Abstract
Objective
To better define the immunologic character of the T cell infiltrate in lupus nephritis.
Methods
We performed double immunohistochemical staining and clonotypic T cell receptor (TCR) beta-chain sequencing in multiple anatomic regions isolated by laser-capture microdissection from renal biopsies.
Results
SLE kidneys have a variably patterned and often extensive infiltrate of predominantly clonally expanded T cells of CD4 and CD8 lineages. CD4 T cells were prominent in nearly two-thirds of SLE biopsies, and distributed as broad periglomerular aggregates or intermixed with CD8 T cells forming periglomerular caps. Sequencing of the T cell TCR from periglomerular regions showed a predominance of clonally expanded T cells. The CD8 T cells, which were present in all biopsies, often adhered to Bowman's capsule and infiltrated the tubular epithelium. They exhibited features that suggest participation in an adaptive immune response: differentiation into CD28null memory-effector phenotype, trafficking of the same expanded clonotype to different regions of the kidney and to the peripheral blood, and clonal persistence for years in repeat biopsies. CD8 T cell tubulitis was especially associated with progressive changes.
Conclusions
The immunological characteristics of the infiltrating CD4 and CD8 T cells in the lupus kidney indicate they have the potential to mediate injury, which may be relevant to development of progressive renal failure. Whereas the oligoclonality of the CD4 T cell infiltrate is consistent with the paradigm of SLE as a class II-associated autoimmune disease, the finding of CD8 T cell clonality and trafficking implies participation in a distinct systemic adaptive immune response.
Introduction
In SLE glomeruli the enriched deposition of autoantibodies and lupus autoantigens formed the basis of the paradigm for the pathogenesis of acute glomerulonephritis(1). However, this mechanism does not adequately account for the development of the chronic abnormalities responsible for end stage renal disease (ESRD), and they raise the question whether additional immunologic mechanisms may operate. Reports have suggested that intrarenal T cells could play a more direct pathogenic role. These observations include the immunophenotypic identification of T cells in lupus kidneys (2-5) and the association between the infiltration of T cells in periglomerular and interstitial regions of the kidney and the expression within laser-captured glomeruli of genes implicated in glomerulosclerosis (6). Analogously, in the NZB/W mouse, intrarenal T cells accumulate during glomerulonephritis (7), while breeding studies in the NZM2328 lupus mouse distinguish between genes governing development of the autoantibody mediated spontaneous acute glomerulonephritis and those mediating T cell dominated chronic nephritis(8, 9).
The lineage of the infiltrating T cell is critical to potential pathogenic mechanisms. One anticipates finding clonally expanded CD4 T cells because of the abundance of glomerular immune complexes that could be digested and presented by dendritic cells or macrophages to CD4 T cells in the context of MHC class II lupus susceptibility allotypes(10, 11) and because these CD4 T cells could provide help for autoantibody production in the tubulointerstitial compartment(12, 13). Conversely, the presence of CD8 T cells is not explained by a MHC class II-based cognitive recognition process, unless CD8 T cells are secondarily attracted by an innate immune or cytokine mechanisms. CD4 and CD8 T cells have been identified in the lupus kidney (2). D'Agati et al. found CD8 T cells predominated in most biopsies (2), but that CD4 T cell proportions correlated with activity indices. Alexopoulos emphasized the correlation between glomerular function and interstitial CD8 T cells (3). Masutani reported a CD4 T cell predominance (4). Subsequently Couzi et al., reemphasized periglomerular CD8+ T cells (5) and reported the numbers of CD8 T cells correlated with increasing activity indices, serum creatinine, cellular crescents and poor response to therapy. Couzi proposed these CD8 T cells are secondarily recruited by type I interferons from plasmacytoid dendritic cells (5) perhaps activated by ingestion of immune complexes with signaling through TLR7 and TLR9.
While observations point to an association between intrarenal T cells and renal injury, the basic immunologic characteristics of infiltrating T cells have not been sufficiently delineated to elucidate their immunologic significance or potential to exert a more direct pathogenic role. The major question is whether the T cell accumulation in the kidney consists of lymphocytes entering the kidney secondarily and non-clonally in response to injury and inflammation by innate or chemokine mechanisms(5), or whether T cells directly mediate tissue injury through their activation, differentiation to memory-effector phenotype(14, 15), and clonal expansion in an adaptive immune response characterized by cognitive interactions with the clonotypic T cell receptor (TCR) and effector functions(16). The differing immunologic characteristics of these two primary scenarios can be distinguished by immunophenotyping, which delineates lineage and differentiation stage, and TCR repertoire analysis(16), which defines the clonal composition of the T cell infiltrate through structural features of the clonotypic T cell TCR β-chain in different sites of renal involvement isolatable by laser capture microdissection.
Accordingly, we sought to delineate the immunologic features of the T cells infiltrating the various regions of the kidney in a retrospective cross sectional study of different classes of severe lupus nephritis with particular emphasis on characterizing the presence and features of the CD8 T cells. We explored the feasibility of combining several methods to search for clues about the nature of the immune processes responsible for the accumulation of the intra-renal T cells. A preliminary report of this work has appeared in abstract form (17).
Materials and Methods
Study population
Cases were classified as SLE according to ACR criteria(18). The study subjects participated voluntarily and gave informed consent, or residual archival renal biopsy tissue was used. The study followed institutional guidelines and was approved by the Columbia University IRB.
Flow cytometry on peripheral blood and immunohistochemistry on renal biopsies
Purified peripheral blood CD4 and CD8 T cells from five cases were sequentially positively selected using magnetic beads (Dynal) and cell sorting (FACSAria, BD) as described(19). 25 biopsies including 6 cases with repeat biopsies were studied by 3-color immunohistochemistry on paraffin sections to study patterns of T cell anatomic distribution and immunophenotype. For immunostaining, 3μ paraffin sections from residual biopsies were steamed and stained with the first primary antibody from either rabbit anti-human CD3 or CD4 (Cell Marque Inc, CA), or goat anti-human CD28 (R&D Systems, MN) or monoclonal mouse anti-human CD8 (DAKO) as described(19). This was followed by a species-specific secondary biotinylated antibody and Alexa Fluo 555 Streptavidin (Vector Laboratories). After blocking, second stage staining was similarly performed followed by fluorescein-conjugated avidin, and DAPI counterstaining. The three fluorescent images taken at different wavelengths on a Zeiss Axiovert 200, running Axiovision 4.5, were merged. At least four to six merged and unmerged images were examined to count the proportion and phenotype of T cells in the samples.
T cell repertoire β-chain length distribution analysis to determine the extent of clonal expansions in the repertoire of infiltrating T cells
RNA from renal biopsy sections or peripheral blood T cell subsets was extracted and reverse-transcribed as described(19, 20). For some biopsies adequate residual frozen material was not available for this retrospective study. The third complementarity determining region (CDR3) region within the TCR β-chain and its flanking sequences were amplified either in 24 separate polymerase chain reactions, using forward primers specific for each TCR β-chain variable region (Vβ) family(21), or using degenerate primers that reacted with all BV families. The IMGT and Arden nomenclature for variable region families were used(21-23). Cloning and nucleotide sequencing of amplified β-chain CDR3 regions were performed as described.(19, 21) CDR3 sequences were edited and aligned (Geneious) and analyzed using IMGT/V-QUEST criteria.(22) Representative sequences were deposited in Genbank. (http://www.ncbi.nlm.nih.gov/genbank) To describe repertoire diversity for the sequences in each Vβ family analyzed, the Simpson's diversity index (SDI) was calculated as described, using 20-96 sequences from amplifications with primers specific for four or more BV families(24). The SDI ranges from 0 to 1, with 1 representing maximal polyclonal diversity, and 0 representing a single monoclonal expansion. To examine the distribution and trafficking of T cell clones, we used laser capture microdissection to isolate periglomerular, glomerular, intertubular and tubular compartments (6). For the SDI calculations 9 cases were studied after laser capture for T cells infiltrating the periglomerular interstitium and 11 cases for the intertubular interstitium. Interpretation of clonal trafficking was based on the distribution of particular clones across the anatomic regions of the kidney, which provides an important insight into the immunologic characteristics of a T cell. Clones engaged in peptide-driven recognition are likely to exhibit clonal expansions in a given site, be prevalent in two or more spatially distinct but anatomically similar sites, and persistently represented across the repertoire in these sites over time in repeat biopsies.
Anatomic Pathology
The glomerular pathologic findings were specified according to the ISN/RPS classification of lupus nephritis(25). Activity and Chronicity indices were defined according to Austin (26).
Statistical Analysis
The Wilcoxon rank sum test was used for comparisons. A t-test was used to compare the means of independent or coordinate samples. Pearson correlation coefficients were calculated. A two-tailed p-value < 0.05 was considered significant. SPSS 17 software was used for all analyses (SPSS, Chicago, Il).
Results
Clinical and pathological features of study cases
Table 1 summarizes the main points of the clinical and pathologic features of the cases. All patients met ACR SLE classification criteria.
Table 1.
Clinical and pathological information
| Clinical data at time of biopsy |
Biopsy Findings |
Post Biopsy |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case No. | Age Onset SLE | Sex | Duration disease (Yrs) | Yr Bx | Age (Yrs) | anti DNA titer (<10) | C3 (83-177) | C4 (16-47) | CH50 (60-144) | Serum creat. (1.0-1.2) | Ur Pr | ISN/RPS Class | AI | CI | TBM Dep | Therapy | Follow up (yrs) | Final Outcome Status |
| 1 | 48 | M | 5 | 2007 | 51 | 48 | 12 | 122 | 2.7 | 4 | V | 1 | 2 | 0 | MMF+Pred | 3 | 2 | |
| 2 | 24 | F | 19 | 2007 | 40 | 9766 | 48 | 9 | 60 | 0.9 | 3 | IV-G & V | 18 | 3 | + | Imuran+Pred | ||
| 2 | 24 | F | 19 | 2008 | 41 | 754 | 42 | 16 | 45 | 3.3 | 3 | IV-G & V | 6 | 10 | + | CTX,MMF+Pred | 4 | 3 |
| 3 | 32 | F | 8 | 2004 | 32 | 2087 | 38 | 3 | 3 | 1.5 | 3 | III & V | 6 | 2 | + | MMF+Pred | ||
| 3 | 32 | F | 8 | 2007 | 36 | 872 | 64 | 9 | 24 | 1.9 | 2 | IV-S | 17 | 3 | + | CTX,MMF+Pred | 7 | 2 |
| 4 | 21 | M | 5 | 2006 | 22 | 3153 | 37 | 11 | 19 | 0.6 | 2 | IV-S | 8 | 0 | 0 | MMF+CSA+Pred | 4 | 0 |
| 5 | 33 | F | 16 | 2005 | 44 | 1770 | 53 | 20 | 93 | 0.8 | 3 | III & V | 6 | 3 | + | MMF+Pred | 5 | 0 |
| 6 | 20 | F | 7 | 2006 | 23 | 1513 | 32 | 5 | 1.6 | 2 | III & V | 3 | 7 | + | Imuran+Pred | 5 | 2 | |
| 7 | 11 | F | 16 | 2003 | 19 | 185 | 47 | 6 | 38 | 1.2 | 3 | III & V | 3 | 6 | + | CTX+Pred+CSA | ||
| 7 | 11 | F | 16 | 2005 | 21 | 14 | 59 | 13 | 1.3 | 2 | III & V | 3 | 9 | + | CTX+Rituximab,MMF | 4 | 1 | |
| 8 | 8.5 | F | 9.5 | 2003 | 11 | 17026 | 33 | 5 | 8 | 0.5 | 3 | IV-G & V | 18 | 3 | + | CTX,MMF+Pred | 8 | 0 |
| 9 | 20 | F | 7 | 2006 | 22 | 456 | 98 | 19 | 117 | 2.3 | 3 | III & V | 8 | 4 | + | CTX,MMF+Pred | 3 | 1 |
| 10 | 42 | F | 12 | 2006 | 49 | 1919 | 51 | 9 | 56 | 1.8 | 3 | IV-S | 12 | 6 | + | CTX+Pred | 5 | 1 |
| 11 | 14 | M | 5 | 2007 | 15 | 7 | 52 | 6 | 49 | 0.7 | 3 | III & V | 6 | 0 | 0 | CTX+Pred | 4 | 0 |
| 12 | 36 | F | 7 | 2005 | 37 | 1346 | 60 | 11 | 1.7 | 3 | V | 1 | 2 | 0 | CTX,MMF+Pred | 5 | 0 | |
| 13 | 17 | F | 12 | 2004 | 20 | 9216 | 40 | 6 | 0 | 0.6 | 1 | IV-G & V | 12 | 1 | 0 | MMF+Pred | ||
| 13 | 17 | F | 12 | 2004 | 22 | 2180 | 44 | 6 | 22 | 0.8 | 3 | IV-G & V | 15 | 4 | + | Imuran+Pred,CTX | ||
| 13 | 17 | F | 12 | 2005 | 23 | 903 | 103 | 25 | 67 | 1.2 | 3 | IV-G & V | 14 | 5 | 0 | MMF+Rituximab | ||
| 13 | 17 | F | 12 | 2008 | 26 | 46 | 53 | 18 | 147 | 5.2 | 3 | IV-G & V | 9 | 9 | 0 | MMF+Pred | 9 | 3 |
| 14 | 17 | F | 6 | 2007 | 19 | 376 | 54 | 7 | 86 | 2.7 | 3 | IV-S & V | 7 | 9 | + | Pred | 3 | 1 |
| 15 | 20 | F | 13 | 2006 | 29 | 180 | 66 | 13 | 36 | 2 | 3 | IV-G & V | 13 | 3 | 0 | CTX,MMF+Pred | ||
| 15 | 20 | F | 13 | 2009 | 32 | 11 | 91 | 28 | 157 | 5.4 | 2 | IV-G & V | 6 | 11 | 1 | MMF+Pred | 5 | 3 |
| 16 | 12 | M | 14 | 2002 | 17 | 1027 | 65 | 6 | 91 | 0.8 | 1 | IV-S | 8 | 2 | + | CTX+Pred | 3 | 0 |
| 17 | 20 | F | 7 | 2006 | 22 | 0.5 | 2 | III | 4 | 0 | + | Pred | 1 | |||||
| 17 | 20 | F | 7 | 2007 | 23 | 155 | 52 | 3 | 0.5 | 3 | IV-G & V | 13 | 3 | + | MMF+Pred | 0 | N/A | |
Duration disease (Yrs) to present; Serum creat.=serum creatinine mg/dl;Ur Pr=Urinary Protein, 0-4+; AI,CI= activity and chronicity indices; TBM dep=Tubular-basement membrane Ig deposits; CTX=cyclophosphamide; CSA=Cyclosporine; MMF=Mycophenolate mofetil; Pred=Prednisone; Final Outcome status, 0=normal renal function with persistent proteinuria, 1=serum creatinine elevated over U.L.N. ≥1.0mg/dl females, ≥1.2mg/dl males, but less than doubling, 2=doubling serum creatinine, 3 ESRD, S/P transplant, N/A not available.
Different patterns of T cell distribution in the kidney
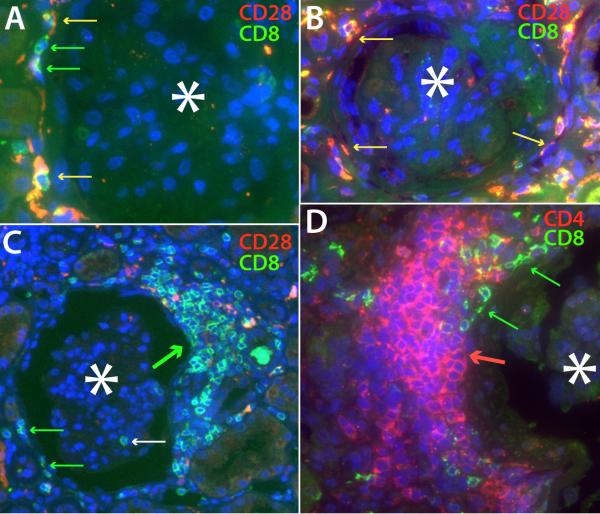
Figures 1 and 2 illustrate T cells distribution patterns in different regions of the kidney. There were four main periglomerular patterns: Adherent, T cells were scattered but arrayed closely around the glomerular Bowman's capsule, with some lymphocytes tightly flattened against the outer capsular membrane, suggesting an effector T cell in an immunologic synapse(27) (Figure 1, A, B, and D, and Table 2). They were mainly CD8+CD28null memory-effectors, Figure 1, A, but occasionally were CD8+CD28+ (Figure 1B, table 2). Less frequently CD4 T cells exhibited the same distribution (Figure 2D). The glomerulus rarely contained T cells, although when present, they were CD8+CD28null phenotype, and were correlated with the presence of adherent CD8 T cells (r=0.572, p=0.003). Focal cap, a discrete cluster of T cells at a circumscribed region of the glomerular capsule (Figure 1C). They almost exclusively consisted of CD8 lineage T cells. Uncommonly CD4 cells formed similar caps, and their presence was correlated with periglomerular adherent CD4 T cells (r=0.65, p=0.004). Mixed CD4 and CD8 T cell caps comprised the third pattern (Figure 2A and 2D). They were often larger than focal caps, and more circumferential, (Figure 2A) sometimes filling regions between two or more closely situated glomeruli and their surrounding tubules. Mixed caps were especially correlated with the presence of CD4 periglomerular adherent cells (r=0.51, p=0.012) and CD4 focal caps (r=0.46, p=0.025). These occurred within the mixed cap or separately. The fourth pattern was Aggregate, a large infiltration of more evenly distributed and sometimes loosely spaced CD4 T cells not clearly related to a renal structure (Figure 1D) and sometimes contiguous with a perivascular CD4 T cell cuff, or extending into the intertubular region. There were no CD8 preponderant aggregates. When CD8 T cells were identified in glomeruli with CD4 T cell aggregates, the CD8 T cells were not diffusely admixed with the CD4 T cells of the aggregate, as in a mixed cap (Figure 2A and D), but were found coexisting in focal cap or adherent patterns (Figure 1D, Table 2).
Figure 1.
Patterns of T cell distribution in the periglomerular and intertubular regions are shown using double staining for either CD8 (green) and CD28 (red) or CD4 (red) and CD8 (green). Co-localization in these merged images produces a yellow to fuchsia color, depending on relative intensities. For all images, an asterisk is placed in the center of the glomerulus, whose location is visible by autofluorescence and DAPI nuclear counterstain. A. Two CD8+CD28null T cells (green arrow) and, a few CD8+CD28+ T cells (yellow arrows) appear closely adherent to the exterior of Bowman's capsule. B. CD8+CD28+ T cells (yellow arrows) are closely adherent to and flattened against the glomerular capsule, suggesting an “immunologic synapse”. C. A larger cap-like accumulation of CD8+CD28null T cells outside Bowman's capsule (thick green arrow) surrounds a portion of a glomerulus. A few lymphocytes outside the cap appear closely adherent to the glomerular capsule (green arrows). The white arrow marks an intraglomerular CD8 T cell. D. A large T cell aggregate predominantly composed of CD4 T cells surrounds much of a glomerulus, while a smaller independent cap-like subcluster of CD8 T cells appears more closely adherent to the glomerular capsule (green arrows).
Figure 2. Low power views illustrate various T cell distribution patterns.
Images are stained as in figure 1. A. A large region of case 11 containing multiple glomeruli shows confluent mixed CD4-CD8 T cell periglomerular caps, extending as intertubular infiltrates. Some CD4 T cells (red arrows) and CD8 T cells (green arrows) adhere closely to the exterior of Bowman's capsule. There are multiple foci of CD8 tubulitis (white arrows), whereas CD4 tubulitis is extremely rare (blue arrow). B. A biopsy in 2005 from case 13 shows tubules with numerous adherent or intraepithelial CD8 T cells (white arrows). A few loosely dispersed CD4 T cells are also present in the intertubular region. C. Double staining for CD4 and CD3 distinguishes macrophages (CD4+ CD3-) (Orange arrows) from T cells. Most of the cells in this field are CD8 T cells (green staining). CD4 T cells that coexpress CD3 range in color from yellow to fuchsia (yellow arrows). D. An earlier biopsy in 2004 from case 13 shows a portion of a small mixed cap of CD4 and CD8 T cells with adherent CD4 T cells (red arrows) and adherent CD8 T cells (green arrow).
Table 2.
Patterns of CD4 and CD8 T Cell Distribution in Different Biopsies Compared to Assessment of TCR β-Chain Sequence Diversity
| Periglomerular |
Tubules |
β-Chain Sequence Diversity |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CD8 | CD4 | CD8 | CD4 | CD8 | CD4 | CD8/CD4 | CD8 | CD4 | CD8 | CD4 | CD8 | CD4 | CD8 | CD4 | Simpson Diversity Index | |||
| Case No. | Yr Bx | Intra-Glomerular | Aggregate | Focal Cap | Mixed Cap | Adherent | Intertubular interstitium | Tubulitis | Adherent basal Tubule | Peri-glomerular interstitium | Peri-tubular interstitium | |||||||
| 1 | 2007 | 0 | 0 | 0 | 3 | 0 | 2 | 0 | 1 | 2 | 0.5 | 4 | 1 | 0 | 0 | 0 | ||
| 2 | 2007 | 0 | 0 | 0 | 3 | 0 | 1 | 1 | 0 | 2 | 0 | 1 | 0 | 0 | 0 | 0 | ||
| 2 | 2008 | 0 | 0 | 0 | 3 | 2 | 4 | 3 | 1 | 4 | 0.5 | 3 | 4 | 0 | 1 | 0 | ||
| 3 | 2004 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.5 | 0 | 0 | 1 | 0 | ||
| 3 | 2007 | 2 | 0 | 0 | 4 | 2 | 2 | 0 | 2 | 1 | 2 | 3 | 3 | 0.5 | 2 | 0.5 | ||
| 4 | 2006 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0.5 | 0 | 2 | 1 | 1 | 0 | 1 | 0 | 0 | 0 |
| 5 | 2005 | 0 | 0 | 0 | 3 | 1 | 0 | 0 | 2 | 0 | 3 | 1 | 2 | 0 | 0 | 0 | 0.86 | |
| 6 | 2006 | 2 | 0 | 0 | 3 | 2 | 0 | 0 | 1 | 0 | 2 | 2 | 3 | 0 | 2 | 0 | 0 | 0.75 |
| 7 | 2003 | 0.5 | 0 | 0 | 2 | 1 | 0.5 | 0 | 3 | 0 | 3 | 2 | 2 | 0 | 2 | 0 | ||
| 7 | 2005 | 0 | 0 | 0 | 2 | 2 | 0 | 2 | 2 | 1 | 2 | 0.5 | 3 | 0 | 1 | 0 | ||
| 8 | 2003 | 0 | 0 | 0 | 2 | 2 | 1 | 0 | 2 | 0.5 | 2 | 0.5 | 0 | 0 | 0 | 0 | 0.75 | 0.86 |
| 9 | 2006 | 0.5 | 0 | 0 | 2 | 0 | 0 | 0 | 2 | 0 | 1 | 2 | 3 | 0.5 | 1 | 0 | 0.53 | 0.74 |
| 10 | 2006 | 3 | 0 | 0 | 1 | 0 | 0 | 0 | 3 | 0 | 3 | 3 | 2 | 0 | 2 | 0 | ||
| 11 | 2007 | 1 | 0 | 0 | 0 | 2 | 2 | 4 | 2 | 4 | 3 | 4 | 4 | 1 | 3 | 1 | 0.59 | |
| 12 | 2005 | 1 | 0 | 0 | 0 | 2 | 3 | 3 | 1 | 0 | 1.5 | 1.5 | 1 | 0 | 1 | 0 | 0.56 | |
| 13 | 2004 | 1 | 0 | 0 | 2 | 3 | 3 | 2 | 2 | 2 | 4 | 0 | 1 | 0 | 2 | 0 | ||
| 13 | 2005 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 1 | 0 | 3 | 0.5 | 4 | 0.5 | 3 | 0 | 0.31 | |
| 13 | 2008 | 0 | 0 | 0 | 0 | 0 | 2 | 2 | 1 | 0 | 2 | 2 | 4 | 0 | 0 | 0 | 0.69 | 0.61 |
| 14 | 2007 | 2 | 0 | 0 | 0 | 2 | 0 | 3 | 3 | 0.5 | 4 | 1 | 4 | 1 | 3 | 0 | 0 | 0.64 |
| 15 | 2006 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0.73 | 0.82 |
| 15 | 2009 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 2 | 3 | 4 | 3 | 4 | 1 | 3 | 1 | ||
| 16 | 2002 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 2 | 0 | 0.5 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 17 | 2006 | 2 | 0 | 0 | 0 | 2 | 1 | 0 | 3 | 1 | 1 | 0.5 | 2 | 1 | 0 | 0 | ||
| 17 | 2007 | 3 | 0 | 0 | 0 | 4 | 0.5 | 0.5 | 3 | 0 | 3 | 1 | 3 | 0 | 3 | 0 | 0.77 | |
Semiquantitative grading (0-4+) of the number and extent of infiltrating T cells in each pattern of distribution, 0=absent, 0.5=trace, 1=mild, 2=moderate, 3=severe and focal, 4 severe and diffuse; The Simpson Diversity Index summarizes the number and extent of clonal expansions demonstrated by sequencing, 0=a single clone, 1=a polyclonal repertoire.
In the tubular region T cells were distributed in three patterns: Diffuse intertubular, where widely distributed CD4 or CD8 T cells infiltrated the intertubular interstitium without a particular pattern of additional localization (Table 2). Adherent intertubular where T cells appeared adherent to basal portions of the tubular epithelium (Figure 2A and B), a pattern predominantly exhibited by CD8 T cells; and intratubular or tubulitis, a pattern of localization almost exclusively shown by CD8 T cells (Figure 2A and B). CD8 tubulitis was closely correlated with adherent intertubular (r=0.53, p=0.007) and diffuse intertubular (r=0.626, p=0.001) CD8 T cell infiltration, emphasizing a close relationship among these CD8 T cells. Relatively rare and isolated instances of CD4 T cell tubulitis were encountered (Figure 2A). Interestingly the few cases with CD4 tubulitis also mainly exhibited CD4 adherent basal tubular cells (r=0.756, p=0.0001) and adherent periglomerular CD4 T cells (r=0.52, p=0.01). The presence of mixed caps also correlated with CD4 tubulitis (r=0.6, p=0.002) and adherent basal tubular CD4 T cells (r=0.5, p=0.015) and importantly with CD8 tubulitis (r=0.47, p=0.02). In samples where CD4 and CD3 reagents were used, occasionally CD4+CD3- myeloid lineage cells were identified in the periglomerular or intertubular region by staining only for the red fluorochrome in an unmerged and merged image, and as also suggested by their convoluted nuclei, and abundant cytoplasm (Figure 2C).
Associations in progressive renal disease
In the five cases (2, 3, 7, 13, and 15) with progressive renal disease where repeat biopsies were available and the serum creatinine increased in the interval between biopsies (Table 1), comparison of the pattern of T cells infiltration between the two biopsies (Table 2) demonstrated a significant increase in the number and size of mixed CD8-CD4 periglomerular caps (mean score 0.6 vs 2.4, p=0.027) and the extent of CD8 tubulitis (mean score 0.8 vs. 3.6, p=0.003). (One tailed t test, correlated samples) Some cases with marked increases in mixed CD8-CD4 caps also had striking increases in CD4 periglomerular adherent cells, while some cases developing extensive CD8 T cell tubulitis exhibited increases in CD8 peritubular adherent cells.
Comparison of patterns of T cell distribution in different biopsies
Table 2, illustrates that biopsies varied considerably in their patterns of T cell distribution, usually exhibiting more than one pattern. Three main groups were evident: one where CD4 periglomerular aggregates predominated, Table 2; a second group where CD4 and CD8 T cell mixed caps predominated; and a third smaller group where CD8 T cells predominated. However, CD8 T cell infiltration was found in all biopsies. The samples exhibiting a predominance of CD4 periglomerular aggregates or mixed caps also usually had a significant presence of CD8 T cells in different patterns (Table 2). These patterns usually were fairly uniform across the sections, but occasionally varied. In case 2(Figure 1D) of the first group, CD4 T cells were present in a periglomerular aggregate and a diffuse intertubular pattern. CD8 T cells were infrequent, but interestingly, the few CD8 T cells are often present as periglomerular adherent T cells (Figure 1D), in contrast to CD4 T cells.
Case 11 (Figure 2A and C), representative of the second group, is characterized by a predominant pattern of mixed CD4 and CD8 T cell caps. Additional patterns of CD4 T cell distribution, variably distinct from the mixed T cell cap are present. In addition case 11 has a considerable proportion of CD8 T cells present in a combination of periglomerular, basal tubular adherent and intratubular, “tubulitis” patterns (Figure 2A and Table 2). Interestingly these patterns (Figure 2D and 2B) characterize the four consecutive biopsies of case 13 culminating in ESRD.
In contrast, the third group, consisting of cases 16 and 17, (Figure 1C) illustrates a marked preponderance of CD8 T cells with very few CD4 T cells and no CD4 aggregates or mixed T cell caps. The CD8 T cells are in a pattern of periglomerular adherent and/or focal caps, with varying tubular involvement, reflecting the clustering of significant correlation values for these patterns within the overall correlation matrix for CD8 T cells.
TCR β-chain sequences show that a small number of clones account for most of kidney-infiltrating T cells
Figure 3 uses histograms of the distribution of TCR β-chain lengths obtained by TCR β-chain sequencing of a pool of 4 sections of the whole biopsy from case 16 to illustrate that the overall repertoire of infiltrating αβT cells is composed of a limited number (n=28) of expanded clones varying in number from 2 to 5 clones per BV family. Often several clones occur at the same CDR3 length. To better describe the relative preponderance and distribution of clones, multiplex PCR analysis with degenerate primers that react with all BV families was used. Clones were only identified in 8 BV families indicative of selective clonal entry. The mean Simpson diversity index (SDI) for this entire repertoire is 0.552, consistent with considerable oligoclonality. Staining of case 16 (not illustrated) revealed that the T cell infiltration consisted of small numbers of CD28nullCD8+T cells mainly located in focal caps similar to those illustrated (Figure 1C, Table 2). Supporting the inference that CD8 T cells accounted for much of the sequenced sample, the dominant BV7 T cell clone at CDR3 length=9 was found shared with the peripheral blood CD8 subset as indicated by the black filled rectangle (Figure 3). This result is representative of 3 additional kidney biopsies, cases 3, 8 and 15, studied by TCR β-chain sequencing, which also showed the infiltrating repertoire of T cells consisted of a small number of T cell clones, (data not illustrated).
Figure 3.
The TCR β-chain nucleotide sequences of different clones are summarized in histograms according to their CDR3 region length, and the size of the clones, as represented by the number of identical clonotype sequences, is shown by the size of each rectangle. A. 28 variably expanded clones in 8BV families comprise the repertoire of αβ T cells present in a renal biopsy of case 16. The black-filled rectangle in the BV7 repertoire indicates a clone shared with the peripheral blood CD8 subset. The distribution of a reference polyclonal CD4 T cell repertoire is shown with gray fill. B. αβ T cell clones in laser-captured intertubular interstitium of case 8. Several clones are present at the same CDR3 length. The black-filled rectangle in the BV3 repertoire indicates a clone shared with the peripheral blood CD8 subset. C. A BV3 αβT cell clone in 5 anatomic regions obtained by laser-capture in case 13 in 2008 is identical to a clone present in the CD8 peripheral blood in 2002. D. Presence of the same αβ T cell clone in two anatomic regions of a renal biopsy in case 13 and its persistence in the kidney over a six-year period.
TCR β-chain sequences from regions of the lupus kidney reveal that the repertoire of kidney-infiltrating T cells is composed of a small number of clones
Laser capture of the biopsy intertubular interstitium was performed to investigate the number and distribution of clones in a defined anatomical region. Using TCR β-chain sequencing, Figure 3B demonstrates the oligoclonal repertoire in case 8, which was selected to illustrate the most multiclonal intertubular sample. SDI=0.86 (Table 2). The intertubular interstitial repertoire consisted of 94 sequences that were grouped into 32 clones of which 9 were single sequence clones and 23 were multisequence expanded clones. Clones were present in 9 BV families, of which 6 are illustrated. Immunostaining in the intertubular interstitium of this case revealed a predominance of CD28nullCD8+ T cells, suggesting the majority of T cells characterized by sequencing was likely to be of CD8 lineage and memory-effector phenotype, consistent with the clonal expansions. Supporting this interpretation was the finding that the BV3 clone CASSRGVYEQY at position CDR3=8 is present as an expanded clone (8 sequences) in the peripheral blood CD8 subset (Table 3). Laser capture and sequencing of the intertubular interstitium in 4 other Group 1 biopsies (48 to 96 sequences for each BV family studied) (cases 4, 5, 6 and 9), gave similar results with a lower SDI, indicating a substantially oligoclonal repertoire, mean = 0.59 (Table 2). Analysis of the laser captured intertubular interstitium for samples in the second and third case groups also demonstrated that the repertoire of infiltrating T cells was highly oligoclonal, (Table 2). Table 2 illustrates that the SDI for the laser captured periglomerular interstitium for five samples of the first group of cases with predominantly CD4 aggregate pattern in this site ranged from 0 to 0.86 (mean 0.43), also indicative of a fairly high degree of oligoclonality. Indeed an SDI=0 was found. Interestingly, the repertoire was also oligoclonal, SDI=0.77, in the periglomerular interstitium of case 17 containing an almost exclusive representation of CD8+CD28null memory effector T cells present as focal caps or periglomerular adherent cells, without a major CD4 T cell presence.
Table 3.
Identical Clonotypes in Different Sites Indicating Clonal Trafficking Between Subcompartments of the Kidney and Between Kidney and Blood*
| T Cell Receptor β-Chain Structure |
|||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case | Sample year | Sample Type | Size of Clone (#seq.) | CDR3 Length | CDR3 Region Sequence and Analysis | TRBV Element (BV) | TRBD Element | TRBJ Element | |||||||||||||||||||
| 6 | 2006 | Periglom. | 30 | 12 | TGT | GCC | AGT | AGT | Agg | gGG | GGG | Cag | ggC | TAT | GGC | TAC | ACC | TRBV19 | 1*01 | 1-2*01 | |||||||
| Interstitium | C | A | S | S | R | G | G | Q | G | Y | G | Y | T | (17) | |||||||||||||
| 2006 | Intertubular | 14 | 12 | TGT | GCC | AGT | AGT | Agg | gGG | GGG | Cag | ggC | TAT | GGC | TAC | ACC | TRBV19 | 1*01 | 1-2*01 | ||||||||
| Interstitium | C | A | S | S | R | G | G | Q | G | Y | G | Y | T | (17) | |||||||||||||
| 15 | 2006 | Periglom. | 4 | 12 | TGT | GCC | AGT | AGT | ATt | GGG | ACA | GGC | TCC | TAC | GAG | CAG | TAC | TRBV19 | 1*01 | 2-7*01 | |||||||
| Interstitium | C | A | S | S | I | G | T | G | S | Y | E | Q | Y | (17) | |||||||||||||
| 2006 | Intertubular | 1 | 12 | TGT | GCC | AGT | AGT | ATt | GGG | ACA | GGC | TCC | TAC | GAG | CAG | TAC | TRBV19 | 1*01 | 2-7*01 | ||||||||
| Interstitium | C | A | S | S | I | G | T | G | S | Y | E | Q | Y | (17) | |||||||||||||
| 13 | 2004 | Periglom. | 2 | 13 | TGT | GCC | AGC | Agg | cta | GAC | Aat | gtc | cca | aAC | AAT | GAG | CAG | TTC | TRBV7-3 | 1*01 | 2-1*01 | ||||||
| Interstitium | C | A | S | R | L | D | N | A | P | N | N | E | Q | F | (6S1) | ||||||||||||
| 2004 | Intertubular | 50 | 13 | TGT | GCC | AGC | Agg | cta | GAC | Aat | gtc | cca | aAC | AAT | GAG | CAG | TTC | TRBV7-3 | 1*01 | 2-1*01 | |||||||
| Interstitium | C | A | S | R | L | D | N | A | P | N | N | E | Q | F | (6S1) | ||||||||||||
| 13 | 2008 | Periglom. | 3 | 13 | TGT | GCC | AGC | Agg | cta | GAC | Aat | gtc | cca | aAC | AAT | GAG | CAG | TTC | TRBV7-3 | 1*01 | 2-1*01 | ||||||
| Interstitium | C | A | S | R | L | D | N | A | P | N | N | E | Q | F | (6S1) | ||||||||||||
| 2008 | Tubules | 5 | 13 | TGT | GCC | AGC | Agg | cta | GAC | Aat | gtc | cca | aAC | AAT | GAG | CAG | TTC | TRBV7-3 | 1*01 | 2-1*01 | |||||||
| C | A | S | R | L | D | N | A | P | N | N | E | Q | F | (6S1) | |||||||||||||
| 13 | 2008 | Periglom. | 14 | 19 | TGT | GCC | AGC | AGT | TTA | Ttt | ttg | gcc | aac | cgt | cGG | GAC | AGG | GGt | tCA | GAT | ACG | CAG | TAT | TTT | TRBV28 | 1*01 | 2-3*01 |
| Interstitium | C | A | S | S | L | F | L | A | N | R | R | D | R | G | S | D | T | Q | Y | F | (3) | ||||||
| 2008 | Intertubular | 20 | 19 | TGT | GCC | AGC | AGT | TTA | Ttt | ttg | gcc | aac | cgt | cGG | GAC | AGG | GGt | tCA | GAT | ACG | CAG | TAT | TTT | TRBV28 | 1*01 | 2-3*01 | |
| Interstitium | C | A | S | S | L | F | L | A | N | R | R | D | R | G | S | D | T | Q | Y | F | (3) | ||||||
| 2008 | Intertubular | 14 | 19 | TGT | GCC | AGC | AGT | TTA | Ttt | ttg | gcc | aac | cgt | cGG | GAC | AGG | GGt | tCA | GAT | ACG | CAG | TAT | TTT | TRBV28 | 1*01 | 2-3*01 | |
| Interstitium | C | A | S | S | L | F | L | A | N | R | R | D | R | G | S | D | T | Q | Y | F | (3) | ||||||
| 2008 | Tubules | 6 | 19 | TGT | GCC | AGC | AGT | TTA | Ttt | ttg | gcc | aac | cgt | cGG | GAC | AGG | GGt | tCA | GAT | ACG | CAG | TAT | TTT | TRBV28 | 1*01 | 2-3*01 | |
| C | A | S | S | L | F | L | A | N | R | R | D | R | G | S | D | T | Q | Y | F | (3) | |||||||
| 2008 | Glomerulus | 1 | 19 | TGT | GCC | AGC | AGT | TTA | Ttt | ttg | gcc | aac | cgt | cGG | GAC | AGG | GGt | tCA | GAT | ACG | CAG | TAT | TTT | TRBV28 | 1*01 | 2-3*01 | |
| C | A | S | S | L | F | L | A | N | R | R | D | R | G | S | D | T | Q | Y | F | (3) | |||||||
| 2002 | PB CD8 | 1 | 17 | TGT | GCC | AGC | AGT | TTA | Ttt | ttg | gcc | aac | cgt | cGG | GAC | AGG | GGt | tCA | GAT | ACG | CAG | TAT | TTT | TRBV28 | 1*01 | 2-3*01 | |
| C | A | S | S | L | F | L | A | N | R | R | D | R | G | S | D | T | Q | Y | F | (3) | |||||||
| 13 | 2008 | Glomerulus | 1 | 15 | TGT | GCC | AGC | AGC | TTa | ttc | cgt | GGG | ACA | GGG | caa | ACC | GGG | GAG | CTG | TTT | TTT | TRBV5-1 | 1*01 | 2-2*01 | |||
| C | A | S | S | L | F | R | G | T | G | Q | T | G | E | L | F | F | (5S1) | ||||||||||
| 2008 | Intertubular | 4 | 15 | TGT | GCC | AGC | AGC | TTa | ttc | cgt | GGG | ACA | GGG | caa | ACC | GGG | GAG | CTG | TTT | TTT | TRBV5-1 | 1*01 | 2-2*01 | ||||
| Interstitium | C | A | S | S | L | F | R | G | T | G | Q | T | G | E | L | F | F | (5S1) | |||||||||
| 8 | 2003 | Kidney | 2 | 8 | TGT | GCC | AGC | Agc | CGG | Ggc | gtg | TAC | GAG | CAG | TAC | TRBV28 | 2*01 | 2-7*01 | |||||||||
| Biopsy | C | A | S | S | R | G | V | Y | E | Q | Y | (3) | |||||||||||||||
| 2003 | PB CD8 | 8 | 8 | TGT | GCC | AGC | Agc | CGG | Ggc | gtg | TAC | GAG | CAG | TAC | TRBV28 | 2*01 | 2-7*01 | ||||||||||
| C | A | S | S | R | G | V | Y | E | Q | Y | (3) | ||||||||||||||||
| 16 | 2002 | Kidney | 1 | 9 | TGC | GCC | AGC | AGC | CCG | GGA | ata | ggt | ACT | GAA | GCT | TTC | TRBV4 | 2*02 | 1-1*01 | ||||||||
| Biopsy | C | A | S | S | P | G | I | G | T | E | A | F | (7S1) | ||||||||||||||
| 2002 | PB CD8 | 19 | 9 | TGC | GCC | AGC | AGC | CCG | GGA | ata | ggt | ACT | GAA | GCT | TTC | TRBV4 | 2*02 | 1-1*01 | |||||||||
| C | A | S | S | P | G | I | G | T | E | A | F | (7S1) | |||||||||||||||
The IMGT CDR3 length is shown in terms of number of amino acids. The nucleotide sequence of the CDR3 region, arranged in codons, is represented in upper case when the nucleotides are germline encoded. The germline D element residues are underlined. Non-germline encoded N and P diversity nucleotides are represented as lower case letters. The single letter amino acid code for each codon is indicated. The TRBV designation for the variable element is shown along with the Arden et al. designation in parentheses.
Persistence of T cell clones shared between multiple regions of the kidney, or between kidney and peripheral blood
We next compared the distribution of clones in different anatomic regions of the biopsy to assess whether the same clonotype trafficked to different regions. Figure 3C illustrates an interesting instance where a BV3 clone, CASSLFLANRRDRDRGSDTQYF, shown as a filled rectangle, was found in five anatomic regions in the 2008 biopsy of case 13, studied shortly before development of ESRD. The periglomerular interstitium had the greatest number of different additional clones, while the other regions of the kidney contained only the shared clone and one other BV3 clone. Staining of this case revealed mainly CD28nullCD8+ T cells within the glomerulus and within the tubules as tubulitis, (Figure 2B, Table 2) suggesting that the predominant clone shared by these sites is likely of CD8 lineage. This inference was confirmed by identifying the same shared clone in the peripheral blood CD8 T cell subset studied 6 years previously, in 2002 at the start of the glomerulonephritis (Figure 3C, Table 3). Case 15 contains two additional examples of shared clonotypes between periglomerular interstitium and the intertubular interstitium, and case 13 also exhibits clonal trafficking between the glomerulus and the intertubular interstitium, (Table 3). Similar sharing of a clonotype between periglomerular and intertubular interstitium was also found for case 6, (Table 3).
Figure 3D shows another example (case 13) where the BV6 family repertoire in the periglomerular interstitium of the biopsy obtained in 2002 consists of a single sequence clone, CASRLDNAPNNEQFF, which is also highly expanded (n=39) in the intertubular interstitium. No other T cell clones were identified at this site. However, in the repeat biopsy of this case 6 years later in 2008, the same shared clonotype is still identified in the periglomerular interstitium, and as a predominant clone in another laser-captured sample of tubules. The staining of the tubules at the time of the biopsy (not shown) revealed only CD28nullCD8+ T cell present as tubulitis similar to that present in 2005 (Figure 2B). In each of the other instances where a clone was found shared between two regions in an initial biopsy, the same clone was found in a subsequent repeat biopsy. In contrast, most of the unshared clones were not found in repeat biopsies, suggesting that clones that traffic to multiple sites persist in the kidney.
Discussion
This exploratory study sought to better define the immunologic character of the CD4 and CD8 T cell infiltrate in different classes of lupus nephritis and provide insights into mechanisms of chronic glomerulonephritis and ESRD. We found that SLE kidneys have a variable and often-extensive infiltrate of clonally expanded T cells present in several different patterns. CD4 T cells were prominent in periglomerular distribution in over two thirds of the biopsies and upon sequencing of these periglomerular regions, clonally expanded T cells predominated, Table 2. The CD4 clonal expansions are compatible with the paradigm of lupus as a class II-associated autoimmune disease with the clones possibly reflecting CD4 T cell help for autoantibodies, and likely relating to those previously described(13). Massengill and colleagues were the first to report oligoclonal expansions of T cells in lupus nephritis(28), and this was confirmed by Murata et al.(29). However they found no evidence of clonal sharing with the blood, and did not determine whether these expansions were members of the CD4 or CD8 T cell subset or how they related to lupus nephritis.
CD8 T cells were found in all biopsies and predominated in two of the 17 cases. Intriguingly, with the notable exception of the mixed CD4 and CD8 T cell periglomerular caps, the distribution of CD8 and CD4 T cells were largely anatomically separate. However, a highly unexpected result was the extent to which CD8 T cells exhibited characteristics of participating in an adaptive immune response. This included differentiation to CD28null memory-effector phenotype(14, 15), trafficking of the same expanded clonotype to different regions of the kidney, persistence of the same clonotype for years in repeat biopsies, and trafficking of members of the expanded clone between sites in the kidney. Additionally, the intrarenal trafficking of the same clonotypes identified as a CD8 T cell subset of peripheral blood in 3 of 5 cases studied at the time of the initial biopsy (Table 3) implicates infiltration of T cells into the kidney as part of a broader CD8 T cell response. The results are inconsistent with the hypothesis that numerous unexpanded polyclonal CD8 T cells secondarily enter the kidney as a generalized response to inflammation, and instead suggest that this lineage is actively involved in an adaptive immunologic axis in the lupus kidney. Moreover, the CD8 T cell infiltrate adherent to basal tubular cells and CD8 tubulitis were significantly negatively correlated with anti dsDNA titres, (-0.463, p=0.03 and -0.533, p=0.0.011, respectively), further emphasizing that CD8 T cells are likely not engaged in an autoimmune response to nuclear antigens. Reciprocally, the highest mean anti dsDNA titres, 3043, were in the group exhibiting CD4 periglomerular aggregates (Table 1).
The pattern of CD8+CD28null T cells closely adherent to periglomerular or tubular epithelium suggests the profile of a CD8 effector cell in an immunologic synapse with its target (27). The clustering on immunostaining of the different CD8 T cell patterns together with the clonotype localization and traffic provided by laser capture microdissection suggests a common autoantigen is expressed by these different cell types that both drives this CD8 T cell response and is the target of its effector function.
The most likely model envisioned to account for the CD8 T cell clonal response is a separate autoimmune response to renal peptides presented in the context of class I MHC allotypes induced by the presence of intrarenal inflammation. Alternatively, the CD8 T cell response could be analogous to the activated intraepithelial CD8 T cell expansions in celiac disease, where the intraepithelial CD8 T cells are cognitively unrelated to gliadin or the CD4 transglutamase autoantigens of celiac disease, but mediate the destruction of the intestinal epithelial cells that results in sprue(30, 31).
The scope of this exploratory study was limited by its retrospective design because relatively small amounts of residual tissue were available for study and paired blood samples were often unavailable. Furthermore, the study was not structured to examine clinical outcome and correlations, but it was still notable that 70% of cases progressed to some renal insufficiency, and that classes III, IV and V did not differ markedly in the characteristics of their T cell infiltration.
Within the scope of these limitations, in 5 cases with repeat biopsies showing worsening renal failure based on serum creatinine elevation (Table 1), we found significant increases in the number and size of mixed CD8-CD4 periglomerular caps (p=0.027) and especially in the extent of CD8 tubulitis (p=0.003) (Table 2) from those of the initial biopsy, further emphasizing the relationship of these two immunologic features and the need to explore their role in indicating the presence of, and mediating, progressive chronic changes. Moreover across all cases CD8 T cell tubulitis was correlated with interstitial infiltration on pathologic examination (p=0.031, data not shown)
The persistence of the same expanded clones for up to 6 years emphasizes the sustained nature of the immunologic drive underlying the CD8 T cell clonal expansion in lupus. This suggests that particular clones detected years earlier in a temporally distant blood sample remain and expand in the kidney over the intervening years in parallel with progressive renal failure despite the abundant administration of standard immunosuppressive therapy mainly targeting the autoantibody response. These immunological characteristics of the infiltrating T cells, particularly of the CD8 lineage, present either in mixed periglomerular caps or as tubulitis, suggest that they may mediate progressive glomerular and tubulointerstitial injury independent of the classic immune recognition events intrinsic to SLE. Based on these findings, a more extensive prospective study of clinical outcome and effect of current therapy on the T cell infiltrate appears warranted.
Acknowledgments
Supported in part by USPHS grants AI046132 and AI055704
Footnotes
The Authors have no financial conflicts of interest
References
- 1.Agnello V, Koffler D, Kunkel HG. Immune complex systems in the nephritis of systemic lupus erythematosus. Kidney Int. 1973;3:90–99. doi: 10.1038/ki.1973.15. [DOI] [PubMed] [Google Scholar]
- 2.D'Agati VD, Appel GB, Estes D, Knowles DM, 2nd, Pirani CL. Monoclonal antibody identification of infiltrating mononuclear leukocytes in lupus nephritis. Kidney Int. 1986;30:573–581. doi: 10.1038/ki.1986.223. [DOI] [PubMed] [Google Scholar]
- 3.Alexopoulos E, Seron D, Hartley RB, Cameron JS. Lupus nephritis: correlation of interstitial cells with glomerular function. Kidney Int. 1990;37:100–109. doi: 10.1038/ki.1990.14. [DOI] [PubMed] [Google Scholar]
- 4.Masutani K, Akahoshi M, Tsuruya K, Tokumoto M, Ninomiya T, Kohsaka T, Fukuda K, Kanai H, Nakashima H, Otsuka T, et al. Predominance of Th1 immune response in diffuse proliferative lupus nephritis. Arthritis Rheum. 2001;44:2097–2106. doi: 10.1002/1529-0131(200109)44:9<2097::AID-ART360>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 5.Couzi L, Merville P, Deminiere C, Moreau JF, Combe C, Pellegrin JL, Viallard JF, Blanco P. Predominance of CD8+ T lymphocytes among periglomerular infiltrating cells and link to the prognosis of class III and class IV lupus nephritis. Arthritis Rheum. 2007;56:2362–2370. doi: 10.1002/art.22654. [DOI] [PubMed] [Google Scholar]
- 6.Peterson KS, Huang JF, Zhu J, D'Agati V, Liu X, Miller N, Erlander M, Jackson MR, Winchester RJ. Characterization of Heterogeneity in the Molecular Pathogenesis of Lupus Nephritis from Transcriptional Profiles of Laser-Captured Glomeruli. J. Clin. Invest. 2004;113:1722–1733. doi: 10.1172/JCI19139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schiffer L, Sinha J, Wang X, Huang W, von Gersdorff G, Schiffer M, Madaio MP, Davidson A, Von Gonsdorff G. Short term administration of costimulatory blockade and cyclophosphamide induces remission of systemic lupus erythematosus nephritis in NZB/W F1 mice by a mechanism downstream of renal immune complex deposition. J. Immunol. 2003;171:489–497. doi: 10.4049/jimmunol.171.1.489. [DOI] [PubMed] [Google Scholar]
- 8.Bagavant H, Fu SM. New insights from murine lupus: disassociation of autoimmunity and end organ damage and the role of T cells. Curr. Opinion Rheum. 2005;17:523–528. doi: 10.1097/01.bor.0000169361.23325.1e. [DOI] [PubMed] [Google Scholar]
- 9.Fu SM, Deshmukh US, Gaskin F. Pathogenesis of systemic lupus erythematosus revisited: End organ resistance to damage, autoantibody initiation and diversification, and HLA-DR. J Autoimmun. 2011;37:104–112. doi: 10.1016/j.jaut.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gibofsky A, Winchester RJ, Patarroyo M, Fotino M, Kunkel HG. Disease associations of the Ia-like human alloantigens. Contrasting patterns in rheumatoid arthritis and systemic lupus erythematosus. J. Ex. Med. 1978;148:1728–1732. doi: 10.1084/jem.148.6.1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Graham RR, Ortmann W, Rodine P, Espe K, Langefeld C, Lange E, Williams A, Beck S, Kyogoku C, Moser K, et al. Specific combinations of HLA-DR2 and DR3 class II haplotypes contribute graded risk for disease susceptibility and autoantibodies in human SLE. Eur.J. Hum. Gen. 2007;15:823–830. doi: 10.1038/sj.ejhg.5201827. [DOI] [PubMed] [Google Scholar]
- 12.Steinmetz OM, Velden J, Kneissler U, Marx M, Klein A, Helmchen U, Stahl RA, Panzer U. Analysis and classification of B-cell infiltrates in lupus and ANCA-associated nephritis. Kidney Int. Suppl. 2008;74:448–457. doi: 10.1038/ki.2008.191. [DOI] [PubMed] [Google Scholar]
- 13.Chang A, Henderson SG, Brandt D, Liu N, Guttikonda R, Hsieh C, Kaverina N, Utset TO, Meehan SM, Quigg RJ, et al. In situ B cell-mediated immune responses and tubulointerstitial inflammation in human lupus nephritis. J. Immunol. 2011;186:1849–1860. doi: 10.4049/jimmunol.1001983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu. Rev. Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 15.Speiser DE, Valmori D, Rimoldi D, Pittet MJ, Lienard D, Cerundolo V, MacDonald HR, Cerottini JC, Romero P. CD28-negative cytolytic effector T cells frequently express NK receptors and are present at variable proportions in circulating lymphocytes from healthy donors and melanoma patients. Eur. J. Immunol. 1999;29:1990–1999. doi: 10.1002/(SICI)1521-4141(199906)29:06<1990::AID-IMMU1990>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 16.Turner SJ, Doherty PC, McCluskey J, Rossjohn J. Structural determinants of T-cell receptor bias in immunity. Nat. Rev. Immunol. 2006;6:883–894. doi: 10.1038/nri1977. [DOI] [PubMed] [Google Scholar]
- 17.Wiesendanger M, D'Agati VD, Winchester RJ. Intrarenal T cells in Class III and IV Lupus Nephritis: Correlates of Clonal Diversity and Trafficking Pattern (479). Arthritis Rheum. 2008;58 [Google Scholar]
- 18.Tan EM, Cohen AS, Fires J, Mazi AT, McShane D, Rothfield NF, Schaller JG, Talal N, Winchester RJ. The 1982 revised criteria for the classification of systemic lupus erythematosus (SLE). Arthritis Rheum. 1982;25:1272–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 19.Winchester R, Wiesendanger M, O Brien W, Zhang H-Z, Maurer MS, Gillam LD, Schwartz A, Marboe C, Stewart AS. Circulating activated and effector memory T cells are associated with calcification and clonal expansions in bicuspid and tricuspid valves of calcific aortic stenosis. J. Immunol. 2011;187:1006–1014. doi: 10.4049/jimmunol.1003521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Curran SA, FitzGerald OM, Costello PJ, Selby JM, Kane DJ, Bresnihan B, Winchester R. Nucleotide sequencing of psoriatic arthritis tissue before and during methotrexate administration reveals a complex inflammatory T cell infiltrate with very few clones exhibiting features that suggest they drive the inflammatory process by recognizing autoantigens. J. Immunol. 2004;172:1935–1944. doi: 10.4049/jimmunol.172.3.1935. [DOI] [PubMed] [Google Scholar]
- 21.Wu HD, Maurer M, Friedman RA, Marboe CC, Ruiz-Vazquez EM, Ramakrishnan R, Schwartz A, Tilson MD, Stuart AS, Winchester R. The Lymphocytic Infiltration In Calcific Aortic Stenosis Predominantly Consists of Clonally Expanded T Cells. J. Immunol. 2007;178:5329–5339. doi: 10.4049/jimmunol.178.8.5329. [DOI] [PubMed] [Google Scholar]
- 22.Brochet X, Lefranc MP, Giudicelli V. IMGT/V-QUEST: the highly customized and integrated system for IG and TR standardized V-J and V-D-J sequence analysis. Nucleic Acids Res. 2008;36:W503–508. doi: 10.1093/nar/gkn316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arden B, Clark SP, Kabelitz D, Mak TW. Human T-cell receptor variable gene segment families. Immunogen. 1995;42:455–500. doi: 10.1007/BF00172176. [DOI] [PubMed] [Google Scholar]
- 24.Venturi V, Kedzierska K, Turner SJ, Doherty PC, Davenport MP. Methods for comparing the diversity of samples of the T cell receptor repertoire. J Immunol. Meth. 2007;321:182–195. doi: 10.1016/j.jim.2007.01.019. [DOI] [PubMed] [Google Scholar]
- 25.Weening JJ, D'Agati VD, Schwartz MM, Seshan SV, Alpers CE, Appel GB, Balow JE, Bruijn JA, Cook T, Ferrario F, et al. The classification of glomerulonephritis in systemic lupus erythematosus revisited. J. Am. Soc. Neph. 2004;15:241–250. doi: 10.1097/01.asn.0000108969.21691.5d. [DOI] [PubMed] [Google Scholar]
- 26.Austin H.A.d., Muenz LR, Joyce KM, Antonovych TT, Balow JE. Diffuse proliferative lupus nephritis: identification of specific pathologic features affecting renal outcome. Kidney Int. 1984;25:689–695. doi: 10.1038/ki.1984.75. [DOI] [PubMed] [Google Scholar]
- 27.Springer TA, Dustin ML, Kishimoto TK, Marlin SD. The lymphocyte function-associated LFA-1, CD2, and LFA-3 molecules: cell adhesion receptors of the immune system. Annu. Rev. Immunol. 1987;5:223–252. doi: 10.1146/annurev.iy.05.040187.001255. [DOI] [PubMed] [Google Scholar]
- 28.Massengill SF, Goodenow MM, Sleasman JW. SLE nephritis is associated with an oligoclonal expansion of intrarenal T cells. Am. J. Kidney Dis. 1998;31:418–426. doi: 10.1053/ajkd.1998.v31.pm9506678. [DOI] [PubMed] [Google Scholar]
- 29.Murata H, Matsumura R, Koyama A, Sugiyama T, Sueishi M, Shibuya K, Tsutsumi A, Sumida T. T cell receptor repertoire of T cells in the kidneys of patients with lupus nephritis. Arthritis Rheum. 2002;46:2141–2147. doi: 10.1002/art.10432. [DOI] [PubMed] [Google Scholar]
- 30.Jabri B, de Serre NP, Cellier C, Evans K, Gache C, Carvalho C, Mougenot JF, Allez M, Jian R, Desreumaux P, et al. Selective expansion of intraepithelial lymphocytes expressing the HLA-E- specific natural killer receptor CD94 in celiac disease. Gastroenterology. 2000;118:867–879. doi: 10.1016/S0016-5085(00)70173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meresse B, Curran SA, Ciszewski C, Orbelyan G, Setty M, Bhagat G, Lee L, Tretiakova M, Semrad C, Kistner E, et al. Reprogramming of CTLs into natural killer-like cells in celiac disease. J. Ex. Med. 2006;203:1343–1355. doi: 10.1084/jem.20060028. [DOI] [PMC free article] [PubMed] [Google Scholar]