.16) | 0.83 (0.61-1.15) | |
| Placebo | 42 | 2.5 (1.4 – 4.7) | 2.9 (1.7 – 5.1) | 1.14 (0.88 – 1.49) | ||
|
| ||||||
| Active HT | 0.08 | |||||
| No | ||||||
| CaD | 87 | 2.7 (1.9 – 3.8) | 2.3 (1.6 – 3.3) | 0.86 (0.70 – 1.07) | 0.82 (0.61 - 1.11) | |
| Placebo | 86 | 1.9 (1.3 – 2.8) | 2.0 (1.4 – 2.9) | 1.05 (0.85 – 1.29) | ||
| Yes | ||||||
| CaD | 92 | 5.1 (3.6 – 7.1) | 5.6 (4.3 – 7.3) | 1.10 (0.93 – 1.30) | 1.16 (0.92 - 1.45) | |
| Placebo | 65 | 4.7 (3.2 – 6.9) | 4.5 (3.0 – 6.6) | 0.95 (0.83 – 1.09) | ||
|
| ||||||
| Gail Risk Score | 0.23 | |||||
| < 1.25 | ||||||
| CaD | 109 | 3.3 (2.4 – 4.5) | 3.1 (2.3 – 4.2) | 0.95 (0.80 – 1.14) | 0.90 (0.70 - 1.15) | |
| Placebo | 89 | 3.0 (2.1 – 4.2) | 3.1 (2.3 – 4.3) | 1.06 (0.90 – 1.25) | ||
| 1.25 – < 1.75 | ||||||
| CaD | 42 | 4.2 (2.3 – 7.6) | 4.2 (2.5 – 7.1) | 1.02 (0.75 – 1.37) | 1.01 (0.65 - 1.57) | |
| Placebo | 35 | 1.9 (0.9 – 3.7) | 1.9 (1.0 – 3.6) | 1.00 (0.72 – 1.39) | ||
| ≥ 1.75 | ||||||
| CaD | 28 | 5.2 (3.2 – 8.5) | 5.4 (3.3 – 8.7) | 1.03 (0.81 – 1.30) | 1.22 (0.84 - 1.78) | |
| Placebo | 27 | 4.2 (2.5 – 7.3) | 3.6 (1.8 – 7.0) | 0.84 (0.62 – 1.14) | ||
|
| ||||||
| Race/Ethnicity | 0.11 | |||||
| White | ||||||
| CaD | 82 | 2.9 (1.9 – 4.3) | 3.3 (2.4 – 4.7) | 1.17 (0.95 – 1.42) | 1.18 (0.90 - 1.55) | |
| Placebo | 78 | 2.9 (1.9 – 4.3) | 2.8 (1.9 – 4.1) | 0.99 (0.82 – 1.19) | ||
| Black | ||||||
| CaD | 65 | 4.4 (3.0 – 6.6) | 3.6 (2.4 – 5.4) | 0.81 (0.63 – 1.03) | 0.76 (0.55 - 1.06) | |
| Placebo | 54 | 2.6 (1.6 – 4.1) | 2.7 (1.8 – 4.3) | 1.06 (0.86 – 1.30) | ||
| Hispanic | ||||||
| CaD | 25 | 4.3 (2.6 – 7.2) | 4.1 (2.5 – 6.6) | 0.95 (0.74 – 1.23) | 0.85 (0.52 - 1.40) | |
| Placebo | 14 | 2.3 (0.8 – 6.4) | 2.6 (1.1 – 5.9) | 1.12 (0.66 – 1.89) | ||
|
| ||||||
| BMI (kg/m2) | 0.31 | |||||
| Normal (<25) | ||||||
| CaD | 35 | 6.8 (4.3-10.7) | 5.5 (3.3 – 9.3) | 0.81 (0.66 – 1.01) | 0.79 (0.58 - 1.06) | |
| Placebo | 37 | 7.5 (4.9-11.5) | 7.7 (5.0-12.0) | 1.04 (0.83 – 1.29) | ||
| Overweight (25-<30) | ||||||
| CaD | 63 | 3.8 (2.5 – 5.8) | 3.5 (2.3 – 5.5) | 0.94 (0.73 – 1.20) | 1.02 (0.71-1.46) | |
| Placebo | 50 | 4.2 (2.8 – 6.4) | 3.9 (2.5 – 6.0) | 0.92 (0.71 – 1.20) | ||
| Obese (≥ 30) | ||||||
| CaD | 81 | 2.8 (1.9 – 4.2) | 3.1 (2.3 – 4.2) | 1.09 (0.89 – 1.34) | 1.03 (0.77-1.38) | |
| Placebo | 63 | 1.2 (0.8 – 1.8) | 1.2 (0.8 – 1.8) | 1.06 (0.86 – 1.30) | ||
|
| ||||||
| Solar Irradiation (Langleys) | 0.13 | |||||
| < 350 | ||||||
| CaD | 34 | 2.4 (1.1 – 5.0) | 3.2 (1.8 – 5.9) | 1.36 (0.92 – 2.00) | 1.53 (1.00 - 2.32) | |
| Placebo | 48 | 2.7 (1.6 – 4.5) | 2.4 (1.4 – 4.1) | 0.89 (0.71 – 1.12) | ||
| 350 - < 400 | ||||||
| CaD | 94 | 3.9 (2.8 – 5.5) | 3.5 (2.5 – 4.8) | 0.88 (0.73 – 1.06) | 0.78 (0.58 - 1.04) | |
| Placebo | 62 | 2.5 (1.6 – 3.9) | 2.8 (1.9 – 4.1) | 1.13 (0.91 – 1.40) | ||
| ≥ 400 | ||||||
| CaD | 51 | 4.5 (3.1 – 6.6) | 4.3 (3.0 – 6.2) | 0.96 (0.80 – 1.14) | 0.98 (0.74 - 1.31) | |
| Placebo | 41 | 3.7 (2.2 – 6.2) | 3.6 (2.2 – 5.9) | 0.97 (0.76 – 1.24) | ||
|
| ||||||
| Category of Mammographic Density | 0.42 | |||||
| < 1% | ||||||
| CaD | 35 | 0.3 (0.2 – 0.4) | 0.5 (0.3 – 0.7) | 1.78 (1.14 – 2.78) | 1.23 (0.70 - 2.18) | |
| Placebo | 39 | 0.3 (0.2 – 0.4) | 0.4 (0.3 – 0.6) | 1.45 (0.99 – 2.10) | ||
| 1% – < 10% | ||||||
| CaD | 87 | 3.6 (3.2 – 4.2) | 3.2 (2.5 – 4.0) | 0.87 (0.73 – 1.04) | 0.97 (0.76 - 1.24) | |
| Placebo | 71 | 3.3 (2.8 – 3.9) | 3.0 (2.4 – 3.8) | 0.90 (0.76 – 1.01) | ||
| ≥ 10% | ||||||
| CaD | 57 | 19.2 (17.3-21.5) | 15.5 (13.1-18.4) | 0.81 (0.70 – 0.92) | 0.93 (0.77 - 1.13) | |
| Placebo | 41 | 19.3 (16.7-22.4) | 16.7 (13.7-20.4) | 0.86 (0.76 – 0.98) | ||
E+P = conjugated equine estrogen plus medroxyprogesterone acetate; E-alone = conjugated equine estrogen; HT hormone therapy; CaD calcium with vitamin D; DM = dietary modification; BMI = body mass index;
Geometric mean
P for interaction
In sensitivity analyses limited to women who were adherent (taking at least 80% of CaD supplements and HT medication), differences between groups were slightly greater. Mean mammographic density decreased 0.49% in the CaD group and increased 0.11% in the placebo group (ratio of geometric means comparing one year change in density in CaD supplementation and placebo groups = 0.83; 95% CI = 0.86 – 1.11; P = 0.21). Results from analyses stratified by HT treatment arm were also somewhat stronger than in the analysis of all participants. Among HT trial members assigned to placebo, the ratio of geometric mean density comparing CaD and placebo groups was 0.67 (95% CI = 0.41 – 1.07). In contrast, among women in the HT active arm, the ratio of geometric mean density comparing CaD and placebo groups was 1.07 (95% CI = 0.79 – 1.47; P for interaction = 0.12).
Discussion
In this study of postmenopausal women, daily supplementation of 1000 mg of elemental calcium and 400 IU of vitamin D3 for one year did not affect mammographic density as compared to placebo.
We observed some evidence that vitamin D and calcium supplementation was associated with slightly lower mammographic density in postmenopausal women not currently using HT and slightly higher density in women using HT, but the test for interaction was not statistically significant (P interaction = 0.08). Given our small sample size, especially for subgroup analyses and tests for interactions, this finding may be due to chance. Alternatively, in HT users modest changes related to vitamin D and calcium supplementation may be masked by changes associated with hormone use. Finally, a potential interaction may exist between estrogens and vitamin D as they relate to mammographic density and perhaps to breast cancer risk also. While interaction with hormone therapy has not been observed in previous studies of supplemental calcium and vitamin D and postmenopausal breast cancer, including the WHI CaD trial,(6) few studies have evaluated this.(16) Potential biologic mechanisms supporting an interaction between estrogen and vitamin D include competition for cell membrane megalin receptors, which play a role in the endocytosis of sex steroid hormones and vitamins, and effects of estrogens on circulating levels of calbindin, a binding protein regulating intracellular free calcium levels and effecting cell proliferation.(41) However, it is interesting to note that inverse relationships between vitamin D, calcium and mammographic density have been more consistently observed in studies of premenopausal women,(20, 23, 24, 26) in whom circulating levels of estrogens and mean mammographic density are higher than in postmenopausal women. Additional studies of vitamin D, breast density and breast cancer should further explore the potential interaction between vitamin D and estrogen-related factors including menopausal status and HT use when possible.
The mean mammographic density among participants in our population (arithmetic mean = 8.4%; SD = 10.2%) was considerably lower than in previous studies of postmenopausal women, perhaps because our participants were somewhat older than women in other studies(16, 20, 23) and because HT trial participants were required to be off hormones for at least three months prior to randomization. Studies observing the strongest relationships between density and vitamin D and/or calcium intake generally evaluated women with higher mammographic densities.(17, 19) An effect of vitamin D on mammographic density may not be detectable in women who already have very low percent density due to a ‘floor effect’. We did not find an effect of supplementation in subgroup analyses of women with higher mean density including younger women, HT users, or those with baseline density ≥10%, possibly due to the small sample size for these analyses. Additionally, it is possible that one year of supplementation was insufficient to affect mammographic density, though significant differences in density were observable after one year of hormone treatment in both the WHI E+P trial (mean density increased from 3.9% to 9.6% over 1 year in the E+P active group) and E-alone trials (mean density increased from 6.8% to 8.4% over 1 year in the E-alone active group).(36, 37)
The dose of vitamin D tested in our study, 400 IU/day, may have been insufficient to modify breast density within one year. It has been proposed that in the absence of sunlight exposure, vitamin D intake of 1700- 2000 IU per day is necessary to achieve 25(OH)D levels of 75 nmol/L (30 ng/dL), which may be needed to lower breast cancer risk.(42, 43) The dose used in our study, combined with background personal use of vitamin D supplements and dietary intake, is consistent with current Institute of Medicine guidelines (i.e., 600 IU/day for women ≤ 70 years, 800 IU/day for women >70 years) (44). While differences in actual vitamin D intake between intervention groups may have been insufficient to detect an effect on density, overall levels of intake in our study are comparable with those of previous studies that did observe a relation with mammographic density.(17, 23, 26) Furthermore, in the CaD trial, vitamin D and calcium supplementation was associated with significantly lower risk of breast cancer in women consuming <200 IU/day of vitamin D at baseline (0.79; 95% CI = 0.65 – 0.97) but significantly increased in women consuming ≥600 IU/day (1.34; 95% CI = 1.01 – 1.78; P interaction = 0.003).(6) These findings do not support the hypothesis that higher doses of vitamin D than ours are necessary to modify mammographic density and breast cancer risk among older postmenopausal women.
To our knowledge, this is the first observation of the effect of calcium and vitamin D supplementation on mammographic density in the context of a randomized clinical trial. While we observed no effect of 400 IU/day of vitamin D3 along with 1000 mg/day of elemental calcium on mammographic density after approximately one year in this small study, questions persist concerning the potential for interaction between vitamin D and estrogen and warrant further investigation.
ACKNOWLEDGEMENTS
Women’s Health Initiative Investigators Program Office: (National Heart, Lung, and Blood Institute, Bethesda, Maryland) Elizabeth Nabel, Jacques Rossouw, Shari Ludlam, Joan McGowan, Leslie Ford, and Nancy Geller.
Clinical Coordinating Center: (Fred Hutchinson Cancer Research Center, Seattle, WA) Ross Prentice, Garnet Anderson, Andrea LaCroix, Charles L. Kooperberg, Ruth E. Patterson, Anne McTiernan; (Medical Research Labs, Highland Heights, KY) Evan Stein; (University of California at San Francisco, San Francisco, CA) Steven Cummings.
Clinical Centers: (Albert Einstein College of Medicine, Bronx, NY) Sylvia Wassertheil-Smoller; (Baylor College of Medicine, Houston, TX) Aleksandar Rajkovic; (Brigham and Women’s Hospital, Harvard Medical School, Boston, MA) JoAnn E. Manson; (Brown University, Providence, RI) Charles B. Eaton; (Emory University, Atlanta, GA) Lawrence Phillips; (Fred Hutchinson Cancer Research Center, Seattle, WA) Shirley Beresford; (George Washington University Medical Center, Washington, DC) Lisa Martin; (Los Angeles Biomedical Research Institute at Harbor- UCLA Medical Center, Torrance, CA) Rowan Chlebowski; (Kaiser Permanente Center for Health Research, Portland, OR) Yvonne Michael; (Kaiser Permanente Division of Research, Oakland, CA) Bette Caan; (Medical College of Wisconsin, Milwaukee, WI) Jane Morley Kotchen; (MedStar Research Institute/Howard University, Washington, DC) Barbara V. Howard; (Northwestern University, Chicago/Evanston, IL) Linda Van Horn; (Rush Medical Center, Chicago, IL) Henry Black; (Stanford Prevention Research Center, Stanford, CA) Marcia L. Stefanick; (State University of New York at Stony Brook, Stony Brook, NY) Dorothy Lane; (The Ohio State University, Columbus, OH) Rebecca Jackson; (University of Alabama at Birmingham, Birmingham, AL) Cora E. Lewis; (University of Arizona, Tucson/Phoenix, AZ) Cynthia A Thomson; (University at Buffalo, Buffalo, NY) Jean Wactawski-Wende; (University of California at Davis, Sacramento, CA) John Robbins; (University of California at Irvine, CA) F. Allan Hubbell; (University of California at Los Angeles, Los Angeles, CA) Lauren Nathan; (University of California at San Diego, LaJolla/Chula Vista, CA) Robert D. Langer; (University of Cincinnati, Cincinnati, OH) Margery Gass; (University of Florida, Gainesville/Jacksonville, FL) Marian Limacher; (University of Hawaii, Honolulu, HI) J. David Curb; (University of Iowa, Iowa City/Davenport, IA) Robert Wallace; (University of Massachusetts/Fallon Clinic, Worcester, MA) Judith Ockene; (University of Medicine and Dentistry of New Jersey, Newark, NJ) Norman Lasser; (University of Miami, Miami, FL) Mary Jo O’Sullivan; (University of Minnesota, Minneapolis, MN) Karen Margolis; (University of Nevada, Reno, NV) Robert Brunner; (University of North Carolina, Chapel Hill, NC) Gerardo Heiss; (University of Pittsburgh, Pittsburgh, PA) Lewis Kuller; (University of Tennessee Health Science Center, Memphis, TN) Karen C. Johnson; (University of Texas Health Science Center, San Antonio, TX) Robert Brzyski; (University of Wisconsin, Madison, WI) Gloria E. Sarto; (Wake Forest University School of Medicine, Winston-Salem, NC) Mara Vitolins; (Wayne State University School of Medicine/Hutzel Hospital, Detroit, MI) Michael Simon.
Financial Support: The WHI program is funded by the National Heart, Lung and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts N01WH22110, 24152, 32100-2, 32105-6, 32108-9, 32111-13, 32115, 32118-32119, 32122, 42107-26, 42129-32, and 44221. The Mammogram Ancillary Study was funded by the National Cancer Institute (CA7601704).
Footnotes
Conflicts of Interest: Dr. JoAnn E. Manson and colleagues at Brigham and Women’s Hospital, Harvard Medical School, are recipients of funding from the National Institutes of Health to conduct the VITamin D and OmegA-3 TriaL (VITAL), a large-scale randomized trial of vitamin D and omega-3s in the prevention of cancer and cardiovascular disease. No additional conflicts declared.
References
- 1.Yin L, Grandi N, Raum E, Haug U, Arndt V, Brenner H. Meta-analysis: Serum vitamin D and breast cancer risk. Eur J Cancer. 2010 Aug;46(12):2196–205. doi: 10.1016/j.ejca.2010.03.037. [DOI] [PubMed] [Google Scholar]
- 2.Chen P, Hu P, Xie D, Qin Y, Wang F, Wang H. Meta-analysis of vitamin D, calcium and the prevention of breast cancer. Breast Cancer Res Treat. 2010 Jun;121(2):469–77. doi: 10.1007/s10549-009-0593-9. [DOI] [PubMed] [Google Scholar]
- 3.Bertone-Johnson ER, Chen WY, Holick MF, Hollis BW, Colditz GA, Willett WC, et al. Plasma 25- hydroxyvitamin D and 1,25-dihydroxyvitamin D and risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 2005 Aug;14(8):1991–7. doi: 10.1158/1055-9965.EPI-04-0722. [DOI] [PubMed] [Google Scholar]
- 4.Engel P, Fagherazzi G, Boutten A, Dupre T, Mesrine S, Boutron-Ruault MC, et al. Serum 25(OH) vitamin D and risk of breast cancer: A nested case-control study from the French E3N cohort. Cancer Epidemiol Biomarkers Prev. 2010 Sep;19(9):2341–50. doi: 10.1158/1055-9965.EPI-10-0264. [DOI] [PubMed] [Google Scholar]
- 5.Hjartaker A, Thoresen M, Engeset D, Lund E. Dairy consumption and calcium intake and risk of breast cancer in a prospective cohort: The Norwegian Women and Cancer Study. Cancer Causes Control. 2010 Nov;21(11):1875–85. doi: 10.1007/s10552-010-9615-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chlebowski RT, Johnson KC, Kooperberg C, Pettinger M, Wactawski-Wende J, Rohan T, et al. Calcium plus vitamin D supplementation and the risk of breast cancer. J Natl Cancer Inst. 2008 Nov 19;100(22):1581–91. doi: 10.1093/jnci/djn360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCullough ML, Stevens VL, Patel R, Jacobs EJ, Bain EB, Horst RL, et al. Serum 25-hydroxyvitamin D concentrations and postmenopausal breast cancer risk: A nested case control study in the Cancer Prevention Study-II Nutrition Cohort. Breast Cancer Res. 2009;11(4):R64. doi: 10.1186/bcr2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freedman DM, Chang SC, Falk RT, Purdue MP, Huang WY, McCarty CA, et al. Serum levels of vitamin D metabolites and breast cancer risk in the prostate, lung, colorectal, and ovarian cancer screening trial. Cancer Epidemiol Biomarkers Prev. 2008 Apr;17(4):889–94. doi: 10.1158/1055-9965.EPI-07-2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bertone-Johnson ER. Prospective studies of dietary vitamin D and breast cancer: More questions raised than answered. Nutr Rev. 2007 Oct;65(10):459–66. doi: 10.1111/j.1753-4887.2007.tb00271.x. [DOI] [PubMed] [Google Scholar]
- 10.Bertone-Johnson ER. Vitamin D and breast cancer. Ann Epidemiol. 2009 Jul;19(7):462–7. doi: 10.1016/j.annepidem.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 11.McCormack VA, dos Santos Silva I. Breast density and parenchymal patterns as markers of breast cancer risk: A meta-analysis. Cancer Epidemiol Biomarkers Prev. 2006 Jun;15(6):1159–69. doi: 10.1158/1055-9965.EPI-06-0034. [DOI] [PubMed] [Google Scholar]
- 12.Boyd NF, Martin LJ, Yaffe MJ, Minkin S. Mammographic density: A hormonally responsive risk factor for breast cancer. J Br Menopause Soc. 2006 Dec;12(4):186–93. doi: 10.1258/136218006779160436. [DOI] [PubMed] [Google Scholar]
- 13.Welsh J. Vitamin D metabolism in mammary gland and breast cancer. Mol Cell Endocrinol. 2011 Jun 12; doi: 10.1016/j.mce.2011.05.020. [DOI] [PubMed] [Google Scholar]
- 14.Cui Y, Rohan TE. Vitamin D, calcium, and breast cancer risk: A review. Cancer Epidemiol Biomarkers Prev. 2006 Aug;15(8):1427–37. doi: 10.1158/1055-9965.EPI-06-0075. [DOI] [PubMed] [Google Scholar]
- 15.Bertone-Johnson ER, Chlebowski RT, Manson JE, Wactawski-Wende J, Aragaki AK, Tamimi RM, et al. Dietary vitamin D and calcium intake and mammographic density in postmenopausal women. Menopause. 2010 Nov-Dec;17(6):1152–60. doi: 10.1097/gme.0b013e3181e102d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Green AK, Hankinson SE, Bertone-Johnson ER, Tamimi RM. Mammographic density, plasma vitamin D levels and risk of breast cancer in postmenopausal women. Int J Cancer. 2010 Aug 1;127(3):667–74. doi: 10.1002/ijc.25075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berube S, Diorio C, Verhoek-Oftedahl W, Brisson J. Vitamin D, calcium, and mammographic breast densities. Cancer Epidemiol Biomarkers Prev. 2004 Sep;13(9):1466–72. [PubMed] [Google Scholar]
- 18.Brisson J, Berube S, Diorio C, Sinotte M, Pollak M, Masse B. Synchronized seasonal variations of mammographic breast density and plasma 25-hydroxyvitamin D. Cancer Epidemiol Biomarkers Prev. 2007 May;16(5):929–33. doi: 10.1158/1055-9965.EPI-06-0746. [DOI] [PubMed] [Google Scholar]
- 19.Tseng M, Byrne C, Evers KA, Daly MB. Dietary intake and breast density in high-risk women: A cross- sectional study. Breast Cancer Res. 2007;9(5):R72. doi: 10.1186/bcr1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thomson CA, Arendell LA, Bruhn RL, Maskarinec G, Lopez AM, Wright NC, et al. Pilot study of dietary influences on mammographic density in pre- and postmenopausal hispanic and non-hispanic white women. Menopause. 2007 Mar-Apr;14(2):243–50. doi: 10.1097/01.gme.0000235362.72899.7b. [DOI] [PubMed] [Google Scholar]
- 21.Vachon CM, Kushi LH, Cerhan JR, Kuni CC, Sellers TA. Association of diet and mammographic breast density in the minnesota breast cancer family cohort. Cancer Epidemiol Biomarkers Prev. 2000 Feb;9(2):151–60. [PubMed] [Google Scholar]
- 22.Knight JA, Vachon CM, Vierkant RA, Vieth R, Cerhan JR, Sellers TA. No association between 25- hydroxyvitamin D and mammographic density. Cancer Epidemiol Biomarkers Prev. 2006 Oct;15(10):1988–92. doi: 10.1158/1055-9965.EPI-06-0241. [DOI] [PubMed] [Google Scholar]
- 23.Berube S, Diorio C, Masse B, Hebert-Croteau N, Byrne C, Cote G, et al. Vitamin D and calcium intakes from food or supplements and mammographic breast density. Cancer Epidemiol Biomarkers Prev. 2005 Jul;14(7):1653–9. doi: 10.1158/1055-9965.EPI-05-0068. [DOI] [PubMed] [Google Scholar]
- 24.Diorio C, Berube S, Byrne C, Masse B, Hebert-Croteau N, Yaffe M, et al. Influence of insulin-like growth factors on the strength of the relation of vitamin D and calcium intakes to mammographic breast density. Cancer Res. 2006 Jan 1;66(1):588–97. doi: 10.1158/0008-5472.CAN-05-1959. [DOI] [PubMed] [Google Scholar]
- 25.Diorio C, Sinotte M, Brisson J, Berube S, Pollak M. Vitamin D pathway polymorphisms in relation to mammographic breast density. Cancer Epidemiol Biomarkers Prev. 2008 Sep;17(9):2505–8. doi: 10.1158/1055-9965.EPI-08-0493. [DOI] [PubMed] [Google Scholar]
- 26.Colangelo LA, Chiu BCH, Lopez P, Scholtens D, Willis LC, Hendrick R Edward, et al. A pilot study of vitamin D, calcium, and percent breast density in hispanic women. Nutr Research. 2006;26:11–5. [Google Scholar]
- 27.Chai W, Maskarinec G, Cooney RV. Serum 25-hydroxyvitamin D levels and mammographic density among premenopausal women in a multiethnic population. Eur J Clin Nutr. 2010;64(6):652–4. doi: 10.1038/ejcn.2010.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sprague BL, Trentham-Dietz A, Gangnon RE, Buist DS, Burnside ES, Bowles EJ Aiello, et al. The vitamin D pathway and mammographic breast density among postmenopausal women. Breast Cancer Res Treat. 2011 doi: 10.1007/s10549-011-1726-5. doi:10.1007/s10549-011-1726-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Masala G, Ambrogetti D, Assedi M, Giorgi D, Del Turco MR, Palli D. Dietary and lifestyle determinants of mammographic breast density. A longitudinal study in a Mediterranean population. Int J Cancer. 2006 Apr 1;118(7):1782–9. doi: 10.1002/ijc.21558. [DOI] [PubMed] [Google Scholar]
- 30.Mishra G, McCormack V, Kuh D, Hardy R, Stephen A, dos Santos Silva I. Dietary calcium and vitamin D intakes in childhood and throughout adulthood and mammographic density in a British birth cohort. Br J Cancer. 2008 Nov 4;99(9):1539–43. doi: 10.1038/sj.bjc.6604697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results from the Women’s Health Initiative Randomized Controlled Trial. JAMA. 2002 Jul 17;288(3):321–33. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 32.Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SA, Black H, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: The Women’s Health Initiative Randomized Controlled Trial. JAMA. 2004 Apr 14;291(14):1701–12. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 33.The Women’s Health Initiative Study Group Design of the Women’s Health Initiative Clinical Trial and Observational Study. Control Clin Trials. 1998 Feb;19(1):61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 34.Jackson RD, LaCroix AZ, Cauley JA, McGowan J. The Women’s Health Initiative Calcium-vitamin D Trial: Overview and baseline characteristics of participants. Ann Epidemiol. 2003 Oct;13(9 Suppl):S98–106. doi: 10.1016/s1047-2797(03)00046-2. [DOI] [PubMed] [Google Scholar]
- 35.Jackson RD, LaCroix AZ, Gass M, Wallace RB, Robbins J, Lewis CE, et al. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006 Feb 16;354(7):669–83. doi: 10.1056/NEJMoa055218. [DOI] [PubMed] [Google Scholar]
- 36.McTiernan A, Chlebowski RT, Martin C, Peck JD, Aragaki A, Pisano ED, et al. Conjugated equine estrogen influence on mammographic density in postmenopausal women in a substudy of the Women’s Health Initiative Randomized Trial. J Clin Oncol. 2009 Dec 20;27(36):6135–43. doi: 10.1200/JCO.2008.21.7166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McTiernan A, Martin CF, Peck JD, Aragaki AK, Chlebowski RT, Pisano ED, et al. Estrogen-plus-progestin use and mammographic density in postmenopausal women: Women’s Health Initiative Randomized Trial. J Natl Cancer Inst. 2005 Sep 21;97(18):1366–76. doi: 10.1093/jnci/dji279. [DOI] [PubMed] [Google Scholar]
- 38.Boyd NF, Byng JW, Jong RA, Fishell EK, Little LE, Miller AB, et al. Quantitative classification of mammographic densities and breast cancer risk: Results from the Canadian National Breast Screening Study. J Natl Cancer Inst. 1995 May 3;87(9):670–5. doi: 10.1093/jnci/87.9.670. [DOI] [PubMed] [Google Scholar]
- 39.Patterson RE, Kristal AR, Tinker LF, Carter RA, Bolton MP, Agurs-Collins T. Measurement characteristics of the Women’s Health Initiative food frequency questionnaire. Ann Epidemiol. 1999 Apr;9(3):178–87. doi: 10.1016/s1047-2797(98)00055-6. [DOI] [PubMed] [Google Scholar]
- 40.Millen AE, Pettinger M, Freudenheim JL, Langer RD, Rosenberg CA, Mossavar-Rahmani Y, et al. Incident invasive breast cancer, geographic location of residence, and reported average time spent outside. Cancer Epidemiol Biomarkers Prev. 2009 Feb;18(2):495–507. doi: 10.1158/1055-9965.EPI-08-0652. [DOI] [PubMed] [Google Scholar]
- 41.Ding EL, Mehta S, Fawzi WW, Giovannucci EL. Interaction of estrogen therapy with calcium and vitamin D supplementation on colorectal cancer risk: Reanalysis of Women’s Health Initiative Randomized Trial. Int J Cancer. 2008 Apr 15;122(8):1690–4. doi: 10.1002/ijc.23311. [DOI] [PubMed] [Google Scholar]
- 42.Garland CF, Gorham ED, Mohr SB, Grant WB, Giovannucci EL, Lipkin M, et al. Vitamin D and prevention of breast cancer: Pooled analysis. J Steroid Biochem Mol Biol. 2007 Mar;103(3-5):708–11. doi: 10.1016/j.jsbmb.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 43.Vieth R, Bischoff-Ferrari H, Boucher BJ, Dawson-Hughes B, Garland CF, Heaney RP, et al. The urgent need to recommend an intake of vitamin D that is effective. Am J Clin Nutr. 2007 Mar;85(3):649–50. doi: 10.1093/ajcn/85.3.649. [DOI] [PubMed] [Google Scholar]
- 44.Institute of Medicine . Dietary reference intakes for calcium and vitamin D. National Academies Press; Washington, DC: 2010. [PubMed] [Google Scholar]