Abstract
Auditory experience is critical for the acquisition and maintenance of learned vocalizations in both humans and songbirds. Despite the central role of auditory feedback in vocal learning and maintenance, where and how auditory feedback affects neural circuits important to vocal control remain poorly understood. Recent studies of singing birds have uncovered neural mechanisms by which feedback perturbations affect vocal plasticity and also have identified feedback-sensitive neurons at or near sites of auditory and vocal motor interaction. Additionally, recent studies in marmosets have underscored that even in the absence of vocal learning, vocalization remains flexible in the face of changing acoustical environments, pointing to rapid interactions between auditory and vocal motor systems. Finally, recent studies show that a juvenile songbird’s initial auditory experience of a song model has long-lasting effects on sensorimotor neurons important to vocalization, shedding light on how auditory memories and feedback interact to guide vocal learning.
Introduction
Vocal behaviors offer several advantages for exploring how sensory feedback affects the brain and behavior. First, some vocalizations, including human speech and the courtship songs of certain passerine (i.e., perching) birds, are learned in a process that uses vocalization-related feedback to match vocal output to a memorized acoustic model (for a review, see [1]). Second, because songbirds are one of the few non-human animals that learn their vocalizations and because the neural circuitry for birdsong is well delineated, the songbird is an exceptional organism in which to explore how the brain harnesses feedback to learn complex sequences of vocal gestures. While the behavioral importance of auditory feedback to vocal learning is well characterized, the neural mechanisms through which auditory feedback shapes vocal output remain poorly understood. Third, even in the absence of vocal learning, many animals can adjust their vocalizations to compensate for changing acoustical environments, for example, by modulating vocal intensity to combat environmental noise or by controlling vocal timing to avoid overlap with other callers [2–6]. In addition to distinguishing self-generated vocalizations from environmental noise, however, vocal learners face the challenge of matching performance-related feedback to a memorized acoustic model. Thus, many neural mechanisms of feedback processing identified in songbirds will be common to all vocal species, while others may be suited specifically to the challenges of vocal learning.
This review addresses recent research in songbirds that examines how auditory feedback affects learned vocal performance. We also consider recent research that examines behavioral and neural aspects of vocalization-related feedback processing in animals, such as marmosets, which produce complex, unlearned vocalizations. Finally, recent studies in songbirds that examine the effects of early experience with an acoustic model on vocal motor circuits are considered in the context of how auditory feedback and auditory memories interact to guide vocal learning.
Mechanisms for feedback-dependent vocal plasticity in songbirds
The role of auditory feedback in songbird vocal learning and maintenance
Juvenile songbirds learn to sing by listening to and memorizing one or more tutor songs (i.e., sensory learning) that they subsequently copy through an often-prolonged period of vocal practice (i.e., sensorimotor learning) [1,7,8]. The “plastic” songs produced during sensorimotor learning are more variable and dependent on auditory feedback than the stereotyped, “crystallized” songs of adults [1,7,8]. The adults of some species, including the zebra finch and the Bengalese finch, also require feedback to maintain stable songs, and deafening in adults of these species causes the spectral and temporal features of their songs to degrade [9–11]. The highly stereotyped structure of these birds’ crystallized songs provides an extremely sensitive backdrop on which to detect feedback-dependent changes. Consequently, most recent studies examining how feedback affects song have used adult zebra or Bengalese finches as experimental subjects.
In addition to deafening, online signal processing methods have been used to selectively disrupt singing-related auditory feedback with either broadband noise or delayed auditory feedback (collectively referred to as distorted auditory feedback, or DAF). When adult zebra finches are exposed to DAF for several days, their song degrades and then recovers when DAF is discontinued [12,13]. In addition to its reversibility, DAF is a highly powerful experimental tool because it can be applied in a precisely targeted manner. For instance, when DAF is targeted to only a single ~100 msec syllable in a polysyllabic song, that syllable degrades whereas flanking syllables are unaffected, indicating that feedback-dependent vocal modulation is temporally precise [12,13]. Furthermore, because the adult song still exhibits slight variability, DAF can also be applied in a contingent manner, for example, by targeting only those syllable renditions that fall above or below a certain pitch threshold (Figure 1A) [13]. When adult finches are exposed to contingent DAF, the pitch of the targeted syllable shifts down or up adaptively, subsequently minimizing feedback interference [13, 14••, 15••].
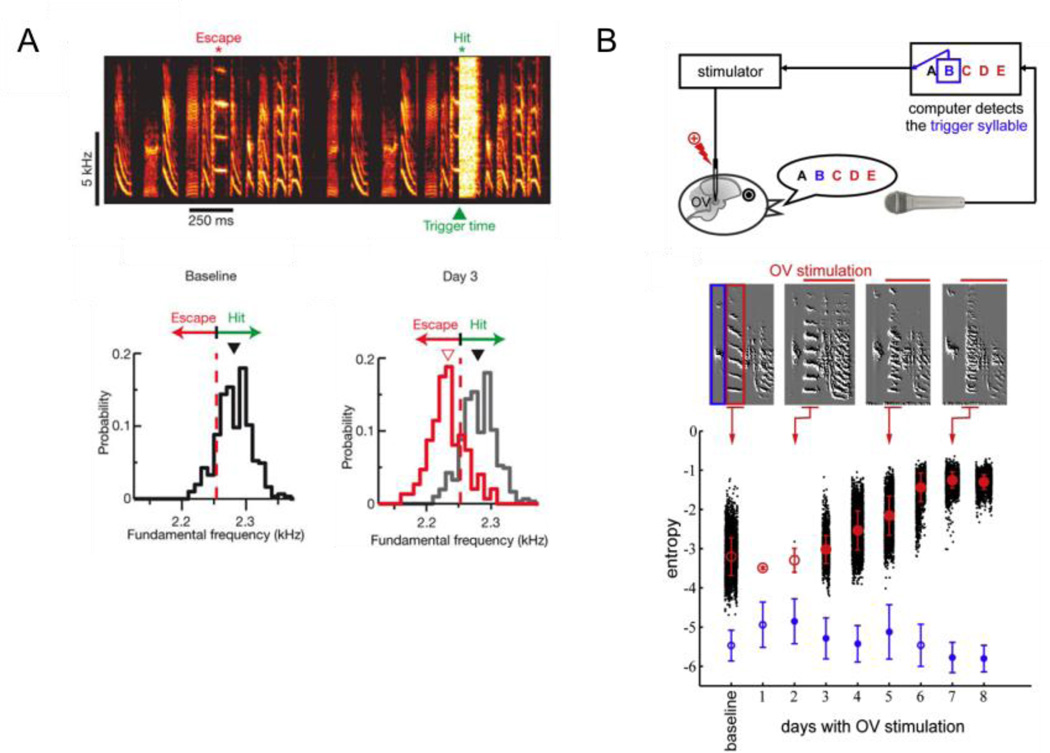
Figure 1. Online signal processing methods can be used to disrupt singing-related auditory feedback and drive changes to song.
A) In a contingent reinforcement protocol, syllable renditions falling above a threshold pitch (i.e., hits, represented in green) are accompanied by playback of a noise burst. Over several hours, the bird increases the proportion of renditions that fall below threshold (i.e., escapes, represented in red), pointing to an adaptive process driven by perturbation of auditory feedback. Reprinted with permission from [13]. B) Singing-triggered microstimulation in the auditory thalamus (OV) slowly increases the spectral entropy of the target syllable (syllable C, red), whereas the entropy of a preceding trigger syllable (syllable B, blue) not blanketed by microstimulation remains unaffected. Reprinted with permission from [16•].
A consistent observation is the relatively slow (~hours to days) time course over which DAF elicits changes in spectral and temporal features of song. One possibility is that song changes slowly because of a failure to completely mask vocalization-related feedback, which could enable the bird to partially disambiguate its voice from the DAF signal. However, song also changes slowly when singing-triggered microstimulation is applied directly to the bird’s analogue of the thalamic medial geniculate body (i.e., OV, Figure 1B) [16•], suggestive of an underlying “stickiness” to the song motor program. This slow effect of altered feedback on song contrasts with the very rapid feedback-dependent compensation in pitch observed in humans and bats [17,18] and also the rapid alterations in vocal intensity exhibited by a wide range of animals, including human, whales, and songbirds ([2,4,6], see below).
Neural mechanisms for feedback-dependent vocal plasticity
The neural circuitry for song (i.e., the song system) comprises spatially distributed, interconnected, and compact (~1 mm diameter) brain nuclei. This distributed and nodal architecture is suitable for targeted manipulation of neural activity, facilitating the analysis of how disrupted feedback perturbs vocalization. The song system can be divided into two functionally distinct pathways (Figure 2). The song motor pathway (SMP) is obligatory for singing and the anterior forebrain pathway (AFP) is necessary for sensorimotor learning but not crystallized song production [19–22]. The output of the AFP, the nucleus LMAN, projects to the song motor nucleus RA in the SMP, which serves as a locus where the AFP can influence song motor commands. More specifically, the AFP plays a critical role in translating changes in auditory feedback to changes in song output, as evidenced by the finding that lesions of LMAN can block song degradation induced by deafening [23] and other manipulations of auditory feedback ([24], see below).
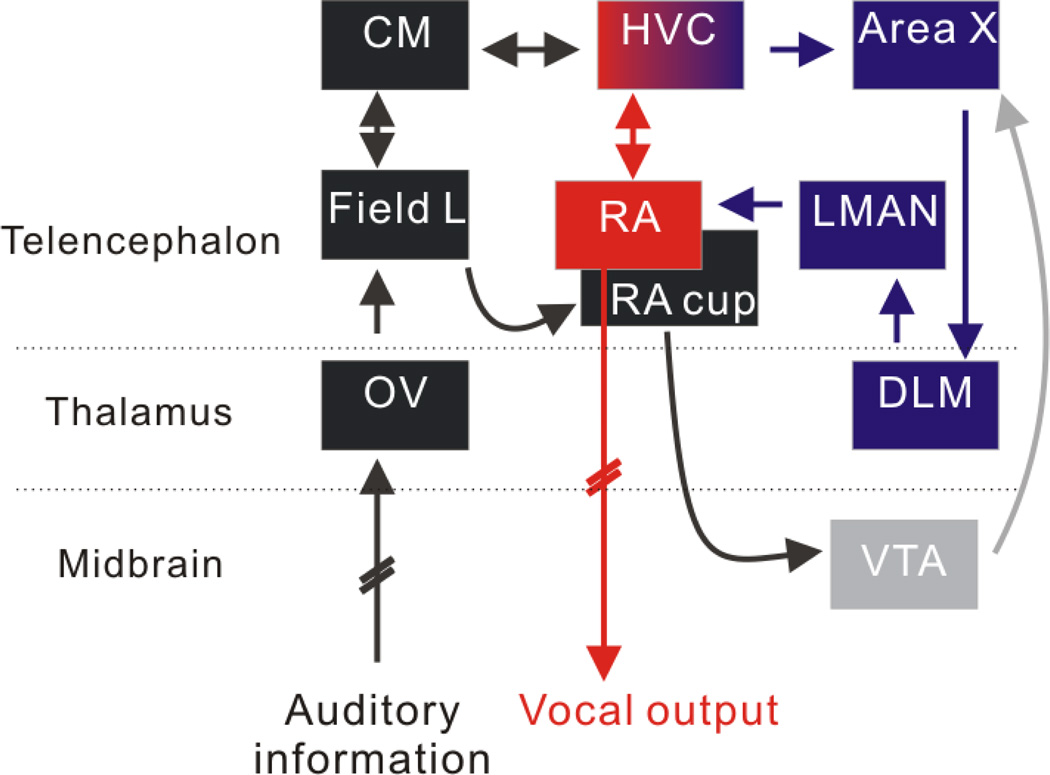
Figure 2. Simplified schematic of songbird auditory and vocal control regions.
Ascending auditory information (black) is relayed through the hindbrain and midbrain to the thalamic nucleus OV and then to the auditory telencephalic area Field L. Field L is reciprocally connected with the secondary auditory area CM and also projects to the RA cup. The song system (red and blue) consists of the two distinct neural pathways. The song motor pathway (SMP, red) includes HVCRA neurons, the downstream song premotor nucleus RA, and vocal-respiratory targets in the brainstem that control song production. The anterior forebrain pathway (AFP, blue) includes HVCAFP neurons, the striatal region Area X, the thalamic nucleus DLM, and the telencephalic nucleus LMAN, which provides input to the SMP via its projection to RA. The ventral tegmental area (VTA) is shown in gray. Hatched lines indicate that intervening brain regions have been omitted from the schematic for clarity. Neurons acutely sensitive to DAF have been detected in all of the auditory areas shown in black ([16•,33••], Las and Fee, personal communication) and also in HVC [36].
Contingent DAF techniques have proven critical in identifying neural mechanisms for feedback-dependent vocal plasticity. Two recent and elegantly integrative studies have combined reversible inactivation of LMAN with contingent DAF to systematically probe how the AFP contributes to feedback-dependent changes in song performance. Using reverse microdialysis methods to focally inactivate LMAN in adult zebra finches, Andalman and Fee [14••] measured the AFP’s contributions to song performance before and during exposure to contingent DAF. Prior to DAF, inactivating LMAN had little or no effect on song, consistent with the notion that crystallized song is driven solely by the SMP. However, once contingent DAF began to trigger changes to the targeted syllable’s pitch, inactivation of LMAN caused an immediate reversion toward the baseline pitch. Warren et al. [15••] made nearly identical observations in adult Bengalese finches and also observed a similar effect by infusing an NMDA receptor antagonist into RA, which selectively blocks LMAN synapses on RA neurons [25]. This latter finding indicates that LMAN affects song through RA, and not through other pathways to which LMAN contributes. Together, these studies show that LMAN adaptively biases motor output to minimize feedback perturbation, indicating that LMAN is a vehicle for feedback-dependent vocal plasticity.
These studies also reveal that when contingent DAF is applied over consecutive days, a portion of the vocal shift persists despite LMAN inactivation. This process of vocal consolidation can take one to two days, indicating that feedback-dependent vocal plasticity in songbirds operates over at least two different timescales (hours and days), presumably by engaging different synaptic mechanisms. This slower AFP-independent consolidation of feedback-dependent plasticity most likely involves changes to the synapses that link HVC to RA neurons. Interestingly, HVC-RA synapses in juvenile zebra finches undergo slow consolidation over the course of sensorimotor learning [26], raising the possibility that acute interactions between LMAN and HVC synapses on RA neurons drive adaptive song variability that is translated more slowly into long-lasting changes in HVC-RA synapses and vocal performance.
Contingent DAF involves singing-triggered playback of noise, which potentially is an aversive stimulus, whereas sensorimotor learning employs intrinsic error correction processes. This raises the issue of whether contingent DAF engages mechanisms similar to those used for sensorimotor learning in juveniles or error correction in adults. Warren et al. [15••] addressed this question by monitoring LMAN’s role in song recovery following prolonged exposure to contingent DAF. Indeed, when DAF was discontinued, the pitch of the target syllable recovered slowly over one or two weeks to the baseline value. During this recovery period, LMAN inactivation actually pushed the syllable’s pitch further away from its pre-DAF baseline value, indicating that the AFP actively drives recovery. Thus, LMAN appears to operate similarly to modulate vocalization in response to external reinforcement and when vocal performance has strayed from a baseline state. Although these experiments involved adult birds, they suggest that LMAN could function similarly during sensorimotor learning to enable the juvenile to match the tutor’s song. Moreover, the full recovery of syllable pitch points to the persistence of a highly stable internal song representation against which feedback is compared.
Auditory feedback processing in the songbird brain
An important and as yet unmet goal is to identify how neurons that are acutely sensitive to feedback perturbation affect song. While LMAN’s role in feedback-dependent plasticity and the detection of song-evoked auditory responses in the LMAN of anesthetized birds [27] make it a possible site for this sensorimotor interaction, the singing-related activity of LMAN neurons is not acutely sensitive to DAF [28]. These findings raise the possibility that the acute feedback sensors lie upstream of LMAN, and their activity alters LMAN activity only slowly and in a cumulative manner. In support of this idea, cutting the vocal nerve, which drives immediate spectral distortion and slower temporal reorganization of song, also causes the auditory selectivity of LMAN neurons to shift slowly (> 1 week) toward the spectrally-distorted song, and these shifts precede temporal degradation [29]. Taken together, these studies suggest that changes to auditory feedback may act on LMAN neurons over relatively long timescales that parallel the delayed effects of feedback perturbation on vocal output.
These various findings have focused the search for feedback-sensing neurons to areas upstream of LMAN (Figure 2). The only song motor nucleus known to receive direct inputs from auditory brain areas is HVC, and inactivating HVC abolishes auditory responses in LMAN [29]. Although AFP-projecting HVC neurons (i.e., HVCAFP) are active during singing and in response to song playback [30,31], their singing-related activity is unaffected by DAF [31,32]. In contrast, neurons acutely sensitive to DAF have been detected in the auditory telencephalic region CM [33••], which supplies direct auditory input to HVC [34], and in the auditory thalamus (OV) [16•], which supplies indirect auditory input to both CM and HVC. Intriguingly, some CM neurons only fire action potentials when song is accompanied by DAF, consistent with the idea that they are part of a circuit that detects vocal error [33••]. Moreover, singing-triggered microstimulation in OV causes song to degrade [16•], providing a causal link between feedback-sensing neurons and feedback-dependent changes to song. Despite this recent progress, the exact sites of auditory-vocal interaction remain undetermined, because OV supplies auditory drive to the entire auditory telencephalon and CM projects to other parts of the auditory telencephalon as well as to HVC.
A current goal is to identify where the feedback signal travels between the auditory forebrain and the song system, particularly the AFP. One possibility is that it travels directly from CM to HVC, but altered feedback only affects HVCAFP neuronal properties over timescales too long to measure with chronic electrophysiological recording techniques (~10s of minutes). Consistent with this view, a recent two-photon imaging study in adult zebra finches found that deafening drives decreases in the size of dendritic spines on HVCAFP neurons over the course of a few days (Tschida and Mooney, unpublished]. Importantly, these structural changes are specific to HVCAFP neurons, emerge prior to the onset of deafening-induced vocal deterioration, and are paralleled by electrophysiological weakening of synapses onto HVCX neurons. Furthermore, the singing-related activity of putative HVC interneurons, which are known to innervate HVCAFP neurons [35], is suppressed by DAF on a trial-by-trial basis [36]. These findings raise the possibility that HVC interneurons are acute sensors of auditory feedback that act to slowly alter HVC’s output to the AFP. A second and not mutually exclusive possibility is that feedback evaluation of song performances enters the AFP independently of HVC, perhaps via the ventral tegmental area (VTA), a region implicated in reinforcement learning [37] and that innervates the striatal portion of the AFP (Figure 2) [38]. Indeed, Las and Fee (personal communication) recently characterized VTA-projecting neurons in the zebra finch auditory telencephalon that show singing-related activity that is sensitive to DAF. An important and challenging experimental goal will be to determine whether selectively manipulating the activity of feedback-sensing CM neurons, HVC neurons, or VTA-projecting neurons is sufficient to induce song plasticity.
Auditory feedback and dynamic modulation of innate vocalizations
Whether learned or innate, vocalizations enable the caller to communicate to a listening audience for diverse purposes, including courtship, maintaining group cohesion, and warning of impending threat from predators. To reach their audience, callers employ various strategies in response to acoustic interference, including increasing vocal intensity to overcome ambient noise (i.e., the Lombard effect), altering vocal onset to minimize overlap with other callers, and truncating vocalization altogether. Even in the absence of vocal learning, these “reflexive” behaviors indicate that the auditory system must be able to evaluate vocalization-related auditory feedback relative to extraneous sounds and rapidly modulate vocal performance.
Outside the songbird, the clearest picture of how auditory neurons monitor vocalization-related feedback derives from studies in marmosets, a New World monkey with a rich repertoire of innate calls. In freely vocalizing marmosets, most (~75%) neurons in the primary auditory cortex are suppressed during vocalization, and this suppression begins up to 200 msec prior to vocal onset, suggesting that it is driven by vocal premotor activity [39–41]. Interestingly, a recent pre-operative mapping study in humans using implanted intracranial electrodes reported similar suppressive effects of vocalization in the human auditory cortex [42]. A plausible substrate for vocal motor-driven auditory suppression is a direct projection from frontal cortical areas to the primary auditory cortex, which could convey vocalization-related corollary discharge to the auditory system. In the songbird, an analogous projection from HVC into CM [43] may account for putative corollary discharge activity that can be detected in the auditory telencephalon [33••], with the distinction that singing predominantly elevates rather than suppresses activity in the bird’s auditory telencephalon. Vocal motor information also could enter at other levels of the auditory system, as brainstem areas have been identified that are important to rapid feedback-dependent modulation of bat ultrasonic navigation cries [44]. Therefore, an important goal is to identify the sources of corollary discharge that can be detected in the auditory telencephalon of both songbirds and primates.
It will also be important to determine the functional significance of vocalization-related corollary discharge in auditory areas. Although feedforward interactions from vocal premotor to auditory areas are central to forward models of human speech production [45], their significance in non-human primates remains mysterious. One clue is that auditory cortical neurons in the vocalizing marmoset, though suppressed, display heightened sensitivity to feedback perturbation [41], which could facilitate rapid modulation of vocal performance in changing acoustical environments. Because marmosets exhibit a robust Lombard effect, an important goal is to examine how the auditory cortex functions in response to the combination of acoustic noise and vocalization-related feedback. Preliminary studies indicate that masking noise reduces vocalization-related suppression in the marmoset auditory cortex, and that as the animal increases its vocal intensity, additional changes in auditory cortical activity compensate for the effects of the masking noise [5]. The origins of these effects on auditory cortical activity and its influence on vocal control remain unknown but are important questions to resolve.
While it is important to distinguish rapid auditory-dependent vocal compensation, as evinced by the Lombard effect, from slower and more complex forms of vocal learning, it is also useful to consider whether these different forms of feedback-dependent plasticity employ similar underlying neural mechanisms. In this light, Bengalese finches display a Lombard effect [46] and other rapid vocal changes in response to feedback perturbation, such as abrupt song terminations and changes in song duration [47]. Male finches sing highly stereotyped directed songs to females and more variable undirected songs when housed alone, and the increased variability of undirected song reflects heightened bursting activity of LMAN neurons [48]. This social context-dependent switch in song performance, though acoustically subtle, provides an experimental tool for dynamically switching between AFP-dependent and independent forms of singing. Notably, the Lombard effect and other rapid vocal effects of feedback perturbation are strongly reduced during directed song [46,49]. These findings raise the possibility that the AFP may serve a wide variety of feedback-dependent processes, ranging from rapid compensatory vocal modulation to slower processes of vocal imitation. Perhaps vocal learning exploits neural mechanisms that control reflexive forms of sensory feedback-dependent modulation, as theorized for volitional control of limb movements [50].
Effects of early acoustic experience on vocal premotor circuits
In addition to distinguishing self-produced vocalizations from environmental sounds, vocal learners face the unique challenge of matching vocalization-related auditory feedback to a memorized acoustic model. This matching process is thought to involve a comparator circuit that generates adaptive error signals when feedback fails to match the model. Although the nature of the comparator remains enigmatic, a complete understanding of how auditory feedback guides vocal learning will require understanding how and where the song model is represented in the brain and how this representation interacts with feedback-sensing neurons. For songbirds, the prevailing view is that the song model is stored in auditory areas outside the song system [51], and it is reasonable to assume that the representation of the tutor song depends at least in part on auditory neurons [52]. However, several recent studies indicate that early auditory experience of a tutor song rapidly alters the structure and function of SMP nuclei, specifically HVC and RA, implicating vocal premotor regions in the formation and storage of song memories.
Two recent studies indicate that early experience with a tutor song model exerts rapid and long-lasting effects on HVC. Using in vivo two-photon imaging methods to visualize dendritic spines on HVC projection neurons, Roberts et al. [53••] found that spines were smaller and more labile in juvenile zebra finches raised in isolation from a tutor. Notably, one day of tutor song experience led to a rapid increase in spine size, density and stability (Figure 3A). Moreover, these structural changes were accompanied by enhancement of spontaneous synaptic activity, consistent with synaptic potentiation. Importantly, the magnitude of these rapid (~24h) structural changes correlated with the quality of tutor song imitation measured 30–45 d later, suggesting that synaptic strengthening in HVC helps to encode the tutor song memory. Further support for this idea comes from the finding that early tutor song experience can exert long-lasting effects on the auditory properties of HVC neurons. Juvenile swamp sparrows memorize and copy a dozen or more tutor songs during development, only a few of which are retained in their adult repertoire [54]. When anesthetized adult swamp sparrows were exposed to playback of their various tutor songs, their HVC neurons exhibited strong and selective responses for tutor songs that were copied only transiently during sensorimotor learning or, in some cases, apparently never copied at all [55•]. Taken together, these studies suggest that tutor song experience can exert long-lasting effects on HVC even in the absence of extensive vocal rehearsal, implicating HVC in the formation and storage of tutor song memories. The juxtaposition of tutor memories with feedback-sensitive neurons in HVC ([36], Tschida and Mooney, unpublished) advances HVC as one plausible site for comparison between these two song representations.
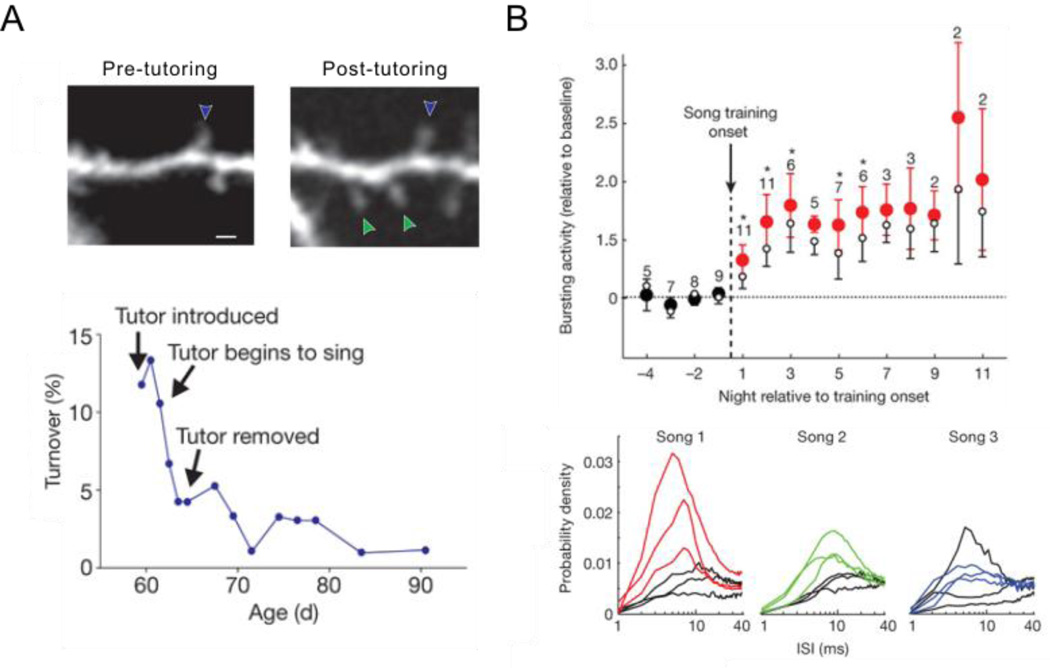
Figure 3. Early auditory experience with a tutor song model rapidly affects vocal premotor neurons.
A) Auditory experience of a tutor song rapidly alters dendritic spines on HVC projection neurons. Top panel: representative in vivo multiphoton images of an HVC neuron from a juvenile bird show that tutor song experience increases spine density (blue arrowhead indicates a spine that was stable over the first day of tutoring; green arrowheads indicate spines that were gained following tutoring). Bottom panel: levels of spine turnover in a juvenile’s HVC decrease rapidly after the tutor begins to sing. Adapted with permission from [53••]. B) Initial tutor song experience drives increased bursting activity in RA neurons (top panel), the temporal pattern of which depends on the tutor song heard (bottom panel). Adapted with permission from [57•].
Additional experiments indicate that tutor song experience also has rapid effects on song system neurons downstream from HVC. Inspired by the observation that the spontaneous activity of RA neurons in sleeping adult zebra finches carries information about daytime singing experience [56], Shank and Margoliash [57•] made neural recordings in head-fixed, sleeping isolate juvenile finches to test the effects of daytime tutor song experience on RA activity. Daytime tutor song experience drove changes in the bursting of RA neurons on the subsequent night, the temporal pattern of which was specific to the tutor song heard by the juvenile (Figure 3B). Conspicuously, the juvenile had to sing after tutoring for RA bursting activity to change, suggesting that this change results from a comparison between auditory feedback and the song model. Because these two types of information could be compared in more than one brain region, an important goal of future research is to identify potential comparators and determine whether they generate error signals used to drive changes in vocal performance.
Conclusions
Recent advances have improved our understanding of the neural mechanisms for the implementation of feedback-dependent vocal plasticity in songbirds, and feedback-sensitive neurons have been identified in both auditory and sensorimotor brain areas. Despite this progress, a key remaining challenge is the identification of specific auditory-vocal interfaces through which feedback information can enable changes in vocal output. Even in the absence of vocal learning, many animals are capable of dynamically modulating vocal output in response to acoustic interference. Recent studies in marmosets have demonstrated changes in the activity of auditory cortical neurons that correlate with this behavioral sensitivity and are indicative of crosstalk between cortical vocal premotor and auditory areas. An important next step in both songbirds and marmosets will be to delineate circuits that convey vocalization-related corollary discharge signals to the auditory system and to test the behavioral relevance of changes in activity of feedback-sensing neurons to changes in vocal dynamics. Finally, recent studies in songbirds indicate that early auditory experience with song models drives rapid, dramatic, and long-lasting changes in song premotor circuits. It remains an important goal to identify how, where, and over what time course auditory feedback interacts with the neural representation of tutor song models to guide vocal imitation.
Highlights.
Feedback-sensing auditory and sensorimotor neurons have been detected in songbirds.
Specific sensory-motor interfaces where feedback influences song remain enigmatic.
Flexibility in innate vocalizations points to rapid auditory-vocal interactions.
Early experience of acoustic models drives rapid changes in vocal premotor circuits.
Song models and feedback might interact at premotor sites to guide vocal learning.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mooney R. Neural mechanisms for learned birdsong. Learn Mem. 2009;16:655–669. doi: 10.1101/lm.1065209. [DOI] [PubMed] [Google Scholar]
- 2.Cynx J, Lewis R, Tavel B, Tse H. Amplitude regulation of vocalizations in noise by a songbird, Taeniopygia guttata. Anim Behav. 1998;56:107–113. doi: 10.1006/anbe.1998.0746. [DOI] [PubMed] [Google Scholar]
- 3.Egnor SE, Wickelgren JG, Hauser MD. Tracking silence: adjusting vocal production to avoid acoustic interference. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2007;193:477–483. doi: 10.1007/s00359-006-0205-7. [DOI] [PubMed] [Google Scholar]
- 4.Lane HTB. The Lombard sign and the role of hearing in speech. J Speech Hear Res. 1971;14:677–709. [Google Scholar]
- 5.Roy S, Miller CT, Gottsch D, Wang X. Vocal control by the common marmoset in the presence of interfering noise. J Exp Biol. 2011;214:3619–3629. doi: 10.1242/jeb.056101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scheifele PM, Andrew S, Cooper RA, Darre M, Musiek FE, Max L. Indication of a Lombard vocal response in the St. Lawrence River Beluga. J Acoust Soc Am. 2005;117:1486–1492. doi: 10.1121/1.1835508. [DOI] [PubMed] [Google Scholar]
- 7.Immelman K. Song development in the zebra finch and other estrildid finches. In: Hinde RA, editor. Bird Vocalizations. Cambridge University Press; 1969. pp. 61–77. [Google Scholar]
- 8.Marler P. A comparative approach to vocal learning: Song development in white-crowned sparrows. Journal of Comparative and Physiological Psychology. 1970;71:1–25. [Google Scholar]
- 9.Nordeen KW, Nordeen EJ. Auditory feedback is necessary for the maintenance of stereotyped song in adult zebra finches. Behav Neural Biol. 1992;57:58–66. doi: 10.1016/0163-1047(92)90757-u. [DOI] [PubMed] [Google Scholar]
- 10.Okanoya K, Yamaguchi A. Adult Bengalese finches (Lonchura striata var. domestica) require real-time auditory feedback to produce normal song syntax. J Neurobiol. 1997;33:343–356. [PubMed] [Google Scholar]
- 11.Woolley SM, Rubel EW. Bengalese finches Lonchura Striata domestica depend upon auditory feedback for the maintenance of adult song. J Neurosci. 1997;17:6380–6390. doi: 10.1523/JNEUROSCI.17-16-06380.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leonardo A, Konishi M. Decrystallization of adult birdsong by perturbation of auditory feedback. Nature. 1999;399:466–470. doi: 10.1038/20933. [DOI] [PubMed] [Google Scholar]
- 13.Tumer EC, Brainard MS. Performance variability enables adaptive plasticity of 'crystallized' adult birdsong. Nature. 2007;450:1240–1244. doi: 10.1038/nature06390. [DOI] [PubMed] [Google Scholar]
- 14. Andalman AS, Fee MS. A basal ganglia-forebrain circuit in the songbird biases motor output to avoid vocal errors. Proc Natl Acad Sci U S A. 2009;106:12518–12523. doi: 10.1073/pnas.0903214106.. The authors combine contingent DAF and reversible inactivation to demonstrate the role of a cortico-basal ganglia (BG) pathway in driving adaptive changes in song performance. This study suggests that LMAN, the output of the cortico-BG pathway is a source of biased variability that enables the bird to shift vocal pitch to escape singing-triggered noise playback.
- 15. Warren TL, Tumer EC, Charlesworth JD, Brainard MS. Mechanisms and time course of vocal learning and consolidation in the adult songbird. J Neurophysiol. 2011;106:1806–1821. doi: 10.1152/jn.00311.2011.. Using a combination of contingent DAF and reversible inactivation, the authors show that LMAN mediates its adaptive effect on song performance via the synapses it makes on song premotor neurons. The authors also find that recovery from prolonged exposure to DAF depends on LMAN activity to “push” song pitch back to the baseline state.
- 16. Lei H, Mooney R. Manipulation of a central auditory representation shapes learned vocal output. Neuron. 2010;65:122–134. doi: 10.1016/j.neuron.2009.12.008.. Using singing-triggered microstimulation in the auditory thalamus to focally disrupt central auditory processing, the authors show that song destabilization in response to central perturbation of feedback is slow and that vocal “mistakes” are not coupled to stimulation on a trial by trial basis, suggesting that the coupling between the auditory and vocal motor systems is weak and/or indirect.
- 17.Elman JL. Effects of frequency-shifted feedback on the pitch of vocal productions. J Acoust Soc Am. 1981;70:45–50. doi: 10.1121/1.386580. [DOI] [PubMed] [Google Scholar]
- 18.Schnitzler HU. Die Ultraschallortungslaute der Hufeisennasen-Fledermause (Chiroptera, Rhinolophidae) in verschiedenen Orientierungssituationen. Z Vergl Physiol. 1968;57:376–408. [Google Scholar]
- 19.Bottjer SW, Miesner EA, Arnold AP. Forebrain lesions disrupt development but not maintenance of song in passerine birds. Science. 1984;224:901–903. doi: 10.1126/science.6719123. [DOI] [PubMed] [Google Scholar]
- 20.Nottebohm F, Kelley DB, Paton JA. Connections of vocal control nuclei in the canary telencephalon. J Comp Neurol. 1982;207:344–357. doi: 10.1002/cne.902070406. [DOI] [PubMed] [Google Scholar]
- 21.Nottebohm F, Stokes TM, Leonard CM. Central control of song in the canary, Serinus canarius. J Comp Neurol. 1976;165:457–486. doi: 10.1002/cne.901650405. [DOI] [PubMed] [Google Scholar]
- 22.Scharff C, Nottebohm F. A comparative study of the behavioral deficits following lesions of various parts of the zebra finch song system: implications for vocal learning. J Neurosci. 1991;11:2896–2913. doi: 10.1523/JNEUROSCI.11-09-02896.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brainard MS, Doupe AJ. Interruption of a basal ganglia-forebrain circuit prevents plasticity of learned vocalizations. Nature. 2000;404:762–766. doi: 10.1038/35008083. [DOI] [PubMed] [Google Scholar]
- 24.Williams H, Mehta N. Changes in adult zebra finch song require a forebrain nucleus that is not necessary for song production. J Neurobiol. 1999;39:14–28. [PubMed] [Google Scholar]
- 25.Mooney R. Synaptic basis for developmental plasticity in a birdsong nucleus. J Neurosci. 1992;12:2464–2477. doi: 10.1523/JNEUROSCI.12-07-02464.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kittelberger JM, Mooney R. Lesions of an avian forebrain nucleus that disrupt song development alter synaptic connectivity and transmission in the vocal premotor pathway. J Neurosci. 1999;19:9385–9398. doi: 10.1523/JNEUROSCI.19-21-09385.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Doupe AJ, Konishi M. Song-selective auditory circuits in the vocal control system of the zebra finch. Proc Natl Acad Sci U S A. 1991;88:11339–11343. doi: 10.1073/pnas.88.24.11339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leonardo A. Experimental test of the birdsong error-correction model. Proc Natl Acad Sci U S A. 2004;101:16935–16940. doi: 10.1073/pnas.0407870101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roy A, Mooney R. Auditory plasticity in a basal ganglia-forebrain pathway during decrystallization of adult birdsong. J Neurosci. 2007;27:6374–6387. doi: 10.1523/JNEUROSCI.0894-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fujimoto H, Hasegawa T, Watanabe D. Neural coding of syntactic structure in learned vocalizations in the songbird. J Neurosci. 2011;31:10023–10033. doi: 10.1523/JNEUROSCI.1606-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prather JF, Peters S, Nowicki S, Mooney R. Precise auditory-vocal mirroring in neurons for learned vocal communication. Nature. 2008;451:305–310. doi: 10.1038/nature06492. [DOI] [PubMed] [Google Scholar]
- 32.Kozhevnikov AA, Fee MS. Singing-related activity of identified HVC neurons in the zebra finch. J Neurophysiol. 2007;97:4271–4283. doi: 10.1152/jn.00952.2006. [DOI] [PubMed] [Google Scholar]
- 33. Keller GB, Hahnloser RH. Neural processing of auditory feedback during vocal practice in a songbird. Nature. 2009;457:187–190. doi: 10.1038/nature07467.. By combining chronic electrophysiological recordings and singing-triggered DAF, the authors identify feedback-sensing neurons in the auditory telencephalon of juvenile zebra finches and also uncover evidence of singing-related corollary discharge. Interestingly, some neurons were only active when singing was accompanied by DAF, suggesting that they could participate in an error correction process.
- 34.Bauer EE, Coleman MJ, Roberts TF, Roy A, Prather JF, Mooney R. A synaptic basis for auditory-vocal integration in the songbird. J Neurosci. 2008;28:1509–1522. doi: 10.1523/JNEUROSCI.3838-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mooney R, Prather JF. The HVC microcircuit: the synaptic basis for interactions between song motor and vocal plasticity pathways. J Neurosci. 2005;25:1952–1964. doi: 10.1523/JNEUROSCI.3726-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sakata JT, Brainard MS. Online contributions of auditory feedback to neural activity in avian song control circuitry. J Neurosci. 2008;28:11378–11390. doi: 10.1523/JNEUROSCI.3254-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schultz W. Behavioral dopamine signals. Trends Neurosci. 2007;30:203–210. doi: 10.1016/j.tins.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 38.Lewis JW, Ryan SM, Arnold AP, Butcher LL. Evidence for a catecholaminergic projection to area X in the zebra finch. J Comp Neurol. 1981;196:347–354. doi: 10.1002/cne.901960212. [DOI] [PubMed] [Google Scholar]
- 39.Eliades SJ, Wang X. Sensory-motor interaction in the primate auditory cortex during self-initiated vocalizations. J Neurophysiol. 2003;89:2194–2207. doi: 10.1152/jn.00627.2002. [DOI] [PubMed] [Google Scholar]
- 40.Eliades SJ, Wang X. Dynamics of auditory-vocal interaction in monkey auditory cortex. Cereb Cortex. 2005;15:1510–1523. doi: 10.1093/cercor/bhi030. [DOI] [PubMed] [Google Scholar]
- 41.Eliades SJ, Wang X. Neural substrates of vocalization feedback monitoring in primate auditory cortex. Nature. 2008;453:1102–1106. doi: 10.1038/nature06910. [DOI] [PubMed] [Google Scholar]
- 42.Greenlee JD, Jackson AW, Chen F, Larson CR, Oya H, Kawasaki H, Chen H, Howard MA., 3rd Human auditory cortical activation during self-vocalization. PLoS One. 2011;6:e14744. doi: 10.1371/journal.pone.0014744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Akutagawa E, Konishi M. New brain pathways found in the vocal control system of a songbird. J Comp Neurol. 2010;518:3086–3100. doi: 10.1002/cne.22383. [DOI] [PubMed] [Google Scholar]
- 44.Smotherman M, Zhang S, Metzner W. A neural basis for auditory feedback control of vocal pitch. J Neurosci. 2003;23:1464–1477. doi: 10.1523/JNEUROSCI.23-04-01464.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hickok G, Houde J, Rong F. Sensorimotor integration in speech processing: computational basis and neural organization. Neuron. 2011;69:407–422. doi: 10.1016/j.neuron.2011.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kobayasi KI, Okanoya K. Context-dependent song amplitude control in Bengalese finches. Neuroreport. 2003;14:521–524. doi: 10.1097/00001756-200303030-00045. [DOI] [PubMed] [Google Scholar]
- 47.Sakata JT, Brainard MS. Real-time contributions of auditory feedback to avian vocal motor control. J Neurosci. 2006;26:9619–9628. doi: 10.1523/JNEUROSCI.2027-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kao MH, Wright BD, Doupe AJ. Neurons in a forebrain nucleus required for vocal plasticity rapidly switch between precise firing and variable bursting depending on social context. J Neurosci. 2008;28:13232–13247. doi: 10.1523/JNEUROSCI.2250-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sakata JT, Brainard MS. Social context rapidly modulates the influence of auditory feedback on avian vocal motor control. J Neurophysiol. 2009;102:2485–2497. doi: 10.1152/jn.00340.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Scott SH. Optimal feedback control and the neural basis of volitional motor control. Nat Rev Neurosci. 2004;5:532–546. doi: 10.1038/nrn1427. [DOI] [PubMed] [Google Scholar]
- 51.Bolhuis JJ, Gahr M. Neural mechanisms of birdsong memory. Nat Rev Neurosci. 2006;7:347–357. doi: 10.1038/nrn1904. [DOI] [PubMed] [Google Scholar]
- 52.London SE, Clayton DF. Functional identification of sensory mechanisms required for developmental song learning. Nat Neurosci. 2008;11:579–586. doi: 10.1038/nn.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Roberts TF, Tschida KA, Klein ME, Mooney R. Rapid spine stabilization and synaptic enhancement at the onset of behavioural learning. Nature. 2010;463:948–952. doi: 10.1038/nature08759.. Using in vivo imaging in juvenile zebra finches, the authors demonstrate that initial tutor song experience drives the rapid enlargement, stabilization, and growth of dendritic spines on HVC neurons. These structural changes were accompanied by enhanced synaptic activity, consistent with synaptic potentiation, and were predictive of how well the juvenile would ultimately imitate the tutor song, consistent with the idea that synapses in HVC participate in the encoding of tutor song memories.
- 54.Marler P, Peters S. Sparrows learn adult song and more from memory. Science. 1981;213:780–782. doi: 10.1126/science.213.4509.780. [DOI] [PubMed] [Google Scholar]
- 55. Prather JF, Peters S, Nowicki S, Mooney R. Persistent representation of juvenile experience in the adult songbird brain. J Neurosci. 2010;30:10586–10598. doi: 10.1523/JNEUROSCI.6042-09.2010.. The authors systematically tutored juvenile swamp sparrows on a wide array of tutor songs, and then monitored song development to confirm that although copies of many songs were generated during sensorimotor learning, only a few of these songs were retained in the adult repertoire. Nonetheless, recordings made in the HVC of these birds in adulthood detected neurons that were strongly responsive to tutor songs that were only transiently imitated, and more rarely to tutor songs that were apparently never copied at all. These findings suggest that HVC contains a persistent record of early auditory-vocal experience, even when the adult vocal repertoire no longer reports the effects of that experience.
- 56.Dave AS, Margoliash D. Song replay during sleep and computational rules for sensorimotor vocal learning. Science. 2000;290:812–816. doi: 10.1126/science.290.5492.812. [DOI] [PubMed] [Google Scholar]
- 57. Shank SS, Margoliash D. Sleep and sensorimotor integration during early vocal learning in a songbird. Nature. 2009;458:73–77. doi: 10.1038/nature07615.. Neural recordings made in sleeping juvenile birds reveal that daytime exposure to a tutor song generates changes in the bursting activity of song premotor neurons in RA on the following night. The temporal pattern of the bursting activity depended on the acoustic structure of the tutor song and also required daytime singing by the juvenile, suggesting that it is shaped by the activity of a song comparator.