Abstract
Background
Essential Tremor (ET) is among the most prevalent neurological disorders. Growing clinical and neuro-imaging evidence implicates cerebellar dysfunction in the pathogenesis of ET and emerging postmortem studies have identified structural changes in the cerebellum, particularly in Purkinje cells. In this study we systematically quantified focal Purkinje cell dendritic swellings (DS) in 20 ET vs. 19 control brains.
Methods
In each brain, a standard parasagittal neocerebellar tissue block was harvested. DS were quantified in one 7-μm thick section stained with Luxol Fast Blue/Hematoxylin and Eosin (LH&E) and one section stained with Bielschowsky method.
Results
The number of DS were higher in cases than controls by LH&E (1.50 ± 1.79 vs. 0.05 ± 0.23, p = 0.002) and Bielschowsky methods (2.70 ± 3.10 vs. 0.37 ± 0.50, p = 0.002). The number of DS was correlated with the number of torpedoes, and marginally inversely correlated with the number of Purkinje cells.
Discussion
The current study documents and quantifies an additional structural abnormality in the ET cerebellum, adding to the growing list of such changes in this disease. The mechanisms that underlie this and other structural changes observed in ET are currently unknown, and they deserve additional exploration.
Keywords: essential tremor, brain, pathology, Purkinje cell, dendritic swellings, neurodegenerative
Introduction
Essential Tremor (ET) is widely acknowledged to be one of the most prevalent neurological disorders in adults [1]. Aside from a variety of tremors, patients may experience difficulty with gait and balance as well as cognitive impairment [2,3]. There is growing clinical and neuro-imaging evidence implicating cerebellar dysfunction in the pathogenesis of ET [4–7]. Furthermore, an emergence of postmortem studies in recent years has elucidated detectable structural changes in the brains of patients with ET. Most of these changes involve the cerebellum, which is essential for the coordination of fine motor movements [8]. These changes include Purkinje cell loss and increased numbers of heterotopic Purkinje cells; the occurrence of torpedoes, which are six to seven times more abundant in ET than control brains; and the appearance of an unusually dense basket cell axonal plexus [9–11].
In our postmortem studies of the ET cerebellum, we have recently observed some focal swellings in the dendritic processes of Purkinje cells, but no quantitative case-control analysis has been performed until now. One of the most defining characteristics of Purkinje cells is their large and elaborate dendritic arbor, through which they receive most of their synaptic information from climbing and parallel fibers. Interference with this input could directly affect cerebellar function. In this study, we systematically quantified focal dendritic swellings (DS) in Purkinje cells in ET patients vs. age-matched controls.
Methods
The study was conducted at the Essential Tremor Centralized Brain Repository (ETCBR) of the New York Brain Bank (NYBB) at Columbia University Medical Center. The 39 brains included 20 ET cases and 19 available non-diseased controls who were frequency- rather than individually-matched based on age. All ET cases were diagnosed by their treating neurologist and the ET diagnosis was confirmed using ETCBR criteria by a second neurologist specializing in movement disorders (E.D.L.) [9].
During life, demographic and clinical data were collected through a series of semi-structured questionnaires. Heavy ethanol use was defined previously as consumption of an average of four or more standard drinks (15 ml of absolute ethanol) per day for a man, or three or more per day for a woman, at any point in their lives [12]. Data on lifetime exposure to medications known to cause cerebellar damage (e.g., lithium, diphenylhydantoin) were collected. Most ET cases also underwent a standardized videotaped neurological examination, which included an assessment of postural tremor (sustained arm extension), five tests of kinetic tremor (pouring, drinking, using spoon, finger-nose-finger maneuver, and drawing spirals), and head and voice tremor. Each of these six tests of postural and kinetic tremor was performed with each arm (twelve tests total). Videotaped action tremor was rated by a senior neurologist specializing in movement disorders (E.D.L.) during each test using a scale from 0 (no tremor) to 3 (large amplitude tremor) [13], resulting in a total tremor score (range = 0 – 36).
Age-matched control brains were normal elderly control subjects from the NYBB, derived from the Alzheimer’s Disease Research Center and the Washington Heights Inwood Columbia Aging Project; they were free of clinical diagnoses of Alzheimer’s disease (AD), ET, or Parkinson’s disease (PD) and without neuropathological diagnoses of neurodegenerative disease. By design, none of the case or control brains had brainstem or cortical Lewy bodies. The NYBB operates under approval of the IRB of Columbia University Medical Center.
As previously described, all brains underwent a complete neuropathological assessment at the NYBB [9]. All brains had standardized measurements of brain weight (grams), postmortem interval (PMI, hours between death and placement of brain in a cold room or upon ice), Braak and Braak AD staging for neurofibrillary tangles [14, 15], Braak PD staging [16], and Consortium to Establish a Registry for AD (CERAD) ratings for neuritic plaques [17].
As described, a standard 3 × 20 × 25 mm parasagittal, formalin-fixed, tissue block was harvested from the neocerebellum [18]; the block included the cerebellar cortex, white matter and dentate nucleus. For the quantification of DS, in each ET case and control, two sequential 7 μm thick paraffin sections were obtained. One of them was stained with Luxol Fast Blue counterstained with Hematoxylin and Eosin (LH&E) and the other was stained with a Bielschowsky silver method. As described in other publications [19–23], DS were defined as rounded-to-ovoid masses in the molecular layer that were associated with Purkinje cell dendritic processes; these swellings had a glassy eosinophilic appearance on LH&E and were dark brown-to-black on Bielschowsky stain. DS were enumerated in each slide, and the DS count represents the number of such swellings in the entire slide. The DS counts were initially performed by a trained technician (M.Y.) and then confirmed by a senior neuropathologist (P.L.F.); both were blinded to diagnosis, clinical information, torpedo counts and Purkinje cell counts. In addition, a senior neuropathologist (P.L.F.) who was blinded to all clinical information counted torpedoes throughout the entire LH&E and Bielschowsky stained sections, and counted and averaged Purkinje cells in five 100x fields (LH&E), as previously described [18]. As previously described [10], a semiquantitative rating of the appearance of the basket cell plexus surrounding Purkinje cell bodies throughout Bielschowsky preparations was carried out by a senior neuropathologist (P.L.F.) who was blinded to all clinical information. The following scale was used: 0 (few, or no discernible processes); 1 (sparse number of processes); 2 (moderate number of processes); and 3 (dense tangle of processes). In some instances, the rater used intermediate values (0.5, 1.5, and 2.5).
All statistical analyses were performed in SPSS (version 18.0). The number of DS on LH&E stained sections was not normally distributed (Kolmogorov-Smirnov test z = 2.32, p < 0.001) nor was the number of DS on Bielschowsky stained sections (Kolmogorov-Smirnov test z = 2.08, p <0.001). Therefore, both means and medians were reported and non-parametric test statistics were used when analyzing these variables.
In the planning phase of the study, the a priori power calculations indicated that a targeted sample size of 20 ET cases and 20 controls would provide adequate power (86.9%) to detect a case control difference of 50%, assuming alpha = 0.05 and that the controls had 1 ± 0.5 DS per slide. A sample size of 19 in each group would provide 85.1% power, which was still adequate to detect this difference.
Results
The 20 ET cases and 19 controls were similar in age and gender; they had similar brain weights and CERAD plaque scores (Table 1). The PMI was shorter and Braak staging was higher in ET cases than controls (Table 1). Tremor duration in ET cases (mean = 43.1 years) ranged from 5 – 80 years (Table 1). All cases and controls had a Braak PD staging of 0.
Table 1.
Clinical Characteristics of 20 ET Cases and 19 Controls
| ET Cases | Controls | Significance | |
|---|---|---|---|
| Age (years) | 81.8 ± 7.7 | 79.4 ± 9.8 | p = 0.40 a |
|
| |||
| Female gender | 13 (65.0%) | 9 (47.4%) | p = 0.27 b |
|
| |||
| Tremor duration (years) | 43.1 ± 23.2 | Not applicable | Not applicable |
|
| |||
| Postmortem interval (hours) | 3.4 ± 3.1 | 7.7 ± 7.5 | p = 0.03 a |
|
| |||
| Brain weight (grams) | 1245.7 ± 136.5 | 1243.7 ± 122.2 | p = 0.40 a |
|
| |||
| CERAD plaque score | p = 0.43 b | ||
| 0 | 10 (50.0%) | 13 (68.4%) | |
| A | 7 (35.0%) | 5 (26.3%) | |
| B | 3 (15.0%) | 1 (5.3%) | |
| C | 0 (0.0%) | 0 (0.0%) | |
|
| |||
| Braak AD stage | p = 0.02 b | ||
| 0 | 1 (5.0%) | 6 (31.6%) | |
| 1 | 7 (35.0%) | 7 (36.8%) | |
| 2 | 6 (30.0%) | 6 (31.6%) | |
| 3 | 6 (30.0%) | 0 (0.0%) | |
| 4 | 0 (0.0%) | 0 (0.0%) | |
| 5 | 0 (0.0%) | 0 (0.0%) | |
| 6 | 0 (0.0%) | 0 (0.0%) | |
|
| |||
| Torpedo count (LH&E) | 14.5 ± 14.2 | 3.0 ± 3.7 | p = 0.002 a |
|
| |||
| Torpedo count (Bielschowsky) | 29.3 ± 31.3 | 6.4 ± 10.4 | p = 0.005 a |
|
| |||
| Purkinje cell count (LH&E) | 36.1 ± 8.8 | 45.5 ± 13.9 | p = 0.03 a |
|
| |||
| Basket cell axonal plexus density | 2.2 ± 0.7 | 1.4 ± 0.6 | p = 0.003 c |
All values are mean ± standard deviation or number (percentage).
Student’s t test,
Chi-square or Fisher’s Exact test,
Mann-Whitney test
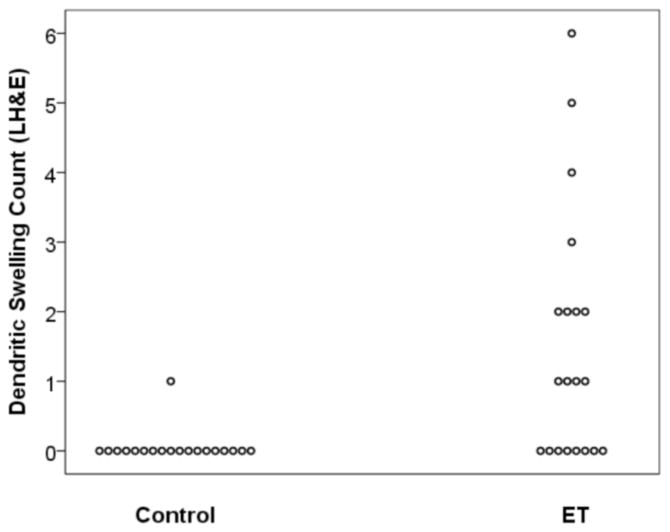
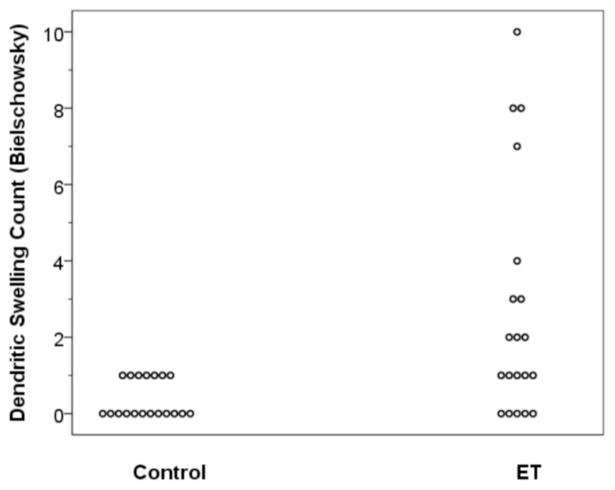
DS were identifiable on both LH&E and Bielschowsky stained sections in the molecular layer, above the Purkinje cell bodies and the basket cell axonal plexus formations (Figures 1 and 2). The number of DS were higher in cases than controls by LH&E (mean ± standard deviation = 1.50 ± 1.79, median = 1.0, range = 0 – 6 in ET cases vs. 0.05 ± 0.23, 0, 0 – 1 in controls)(Mann Whitney z = 3.63, p = 0.002) (Figure 3). Eight (40.0%) ET cases had 2 or more DS by LH&E (i.e., they had a value that was higher than that seen in any of the controls). The number of DS were higher in cases than controls by Bielschowsky method (mean ± standard deviation = 2.70 ± 3.10, median = 1.5, range = 0 – 10 in ET cases vs. 0.37 ± 0.50, 0, 0 – 1 in controls)(Mann Whitney z = 3.21, p = 0.002) (Figure 4). Ten (50.0%) ET cases had 2 or more DS by Bielschowsky method (i.e., they had a value that was higher than that seen in any of the controls).
Figure 1.
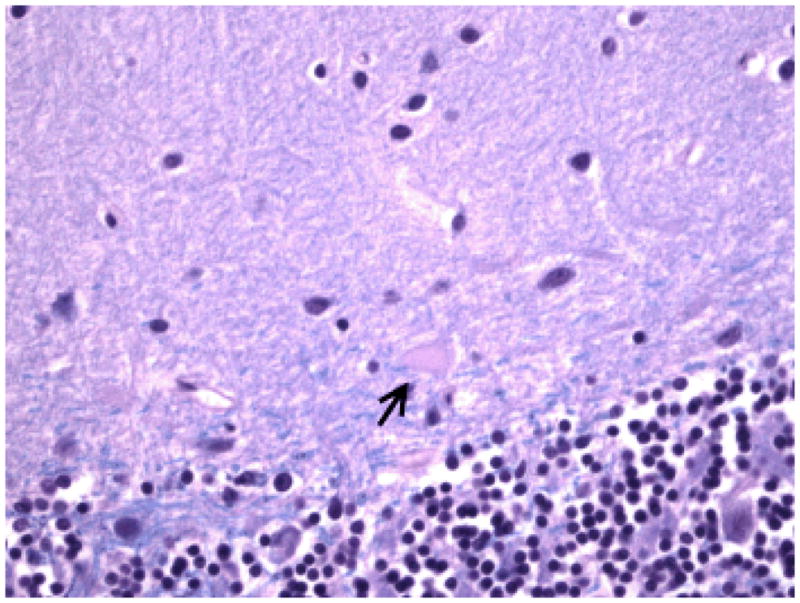
Dendritic swelling (arrow) in an ET case on LH&E-stained cerebellar cortical section (400× magnification).
Figure 2.
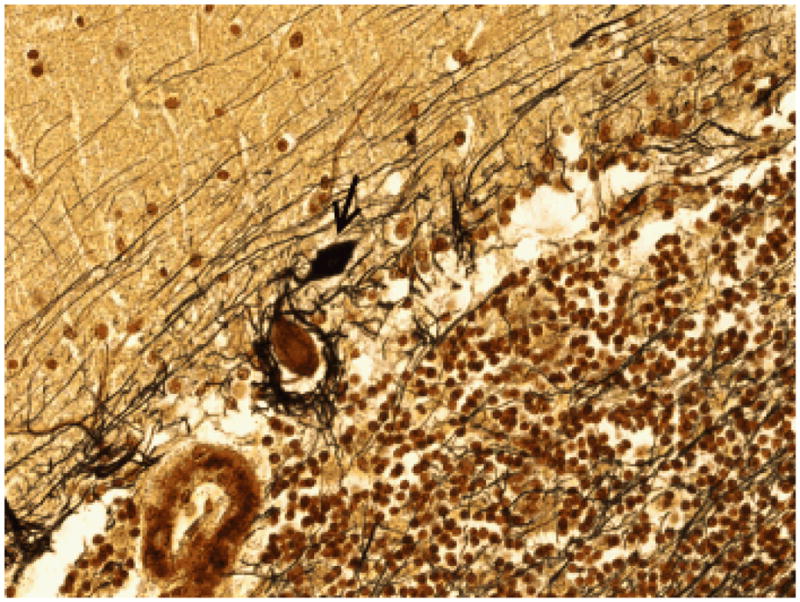
Dendritic swelling (arrow) in an ET case on Bielschowsky-stained cerebellar cortical section (400× magnification). The swelling is in the molecular layer, above the Purkinje cell body and the basket cell axonal plexus. Basket cell axonal plexus density = 3.
Figure 3.
Number of dendritic swellings quantified in LH&E stained sections in ET cases vs. controls.
Figure 4.
Number of dendritic swellings quantified in Bielschowsky stained sections in ET cases vs. controls.
The numbers of DS by LH&E and Bielschowsky method were not correlated with age or brain weight (Spearman’s r, all p > 0.05) (Supplementary Figure 1), or with PMI (respective Spearman’s r = −0.19, p = 0.26 and Spearman’s r = −0.20, p = 0.22), gender (Mann Whitney p values = 0.83 and 0.59), CERAD plaque score (Kruskal Wallis p values = 0.35 and 0.31), or Braak AD stage (Kruskal Wallis p values = 0.08 and 0.44).
The numbers of DS by LH&E and Bielschowsky methods were highly correlated with one another (Spearman’s r = 0.81, p < 0.001), especially in the ET cases (Spearman’s r = 0.86, p < 0.001). The numbers of DS were correlated with the number torpedoes as well as the extent of basket cell process hypertrophy; the number was marginally inversely correlated with the number of Purkinje cells (Table 2). Analyses restricted to ET cases yielded similar correlations, although statistical power was lower (Table 2). In ET cases, the number of DS by LH&E and Bielschowsky methods were not correlated with tremor duration in years (respective r values = −0.16 [p = 0.55] and −0.12 [p = 0.65]) (Supplementary Figure 2).
Table 2.
Correlations Between Dendritic Swellings and (1) Other Cerebellar Features and (2) Braak AD Stage
| Dendritic swellings by LH&E | Dendritic swellings by Bielschowsky method | |
|---|---|---|
|
Analyses Including All ET Cases and Controls
| ||
| Torpedo count (LH&E) | r = 0.63, p< 0.001 | r = 0.59, p< 0.001 |
|
| ||
| Torpedo count (Bielschowsky) | r = 0.57, p < 0.001 | r = 0.55, p < 0.001 |
|
| ||
| Purkinje cell count (LH&E) | r = −0.28, p = 0.11 | r = −0.30, p = 0.09 |
|
| ||
| Basket cell axonal plexus density | r = 0.45, p = 0.007 | r = 0.58, p < 0.001 |
|
| ||
| Braak AD stage | ||
| 0 | 0.14 ± 0.38 [0, 0 – 1] | 0.43 ± 0.79 [0, 0 – 2] |
| 1 | 1.43 ± 1.99 [0.5, 0 – 6] | 2.50 ± 3.55 [1, 0 – 10] |
| 2 | 0.17 ± 0.39 [0, 0 – 1] | 0.92 ± 0.90 [1, 0 – 3] |
| 3 | 1.33 ± 1.63 [1, 0 – 4] | 2.00 ± 2.70 [1, 0 – 1] |
|
| ||
| Analyses Restricted to ET Cases | ||
|
| ||
| Torpedo count (LH&E) | r = 0.58, p = 0.008 | r = 0.61, p = 0.005 |
|
| ||
| Torpedo count (Bielschowsky) | r = 0.48, p = 0.03 | r = 0.60, p = 0.005 |
|
| ||
| Purkinje cell count (LH&E) | r = −0.13, p = 0.59 | r = −0.18, p = 0.47 |
|
| ||
| Basket cell axonal plexus density | r = 0.32, p = 0.21 | r = 0.47, p < 0.059 |
All r values are Spearman’s r.
For the Braak AD stage, the values are mean ± standard deviation [median, minimum -maximum].
The ET cases had a mean total tremor score = 28.5 ± 4.8, 12 had head tremor, 10 had voice tremor and 7 had both. Thirteen had used primidone, 19 had used propranolol and 15 had used another ET medication. None reported a history of head trauma and none were heavy consumers of ethanol or had been exposed to cerebellar toxic medications. ET cases with DS counts of 0 – 1 did not differ from those with higher DS counts with respect to any clinical variables (age, tremor duration, total tremor score, tremor distribution, all p > 0.05).
Discussion
The current study documents and quantifies an additional structural abnormality in the ET cerebellum, adding to a growing list of such changes in this disease. Until very recently, there had been few postmortem studies of ET, and its neuropathological substratum was unknown [9]. Clinical and neuroimaging studies are now providing growing evidence of cerebellar dysfunction in ET [4 – 6] and recent postmortem studies have demonstrated structural changes in the cerebellum, with many of these changes involving the Purkinje cells [8–11].
The present study has shown that DS occur in Purkinje cells of patients diagnosed with ET. While the absolute number of such swellings was modest, in case-control comparisons, there was a significant increase in the number of these DS in ET, both in LH&E stained sections and Bielschowsky stained sections, indicating that a mild increased number of swellings seems to be a feature of diseased brains relative to control brains. Until now, there had been no published data on the DS or other dendritic abnormalities in ET. The presence of these perturbations could have some physiological importance since climbing fibers and parallel fibers have specific target sites on the Purkinje cell dendritic arbor.
We found that there was a correlation between the number of Purkinje cell DS and the number of torpedoes as well as basket cell process hypertrophy. There was also a slight inverse correlation between the number of DS and the number of Purkinje cells. These data suggest that these postmortem structural changes may be connected, reflecting the same underlying degenerative process involving the Purkinje cells and/or their microenvironment.
Purkinje cell DS are not specific to ET and are found in other disorders with cerebellar changes. Thus, they have been observed in a variety of other conditions. These include Menkes disease [19], mucopolysaccharidoses types I, II, and III [20], and spinocerebellar ataxia type 6 [21]. In very early postmortem studies, Uyematsu described focal DS in Purkinje cells in individuals with “senile dementia” [22] and Friede saw abundant DS in amaurotic idiocy and poliodystrophy [23].
This study has several strengths. It is the first systematic quantification of DS in the ET cerebellum. Second, clinically well-characterized cases and controls were carefully age-matched. Third, Purkinje cell DS were quantified in sections stained by two different methods (LH&E and Bielschowsky).
We have documented from postmortem studies that structural changes in the Purkinje cell are linked to ET. In the present study, we sought to quantify focal DS, a previously un-quantified Purkinje cell abnormality, in ET and normal control cerebellar sections. We found that there is a significant increase in the number of DS in ET versus controls and a correlative relationship between DS and other changes of the Purkinje cells. The mechanisms that underlie this and other structural changes observed in ET are currently unknown, and they deserve additional exploration.
Supplementary Material
Acknowledgments
Funding: This work was supported by R01 NS042859 (National Institutes of Health, Bethesda, MD) and by the Claire O’Neil Essential Tremor Research Fund (Columbia University, New York, NY).
Footnotes
Conflicts of Interest: The authors had no conflicts of interest.
References
- 1.Louis ED, Ferreira JJ. How common is the most common adult movement disorder? Update on the worldwide prevalence of essential tremor. Movement Disorders. 2010;25:534–541. doi: 10.1002/mds.22838. [DOI] [PubMed] [Google Scholar]
- 2.Lombardi WJ, Woolston DJ, Roberts JW, Gross RE. Cognitive deficits in patients with essential tremor. Neurology. 2001;57:785–790. doi: 10.1212/wnl.57.5.785. [DOI] [PubMed] [Google Scholar]
- 3.Benito-Leon J, Louis ED, Bermejo-Pareja F, et al. Population-based case-control study of cognitive function in essential tremor. Neurology. 2006;66:69–74. doi: 10.1212/01.wnl.0000192393.05850.ec. [DOI] [PubMed] [Google Scholar]
- 4.Jenkins IH, Bain PG, Colebatch JG, et al. A positron emission tomography study of essential tremor: evidence for overactivity of cerebellar connections. Annals of Neurology. 1993;34:82–90. doi: 10.1002/ana.410340115. [DOI] [PubMed] [Google Scholar]
- 5.Koster B, Deuschl G, Lauk M, Timmer J, Guschlbauer B, Luking CH. Essential tremor and cerebellar dysfunction: Abnormal ballistic movements. Journal of Neurology Neurosurgery and Psychiatry. 2002;73:400–405. doi: 10.1136/jnnp.73.4.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benito-Leon J, Alvarez-Linera J, Hernandez-Tamames JA, Alonso-Navarro H, Jimenez-Jimenez FJ, Louis ED. Brain structural changes in essential tremor: Voxel-based morphometry at 3-tesla. Journal of Neurological Sciences. 2009;287:138–142. doi: 10.1016/j.jns.2009.08.037. [DOI] [PubMed] [Google Scholar]
- 7.Helmchen C, Hagenow A, Miesner J, et al. Eye movement abnormalities in essential tremor may indicate cerebellar dysfunction. Brain. 2003;126:1319–1332. doi: 10.1093/brain/awg132. [DOI] [PubMed] [Google Scholar]
- 8.Axelrad JE, Louis ED, Honig LS, et al. Reduced Purkinje cell number in essential tremor: A postmortem study. Archives of Neurology. 2008;70:1452–1455. doi: 10.1001/archneurol.2007.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Louis ED, Faust PL, Vonsattel JP, et al. Neuropathological changes in essential tremor: 33 cases compared with 21 controls. Brain. 2007;130:3297–3307. doi: 10.1093/brain/awm266. [DOI] [PubMed] [Google Scholar]
- 10.Erickson-Davis CR, Faust PL, Vonsattel JP, et al. “Hairy baskets” associated with degenerative Purkinje cell changes in essential tremor. Journal of Neuropathology and Experimental Neurology. 2010;69:262–271. doi: 10.1097/NEN.0b013e3181d1ad04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuo SH, Erickson-Davis CR, Gillman A, Faust PL, Vonsattel JP, Louis ED. Increased number of heterotopic Purkinje cells in essential tremor. Journal of Neurology Neurosurgery and Psychiatry. 2010 doi: 10.1136/jnnp.2010.213330. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harasymiw JW, Bean P. Identification of heavy drinkers by using the early detection of alcohol consumption score. Alcohol Clin Exp Res. 2001;25:228–35. [PubMed] [Google Scholar]
- 13.Louis ED, Zheng W, Applegate L, Shi L, Factor-Litvak P. Blood harmane concentrations and dietary protein consumption in essential tremor. Neurology. 2005;65:391–6. doi: 10.1212/01.wnl.0000172352.88359.2d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Braak H, Braak E. Diagnostic criteria for neuropathologic assessment of Alzheimer’s disease. Neurobiology of Aging. 1997;18:S85–S88. doi: 10.1016/s0197-4580(97)00062-6. [DOI] [PubMed] [Google Scholar]
- 15.Braak H, Alafuzoff I, Arzberger T, Kretzchmar H, Del Tredici K. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathologica. 2006;112:389–404. doi: 10.1007/s00401-006-0127-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiology of Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 17.Mirra SS. The CERAD neuropathology protocol and consensus recommendations for the postmortem diagnosis of Alzheimer’s disease: a commentary. Neurobiology of Aging. 1997;18:S91–S94. doi: 10.1016/s0197-4580(97)00058-4. [DOI] [PubMed] [Google Scholar]
- 18.Louis ED, Vonsattel JP, Honig LS, Ross GW, Lyons KE, Pahwa R. Neuropathologic findings in essential tremor. Neurology. 2006;66:1756–1759. doi: 10.1212/01.wnl.0000218162.80315.b9. [DOI] [PubMed] [Google Scholar]
- 19.Troost D, van Rossum A, Straks W, Willemse J. Menkes kinky hair disease. II. A clinicopathological report of three cases. Brain Development. 1982;4:115–126. doi: 10.1016/s0387-7604(82)80005-3. [DOI] [PubMed] [Google Scholar]
- 20.Ferrer I, Cusi V, Pineda M, Galorfre E, Vila J. Focal dendritic swellings in Purkinje cells in mucopolysaccharidoses types I, II and III. A Golgi and ultrastructural study. Neuropathology and Applied Neurobiology. 1988;14:315–323. doi: 10.1111/j.1365-2990.1988.tb00891.x. [DOI] [PubMed] [Google Scholar]
- 21.Yang Q, Hashizume Y, Yoshida M, et al. Morphological Purkinje cell changes in spinocerebellar ataxia type 6. Acta Neuropathologica. 2000;100:371–376. doi: 10.1007/s004010000201. [DOI] [PubMed] [Google Scholar]
- 22.Uyematsu S. A study of some peculiar changes found in the oxons and dendrites of the Purkinje cells. Archives of Neurology and Psychiatry. 1921;5:231–269. [Google Scholar]
- 23.Friede RL. An enzyme histochemical study of torpedoes and dendritic swellings in the cerebellum. Acta Neuropathologica. 1965;4:288–292. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.