Abstract
Background
Endogenous cannabinoids such as anandamide and 2-arachidonoylglycerol (2-AG) exert important regulatory influences on neuronal signaling, participate in short- and long-term forms of neuroplasticity, and modulate stress responses and affective behavior in part through the modulation of neurotransmission in the amygdala. Alcohol consumption alters brain endocannabinoid levels, and alcohol dependence is associated with dysregulated amygdalar function, stress responsivity and affective control.
Methods
The consequence of long-term alcohol consumption on the expression of genes related to endocannabinoid signaling was investigated using quantitative RT-PCR analyses of amygdala tissue. Two groups of ethanol-exposed rats were generated by maintenance on an ethanol liquid diet (10%): one group received continuous access to ethanol for 15 days, while the second group was given intermittent access to the ethanol diet (5 days/week for 3 weeks). Control subjects were maintained on an isocaloric ethanol-free liquid diet. To provide an initial profile of acute withdrawal amygdala tissue was harvested following either 6 or 24 hours of ethanol withdrawal.
Results
Acute ethanol withdrawal was associated with significant changes in mRNA expression for various components of the endogenous cannabinoid system in the amygdala. Specifically, reductions in mRNA expression for the primary clearance routes for anandamide and 2-AG (FAAH and MAGL, respectively) were evident, as were reductions in mRNA expression for CB1, CB2 and GPR55 receptors. Although similar alterations in FAAH mRNA were evident following either continuous or intermittent ethanol exposure, alterations in MAGL and cannabinoid receptor-related mRNA (e.g. CB1, CB2, GPR55) were more pronounced following intermittent exposure. In general, greater withdrawal-associated deficits in mRNA expression were evident following 24 versus 6 hours of withdrawal. No significant changes in mRNA expression for enzymes involved in 2-AG biosynthesis (e.g. DAGL-α/β) were found in any condition.
Conclusions
These findings suggest that ethanol dependence and withdrawal are associated with dysregulated endocannabinoid signaling in the amygdala. These alterations may contribute to withdrawal-related dysregulation of amygdalar neurotransmission.
Keywords: ethanol, withdrawal, endocannabinoid system, amygdala
Introduction
Chronic alcohol exposure has been shown to affect the function of several neurotransmitter systems in the central nervous system and these alterations are implicated in the development of tolerance and dependence to ethanol (Koob et al., 1998). Among these systems, a growing amount of evidence points to an important influence of the endogenous cannabinoid system (ECS) in ethanol-related behaviors [for review see (Rodriguez de Fonseca et al., 2005)].
The ECS is a lipid transmitter system, consisting of at least two G protein-coupled receptors, CB1 and CB2, their endogenous ligands N-arachidonoylethanolamine (anandamide) and 2-arachidonoylglycerol (2-AG), and the enzymes that are involved in the regulation and metabolism of anandamide and 2-AG (Ahn et al., 2008; Di Marzo, 2008; Fowler, 2006). The primary pathway for anandamide synthesis is mediated by a specific phospholipase D (NAPE-PLD) (Okamoto et al., 2004), though alternate routes of anandamide formation have also been proposed (Leung et al., 2006; Liu et al., 2008; Liu et al., 2006; Simon and Cravatt, 2006; Simon and Cravatt, 2008). The primary biosynthetic route for 2-AG is mediated by two sn-1-selective DAG lipases, DAGL-α and DAGL-β (Bisogno et al., 2003). Inactivation of endocannabinoid signaling is mediated by cellular reuptake and subsequent intracellular hydrolysis. Fatty acid amide hydrolase (FAAH) and monoacylglycerol lipase (MAGL) have been identified as enzymes primarily responsible for the degradation of anandamide and 2-AG, respectively (Cravatt et al., 1996; Dinh et al., 2002).
Several findings demonstrate that CB1 receptors exert a facilitory influence on ethanol preference and consumption. While CB1 activation increases ethanol consumption in both rats (Colombo et al., 2002) and mice (Wang et al., 2003), CB1 receptor antagonists such as SR141716A decrease ethanol consumption (Arnone et al., 1997; Freedland et al., 2001; Rodriguez de Fonseca et al., 1999). In agreement with these findings, CB1 receptor knockout mice display low ethanol preference and intake compared to wild-type mice (Hungund et al., 2003; Naassila et al., 2004; Thanos et al., 2005; Wang et al., 2003). In situ hybridization studies have shown an increase in CB1 receptors mRNA expression in several brain areas of Marchigian Sardinian alcohol-preferring rats, suggesting an altered function of CB1 receptors in these genetically selected alcohol-preferring rats (Cippitelli et al., 2005). Moreover, alcohol-preferring AA rats are characterized by reduced FAAH expression and function in the prefrontal cortex (PFC) and localized infusion of the FAAH inhibitor URB597 into the PFC increases ethanol consumption by outbred Wistar rats (Hansson et al., 2007).
As occurs with other neurotransmitters (i.e., dopamine, corticotropin-releasing factor, etc.) chronic ethanol exposure induces neuroadaptations in the ECS. These effects of ethanol are dependent on the route of administration, dose, voluntary versus forced administration and the duration of ethanol exposure. Acute intraperitoneal administration of ethanol decreases anandamide but not 2-AG levels in the nucleus accumbens, and does not affect the expression of CB1 receptors, NAPE-PLD or FAAH mRNA expression in various brain regions (Ferrer et al., 2007). However, self-administered ethanol dose-dependently increases 2-AG levels without modifying the levels of anandamide in nucleus accumbens microdialysates (Alvarez-Jaimes et al., 2009; Caille et al., 2007). Long-term alcohol exposure increases both 2-AG and anandamide formation in human neuroblastoma cells and primary cultures of rodent cerebellar granule neurons (Basavarajappa and Hungund, 1999; Basavarajappa et al., 2000; Basavarajappa et al., 2003). Consistently, chronic inhalation of ethanol vapor induces a down-regulation of CB1 receptors and a desensitization of cannabinoid-activated signal transduction (Basavarajappa et al., 1998; Basavarajappa and Hungund, 1999; Vinod et al., 2006), suggesting the induction of overactive endocannabinoid production. Although the exact nature of the neuroadaptive changes in humans are yet to be described, several genetic polymorphisms of endocannabinoid-related genes confirm the existence of a link between these signaling lipids and alcohol abuse. These include polymorphisms and/or mutations of the genes encoding either the CB1 receptors (Comings et al., 1997; Schmidt et al., 2002) or the anandamide degrading enzyme FAAH (Sipe et al., 2002).
One of the most consistent features of alcohol abuse and alcoholism is the presence of anxiety, mostly associated to withdrawal and protracted abstinence. One regional substrate for alcohol-associated anxiety is the amygdala (Koob, 2009; Merlo Pich et al., 1995) which is a crucial subcortical area that integrates reward, emotions and conditioned learning [for review see (Rodriguez de Fonseca and Navarro, 1998)]. Although several studies have demonstrated drug-induced alterations in amygdalar endocannabinoid function (Kamprath et al., 2011; Orio et al., 2009; Rodriguez de Fonseca et al., 1997; Schmidt et al., 2011) there is scarce information on the effects of chronic alcohol on the ECS components in the amygdala. In the present study, we evaluated the effects of alcohol withdrawal following chronic ethanol treatment on the expression of different cannabinoid signaling-related genes in the amygdala. Since there is considerable evidence that repeated cycles of withdrawal contribute to excessive ethanol consumption and progressively worsen withdrawal-associated symptoms (Holter et al., 1998; Lopez and Becker, 2005; Overstreet et al., 2002; Overstreet et al., 2004; Rimondini et al., 2002), we have compared the potential differences existing between continuous and intermittent ethanol exposure on these neuroadaptations.
Materials and methods
Subjects
Male Wistar rats (Charles River, Wilmington, MA, USA) weighing 250–300 g at the beginning of the experiments were housed in groups of two in a humidity and temperature-controlled (22°C) vivarium on a 12 h light/dark cycle (lights off at 10AM). Upon arrival in the vivarium, rats were allowed to acclimatize to the new environment for 7 days before any experimental procedure was performed. The rats had ad libitum access to food and water, except during exposure to an ethanol-containing liquid diet. All procedures were conducted in strict adherence to the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Chronic ethanol treatment
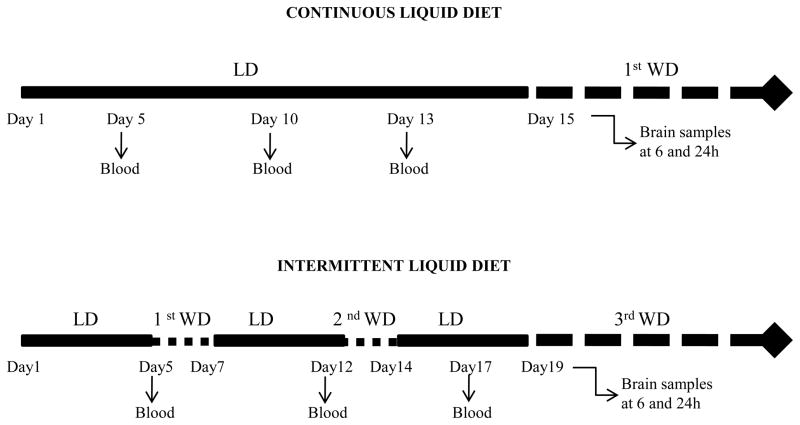
Regular chow diet was removed and replaced by the liquid diet consisting of chocolate flavored Boost liquid nutritional supplement fortified with vitamins and minerals. Rats were separated into two main groups; the chronic continuous diet and the chronic intermittent diet groups (figure 1). The chronic continuous group was separated into two groups; the ethanol group (n=16) received a diet containing 10% (w/v) ethanol and the control group (n=16) received an ethanol-free diet supplemented with sucrose to equalize the caloric intake in both groups. The diet was available 24 h for 15 days. The chronic intermittent group was also separated into ethanol group (n=16) and control group (n=16) and maintained on the diet 5-days per week. On weekends the animals were given ad libitum access to chow and water. This schedule was maintained for 3 weeks. This allowed the animals to undergo two withdrawal periods of 2 days each prior to experiment, which was performed on day 1 during third withdrawal.
Figure 1.
Experimental design used for the different scheduled access protocols: the chronic continuous diet and the chronic intermittent diet. Abbreviations: LD, liquid diet; WD, withdrawal.
The ethanol intake was evaluated every day during maintenance of the liquid diet. The daily ethanol intake data (g/kg) represent the mean of the average of ethanol ingest relative to body mass per cage (2 rats/cage).
On the morning of the final liquid diet day, animals were maintained in their home-cage with access to the regular chow diet and water. Rats were anesthetized by isoflurane inhalation and decapitated 6 and 24 h after removing the liquid diet. Brains were quickly removed, immediately frozen on dry ice and stored at −70°C until determination of the expression of the main components of the endocannabinoid system using quantitative real-time PCR.
Dissection of the amygdala
Brains were sectioned coronally (2 mm slices) in a rat brain matrix. The amygdala was dissected out bilaterally with a 2-mm-diameter punch tool using the rat brain atlas of Paxinos and Watson (Paxinos and Watson, 1998)
Quantitative real-time PCR
Quantitative real-time PCR was used to measure CB1, CB2, NAPE-PLD, FAAH, DAGL-α, DAGL-β and MAGL mRNA levels. Total RNA from amygdala was extracted by using the Trizol method, according the manufacturer’s instructions (Gibco BRL Life Technologies, Baltimore, MD, USA). All RNA samples had A260/280 ratios of 1.8 to 2.0 after purification with RNeasy micro- or minelute cleanup-kit (Quiagen, Hilden, Germany). Purified RNA from each sample and random hexamers were used to generate first strand cDNA using transcriptor reverse transcriptase (Roche Applied Science, Indianapolis, IN, USA). Negative controls included reverse transcription reactions omitting reverse transcriptase. The obtained cDNA was used as the template for quantitative real-time PCR, which was performed in an iCycler system (Bio-Rad, Hercules, CA, USA) using the SYBR Green I detection format (FastStart Universal Master kit, Roche). Each reaction was run in duplicate and contained 2.5 μl of cDNA template, 5 mM Cl2Mg and 0.4–0.5 μM of primers in a final reaction volume of 15 μl. Cycling parameters were 95°C for 5 min to activate DNA polymerase, then 35–45 cycles of 95°C for 10 s, annealing temperature for 15 s (table 1) and a final extension step of 72°C for 15 s in which fluorescence was acquired. Melting curves analysis were performed to ensure only a single product was amplified. β-actin and Cyclophilin (Cyp) were evaluated as endogenous reference gene candidates. Cyp was chosen the house-keeping gene since it was more stable and no significant changes were detected between the different groups in the present study. Thus, gene expression was normalized to Cyp. Primers for PCR reaction (table 1) were designed based on NCBI database sequences of rat reference mRNA and checked for specificity with BLAST software from NCBI website (http://blast.ncbi.nlm.nih.gov/Blast.cgi). Quantification was carried out with a standard curve run at the same time as the samples with each reaction run in duplicate. Absolute values from each sample were normalized with regard to the house-keeping gene Cyp.
Table 1.
Primers sequences used for real time polymerase chain reaction (RT-PCR).
| Gene | Oligosense 5′→3′ | Oligoantisense 5′→3′ | N° access Genbank | Product size (bp) | Annealling T(°C) |
|---|---|---|---|---|---|
| Cyp | AGAAGGCATGAGCATTGTGG | TTACAGGGTATTGCGAGCAG | NM_017101.1 | 189 | 55 |
| CB1 | AGACCTCCTCTACGTGGGCTCG | GTACAGCGATGGCAGCTGCTG | NM_012784.2 | 314 | 58 |
| CB2 | GCAGCCTGCTGCTGACTGCTG | TGCTTTCCAGAGGACATACCC | NM_020543.3 | 284 | 58,9 |
| GPR55 | GGGATACAAGTGCTTCCACA | AAAGGAGACCACGAAGACGA | AF100789 | 226 | 63.3 |
| NAPE-PLD | GGAGCTTATGAGCCAAGGTG | ACTCTCCGTGCTTCAGGATG | AB112351 | 223 | 57 |
| FAAH | GTTACAGAGTGGAGAGCTGTC | GAGGGTTACTGCAGTCAAAGC | NM_024132.3 | 344 | 46.5 |
| DAGL-α | GGGTACCTAATGGCTGCTCA | AGGACTGACCATCCAACCTG | NM_001005886 | 220 | 60,5 |
| DAGL-β | TGATAGGCCCAAAGATGCTG | AAGTCCATTGTGCTCGTCAG | NM_001107120.1 | 161 | 58,9 |
| MAGL | CATGGAGCTGGGGAACACTG | GGAGATGGCACCGCCATGGAG | NM_13852.1 | 240 | 58,1 |
Abbreviations: Cyp, cyclophilin; CB1, cannabinoid receptor type 1; CB2, cannabinoid receptor type 2; GPR55, orphan G protein-coupled receptor GPR55; NAPE-PLD, N-acylphosphatidylethanolamine phospholipase D; FAAH, fatty acid amide hydrolase; DAGL-α/β, diacylglicerol lipase α/β; MAGL, monoacylglicerol lipase
Blood alcohol assay
Blood samples were collected once per week during maintenance of the ethanol liquid diet. Rats were tail-bled within the first 2–3 h after the beginning of the dark cycle. The method consisted of clipping the tail approximately 5 mm from the tip to collect 100 μL of blood into a microtube containing anticoagulant (4 μL heparin; 1000 USP units/mL). Samples were centrifuged at 2000 g for 10 min. Serum was extracted and assayed for ethanol content using the alcohol oxidase method (Analox Instrument LTD, Lunenburg, MA, USA).
Statistical analysis
All data for graphs and tables are expressed as the mean ± SEM. The different experiments included 8–6 animals per group according to the assay. Statistical analysis of results was performed using the computer program GraphPad Prism version 4.0 (GraphPad Software Inc., San Diego, CA, USA). The significance of differences between groups were evaluated by two-way analysis of variance (ANOVA) followed by a post hoc analysis for multiple comparisons (Bonferroni test). A p-value below 0.05 was considered statistically significant.
Results
In the present study, we examined the effect of single and repeated withdrawals after a long-term exposure to alcohol on the ECS in the amygdala of rats. For that purpose, we evaluated changes on the gene expression of relevant components of the ECS, such as the CB1, CB2 and GPR55 receptors, and the synthesis (NAPE-PLD, DAGL-α and DAGL-β) and degradation (FAAH and MAGL) enzymes, measured at 6 and 24 h into withdrawal.
Ethanol consumption and blood alcohol levels
The average daily ethanol intake by rats during the 3 weeks maintenance on continuous and intermittent ethanol-containing liquid diet was 10.34±0.39 g/kg and 10.95±0.42 g/kg, respectively, resulting in average blood alcohol concentrations of 401.8±16.4 mg/dL and 393.94±18.34 mg/dL, respectively (determined from measures taken 1x/week/rat; see Materials and methods).
Effects of single and repeated withdrawal on anandamide-related enzyme mRNA expression
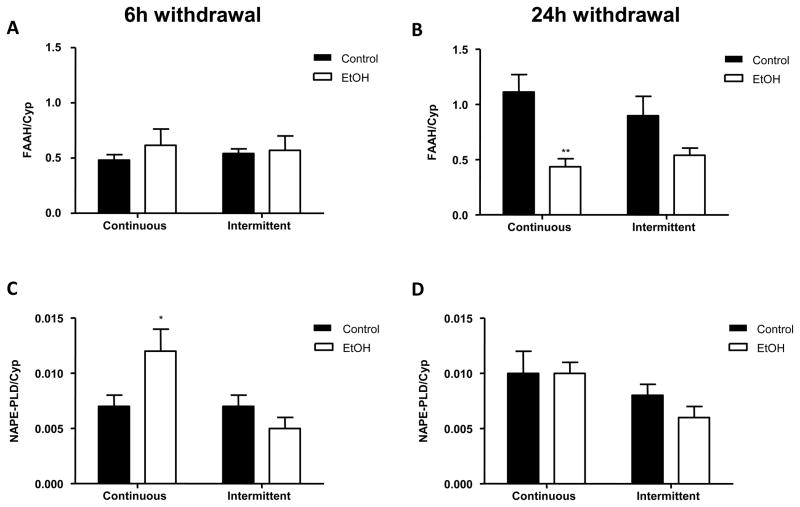
The effects of long-term alcohol exposure on FAAH and NAPE-PLD mRNA levels following 6 and 24 h of withdrawal are shown in figure 2. There was no significant effect of prior ethanol exposure on FAAH expression measured 6 h into withdrawal (F1,24 = 0.4669; n.s.) and also no significant influence of the pattern of ethanol exposure (e.g. continuous vs. intermittent diet; F1,24 = 0.035; n.s.) (figure 2A). In contrast a robust ethanol-related reduction in FAAH gene expression was evident following 24 h withdrawal (F1,28 = 16.53; p < 0.0005), though there was no significant influence of the pattern of ethanol exposure on this effect (pattern of exposure, F1,28 = 0.1901; n.s.; interaction between ethanol and exposure pattern F1,28 = 1.55; n.s.) (figure 2B). Post-hoc analysis revealed a significant 61% reduction in FAAH mRNA in rats given continuous ethanol access (p < 0.01) and a similar (though non-significant) trend was evidence in animals given intermittent ethanol access.
Figure 2.
Effect of a single or repeated withdrawal on mRNA expression of FAAH (A, B) and NAPE-PLD (C, D) in the amygdala. Samples were collected at 6 and 24 h into withdrawal. Absolute values from each sample were normalized with regard to the housekeeping gene cyclophilin. Graphs represent the mean ± SEM (6–8 animals per group). Data were analyzed by two-way ANOVA (alcohol exposure and type of diet) and a Bonferroni post-test. *p < 0.05 and **p < 0.01 denote significant differences compared to the corresponding vehicle-treated group.
Although there was no significant overall effect of ethanol exposure on NAPE-PLD mRNA expression at either withdrawal time (6 h, F1,26 = 1.206; n.s.; 24 h, F1,25 = 0.5077; n.s.), significant effects of exposure pattern were evident (6 h, F1,26 = 6.533; p < 0.05; 24 h, F1,25 = 4.576; p < 0.05) along with a significant interaction between ethanol and pattern of exposure following 6 h (F1,26 = 6.533; p < 0.05) but not 24 h (F1,25 = 0.5077; n.s.) withdrawal (figures 2C, 2D). In the continuous exposure group NAPE-PLD mRNA expression was significantly increased (p < 0.05) following 6 h withdrawal, though this effect was absent after 24 h withdrawal. In contrast, NAPE-PLD mRNA expression tended to be reduced by intermittent ethanol exposure at both withdrawal time points.
Effects of single and repeated withdrawal on 2-AG-related enzymes mRNA expression
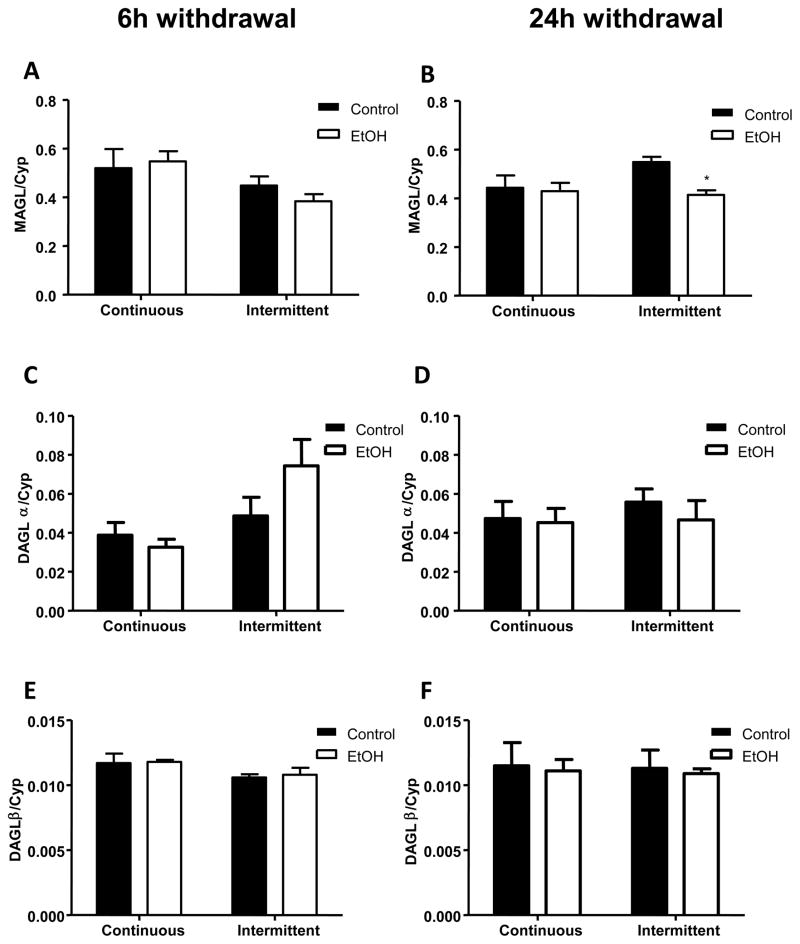
The effects of long-term alcohol exposure on MAGL, DAGL-α and DAGL-β mRNA levels following 6 and 24 h of withdrawal are shown in figure 3. Although no significant effect of ethanol exposure was evident following 6 h withdrawal (F1,28 = 0.1287; n.s), a significant influence of the pattern of ethanol exposure was present (F1,28 = 5.53; p < 0.05) with no interaction between ethanol and exposure pattern (F1,28 = 0.8404; n.s) (figure 3A). By contrast, a significant effect of ethanol exposure was evident following 24 h withdrawal (F1,26 = 4.431; p < 0.05) with a significant 25% reduction in MAGL mRNA levels in the intermittent ethanol exposure group (figure 3B). However, no significant effect of exposure pattern (F1,26 = 1.653; n.s.) or interaction between ethanol and pattern (F1,26 = 2.922; n.s.) were evident at this withdrawal time.
Figure 3.
Effect of a single or repeated withdrawal on mRNA expression of MAGL (A, B), DAGL-α (C, D) and DAGL-β (E, F) in the amygdala. Samples were collected at 6 and 24 h into withdrawal. Absolute values from each sample were normalized with regard to the housekeeping gene cyclophilin. Graphs represent the mean ± SEM (6–8 animals per group). Data were analyzed by two-way ANOVA (alcohol exposure and type of diet) and a Bonferroni post-test. *p < 0.05 denotes significant differences compared to the corresponding vehicle-treated group.
Similar to the effects on MAGL, there was no overall effect of ethanol on DAGL-α mRNA expression after either 6 h (F1,27 = 1.076; n.s.) or 24 h (F1,27 = 0.4458; n.s.) withdrawal (figures 3C, 3D). However, a significant effect of exposure pattern was evident following 6 h withdrawal (F1,27 = 7.460; p < 0.05) though no interaction between ethanol and pattern was present (F1,27 = 2.833; n.s.). There was no effect of exposure pattern on DAGL-α mRNA measured after 24 h withdrawal (F1,27 = 0.3421; n.s.). There were no effects of ethanol exposure (6 h: F1,25 = 0.0837; n.s.; 24 h: F1,27 = 0.1007; n.s.) or pattern of exposure (6 h: F1,25 = 4.099; n.s.; 24 h: F1,27 = 0.0252; n.s.) on DAGL-β mRNA levels(figures 3E, 3F).
Effects of single and repeated withdrawal on CB1, CB2 and GPR55 receptors
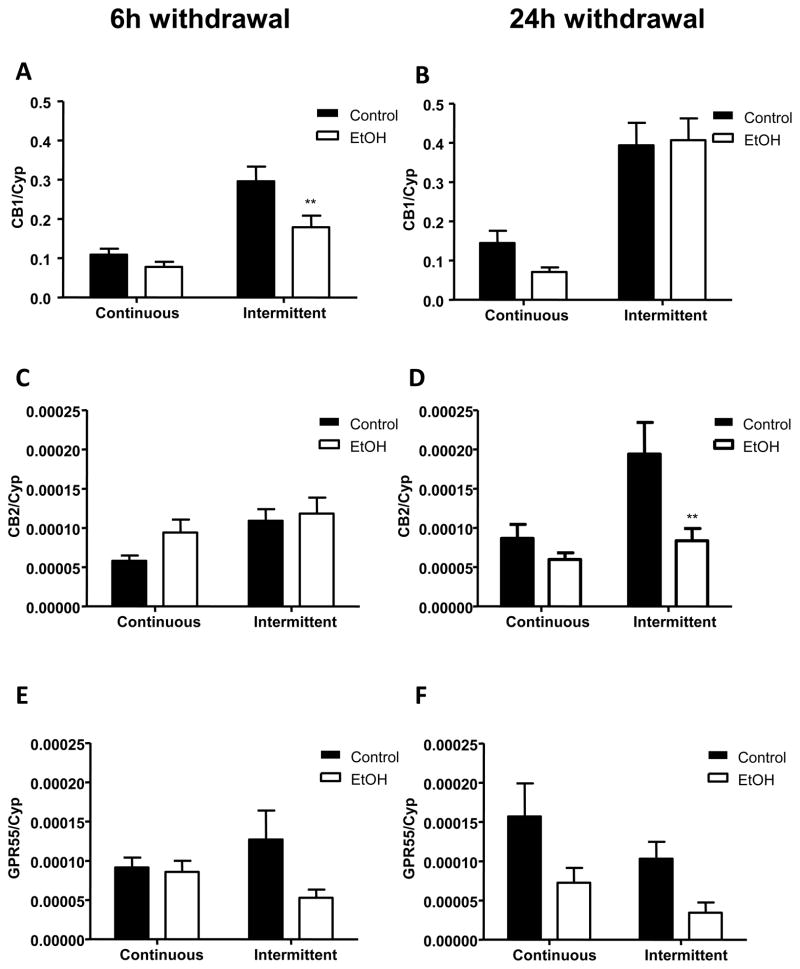
Following 6 h of withdrawal, CB1 receptor mRNA expression was significantly altered as a function of alcohol exposure (F1,27 = 8.846; p < 0.01) and exposure pattern (F1,27 = 33.47; p < 0.0001), though there was no significant interaction between these effects (F1,27 = 2.976; n.s.) (figure 4A). Following 24 h withdrawal, the effect of ethanol on CB1 mRNA was no longer significant (F1,26 = 0.4879; n.s.), though the significant effect of exposure pattern persisted (F1,26 = 44.85; p < 0.0001) and no interaction between these effects was present (F1,27 = 0.9919; n.s.) (figure 4B).
Figure 4.
Effect of a single or repeated withdrawal on mRNA expression of CB1 (A, B), CB2 (C, D) and GPR55 receptors (E, F) in the amygdala. Samples were collected at 6 and 24 h into withdrawal. Absolute values from each sample were normalized with regard to the housekeeping gene cyclophilin. Graphs represent the mean ± SEM (6–8 animals per group). Data were analyzed by two-way ANOVA (alcohol exposure and type of diet) and a Bonferroni post-test. **p < 0.01 denotes significant differences compared to the corresponding vehicle-treated group.
With regard to CB2 receptor mRNA after 6 h withdrawal, although there was a significant influence of exposure pattern (F1,26 = 5.572; p < 0.05) there was no overall effect of prior ethanol exposure (F1,26 = 2.02; n.s.) or interaction between ethanol and exposure pattern (F1,26 = 0.7294; n.s.) (figure 4C). Following 24 h withdrawal there were significant influences of both ethanol exposure (F1,27 = 7.791; p < 0.01) and exposure pattern (F1,27 = 7.104; p < 0.05) but no interaction between these factors (F1,27 = 2.859; n.s.) (figure 4D). At this time point CB2 mRNA expression was significantly reduced in animals given intermittent ethanol exposure (p < 0.01), and a tendency toward reduced levels was evident in animals given continuous ethanol access.
Evaluations of GPR55 mRNA expression revealed that at the 6 h withdrawal time point there was no significant influence of prior ethanol exposure (F1,27 = 3.293; n.s.) or pattern of exposure (F1,27 = 0.00378; n.s.) (figure 4E). However, after 24 h withdrawal a significant influence of prior ethanol exposure was evident (F1,27 = 8.049; p < 0.01) without a significant influence of exposure pattern (F1,27 = 2.918; n.s.) or interaction between ethanol history and exposure pattern (F1,27 = 0.08442; n.s.) (figure 4F). At this time point GPR55 mRNA expression tended to be lower in ethanol exposed rats, regardless of the pattern of exposure the animals received.
Discussion
In the present study we evaluated the effects of chronic ethanol exposure and withdrawal on the amygdalar ECS using two different scheduled access protocols consisting of continuous versus intermittent access to an ethanol liquid diet. The results indicate that alcohol exposure affects gene expression for the primary clearance routes for anandamide and 2-AG as well as the mRNA expression for CB1, CB2 and GPR55 receptors. Specifically, we observed that the first withdrawal after continuous alcohol exposure primarily altered gene expression related to anandamide biosynthesis and clearance, while repeated withdrawals induced by intermittent alcohol exposure also altered gene expression for the 2-AG degrading enzyme MAGL and cannabinoid receptors (CB1, CB2 and GPR55). The average daily ethanol consumption and blood alcohol concentrations did not differ between the continuous and intermittent access groups, and as such the differential effects observed following these exposure protocols likely reflects the influence of singular versus multiple withdrawal periods. The relevance of the present findings is derived from the important influence of endocannabinoid signaling in the regulation of emotionality (Hill and Gorzalka, 2009; Lutz, 2009; Moreira and Wotjak, 2010), and the known involvement of the amygdala in mediating dependence-related disruption of affective state, dependence-related increases in alcohol consumption and relapse behaviors.
Previous observations have described neuroadaptations in the ECS in response to alcohol exposure. Biochemical studies have shown that chronic ethanol administration is associated with elevated formation of both anandamide and 2-AG in SK-N-SH cells and cerebellar granule neurons (Basavarajappa and Hungund, 1999; Basavarajappa et al., 2000; Basavarajappa et al., 2003). In whole animal studies, chronic ethanol vapor inhalation by mice results in a widespread reduction of CB1 receptor density and CB1 agonist-induced G protein activation (Basavarajappa et al., 1998; Basavarajappa and Hungund, 1999; Vinod et al., 2006). However, alcohol exposure appears to produce regionally distinct effects on brain endocannabinoid levels. For example, while alcohol exposure increases tissue anandamide content in limbic forebrain structures and nucleus accumbens (Gonzalez et al., 2002; Gonzalez et al., 2004), significant decreases in anandamide content are observed in the amygdala, hippocampus and prefrontal cortex (Malinen et al., 2009; Rubio et al., 2007). Inconsistent effects of ethanol exposure on tissue anandamide levels in the caudate putamen and 2-AG levels in the prefrontal cortex have also been reported (Malinen et al., 2009; Rubio et al., 2007), and this may result from experimental factors such as rat strain, gender and experimental paradigm employed.
The present results show that withdrawal from chronic alcohol exposure is associated with alterations in the ECS that appear to be reliant both on the nature of exposure and the duration of withdrawal. In our study, rats given continuous access to ethanol liquid diet displayed an increase in mRNA expression for the anandamide biosynthesis enzyme NAPE-PLD at early phases of withdrawal (6 h) and a significant reduction in mRNA expression for the anandamide clearance enzyme FAAH at later withdrawal times (24 h). If these alterations in mRNA expression confer changes in enzyme levels or function, the observed increase in anandamide biosynthesis and decrease in clearance suggests a withdrawal-associated potentiation of anandamide signaling. We suspect that this may reflect a homeostatic response to alcohol-induced reductions in brain interstitial anandamide levels (Ferrer et al., 2007). In contrast to these effects on anandamide, no changes in the expression of 2-AG related genes were evident in the continuous ethanol exposure group. In this regard, the present results are consistent with evidence of decreased anandamide levels with no change in 2-AG levels in rat amygdalar tissue during the early stages of ethanol withdrawal (Rubio et al., 2008). While no significant changes in cannabinoid receptor mRNA expression were evident following 6 h withdrawal from continuous ethanol exposure, there was a tendency toward reduced CB1, CB2 and GPR55 mRNA expression at 24 h withdrawal from continuous exposure. This temporal profile aligns with the onset of decreased FAAH expression at 24 h, but not 6 h of withdrawal from continuous ethanol, which may confer increased anandamide levels at this later stage of acute withdrawal. However, recent evidence that chronic FAAH inhibition does not alter CB1 receptor expression or function (Schlosburg et al., 2010) suggests the presently observed temporal profile for altered FAAH and cannabinoid receptor mRNA expression are not functionally linked. Down-regulation of FAAH expression likely results not only decreased clearance of anandamide but also other N-acylethanolamines including oleoylethanolamide (OEA) and palmitoylethanolamide (PEA). These lipids signal through nuclear PPAR receptors and do not possess significant affinity for classical cannabinoid receptors. However, PEA is a potent agonist at GPR55 receptors (Godlewski et al., 2009) and it is conceivable that increased levels of PEA resulting from decreased clearance by FAAH contribute to reductions in GPR55 mRNA expression present after 24 h ethanol withdrawal.
Withdrawal from intermittent ethanol exposure was generally associated with greater disruptions in ECS gene expression than observed during withdrawal from continuous ethanol exposure. Consistent with abstinence from continuous exposure, expression of FAAH and CB1 mRNA were decreased during withdrawal from intermittent ethanol exposure. In addition, withdrawal from intermittent ethanol exposure was also characterized by significant reductions in mRNA expression for the 2-AG hydrolytic clearance enzyme MAGL as well as mRNA expression for CB2 and GPR55 receptors. Moreover, the onset of reduced CB1 and GPR55 mRNA expression occurred earlier in the progression of withdrawal and in the case of GPR55 this reduction in expression persisted through to the 24 h withdrawal time-point. While a functional correlation between deficits in mRNA expression for endocannabinoid clearance mechanisms and cannabinoid receptors remains to be determined, the present data clearly demonstrate greater disruptions in amygdalar ECS components following repeated versus singular ethanol withdrawals.
In this regard, prior studies have demonstrated a sensitization of stress-induced increases in forebrain and amygdalar tissue 2-AG levels following repeated episodes of stress, and this enhancement of stress-induced 2-AG signaling is linked to the behavioral and physiological habituation to repeated homotypic stress (Patel et al., 2004; Patel et al., 2005b). In light of the stressful nature of alcohol withdrawal the present observation of altered MAGL mRNA expression following repeated but not singular withdrawals is consistent with these prior observations. In this context, recent evidence suggests that the brain ECS is activated in response to anxiogenic situations, and that this activation is part of a negative feedback system that limits the expression of anxiety-like behavior (Gaetani et al., 2003; Haller et al., 2002; Hill and Gorzalka, 2009; Lutz, 2009; Moreira and Wotjak, 2010). Indeed, enhancement of endocannabinoid signaling through inhibition of endocannabinoid clearance mechanisms produces anxiolytic-like behavioral effects in rodents (Bortolato et al., 2006; Kathuria et al., 2003; Rutkowska et al., 2006; Sciolino et al., 2011). In this context, the presently observed withdrawal-related decrease in gene expression for endocannabinoid clearance mechanisms may reflect a homeostatic mechanism to increase endocannabinoid signaling and thereby constrain physiological and behavioral stress responses. Consistent with this hypothesis is evidence that inhibition of FAAH activity reduces alcohol withdrawal-associated anxiety-like behavior (Cippitelli et al., 2008).
An unexpected finding from the present experiment is evidence for a disruption in the circadian cycling that has been characterized for some ECS related genes (Valenti et al., 2004). In the present study samples taken at the 6 h withdrawal time were harvested during the dark phase of the diurnal cycle, while those for the 24 h withdrawal time were harvested during the light phase of the cycle. In ethanol-naïve control animals, we observed that FAAH gene expression is significantly higher during the light phase of the diurnal cycle, in agreement with previous reports of increased FAAH activity during the light phase (Glaser and Kaczocha, 2009; Valenti et al., 2004). However, these diurnal changes in FAAH expression were absent animals previously exposed to chronic ethanol (see figure 2). The mechanism underlying this effect is unknown, though it is possible that dependence-related disruption in glucocorticoid signaling (Little et al., 2008) plays a role given the known influence of glucocorticoids in the regulation of FAAH activity (Waleh et al., 2002). The possible disruption in diurnal FAAH expression or activity may have implications for the well-known disruption of sleep patterns associated with alcohol dependence and withdrawal (Landolt and Gillin, 2001; Trevisan et al., 1998) given that FAAH is the primary clearance route for oleamide that is a sleep-induced fatty acid amide (Boger et al., 1998; Labar and Michaux, 2007). However, the linkage between the present observation of disrupted diurnal cycles of FAAH mRNA expression and dependence-related sleep disruptions is tenuous and certainly requires further investigation.
One important caveat related to the present results is the fact that while our tissue sampling procedure selectively dissected amygdalar tissue, this approach did not differentiate between amygdalar sub-nuclei. This may be an important consideration in light of the differential expression of ECS components within subregions of the amygdala. Immunohistochemical analysis revealed very high levels of CB1 receptors in the lateral amygdala and basolateral amygdala (BLA) (Herkenham et al., 1991; Katona et al., 2001) with no detectable signaling in the central amygdala (CeA). Electrophysiological studies also demonstrated robust CB1 influence in the lateral amygdala and BLA and a lack of CB1 receptor influence in the CeA (Katona et al., 2001). However, other studies have detected faint but consistent immunopositive CB1 signal in the CeA (Kamprath et al., 2011; McDonald and Mascagni, 2001; Patel et al., 2005a; Tsou et al., 1998) and recent work has demonstrated a robust endocannabinoid influence on both excitatory and inhibitory transmission in the CeA (Kamprath et al., 2011; Roberto et al., 2010). Thus, CB1 receptor expression in amygdala is predominantly localized to the BLA with much lower, yet functionally significant levels found in the CeA. The distribution of FAAH and MAGL follows a similar pattern, with a dense presence of these hydrolytic enzymes in the BLA and much less prominent expression in the CeA (Gulyas et al., 2004). Because of this profile of ECS components in the amygdalar nuclei, it is possible that relatively large ethanol-induced changes in the expression of endocannabinoid-related genes in the CeA are overwhelmed by the overall greater presence of ECS genes in the BLA. Because of the more pronounced presence of ECS components in the BLA versus CeA, it is likely that the present findings are largely reflective of ethanol-induced changes in ECS gene expression in the BLA. In other words, even relatively large ethanol-induced changes in the expression of endocannabinoid-related genes in the CeA may be masked by the much greater presence of ECS genes in the BLA.
In this context it is worth emphasizing that the BLA and CeA play distinct roles in mediating the behavioral and physiological effects of ethanol (Huang et al., 2010; Moonat et al., 2011; Radwanska et al., 2008) and chronic ethanol exposure has been shown to induce differential physiological effects in these two amygdalar nuclei (Bertotto et al., 2011; Pandey et al., 2008; Ruggeri et al., 2010; Zhang and Pandey, 2003). Accordingly it will be important in future studies to more closely investigate sub-regional differences in the effects of chronic ethanol on ECS function in the nuclei of the amygdala.
In summary, we report that withdrawal from chronic alcohol exposure is associated with alterations in the amygdalar ECS that appear to be reliant both on the nature of exposure and the duration of withdrawal. In general, exposure to repeated withdrawals was associated with more broad and robust alterations in ECS gene expression (including FAAH, MAGL and cannabinoid receptors) while singular withdrawal was primarily associated only with anandamide-related disruptions in gene expression, and greater withdrawal-associated deficits in mRNA expression were evident following 24 h versus 6 h of withdrawal. These findings provide initial evidence for an involvement of the amygdalar ECS in the manifestation of negative motivational states and enhanced stress responsivity associated with alcohol dependence and withdrawal.
Acknowledgments
Authors’ work has been supported by Red de Trastornos Adictivos UE-FEDER RD06/0001/0000 and grant FIS 07/1226 (Instituto de Salud Carlos III, Ministerio de Ciencia e Innovación); grants PND 049/2009 and PND 2010/143 (Plan Nacional sobre Drogas, Ministerio de Sanidad, Política Social e Igualdad); and Proyectos de Excelencia UE-FEDER CTS-03324 and PAIDI CTS-433 (Consejería de Economía, Innovación y Ciencia, Junta de Andalucía); and NIH grants P60-AA006420, RO1-AA014619 and PO1-DA017259. A. Serrano and P. Rivera contributed equally to the present study.
References
- Ahn K, McKinney MK, Cravatt BF. Enzymatic pathways that regulate endocannabinoid signaling in the nervous system. Chem Rev. 2008;108(5):1687–707. doi: 10.1021/cr0782067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Jaimes L, Stouffer DG, Parsons LH. Chronic ethanol treatment potentiates ethanol-induced increases in interstitial nucleus accumbens endocannabinoid levels in rats. J Neurochem. 2009;111(1):37–48. doi: 10.1111/j.1471-4159.2009.06301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnone M, Maruani J, Chaperon F, Thiebot MH, Poncelet M, Soubrie P, Le Fur G. Selective inhibition of sucrose and ethanol intake by SR 141716, an antagonist of central cannabinoid (CB1) receptors. Psychopharmacology (Berl) 1997;132(1):104–6. doi: 10.1007/s002130050326. [DOI] [PubMed] [Google Scholar]
- Basavarajappa BS, Cooper TB, Hungund BL. Chronic ethanol administration down-regulates cannabinoid receptors in mouse brain synaptic plasma membrane. Brain Res. 1998;793(1–2):212–8. doi: 10.1016/s0006-8993(98)00175-9. [DOI] [PubMed] [Google Scholar]
- Basavarajappa BS, Hungund BL. Chronic ethanol increases the cannabinoidreceptor agonist anandamide and its precursor N-arachidonoylphosphatidylethanolamine in SK-N-SH cells. J Neurochem. 1999;72(2):522–8. doi: 10.1046/j.1471-4159.1999.0720522.x. [DOI] [PubMed] [Google Scholar]
- Basavarajappa BS, Saito M, Cooper TB, Hungund BL. Stimulation of cannabinoid receptor agonist 2-arachidonylglycerol by chronic ethanol and its modulation by specific neuromodulators in cerebellar granule neurons. Biochim Biophys Acta. 2000;1535(1):78–86. doi: 10.1016/s0925-4439(00)00085-5. [DOI] [PubMed] [Google Scholar]
- Basavarajappa BS, Saito M, Cooper TB, Hungund BL. Chronic ethanol inhibits the anandamide transport and increases extracellular anandamide levels in cerebellar granule neurons. Eur J Pharmacol. 2003;466(1–2):73–83. doi: 10.1016/s0014-2999(03)01557-7. [DOI] [PubMed] [Google Scholar]
- Bertotto ME, Maldonado NM, Bignante EA, Gorosito SV, Cambiasso MJ, Molina VA, Martijena ID. Erk activation in the amygdala and hippocampus induced by fear conditioning in ethanol withdrawn rats: Modulation by mk-801. Eur Neuropsychopharmacol. 2011 doi: 10.1016/j.euroneuro.2011.01.001. [DOI] [PubMed] [Google Scholar]
- Bisogno T, Howell F, Williams G, Minassi A, Cascio MG, Ligresti A, Matias I, Schiano-Moriello A, Paul P, Williams EJ, Gangadharan U, Hobbs C, Di Marzo V, Doherty P. Cloning of the first sn1-DAG lipases points to the spatial and temporal regulation of endocannabinoid signaling in the brain. J Cell Biol. 2003;163(3):463–8. doi: 10.1083/jcb.200305129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boger DL, Henriksen SJ, Cravatt BF. Oleamide: an endogenous sleep-inducing lipid and prototypical member of a new class of biological signaling molecules. Curr Pharm Des. 1998;4(4):303–14. [PubMed] [Google Scholar]
- Bortolato M, Campolongo P, Mangieri RA, Scattoni ML, Frau R, Trezza V, La Rana G, Russo R, Calignano A, Gessa GL, Cuomo V, Piomelli D. Anxiolytic-like properties ofthe anandamide transport inhibitor AM404. Neuropsychopharmacology. 2006;31(12):2652–9. doi: 10.1038/sj.npp.1301061. [DOI] [PubMed] [Google Scholar]
- Caille S, Alvarez-Jaimes L, Polis I, Stouffer DG, Parsons LH. Specific alterations of extracellular endocannabinoid levels in the nucleus accumbens by ethanol, heroin, and cocaine self-administration. J Neurosci. 2007;27(14):3695–702. doi: 10.1523/JNEUROSCI.4403-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cippitelli A, Bilbao A, Hansson AC, del Arco I, Sommer W, Heilig M, Massi M, Bermudez-Silva FJ, Navarro M, Ciccocioppo R, de Fonseca FR. Cannabinoid CB1 receptor antagonism reduces conditioned reinstatement of ethanol-seeking behavior in rats. Eur J Neurosci. 2005;21(8):2243–51. doi: 10.1111/j.1460-9568.2005.04056.x. [DOI] [PubMed] [Google Scholar]
- Cippitelli A, Cannella N, Braconi S, Duranti A, Tontini A, Bilbao A, Defonseca FR, Piomelli D, Ciccocioppo R. Increase of brain endocannabinoid anandamide levels by FAAH inhibition and alcohol abuse behaviours in the rat. Psychopharmacology (Berl) 2008;198(4):449–60. doi: 10.1007/s00213-008-1104-0. [DOI] [PubMed] [Google Scholar]
- Colombo G, Serra S, Brunetti G, Gomez R, Melis S, Vacca G, Carai MM, Gessa L. Stimulation of voluntary ethanol intake by cannabinoid receptor agonists in ethanol-preferring sP rats. Psychopharmacology (Berl) 2002;159(2):181–7. doi: 10.1007/s002130100887. [DOI] [PubMed] [Google Scholar]
- Comings DE, Muhleman D, Gade R, Johnson P, Verde R, Saucier G, MacMurray J. Cannabinoid receptor gene (CNR1): association with i.v. drug use. Mol Psychiatry. 1997;2(2):161–8. doi: 10.1038/sj.mp.4000247. [DOI] [PubMed] [Google Scholar]
- Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature. 1996;384(6604):83–7. doi: 10.1038/384083a0. [DOI] [PubMed] [Google Scholar]
- Di Marzo V. Endocannabinoids: synthesis and degradation. Rev Physiol Biochem Pharmacol. 2008;160:1–24. doi: 10.1007/112_0505. [DOI] [PubMed] [Google Scholar]
- Dinh TP, Carpenter D, Leslie FM, Freund TF, Katona I, Sensi SL, Kathuria S, Piomelli D. Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc Natl Acad Sci U S A. 2002;99(16):10819–24. doi: 10.1073/pnas.152334899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer B, Bermudez-Silva FJ, Bilbao A, Alvarez-Jaimes L, Sanchez-Vera I, Giuffrida A, Serrano A, Baixeras E, Khaturia S, Navarro M, Parsons LH, Piomelli D, Rodriguez de Fonseca F. Regulation of brain anandamide by acute administration of ethanol. Biochem J. 2007;404(1):97–104. doi: 10.1042/BJ20061898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler CJ. The cannabinoid system and its pharmacological manipulation--a review, with emphasis upon the uptake and hydrolysis of anandamide. Fundam Clin Pharmacol. 2006;20(6):549–62. doi: 10.1111/j.1472-8206.2006.00442.x. [DOI] [PubMed] [Google Scholar]
- Freedland CS, Sharpe AL, Samson HH, Porrino LJ. Effects ofSR141716A on ethanol and sucrose self-administration. Alcohol Clin Exp Res. 2001;25(2):277–82. [PubMed] [Google Scholar]
- Gaetani S, Oveisi F, Piomelli D. Modulation of meal pattern in the rat by the anorexic lipid mediator oleoylethanolamide. Neuropsychopharmacology. 2003;28(7):1311–6. doi: 10.1038/sj.npp.1300166. [DOI] [PubMed] [Google Scholar]
- Glaser ST, Kaczocha M. Temporal changes in mouse brain fatty acid amide hydrolase activity. Neuroscience. 2009;163(2):594–600. doi: 10.1016/j.neuroscience.2009.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godlewski G, Offertaler L, Wagner JA, Kunos G. Receptors for acylethanolamides-GPR55 and GPR119. Prostaglandins Other Lipid Mediat. 2009;89(3–4):105–11. doi: 10.1016/j.prostaglandins.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez S, Cascio MG, Fernandez-Ruiz J, Fezza F, Di Marzo V, Ramos JA. Changes in endocannabinoid contents in the brain of rats chronically exposed to nicotine, ethanol or cocaine. Brain Res. 2002;954(1):73–81. doi: 10.1016/s0006-8993(02)03344-9. [DOI] [PubMed] [Google Scholar]
- Gonzalez S, Valenti M, de Miguel R, Fezza F, Fernandez-Ruiz J, Di Marzo V, Ramos JA. Changes in endocannabinoid contents in reward-related brain regions of alcohol-exposed rats, and their possible relevance to alcohol relapse. Br J Pharmacol. 2004;143(4):455–64. doi: 10.1038/sj.bjp.0705963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulyas AI, Cravatt BF, Bracey MH, Dinh TP, Piomelli D, Boscia F, Freund TF. Segregation of two endocannabinoid-hydrolyzing enzymes into pre-and postsynaptic compartments in the rat hippocampus, cerebellum and amygdala. Eur J Neurosci. 2004;20(2):441–58. doi: 10.1111/j.1460-9568.2004.03428.x. [DOI] [PubMed] [Google Scholar]
- Haller J, Bakos N, Szirmay M, Ledent C, Freund TF. The effects of genetic and pharmacological blockade of the CB1 cannabinoid receptor on anxiety. Eur J Neurosci. 2002;16(7):1395–8. doi: 10.1046/j.1460-9568.2002.02192.x. [DOI] [PubMed] [Google Scholar]
- Hansson AC, Bermudez-Silva FJ, Malinen H, Hyytia P, Sanchez-Vera I, Rimondini R, Rodriguez de Fonseca F, Kunos G, Sommer WH, Heilig M. Genetic impairment of frontocortical endocannabinoid degradation and high alcohol preference. Neuropsychopharmacology. 2007;32(1):117–26. doi: 10.1038/sj.npp.1301034. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, Rice KC. Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J Neurosci. 1991;11(2):563–83. doi: 10.1523/JNEUROSCI.11-02-00563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, Gorzalka BB. The endocannabinoid system and the treatment of mood and anxiety disorders. CNS Neurol Disord Drug Targets. 2009;8(6):451–8. doi: 10.2174/187152709789824624. [DOI] [PubMed] [Google Scholar]
- Holter SM, Engelmann M, Kirschke C, Liebsch G, Landgraf R, Spanagel R. Long-term ethanol self-administration with repeated ethanol deprivation episodes changes ethanol drinking pattern and increases anxiety-related behaviour during ethanol deprivation in rats. Behav Pharmacol. 1998;9(1):41–8. [PubMed] [Google Scholar]
- Huang MM, Overstreet DH, Knapp DJ, Angel R, Wills TA, Navarro M, Rivier J, Vale W, Breese GR. Corticotropin-releasing factor (CRF) sensitization of ethanol withdrawal-induced anxiety-like behavior is brain site specific and mediated by CRF-1 receptors: relation to stress-induced sensitization. J Pharmacol Exp Ther. 2010;332(1):298–307. doi: 10.1124/jpet.109.159186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hungund BL, Szakall I, Adam A, Basavarajappa BS, Vadasz C. Cannabinoid CB1 receptor knockout mice exhibit markedly reduced voluntary alcohol consumption and lack alcohol-induced dopamine release in the nucleus accumbens. J Neurochem. 2003;84(4):698–704. doi: 10.1046/j.1471-4159.2003.01576.x. [DOI] [PubMed] [Google Scholar]
- Kamprath K, Romo-Parra H, Haring M, Gaburro S, Doengi M, Lutz B, Pape HC. Short-term adaptation of conditioned fear responses through endocannabinoid signaling in the central amygdala. Neuropsychopharmacology. 2011;36(3):652–63. doi: 10.1038/npp.2010.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kathuria S, Gaetani S, Fegley D, Valino F, Duranti A, Tontini A, Mor M, Tarzia G, La Rana G, Calignano A, Giustino A, Tattoli M, Palmery M, Cuomo V, Piomelli D. Modulation of anxiety through blockade of anandamide hydrolysis. Nat Med. 2003;9(1):76–81. doi: 10.1038/nm803. [DOI] [PubMed] [Google Scholar]
- Katona I, Rancz EA, Acsady L, Ledent C, Mackie K, Hajos N, Freund TF. Distribution of CB1 cannabinoid receptors in the amygdala and their role in the control of GABAergic transmission. J Neurosci. 2001;21(23):9506–18. doi: 10.1523/JNEUROSCI.21-23-09506.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Brain stress systems in the amygdala and addiction. Brain Res. 2009;1293:61–75. doi: 10.1016/j.brainres.2009.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Roberts AJ, Schulteis G, Parsons LH, Heyser CJ, Hyytia P, Merlo-Pich E, Weiss F. Neurocircuitry targets in ethanol reward and dependence. Alcohol Clin Exp Res. 1998;22(1):3–9. [PubMed] [Google Scholar]
- Labar G, Michaux C. Fatty acid amide hydrolase: from characterization to therapeutics. Chem Biodivers. 2007;4(8):1882–902. doi: 10.1002/cbdv.200790157. [DOI] [PubMed] [Google Scholar]
- Landolt HP, Gillin JC. Sleep abnormalities during abstinence in alcohol-dependent patients. Aetiology and management. CNS Drugs. 2001;15(5):413–25. doi: 10.2165/00023210-200115050-00006. [DOI] [PubMed] [Google Scholar]
- Leung D, Saghatelian A, Simon GM, Cravatt BF. Inactivation of N-acyl phosphatidylethanolamine phospholipase D reveals multiple mechanisms for the biosynthesis of endocannabinoids. Biochemistry. 2006;45(15):4720–6. doi: 10.1021/bi060163l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little HJ, Croft AP, O’Callaghan MJ, Brooks SP, Wang G, Shaw SG. Selective increases in regional brain glucocorticoid: a novel effect of chronic alcohol. Neuroscience. 2008;156(4):1017–27. doi: 10.1016/j.neuroscience.2008.08.029. [DOI] [PubMed] [Google Scholar]
- Liu J, Wang L, Harvey-White J, Huang BX, Kim HY, Luquet S, Palmiter RD, Krystal G, Rai R, Mahadevan A, Razdan RK, Kunos G. Multiple pathways involved in the biosynthesis of anandamide. Neuropharmacology. 2008;54(1):1–7. doi: 10.1016/j.neuropharm.2007.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Wang L, Harvey-White J, Osei-Hyiaman D, Razdan R, Gong Q, Chan AC, Zhou Z, Huang BX, Kim HY, Kunos G. A biosynthetic pathway for anandamide. Proc Natl Acad Sci U S A. 2006;103(36):13345–50. doi: 10.1073/pnas.0601832103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez MF, Becker HC. Effect of pattern and number ofchronic ethanol exposures on subsequent voluntary ethanol intake in C57BL/6J mice. Psychopharmacology (Berl) 2005;181(4):688–96. doi: 10.1007/s00213-005-0026-3. [DOI] [PubMed] [Google Scholar]
- Lutz B. Endocannabinoid signals in the control of emotion. Curr Opin Pharmacol. 2009;9(1):46–52. doi: 10.1016/j.coph.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Malinen H, Lehtonen M, Hyytia P. Modulation of brain endocannabinoid levels by voluntary alcohol consumption in alcohol-preferring AA rats. Alcohol Clin Exp Res. 2009;33(10):1711–20. doi: 10.1111/j.1530-0277.2009.01008.x. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F. Localization of the CB1 type cannabinoid receptor in the rat basolateral amygdala: high concentrations in a subpopulation of cholecystokinin-containing interneurons. Neuroscience. 2001;107(4):641–52. doi: 10.1016/s0306-4522(01)00380-3. [DOI] [PubMed] [Google Scholar]
- Merlo Pich E, Lorang M, Yeganeh M, Rodriguez de Fonseca F, Raber J, Koob GF, Weiss F. Increase of extracellular corticotropin-releasing factor-like immunoreactivity levels in the amygdala of awake rats during restraint stress and ethanol withdrawal as measured by microdialysis. J Neurosci. 1995;15(8):5439–47. doi: 10.1523/JNEUROSCI.15-08-05439.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moonat S, Sakharkar AJ, Zhang H, Pandey SC. The role of amygdaloid brain-derived neurotrophic factor, activity-regulated cytoskeleton-associated protein and dendritic spines in anxiety and alcoholism. Addict Biol. 2011;16(2):238–50. doi: 10.1111/j.1369-1600.2010.00275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira FA, Wotjak CT. Cannabinoids and anxiety. Curr Top Behav Neurosci. 2010;2:429–50. doi: 10.1007/7854_2009_16. [DOI] [PubMed] [Google Scholar]
- Naassila M, Pierrefiche O, Ledent C, Daoust M. Decreased alcohol self-administration and increased alcohol sensitivity and withdrawal in CB1 receptor knockout mice. Neuropharmacology. 2004;46(2):243–53. doi: 10.1016/j.neuropharm.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Okamoto Y, Morishita J, Tsuboi K, Tonai T, Ueda N. Molecular characterization of a phospholipase D generating anandamide and its congeners. J Biol Chem. 2004;279(7):5298–305. doi: 10.1074/jbc.M306642200. [DOI] [PubMed] [Google Scholar]
- Orio L, Edwards S, George O, Parsons LH, Koob GF. A role for the endocannabinoid system in the increased motivation for cocaine in extended-access conditions. J Neurosci. 2009;29(15):4846–57. doi: 10.1523/JNEUROSCI.0563-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Breese GR. Accentuated decrease in social interaction in rats subjected to repeated ethanol withdrawals. Alcohol Clin Exp Res. 2002;26(8):1259–68. doi: 10.1097/01.ALC.0000023983.10615.D7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Breese GR. Similar anxiety-like responses in male and female rats exposed to repeated withdrawals from ethanol. Pharmacol Biochem Behav. 2004;78(3):459–64. doi: 10.1016/j.pbb.2004.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey SC, Zhang H, Ugale R, Prakash A, Xu T, Misra K. Effector immediate-early gene arc in the amygdala plays a critical role in alcoholism. J Neurosci. 2008;28(10):2589–600. doi: 10.1523/JNEUROSCI.4752-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S, Cravatt BF, Hillard CJ. Synergistic interactions between cannabinoids and environmental stress in the activation of the central amygdala. Neuropsychopharmacology. 2005a;30(3):497–507. doi: 10.1038/sj.npp.1300535. [DOI] [PubMed] [Google Scholar]
- Patel S, Roelke CT, Rademacher DJ, Cullinan WE, Hillard CJ. Endocannabinoid signaling negatively modulates stress-induced activation of the hypothalamic-pituitary-adrenal axis. Endocrinology. 2004;145(12):5431–8. doi: 10.1210/en.2004-0638. [DOI] [PubMed] [Google Scholar]
- Patel S, Roelke CT, Rademacher DJ, Hillard CJ. Inhibition of restraint stress-induced neural and behavioural activation by endogenous cannabinoid signalling. Eur J Neurosci. 2005b;21(4):1057–69. doi: 10.1111/j.1460-9568.2005.03916.x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. New York: Academic Press, Spiral Bound; 1998. [Google Scholar]
- Radwanska K, Wrobel E, Korkosz A, Rogowski A, Kostowski W, Bienkowski P, Kaczmarek L. Alcohol relapse induced by discrete cues activates components of AP-1 transcription factor and ERK pathway in the rat basolateral and central amygdala. Neuropsychopharmacology. 2008;33(8):1835–46. doi: 10.1038/sj.npp.1301567. [DOI] [PubMed] [Google Scholar]
- Rimondini R, Arlinde C, Sommer W, Heilig M. Long-lasting increase in voluntary ethanol consumption and transcriptional regulation in the rat brain after intermittent exposure to alcohol. FASEB J. 2002;16(1):27–35. doi: 10.1096/fj.01-0593com. [DOI] [PubMed] [Google Scholar]
- Roberto M, Cruz M, Bajo M, Siggins GR, Parsons LH, Schweitzer P. The endocannabinoid system tonically regulates inhibitory transmission and depresses the effect of ethanol in central amygdala. Neuropsychopharmacology. 2010;35(9):1962–72. doi: 10.1038/npp.2010.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez de Fonseca F, Carrera MR, Navarro M, Koob GF, Weiss F. Activation of corticotropin-releasing factor in the limbic system during cannabinoid withdrawal. Science. 1997;276(5321):2050–4. doi: 10.1126/science.276.5321.2050. [DOI] [PubMed] [Google Scholar]
- Rodriguez de Fonseca F, Del Arco I, Bermudez-Silva FJ, Bilbao A, Cippitelli A, Navarro M. The endocannabinoid system: physiology and pharmacology. Alcohol Alcohol. 2005;40(1):2–14. doi: 10.1093/alcalc/agh110. [DOI] [PubMed] [Google Scholar]
- Rodriguez de Fonseca F, Navarro M. Role of the limbic system in dependence on drugs. Ann Med. 1998;30(4):397–405. doi: 10.3109/07853899809029940. [DOI] [PubMed] [Google Scholar]
- Rodriguez de Fonseca F, Roberts AJ, Bilbao A, Koob GF, Navarro M. Cannabinoid receptor antagonist SR141716A decreases operant ethanol self administration in rats exposed to ethanol-vapor chambers. Zhongguo Yao Li Xue Bao. 1999;20(12):1109–14. [PubMed] [Google Scholar]
- Rubio M, Fernandez-Ruiz J, de Miguel R, Maestro B, Michael Walker J, Ramos JA. CB1 receptor blockade reduces the anxiogenic-like response and ameliorates the neurochemical imbalances associated with alcohol withdrawal in rats. Neuropharmacology. 2008;54(6):976–88. doi: 10.1016/j.neuropharm.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Rubio M, McHugh D, Fernandez-Ruiz J, Bradshaw H, Walker JM. Short-term exposure to alcohol in rats affects brain levels of anandamide, other N-acylethanolamines and 2-arachidonoyl-glycerol. Neurosci Lett. 2007;421(3):270–4. doi: 10.1016/j.neulet.2007.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggeri B, Braconi S, Cannella N, Kallupi M, Soverchia L, Ciccocioppo R, Ubaldi M. Neuropeptide S receptor gene expression in alcohol withdrawal and protracted abstinence in postdependent rats. Alcohol Clin Exp Res. 2010;34(1):90–7. doi: 10.1111/j.1530-0277.2009.01070.x. [DOI] [PubMed] [Google Scholar]
- Rutkowska M, Jamontt J, Gliniak H. Effects of cannabinoids on the anxiety-like response in mice. Pharmacol Rep. 2006;58(2):200–6. [PubMed] [Google Scholar]
- Sciolino NR, Zhou W, Hohmann AG. Enhancement of endocannabinoid signaling with JZL184, an inhibitor of the 2-arachidonoylglycerol hydrolyzing enzyme monoacylglycerol lipase, produces anxiolytic effects underconditions of high environmental aversiveness in rats. Pharmacol Res. 2011 doi: 10.1016/j.phrs.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlosburg JE, Blankman JL, Long JZ, Nomura DK, Pan B, Kinsey SG, Nguyen PT, Ramesh D, Booker L, Burston JJ, Thomas EA, Selley DE, Sim-Selley LJ, Liu QS, Lichtman AH, Cravatt BF. Chronic monoacylglycerol lipase blockade causes functional antagonism of the endocannabinoid system. Nat Neurosci. 2010;13(9):1113–9. doi: 10.1038/nn.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt K, Krishnan B, Xia Y, Sun A, Orozco-Cabal L, Pollandt S, Centeno M, Genzer K, Gallagher JP, Shinnick-Gallagher P, Liu J. Cocaine withdrawal reduces group I mGluR-mediated long-term potentiation via decreased GABAergic transmission in the amygdala. Eur J Neurosci. 2011;34(2):177–89. doi: 10.1111/j.1460-9568.2011.07769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt LG, Samochowiec J, Finckh U, Fiszer-Piosik E, Horodnicki J, Wendel B, Rommelspacher H, Hoehe MR. Association of a CB1 cannabinoid receptor gene (CNR1) polymorphism with severe alcohol dependence. Drug Alcohol Depend. 2002;65(3):221–4. doi: 10.1016/s0376-8716(01)00164-8. [DOI] [PubMed] [Google Scholar]
- Simon GM, Cravatt BF. Endocannabinoid biosynthesis proceeding through glycerophospho-N-acyl ethanolamine and a role for alpha/beta-hydrolase 4 in this pathway. J Biol Chem. 2006;281(36):26465–72. doi: 10.1074/jbc.M604660200. [DOI] [PubMed] [Google Scholar]
- Simon GM, Cravatt BF. Anandamide biosynthesis catalyzed by the phosphodiesterase GDE1 and detection of glycerophospho-N-acyl ethanolamine precursors in mouse brain. J Biol Chem. 2008;283(14):9341–9. doi: 10.1074/jbc.M707807200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipe JC, Chiang K, Gerber AL, Beutler E, Cravatt BF. A missense mutation in human fatty acid amide hydrolase associated with problem drug use. Proc Natl Acad Sci U S A. 2002;99(12):8394–9. doi: 10.1073/pnas.082235799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanos PK, Dimitrakakis ES, Rice O, Gifford A, Volkow ND. Ethanol self-administration and ethanol conditioned place preference are reduced in mice lacking cannabinoid CB1 receptors. Behav Brain Res. 2005;164(2):206–13. doi: 10.1016/j.bbr.2005.06.021. [DOI] [PubMed] [Google Scholar]
- Trevisan LA, Boutros N, Petrakis IL, Krystal JH. Complications of alcohol withdrawal: pathophysiological insights. Alcohol Health Res World. 1998;22(1):61–6. [PMC free article] [PubMed] [Google Scholar]
- Tsou K, Brown S, Sanudo-Pena MC, Mackie K, Walker JM. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience. 1998;83(2):393–411. doi: 10.1016/s0306-4522(97)00436-3. [DOI] [PubMed] [Google Scholar]
- Valenti M, Vigano D, Casico MG, Rubino T, Steardo L, Parolaro D, Di Marzo V. Differential diurnal variations of anandamide and 2-arachidonoyl-glycerol levels in rat brain. Cell Mol Life Sci. 2004;61(7–8):945–50. doi: 10.1007/s00018-003-3453-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinod KY, Yalamanchili R, Xie S, Cooper TB, Hungund BL. Effect of chronic ethanol exposure and its withdrawal on the endocannabinoid system. Neurochem Int. 2006;49(6):619–25. doi: 10.1016/j.neuint.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Waleh NS, Cravatt BF, Apte-Deshpande A, Terao A, Kilduff TS. Transcriptional regulation of the mouse fatty acid amide hydrolase gene. Gene. 2002;291(1–2):203–10. doi: 10.1016/s0378-1119(02)00598-x. [DOI] [PubMed] [Google Scholar]
- Wang L, Liu J, Harvey-White J, Zimmer A, Kunos G. Endocannabinoid signaling via cannabinoid receptor 1 is involved in ethanol preference and its age-dependent decline in mice. Proc Natl Acad Sci U S A. 2003;100(3):1393–8. doi: 10.1073/pnas.0336351100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Pandey SC. Effects of PKA modulation on the expression of neuropeptide Y in rat amygdaloid structures during ethanol withdrawal. Peptides. 2003;24(9):1397–402. doi: 10.1016/j.peptides.2003.08.008. [DOI] [PubMed] [Google Scholar]