Abstract
Background
The prefrontal cortex (PFC) is critically involved in working memory, cognition and decision-making; processes significantly affected by ethanol. During quiet restfulness or sleep, prefrontal cortical neurons show synaptically-evoked oscillations in membrane potential between hyperpolarized down-states and depolarized up-states. Previous studies from this laboratory used whole-cell electrophysiology and demonstrated that in individual neurons, ethanol inhibited PFC up-states at concentrations associated with behavioral impairment. While those studies monitored activity in one or two neurons at a time, it is likely that in vivo, larger networks of neurons participate in the complex functions of the prefrontal cortex. In the present study, we used imaging and a genetically encoded calcium sensor to examine the effects of ethanol on the activity of multiple neurons simultaneously during up-states.
Methods
Slice cultures of mouse prefrontal cortex were infected with an AAV virus encoding the calcium indicator GCaMP3 whose expression was driven by the neuron-specific synapsin promoter. After 2–3 weeks in culture, a fast CCD-camera imaging system was used to capture changes in GCaMP3 fluorescence before, during and after exposure to ethanol.
Results
PFC neurons displayed robust and reproducible changes in GCaMP3 fluorescence during evoked and spontaneous up-states. Simultaneous whole-cell patch-clamp recording and GCaMP3 imaging verified that neurons transitioned into and out of up-states together. Acute application of ethanol reliably depressed up-state calcium signals with lower doses having a greater effect on up-state duration than amplitude. These effects of ethanol on up-state parameters were reversed during washout.
Conclusions
The results of the present study indicate that ethanol has profound effects on upstate activity in prefrontal neurons and suggest that this action may underlie some of the cognitive impairment associated with acute alcohol intoxication.
Keywords: persistent activity, prefrontal cortex, alcohol, addiction, cognition
Introduction
Ethanol (alcohol) is among the most commonly consumed substances and the manufacturing and distribution of alcohol containing beverages is of major economic importance worldwide. The actions of ethanol on the body are widespread and both adverse and beneficial effects have been observed that are dependent on the amount and frequency of consumption, the target organ/system considered and the genetic makeup of the user (Woodward, 2009). Ethanol’s effects on brain reward and stress pathways are thought to underlie its addictive properties (Kalivas and Volkow, 2005; Koob and Kreek, 2007) and numerous studies have shown that ethanol alters neuronal function in most brain regions studied (McCool, 2010; Weiner and Valenzuela, 2006). Of particular interest in this regard are ethanol’s effects on frontal areas of the brain that sub-serve higher cognitive functions as these processes are often disrupted following acute and chronic exposure to ethanol (Abernathy et al., 2010). Acutely, ethanol causes impairments in memory function, decision-making and risk assessment that likely contribute to the adverse outcomes often associated with intoxication (Weissenborn and Duka, 2003). Deficits in these processes are also often observed in individuals diagnosed with chronic alcohol use disorders (Bechara et al., 2001). The prefrontal cortex (PFC) is critically involved in these types of cognition-based tasks and it exerts top-down integration and control of limbic, sensory and motor areas of the brain (Fuster, 2008).
An important feature of neurons within the prefrontal cortex is their ability to engage in coordinated patterns of network activity that extend across large areas of cortex. For example, during sleep or anesthesia, EEG recordings from prefrontal areas show a slow oscillation in the 0.5–1 Hz frequency range (Steriade et al., 1993). These oscillations arise from synchronized activity of large numbers of neurons and are characterized by periods of sustained neuronal depolarization (up-states) following by electrical silence (down-states). Enhancing oscillatory activity during sleep via transcranial electrical stimulation of prefrontal areas has been demonstrated to improve memory consolidation in humans suggesting that these activity patterns have an important physiological role (Marshall et al., 2006). To study the synaptic processes involved in prefrontal neuron function, single cell electrophysiology has been used in conjunction with brain slices acutely isolated from experimental animals. While powerful, this approach is limited by the small number of neurons that can be simultaneously recorded using whole-cell patch-clamp electrophysiology and by the quiescent nature of neurons present in most acutely isolated brain slices. Dye-based calcium imaging techniques can monitor activity in many cells simultaneously, but this approach is often limited in brain slices by inefficient dye-loading and the indiscriminate nature of cell labeling. In this study, we use a novel genetically encoded calcium sensor that can be expressed selectively in neurons to monitor calcium dynamics in slice cultures of prefrontal cortex. As shown previously, these cultures generate patterns of persistent activity and up-states that resemble those observed in vivo (Plenz and Kitai, 1996; Seamans et al., 2003). The results of the present study reveal that ethanol has profound and widespread effects on up-states in neurons of the prefrontal cortex.
Materials and Methods
Animals
C57BL/6J mouse pups were generated from a breeding colony maintained in the animal vivarium at the Medical University of South Carolina. Adult breeders in the colony were purchased from the Jackson Laboratories (Bar Harbor, ME). All procedures involving animals were conducted under protocols approved by the Institutional Animal Care and Use Committee of the Medical University of South Carolina.
Organotypic slice co-cultures
Slice co-cultures were prepared as previously described (Tu et al., 2007; Woodward and Pava, 2009). Briefly, postnatal day 1–5 C57Bl6/J mouse pups were sacrificed and brains were placed in ice-cold, sucrose-substituted Ringers solution containing (in mM) 200 sucrose, 1.9 KCl, 6 MgCl2, 0.5 CaCl2, 10 glucose, 0.4 ascorbic acid, 25 HEPES (titrated to pH 7.3 with KOH bubbled with 95% O2/5% CO2. Individual coronal sections containing prefrontal cortex including anterior cingulated, prelimbic and infralimbic areas were cut at a thickness of 400 um using a Leica VT-1000 vibratome. Slices were oriented adjacent to one another on a Millipore millicell insert in a 6 well culture dish. Each well contained 1 ml of serum-containing media consisting of 50% basal medium Eagle, 25% Earle’s balanced salt solution, 25% heat-inactivated horse serum with 33 mM glucose, 25 mM HEPES, 170 μM streptomycin and 0.235 mM Glutamax. After 3 days in vitro (DIV), the media was changed to one containing reduced amounts of horse serum (5%). After 14 DIV, 5-fluoro-2-deoxyuridine (4 μM) was added to prevent glial overgrowth. Cell culture dishes were kept in a 37°C humidified incubator equilibrated with 5% CO2 and media was changed every 2–3 days. Cultures were used between 14–23 DIV.
Calcium Imaging and Electrophysiology
Intracellular calcium was monitored using the genetically encoded calcium indicator GCaMP3 (Tian et al., 2009). GCaMP3 was delivered to the cultures via a recombinant AAV2/1 virus with a human synapsin promoter to drive expression selectively in neurons (UPenn Viral Core). After 1–2 DIV, 1–4 uL of the viral stock was added directly to the slice culture under sterile conditions. On the day of recording, the section of the Millipore membrane containing a slice co-culture was carefully removed from the insert and was submerged in a recording chamber. The slice culture was perfused at approximately 2 ml/min with artificial cerebrospinal fluid (ACSF) containing, (in mM) 125 NaCl, 2.5 KCl, 25 NaHCO3, 1.25 NaH2PO4, 1.3 MgCl2, 2 CaCl2, 0.4 ascorbic acid and 10 glucose. ACSF solutions were continuously bubbled with a mixture of 95% O2/5% CO2 and maintained at a temperature of 32–33°C using a flow-through solution heater and heated recording chamber (Warner Instruments, Hamden, CT).
Calcium imaging was performed on a Zeiss FS2 microscope using a NeuroCCD camera system (RedShirt Imaging, Decatur, GA). Up-states were initiated using a concentric bipolar electrode (0.2 ms pulse, 400–750 uA) positioned on the dorsal surface of the PFC slice and full-frame images were acquired at a rate of 125 Hz. In most studies, a series of 5 evoked up-states were captured under control conditions (1 minute inter-event interval) and then the perfusion solution was switched to one containing the test substance for 10 minutes. An additional 5 up-states were then collected at 1 minute intervals while maintaining perfusion of the test substance. This was followed by a 10-minute washout period after which a final series of up-state images were acquired. Optical signals were collected from an 80 × 80 pixel area with illumination provided by a 150W xenon lamp (Cairn Research, Faversham, UK) connected to the scope with a fiber optic cable. Light output from the xenon lamp was filtered using a standard eGFP filter set (excitation 485 nm/emission 525 nm). During each acquisition episode and prior to shutter opening, a five-frame average was collected and this dark background was subtracted from all subsequent images. The camera, light source and data acquisition routines were controlled by Neuroplex software (RedShirt Imaging, Decatur, GA) running on a Dell computer (Round Rock, TX).
In some experiments, simultaneous electrophysiological recordings were conducted in GCaMP3 expressing slices. Patch pipettes (1.5 mm × 1.1 mm; 2–3 MΩ resistance) were filled with an internal recording solution containing (in mM); 130 K-gluconate, 10 KCl, 2 MgCl2, 0.1 EGTA, 10 HEPES, 2 Na2ATP, and 0.3 NaGTP, pH 7.2. Neurons were visualized using Dodt gradient contrast imaging and gigaohm seal formation and breakthrough were conducted under voltage-clamp conditions. After establishing stable whole-cell access, the amplifier was switched to current-clamp mode. In some studies, the perforated-patch technique was used to avoid washout of the GCaMP3 indicator in the patched neuron and both imaging and electrophysiological data were simultaneously collected from the same neuron. For these recordings, amphotericin B was prepared as a stock solution (5.7 mg/ml in DMSO) and was added to the pipette filling solution just prior to use. Following gigaohm seal formation, voltage pulses (−5 mV) were delivered to the neuron to assess amphotericin pore formation and responses were measured using an Axoclamp 700B amplifier (Molecular Devices, Sunnyvale, CA). After pore formation was stable, the amplifier was switched to current-clamp mode and up-states were triggered with the bipolar stimulator. Electrophysiological data were filtered at 2–4 kHz and acquired at 10 kHz using an ITC-16 interface (HEKA Instruments, Bellmore, NY) controlled by AxographX software (Axograph Scientific, Sydney, AUS) running on a Macintosh G4 computer.
Data Analysis
Neuroplex (Redshirt Imaging, Decatur, GA) and ImageJ (NIH) software were used to analyze the GCaMP3 imaging data. Multiple regions of interest corresponding to individual neurons were selected and calcium signals were expressed as %dF/F where dF is the change in the fluorescence value measured relative to a 10-frame average baseline (F) just prior to up-state initiation. Data were analyzed for statistical significance with 1-way Anova using Prism 4 software (GraphPad Software, San Diego, CA). A significance level was set at p<0.05 for all analyses.
Results
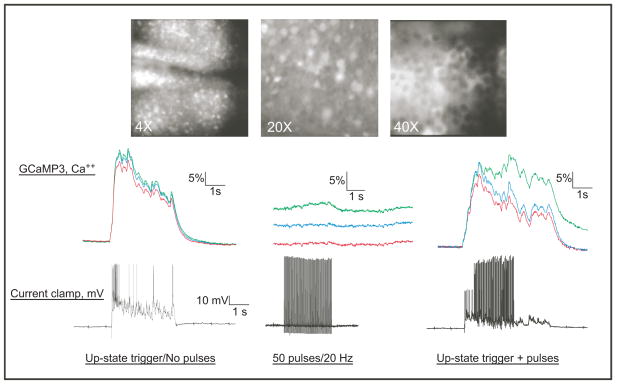
Approximately 4–5 days post-infection, slice cultures displayed widespread expression of the genetically encoded calcium sensor GCaMP3. This signal persisted for the life of the cultures and spontaneous fluctuations in GCaMP3 fluorescence could be observed in slice cultures verifying the presence of spontaneous network activity as previously reported (Seamans et al., 2003; Tu et al., 2007). Figure 1 shows examples of GCaMP3 fluorescence acquired at low (4X), medium (20X) and high (40X) magnification. Each figure represents a single raw image acquired from a different slice culture at a point during an up-state. To monitor the temporal fidelity of the GCaMP3 signal during up-states, the voltage signal from a single GCaMP3 positive neuron was recorded using the perforated patch mode of current clamp while simultaneously acquiring the GCaMP3 fluorescence signal of the entire field at a frame rate of 125 Hz. As shown in the current clamp traces at the bottom of Figure 1, a brief electrical stimulus applied to the slice culture evoked an immediate transition from the down-state resting membrane potential (Vm~−70 mV) to the depolarized up-state (Vm~−55 mV). The up-state persisted for several seconds and was accompanied by bursts of action potentials. The GCaMP3 traces show the calcium signal from the patch-clamped neuron and from several unpatched neighboring neurons. These traces illustrate that up-states occur simultaneously in multiple neurons in the culture and that the GCaMP3 signal accurately tracks up-state electrical activity. Both the onset and offset of the up-state were highly synchronized across the cortical slice (see below and Figure 3 for more detail). To demonstrate whether action potential spiking alone induces a significant increase in the GCaMP3 signal, a series (50 pulses, 20 Hz) of current pulses (1–2 nA, 1 ms) were delivered to the patched neuron during the down-state. As shown in the middle panel of Figure 1, while these pulses induced action potential firing in the current-clamp recording, they generated only a small increase in the GCaMP3 signal from the patched neuron and no change in the unpatched neurons. In contrast, when the same pulse train was delivered during the up-state, the GCaMP3 signal in the patched neuron was boosted over and above that recorded in unpatched neurons. A similar effect was observed when the pulses were delivered at different times during the up-state (data not shown).
Figure 1.
Up-state calcium responses in PFC neurons expressing the genetically encoded calcium sensor GCaMP3. At the top are single raw images showing examples of GCaMP3 fluorescence in different slice cultures imaged at 4X, 20X and 40X magnification. Traces below show changes in intracellular calcium (GCaMP3; %dF/F) and membrane voltage (Vm) from a single patch-clamped neuron. In each set of GCaMP3 traces, the top trace (green) is from the patch-clamped neuron while the other two traces (blue, red) are from neighboring unpatched neurons. Left panel shows calcium and voltage responses during a single evoked up-state; note time-locked initiation and decay of up-state electrical and calcium signals. Middle panel shows calcium and voltage responses to a 20 Hz train of current pulses (50 pulses, 1 nA; 1 msec,) delivered directly to the patched neuron during the down-state. The corresponding GCaMP3 traces are offset vertically to better illustrate the small effect that direct current injection has on the calcium signal in the patch-clamped neuron (top green trace). Right panel shows effect of the same 20 Hz pulse train delivered to the patched neuron during the up-state; note enhanced amplitude and duration of the GCaMP3 signal in the patched neuron (top green trace) as compared to the unpatched neurons.
Figure 3.
Up-state dynamics and ethanol inhibition in GCaMP3 expressing cultures. A) Inset image shows slice culture at 4X magnification and location of 18 regions of interest (ROIs). Dotted line represents the midline of the culture with the dorsal (D) and ventral (V) surfaces marked. Outlined box shows GCaMP3 signal from a single neuron during up-states evoked before, during and after exposure to 44 mM ethanol. Data (mean ± SEM) in graph represent the time to the peak of the up-state calcium response measured for each of the 18 ROIs identified on the image in the absence and presence of ethanol. B) Effects of ethanol on up-state amplitude recorded from 32 different neurons in the same slice culture. Inset graph shows GCaMP3 signal during an up-state from a single neuron before, during and after exposure to 66 mM ethanol. Data (mean ± SEM from five up-states) are taken from a 20X image and show peak calcium responses during evoked up-states collected before (open circles), during (closed squares) and after (open triangles) exposure to 66 mM ethanol.
Up-states in vivo and in vitro are driven by strong glutamatergic inputs onto both pyramidal neurons and interneurons (Sanchez-Vives and McCormick, 2000; Steriade et al., 2001). To characterize how these inputs contribute to the up-state calcium signal, blockers of NMDA and AMPA receptors were washed into the slice. Following a 10-minute application of the NMDA receptor blocker AP5 (100 μM) up-states were eliminated and responses slowly recovered during the washout period (Figure 2A; top traces and Figure 2B). These results are consistent with previous electrophysiological findings demonstrating that NMDA receptors are critical for maintaining up-state activity (Kroener et al., 2009; Seamans et al., 2003; Tu et al., 2007). Blocking AMPA receptors also affected up-state calcium transients but in a qualitatively different way. In the presence of NBQX (20 μM), up-state duration was significantly reduced while up-state amplitude was enhanced by approximately 7.5-fold (Figure 2A; middle traces and Figure 2B). This effect also slowly reversed and the amplitude and duration of evoked up-state calcium signals returned to control-like levels upon washout. To verify that the dramatic change in GCaMP3 fluorescence in the presence of NBQX mirrored that of the electrical signal, the membrane potential of a single neuron in the GCaMP3 expressing culture was monitored by patch-clamp electrophysiology. As shown in the bottom set of traces in Figure 2A, the patch-clamped neuron showed a long-lasting depolarization during the up-state under control conditions. In this particular neuron, the up-state membrane potential was sub-threshold for action potential firing so no spikes were observed. Similar to that observed for GCaMP3, NBQX significantly reduced up-state duration and sharply increased up-state membrane potential so that the voltage signal resembled a large excitatory post-synaptic potential (EPSP). These effects slowly reversed upon washout of NBQX.
Figure 2.
Effects of glutamatergic blockers on up-states in PFC slice cultures. A) Top set of traces (GCaMP3) show changes in calcium during an up-state in the presence of the NMDA antagonist AP5 (100 μM) or the AMPA antagonist NBQX (20 μM). Bottom traces (current-clamp) shows effect of NBQX on up-state electrical activity in a patch-clamped neuron from the same culture as the GCaMP3 signal was obtained. In each set of traces, the left set is data collected under control conditions, the middle during drug application, and the right following washout. Note differences in y-scale bars for the GCaMP3 signal especially for the NBQX responses. B) Summary of effects of AP5 and NBQX on up-state amplitude (left panel) and duration (right panel) as measured by GCaMP3 imaging. For each drug, measurements were taken from 10 different neurons selected at random in each culture under control, drug and washout conditions and data were expressed as percent of control. Data from the neurons was then averaged to yield a group mean and experiments were repeated (N=3 for AP5; N=4 for NBQX) and used to generate the overall mean ± SEM. Symbol(*); value significantly different from control (p<0.05; One-way repeated measures Anova with Dunnett’s post-hoc test).
These results demonstrate that GCaMP3 accurately reports up-state dynamics in single neurons and can be used to monitor activity in a larger set of neurons in the slice culture. We used this approach and determined the effects of ethanol on up-states in multiple neurons simultaneously. In these studies, cultures were infected with the GCaMP3 expressing AAV virus and images were acquired before, during and after acute exposure to ethanol. To illustrate the temporal coherence of up-states across multiple regions of the slice culture, a series of up-states was evoked in a slice culture and the time to the peak of the up-state calcium signal was measured. Figure 3A shows 18 regions of interest (ROI) superimposed over an image acquired at 4X magnification. In this image, the midline of the cortical slice runs across the middle of the image (dotted line) and the stimulus electrode was placed on the dorsal surface of the slice located to the left of the image shown. The corresponding graph plots the time from the stimulus to the peak of the up-state calcium signal for each of the 18 ROI’s and is the average of 5 up-states evoked 1 minute apart. In this example, the peak GCaMP3 signal occurred approximately 380 msec from the stimulus and there was no significant difference in the time to peak at any location measured. In the presence of 44 mM ethanol (approximately 0.2% BEC), the time to peak calcium appeared slightly faster in some regions although this difference was not statistically significant across the entire culture. Nonetheless, as shown in the GCaMP3 traces (Figure 3A, inset), 44 mM ethanol significantly reduced both the duration and amplitude of the evoked up-state and this effect reversed upon washout. Figure 3B shows data collected from a different slice at a 20X magnification where individual neurons could be identified. The inset traces show the GCaMP3 response of an individual neuron during an upstate recorded in the absence and presence of 66 mM ethanol (~0.3% BEC). Note the significant decrease in both up-state duration and amplitude that recovers during washout. The accompanying chart plots the up-state amplitude of the GCaMP3 signal from 32 different neurons located in the prelimbic portion of the slice culture during 5 evoked up-states (1 minute interval) under control, ethanol and washout conditions. In this study, up-state duration for the 5 control up-states averaged 9.63 sec ± 0.84. Following a 10-minute exposure to 66 mM ethanol, up-state amplitude was similarly decreased across all 32 neurons and up-state duration declined to 3.64 sec ± 0.74. Following washout of the ethanol solution, up-state amplitude returned to control values while up-state duration recovered to an average of 7.79 sec ± 1.98.
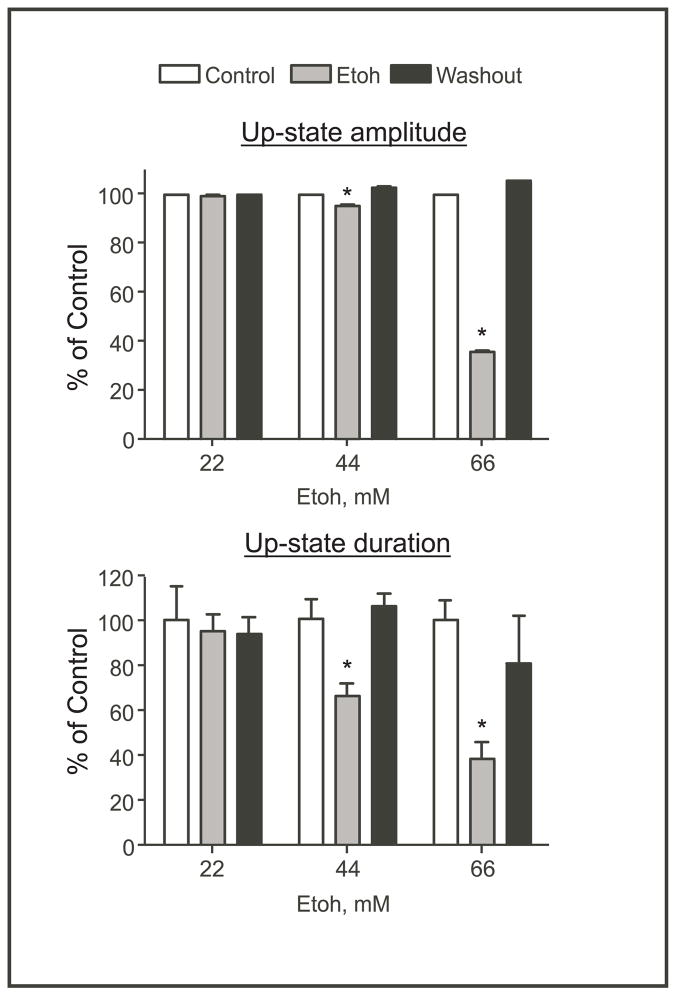
To better establish the relationship between ethanol and up-state parameters, additional experiments were performed in separate GCaMP3 expressing slice cultures using different concentrations of ethanol. Each slice was used for only one concentration of ethanol and data were collected from 32 neurons selected from the prelimbic area of the slice culture. During each experiment, sets of images were acquired at 125 Hz during each of 5 stimulus-evoked upstates (1-minute interval between episodes) collected under control, ethanol and washout conditions (15 up-states total per slice). Values from the five replicates were averaged for each neuron. As individual cultures displayed variability in up-state amplitude, duration and GCaMP3 expression, data from these experiments were expressed as a percent of the averaged control value for each culture. As shown in Figure 4, ethanol at 22 mM had little effect on either upstate amplitude or duration as measured by changes in GCaMP3 fluorescence. Increasing the ethanol concentration to 44 mM significantly reduced the duration of the up-state by approximately 35% while having a small but statistically significant effect on amplitude. At the highest concentration tested (66 mM), ethanol significantly reduced both amplitude and duration by approximately 65%. In each case, the ethanol-induced inhibition of up-state activity returned to control-like levels during washout. Because there is some evidence that L-type calcium channels in certain brain regions may be sensitive to ethanol (Zucca and Valenzuela, 2010), we also tested the effects of the dihydropyridine calcium channel blocker nifedipine on up-state calcium dynamics. In the presence of nifedipine (10 μM) up-state amplitude was 73.4% ± 1.73 of the control value (N=40 neurons from 4 different slice cultures) while up-state duration was slightly but not significantly prolonged (109.9% ± 10.91).
Figure 4.
Concentration-response relationship for ethanol inhibition of up-state calcium responses in GCaMP3 expressing neurons. Summary graphs show effects of ethanol (22, 44, 66 mM) on up-state amplitude (top) and duration (bottom) in GCaMP3 expressing PFC slice cultures. Data are expressed as a mean (±SEM; N=32 neurons each; average of 3 replicates per concentration) percent of the corresponding control value for each ethanol concentration. Symbol(*):value significantly different from corresponding control value (p<0.05, one-way repeated measures Anova.
Discussion
The major finding of the present study is that ethanol, at concentrations associated with behavioral impairment and intoxication, significantly reduced up-state calcium dynamics in multiple neurons maintained in a slice culture model of the prefrontal cortex. These effects were dose-dependent, reversible and were consistently observed across large numbers of neurons within the PFC slice culture. Using whole-cell patch-clamp electrophysiology, Tu et al. (2007) was the first to report that ethanol reduces neuronal activity during up-states in deep layer glutamatergic pyramidal neurons maintained in slice culture. In that study, up-state duration was the most sensitive parameter measured and effects were observed at ethanol concentrations as low as 17 mM; equivalent to the US drink-drive limit of 0.08% blood ethanol concentration. In the present study, 22 mM ethanol, the lowest concentration tested, had only modest effects on up-state duration and no effect on amplitude. This may reflect that in the present study, up-states were evoked by direct electrical stimulation of the slice culture while the results cited from the Tu et al. study came from up-states that arose spontaneously in the cultures. In fact, when up-states were initiated by direct electrical stimulation, Tu et al. reported a slight attenuation of ethanol’s inhibitory effects suggesting differences in the strength of spontaneous and evoked up-states.
In a follow-up study, Woodward and Pava (2009) also used whole-cell electrophysiology and examined the effects of ethanol on up-states from both excitatory pyramidal neurons and fast-spiking interneurons in PFC slice cultures. In that study, dual patch-clamp recordings showed that up-states in these neurons were highly synchronized and that the NMDA receptor blocker MK801 strongly affected up-state dynamics in pyramidal neurons but had relatively little effect on fast-spiking interneurons. Despite this difference in MK801 sensitivity, ethanol inhibited upstates in both pyramidal neurons and interneurons to the same extent. These findings confirm the idea that persistent activity and up-states in the PFC involves inter-connected networks of excitatory and inhibitory neurons (Compte et al., 2003; Durstewitz et al., 2000) and that NMDA receptors on pyramidal neurons are an important source of glutamatergic drive in PFC pyramidal neurons. The essential role of both glutamatergic and GABAergic neurons in upstates is also supported by studies showing that a brief EPSP/IPSP sequence initiates the transition into the up-state (Seamans et al., 2003). This involves sequential activation of synaptic AMPA and GABAA receptors and is followed by activation of NMDA receptors that helps sustain the long-lasting depolarization that characterizes the up-state (Seamans et al., 2003; Wolf et al., 2005).
The differential role of AMPA and NMDA receptors in regulating up-state activity was clearly demonstrated in the present study when recordings were carried out in the presence of receptor selective blockers. Perfusion of cultures with the NMDA antagonist AP5 essentially eliminated the up-state and the associated increase in calcium as measured by GCaMP3 imaging. These results are consistent with those from previous electrophysiological studies showing that blockade of NMDA receptors prevents up-state activity both in vivo and in vitro (Kroener et al., 2009; Seamans et al., 2003). In addition, the lack of an appreciable increase in the GCaMP3 signal in the presence of AP5 also reveals that AMPA receptors that are transiently activated by the stimulus are largely calcium impermeable. Interestingly, blocking AMPA receptors with NBQX also significantly reduced up-state duration, but dramatically enhanced the amplitude of the GCaMP3 signal. This was mirrored by a substantial depolarization in membrane potential from the recorded neuron that resembles that observed in the presence of the GABAA receptor blocker bicucculine (Seamans et al., 2003). During up-states, GABAA receptors normally prevent the membrane potential from reaching values more positive than the chloride reversal potential (~−55–60 mV) thus preventing epileptiform activity and allowing ongoing excitatory activity to induce action potential firing during the up-state. The large depolarization and increase in calcium signal observed in the presence of NBQX suggests that this drug not only blocks fast glutamatergic signaling in pyramidal neurons but may also impair normal GABAergic transmission. A clue as to how this may occur comes from findings from previous studies showing that fast-spiking GABAergic interneurons in the PFC express relatively few NMDA receptors and rely instead upon AMPA receptors for excitation (Wang and Gao, 2009; Woodward and Pava, 2009). By blocking AMPA receptors on these GABAergic interneurons, NBQX would effectively silence them leading to a disinhibition of glutamatergic neurons. Since highly calcium permeable NMDA receptors on pyramidal neurons remain available in the presence of NBQX, glutamate released in response to the electrical stimulus can activate these receptors and combined with reduced GABAergic control of membrane potential induces a large increase in the GCaMP3 calcium signal. The slow kinetics of NMDA receptor currents coupled with the NBQX-mediated block of fast AMPA receptor signaling on both pyramidal and interneurons likely underlies the inability of the slice culture to generate long up-states under these conditions. It should be noted that in previous studies, up-state activity in PFC slice cultures was reported to be completely blocked by the related AMPA antagonist CNQX (Kroener et al., 2009; Seamans et al., 2003). However, unlike NBQX, CNQX also blocks the glycine binding site on the NMDA receptor (Lester et al., 1989; Verdoorn et al., 1989) and when combined, these actions would be expected to prevent up-state activity. Although the above hypothesis can explain the actions of NBQX on up-state dynamics, confirmation of this effect awaits development and use of techniques that can selectively silence sub-populations of GABAergic interneurons while sparing activity in other neuron sub-types. Such an approach using optogenetics is currently being tested in this laboratory.
The different actions of AP5 and NBQX as well as that of the calcium channel blocker nifedipine on up-state activity can be used to infer how ethanol causes its effects. Ethanol’s inhibition of up-state activity more closely resembled that of AP5 and not NBQX or nifedipine as ethanol reduced both up-state duration and amplitude with a greater effect on duration at lower concentrations. This differential effect of ethanol on up-state duration versus amplitude could reflect differences in the ethanol sensitivity of the AMPA, NMDA and GABAA receptors that initiate and shape up-state activity. In a previous study, we showed that pharmacologically isolated AMPA and GABAA-mediated synaptic currents in rat PFC neurons are largely insensitive to concentrations of ethanol up to at least 44 mM (Weitlauf and Woodward, 2008). In contrast, NMDA-mediated EPSCs in those neurons were significantly inhibited at these concentrations of ethanol (Weitlauf and Woodward, 2008). While up-state duration appears particularly sensitive to lower concentrations of ethanol, as concentrations above 44 mM, upstate amplitude is also attenuated. This could reflect actions on other ethanol-sensitive conductances including L-type calcium channels (Zucca and Valenzuela, 2010) since blocking these with nifedipine also partially reduced up-state amplitude. Whether other ethanol-sensitive conductances such as BK potassium channels (Liu et al., 2006) or HCN currents (McDaid et al., 2008; Okamoto et al., 2006) are involved in mediating ethanol’s effect on up-state activity in PFC neurons is not currently known.
The findings from the present imaging study complement and extend those obtained using standard electrophysiological techniques. In particular, calcium imaging can easily monitor activity from large groups of neurons simultaneously and protein-based calcium indicators such as GCaMP3 have additional benefits over traditional fluorescent indicators. Unlike dye-based calcium reporters, GCaMP3 and related indicators can be targeted to discrete cell populations through the use of cell-specific promoters. This improves the signal to noise ratio as neurons and glia differ markedly in their expression of voltage-dependent and ligand-gated calcium permeable ion channels. Although the present study used the synapsin promoter to restrict expression of GCaMP3 to neurons, other promoters (parvalbumin, cholecystokinin, calmodulin kinase II, etc.) can be used to express GCaMP3 in discrete sub-populations of neurons (Borghuis et al., 2011). In addition, advances in viral delivery systems can permit relatively good spatial and temporal control over GCaMP3 expression and when combined with Cre-specific mouse lines, these approaches offer new possibilities for interrogating calcium dynamics in vivo over long time periods (Yizhar et al., 2011). In spite of these advantages, there are some limitations to protein-based calcium sensors. Most notably, GCaMP3 and other genetically encoded indicators typically have slower kinetics than commonly used chemical indicators (eg., Oregon-Green Bapta) and are generally less sensitive to small changes in calcium (Tian et al., 2009). In the current study, this limitation was not a major concern given the relatively long duration and robust nature of evoked up-states observed in the slice cultures. As shown by the simultaneous patch-clamp recording and calcium imaging data, GCaMP3 fluorescence reliably tracked changes in up-state amplitude and duration and was able to detect changes in these parameters during exposure to ethanol. However, limitations in both the physical properties of GCaMP3 and those associated with the standard epifluorescence imaging approach used in this study (eg., collection of out of focus light; dense neuropil staining) did not allow for accurate detection of calcium transients induced by single or small numbers of spikes that occur during the up-state. While confocal imaging approaches can address the out of focus light issue, these systems typically lack the ability to acquire full-field scans at physiologically relevant time scales thus restricting analysis to small sub-cellular domains. Nonetheless, advances in both calcium sensor design and image acquisition performance are rapidly being made and results to date suggest that these approaches will be invaluable in future studies of alcohol action on brain function (Grewe and Helmchen, 2009).
In summary, the results of the current study show that ethanol has profound effects on calcium transients evoked during up-state activity in PFC neurons. The consistent inhibitory effects of ethanol on up-state dynamics across large sections of the slice culture suggest that in vivo, ethanol may significantly affect signal processing within the PFC and thus disrupt the normal functions of this region. These effects may contribute to deficits in PFC-dependent behaviors often observed during acute ethanol intoxication as well as in individuals with a history of alcohol abuse.
Acknowledgments
This work was supported by NIH grants P50AA010761 (including an ARRA supplement), T32AA007474 and F31AA018908.
References Cited
- Abernathy K, Chandler LJ, Woodward JJ. Alcohol and the prefrontal cortex. Int Rev Neurobiol. 2010;91:289–320. doi: 10.1016/S0074-7742(10)91009-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Dolan S, Denburg N, Hindes A, Anderson SW, Nathan PE. Decision-making deficits, linked to a dysfunctional ventromedial prefrontal cortex, revealed in alcohol and stimulant abusers. Neuropsychologia. 2001;39(4):376–89. doi: 10.1016/s0028-3932(00)00136-6. [DOI] [PubMed] [Google Scholar]
- Borghuis BG, Tian L, Xu Y, Nikonov SS, Vardi N, Zemelman BV, Looger LL. Imaging light responses of targeted neuron populations in the rodent retina. J Neurosci. 2011;31(8):2855–67. doi: 10.1523/JNEUROSCI.6064-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compte A, Sanchez-Vives MV, McCormick DA, Wang XJ. Cellular and network mechanisms of slow oscillatory activity (<1 Hz) and wave propagations in a cortical network model. J Neurophysiol. 2003;89(5):2707–25. doi: 10.1152/jn.00845.2002. [DOI] [PubMed] [Google Scholar]
- Durstewitz D, Seamans JK, Sejnowski TJ. Dopamine-mediated stabilization of delay-period activity in a network model of prefrontal cortex. J Neurophysiol. 2000;83(3):1733–50. doi: 10.1152/jn.2000.83.3.1733. [DOI] [PubMed] [Google Scholar]
- Fuster JM. The Prefrontal Cortex. 4. Academic Press; 2008. [Google Scholar]
- Grewe BF, Helmchen F. Optical probing of neuronal ensemble activity. Curr Opin Neurobiol. 2009;19(5):520–9. doi: 10.1016/j.conb.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162(8):1403–13. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Koob G, Kreek MJ. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am J Psychiatry. 2007;164(8):1149–59. doi: 10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroener S, Chandler LJ, Phillips PE, Seamans JK. Dopamine modulates persistent synaptic activity and enhances the signal-to-noise ratio in the prefrontal cortex. PLoS One. 2009;4(8):e6507. doi: 10.1371/journal.pone.0006507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester RA, Quarum ML, Parker JD, Weber E, Jahr CE. Interaction of 6-cyano-7-nitroquinoxaline-2,3-dione with the N-methyl-D-aspartate receptor-associated glycine binding site. Mol Pharmacol. 1989;35(5):565–70. [PubMed] [Google Scholar]
- Liu J, Asuncion-Chin M, Liu P, Dopico AM. CaM kinase II phosphorylation of slo Thr107 regulates activity and ethanol responses of BK channels. Nat Neurosci. 2006;9(1):41–9. doi: 10.1038/nn1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall L, Helgadottir H, Molle M, Born J. Boosting slow oscillations during sleep potentiates memory. Nature. 2006;444(7119):610–613. doi: 10.1038/nature05278. [DOI] [PubMed] [Google Scholar]
- McCool BA. Ethanol modulation of synaptic plasticity. Neuropharmacology. 2010 doi: 10.1016/j.neuropharm.2010.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDaid J, McElvain MA, Brodie MS. Ethanol effects on dopaminergic ventral tegmental area neurons during block of Ih: involvement of barium-sensitive potassium currents. J Neurophysiol. 2008;100(3):1202–10. doi: 10.1152/jn.00994.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto T, Harnett MT, Morikawa H. Hyperpolarization-activated cation current (Ih) is an ethanol target in midbrain dopamine neurons of mice. J Neurophysiol. 2006;95(2):619–26. doi: 10.1152/jn.00682.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plenz D, Kitai ST. Organotypic cortex-striatum-mesencephalon cultures: the nigrostriatal pathway. Neurosci Lett. 1996;209(3):177–80. doi: 10.1016/0304-3940(96)12644-6. [DOI] [PubMed] [Google Scholar]
- Sanchez-Vives MV, McCormick DA. Cellular and network mechanisms of rhythmic recurrent activity in neocortex. Nat Neurosci. 2000;3(10):1027–34. doi: 10.1038/79848. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Nogueira L, Lavin A. Synaptic basis of persistent activity in prefrontal cortex in vivo and in organotypic cultures. Cereb Cortex. 2003;13(11):1242–50. doi: 10.1093/cercor/bhg094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Nunez A, Amzica F. A novel slow (< 1 Hz) oscillation of neocortical neurons in vivo: depolarizing and hyperpolarizing components. J Neurosci. 1993;13(8):3252–65. doi: 10.1523/JNEUROSCI.13-08-03252.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Timofeev I, Grenier F. Natural waking and sleep states: a view from inside neocortical neurons. J Neurophysiol. 2001;85(5):1969–85. doi: 10.1152/jn.2001.85.5.1969. [DOI] [PubMed] [Google Scholar]
- Tian L, Hires SA, Mao T, Huber D, Chiappe ME, Chalasani SH, Petreanu L, Akerboom J, McKinney SA, Schreiter ER, Bargmann CI, Jayaraman V, Svoboda K, Looger LL. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nat Methods. 2009;6(12):875–81. doi: 10.1038/nmeth.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu Y, Kroener S, Abernathy K, Lapish C, Seamans J, Chandler LJ, Woodward JJ. Ethanol inhibits persistent activity in prefrontal cortical neurons. J Neurosci. 2007;27(17):4765–75. doi: 10.1523/JNEUROSCI.5378-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdoorn TA, Kleckner NW, Dingledine R. N-methyl-D-aspartate/glycine and quisqualate/kainate receptors expressed in Xenopus oocytes: antagonist pharmacology. Mol Pharmacol. 1989;35(3):360–8. [PubMed] [Google Scholar]
- Wang HX, Gao WJ. Cell type-specific development of NMDA receptors in the interneurons of rat prefrontal cortex. Neuropsychopharmacology. 2009;34(8):2028–40. doi: 10.1038/npp.2009.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner JL, Valenzuela CF. Ethanol modulation of GABAergic transmission: the view from the slice. Pharmacol Ther. 2006;111(3):533–54. doi: 10.1016/j.pharmthera.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Weissenborn R, Duka T. Acute alcohol effects on cognitive function in social drinkers: their relationship to drinking habits. Psychopharmacology (Berl) 2003;165(3):306–12. doi: 10.1007/s00213-002-1281-1. [DOI] [PubMed] [Google Scholar]
- Weitlauf C, Woodward JJ. Ethanol selectively attenuates NMDAR-mediated synaptic transmission in the prefrontal cortex. Alcohol Clin Exp Res. 2008;32(4):690–8. doi: 10.1111/j.1530-0277.2008.00625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf JA, Moyer JT, Lazarewicz MT, Contreras D, Benoit-Marand M, O'Donnell P, Finkel LH. NMDA/AMPA ratio impacts state transitions and entrainment to oscillations in a computational model of the nucleus accumbens medium spiny projection neuron. J Neurosci. 2005;25(40):9080–95. doi: 10.1523/JNEUROSCI.2220-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward JJ. The Pharmacology of Alcohol. In: Ries RK, Fiellin DA, Miller SA, Saitz R, editors. Principles of Addiction Medicine. 4. Lippincott Williams & Wilkins; Philadelphia: 2009. pp. 85–97. [Google Scholar]
- Woodward JJ, Pava MJ. Effects of ethanol on persistent activity and up-States in excitatory and inhibitory neurons in prefrontal cortex. Alcohol Clin Exp Res. 2009;33(12):2134–40. doi: 10.1111/j.1530-0277.2009.01059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yizhar O, Fenno LE, Davidson TJ, Mogri M, Deisseroth K. Optogenetics in neural systems. Neuron. 2011;71(1):9–34. doi: 10.1016/j.neuron.2011.06.004. [DOI] [PubMed] [Google Scholar]
- Zucca S, Valenzuela CF. Low concentrations of alcohol inhibit BDNF-dependent GABAergic plasticity via L-type Ca2+ channel inhibition in developing CA3 hippocampal pyramidal neurons. J Neurosci. 2010;30(19):6776–81. doi: 10.1523/JNEUROSCI.5405-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]