Abstract
OBJECTIVES
Low-grade colonic mucosal inflammation has been postulated to have an important role in the pathophysiology of irritable bowel syndrome (IBS). The objectives of this study were (i) to identify serum and tissue-based immunological and neuroendocrine markers associated with mucosal inflammation in male (M) and female (F) patients with non-post-infectious IBS (non-PI-IBS) compared with healthy controls and (ii) to assess possible correlations of such markers with IBS symptoms.
METHODS
Sigmoid mucosal biopsies were obtained from 45 Rome II positive IBS patients without a history of PI-IBS (26 F, 35.5% IBS-C, 33.3% IBS-D, 31.1% IBS-A/M) and 41 healthy controls (22 F) in order to measure immunological markers (serum cytokine levels, colonic mucosal mRNA levels of cytokines, mucosal immune cell counts) and neuroendocrine markers associated with mucosal inflammation (corticotropin releasing factor- and neurokinin (NK)-related ligands and receptors, enterochromaffin cells). Symptoms were measured using validated questionnaires.
RESULTS
Of all the serum and mucosal cytokines measured, only interleukin-10 (IL-10) mRNA expression showed a group difference, with female, but not male, patients showing lower levels compared with female controls (18.0 ± 2.9 vs. 29.5 ± 4.0, P = 0.006). Mucosal mRNA expression of NK-1 receptor was significantly lower (1.15 ± 0.19 vs. 2.66 ± 0.56, P = 0.008) in female, but not male, patients compared with healthy controls. No other significant differences were observed.
CONCLUSIONS
Immune cell counts and levels of cytokines and neuropeptides that are associated with inflammation were not significantly elevated in the colonic mucosa of non-PI-IBS patients, and did not correlate with symptoms. Thus, these findings do not support that colonic mucosal inflammation consistently has a primary role in these patients. However, the finding of decreased IL-10 mRNA expression may be a possible biomarker of IBS and warrants further investigation.
INTRODUCTION
Low-grade inflammation or immune activation of the gut has been proposed to have a role in irritable bowel syndrome (IBS) pathophysiology (1–4), and specifically in the development of visceral hypersensitivity and epithelial dysfunction (5 – 7). Reported findings in post-infectious IBS (PI-IBS) as well as in non-PI-IBS or unselected patients generally support this hypothesis. The strongest evidence is from studies of patients with PI-IBS, which have shown increased number colonic lamina propria T lymphocytes (8), and increased mRNA levels of the pro-inflammatory cytokine inter-leukin-1β (IL-1β) in the rectal mucosa compared with patients who do not develop IBS symptoms after a gastroenteritis (9). However, findings have been less consistent in non-PI-IBS patients and unselected patients, particularly in regards to possible correlations of such findings with IBS symptoms. This may be in part related to sample size and increased heterogeneity. Additionally, although IBS occurs in both men and women, most of the studies conducted in IBS have been performed in female patients without a matched sex distribution in the control groups. However, a female predisposition to PI-IBS (10) and sex differences in the physiologic disturbances in IBS, including colonic mucosal mast cell numbers of IBS (11), suggest that sex differences in immune activation may exist in IBS.
In unselected IBS patients, studies have reported increased activated T cells in the blood (12) and increased T lymphocytes (3,11,13) mast cells (2,4,11,13–16), and toll-like receptor expression (17) within the colonic mucosa. Barbara et al. (2) found that the number of mast cells in close proximity to sensory neurons positively correlated with severity and frequency of abdominal pain/discomfort. However, other studies have found similar or lower mast cell numbers in colonic or rectal mucosa in IBS patients compared with healthy controls (3,18,19). In one of these studies, IBS patients had fewer mast cells in the rectum and descending colon and decreased tryptase release regardless of the presence of visceral hypersensitivity in comparison to control subjects (18).
Similar to studies of inflammatory cells in non-PI-IBS and unselected patients, levels of serum cytokines have generally been increased but specific cytokines studied and positive findings have differed (4,12,20–22). There is a lack of evidence to support higher mucosal mRNA or protein levels of pro-inflammatory cytokines in non-PI-IBS (23,24). In female IBS patients, Macsharry et al. (23) demonstrated lower or normal mRNA expression and protein levels of pro-inflammatory cytokines, including IL-1β and IL-6, in colonic tissue from IBS but decreased expression of the anti-inflammatory cytokines IL-10 and TGF-β (transforming growth factor-β)compared with healthy females. In contrast, patients with active inflammatory bowel disease (IBD) had increased chemokines and pro-inflammatory cytokines. Furthermore, we have previously shown lower mRNA levels of IL-2, IL-6, and IL-10 from sigmoid colon biopsies in female IBS patients with diarrhea (IBS-D) compared with healthy females although the two groups were relatively small in size (24).
Studies in IBD have demonstrated that active inflammation is associated with upregulation of mucosal signaling systems, including the corticotropin releasing factor (CRF) (25), neurokinin (NK) (26), and serotonin (5-HT) systems located in enterochromaffin (EC) cells (27). Several studies indicate increased expression of the CRF ligand –receptor system in the colonic mucosa of IBD patients (28–30). Increased expression of NK-1R and altered serotonin signaling have been reported in patients with IBD and in animal models of colitis (26,31). For example, in severe UC (ulcerative colitis), EC cell counts, mucosal 5-HT content, and mRNA expression of tryptophan hydroxylase 1 and SERT (serotonin reuptake transporter) were decreased (32). In contrast, increased EC cell counts have been demonstrated in rectal biopsies of PI-IBS patients (8,14), while normal counts have been found in unspecified or non-PI-IBS (14,32,33). EC cell counts were found to be either normal (non-severe) or decreased (severe) in ulcerative colitis (32). However, mucosal 5-HT content and mRNA expression of tryptophan hydroxylase 1 and the serotonin transporter (SERT) were decreased. It has been hypothesized that SERT expression decreases after intestinal inflammation, resulting in amplification of serotonergic signaling that exerts an increased inflammatory response (27).
Despite the large body of published data, uncertainty remains regarding the following questions: (i) Is the colonic mucosa the source of reported immune activation? (ii) Are IBS symptoms correlated with mucosal immune activation? (iii) Are reported findings related to coexistent psychological symptoms? (iv) Do the reported findings differ between men and women? The current study, performed in a well-characterized sample of male and female IBS patients and matching healthy controls, was aimed to address these questions. A comprehensive set of immune markers, and markers previously shown to be upregulated in association with mucosal inflammation were measured in serum and mucosal biopsies to test the following hypotheses: (i) IBS patients show increased mucosal or systemic expression of pro-inflammatory cytokines, and increased numbers of mucosal lymphocytes and mast cells compared with controls; (ii) IBS patients show altered expression of neuropeptide and neuroendocrine markers (CRF and NK signaling systems, EC and enteroendocrine (EE) cells), which are often associated with mucosal inflammation; and (iii) these peripheral changes correlate with subjective symptoms of IBS.
METHODS
Study subjects
Forty-five IBS patients (26 women and 19 men) and 41 healthy controls (22 women and 19 men) participated in the study. Patients between the ages of 18 and 55 who met entry criteria were recruited from the specialty clinic and community advertisements although subjects were not consecutively enrolled. Age was limited to 55 years since age can affect immune response (34). All IBS patients met the Rome II diagnostic criteria for IBS (35). The diagnosis was confirmed by a gastroenterologist with expertise in IBS (L.C.). Bowel habit subtypes of IBS-D and IBS with constipation (IBS-C) were based on the Rome II subclassiffication criteria. Patients with IBS who did not meet either IBS-D or IBS-C criteria and had a history of alternating diarrhea and constipation were considered to have IBS-A/M. Healthy controls between the ages of 18–55 were recruited by newspaper or internet advertisement from the community, and they did not have a history of IBS, other chronic pain conditions, infectious or inflammatory disorders, or psychiatric illness, and were not taking centrally acting drugs (anxiolytics, narcotics, anti-depressants). None of the subjects had taken corticosteroids or immunosuppressive agents that could affect neuroendocrine and immune function in the past 2 months or had a current history of tobacco or alcohol abuse. For premenopausal women not taking oral contraceptive agents or provera, menstrual cycle phase was determined by the count forward/backward method (menses: first 3 days of menses; follicular: days 4–14; luteal: day 14 to onset of menses). Serum progesterone was collected to help confirm cycle phase. Subjects were compensated $25 for an initial screening visit and $125 for completing the flexible sigmoidoscopy and blood draws for cytokine levels.
Symptom measures
Validated questionnaires were used for IBS symptoms (UCLA[University of California Los Angeles] Bowel Symptom Questionnaire) (36), depression and anxiety (HAD [Hospital Anxiety and Depression] scale) (37), and disease-specific quality of life assessment (38). IBS patients rated the intensity of their IBS symptoms for the past 24 h. Subjects were screened by interview for psychiatric disorders using the structured clinical interview for the DSM-IV (SCID) by a licensed therapist (M.M.) (39).
Study protocol
A flexible sigmoidoscopy to at least 40 cm from the anal verge was performed in the Medical Procedures Unit between 1200 and 1400 hours. Subjects were instructed to use two tap-water enemas as the bowel preparation. Before the sigmoidoscopy, blood samples were drawn for cytokine levels and progesterone level (to confirm menstrual cycle phase). During the sigmoidoscopy, 15 sigmoid colon biopsies were taken at 30 cm from the anal verge.
Serum cytokines
The following cytokines were measured in serum collected from study subjects: IL-1β, IL-6, IL-8, IL-10, IL-12, and TNF-α (tumor necrosis factor-α). In all, 25 μl of MSD (Meso Scale Discovery; Gaithersburg, MD) assay diluent was added into each well of a 96-well plate. The plate was then incubated for 30 min with vigorous shaking (300–1,000 r.p.m.) at room temperature. In all, 25 μl of prepared standard (ranging from 2,500 to 0 pg/ml) or serum sample was dispensed into a separate well of the MSD plate and incubated for 2 h with vigorous shaking (300–1,000 r.p.m.) at room temperature. The plate was then washed 3× with PBS (phosphate-buffered saline) + 0.05% Tween-20. In all, 25μl of detection antibody solution was added into each well of the MSD plate, the plate was sealed and incubated for another 2 h with vigorous shaking (300–1,000 r.p.m.) at room temperature. Followed by washing 3× with PBS + 0.05% Tween-20, 150μl of 2× read buffer T was then added to each well of the MSD plate. The plate was immediately read on the SECTOR(r) Imager (Meso Scale Discovery; Gaithersburg, MD).
Determination of mRNA levels using real-time quantitative reverse transcriptase polymerase chain reaction (TaqMan assay)
For detection of cytokines, CRF-related ligands (CRF; urocortin (Ucn) 1–2), CRF1 receptor (CRF1R) and CRF2 receptor (CRF2R), NK peptides/receptors (TAC1, NK-1R, NK-2R), RNA isolated from sigmoid colon biopsy tissue was reverse transcribed into complementary deoxyribonucleic acid (cDNA) and real-time PCR (polymerase chain reaction) was performed using a 7500 Fast Real-Time PCR system (Applied Biosystems, Foster City, CA). Details of the methodology have been previously described (40,41).
Two primers with amplicon lengths 61 and 113 were used to evaluate CRF1R mRNA levels. The number of PCR cycles, “Ct,” was normalized to arbitrary mRNA units by an 18-s control according to the following formulas: for Ct values <40, log10 (2^[Control Ct − Marker Ct])+10; and for Ct values ≥ 40, the arbitrary mRNA units were set to log10 (2^[Minimum (Control Ct) − 40])+10, if <20% of the samples had Ct values ≥ 40 (otherwise the marker was not analyzed).
Colonic mucosal cellularity
Biopsy specimens were fixed in buffered 10% formalin. Paraffin-embedded specimens were sliced in 4 μm sections with a mictotome, and mounted on slides. Slides were baked at 60 °C for at least 30 min to melt the paraffin. The slides were deparaffinized with xylene and rehydrated through graded ethanol. Endogenous peroxidase activity was blocked with 3 % hydrogen peroxide in methanol for 10 min (except for CD4 lymphocytes). An antigen retrieval technique was applied by boiling the slides for 25 min at 95 °C in 0.01 M sodium citrate buffer, pH = 6.00. For mast cell tryptase (DakoCytomation, Carpinteria, CA; 1:500 dilution), proteinase K, Digest-all-4 enzyme was applied for 5 min at room temperature. Primary antibodies were applied at primary antibody concentration for primary antibody incubation time. The slides were incubated with primary antibodies to CD3 (Biocare Medical, Concord, CA; 1:50 dilution), CD4 (Thermo Scientific, Waltham, MA; 1:10 dilution), and CD8 (DakoCytomation; 1:50 dilution) for T lymphocytes for 45 min. They were subsequently incubated with labeled polymer (secondary antibody) anti-mouse, anti-rabbit for 30 min. The sections were treated with DAB (diaminobenzidine) for 10 min for visualization and then counterstained with hematoxylin. EC and EE cells were identified by staining with antibodies to 5-HT (DakoCytomation; 1:100 dilution) and chromogranin A (CgA; DakoCytomation; 1:1,000 dilution), respectively, similarly as previously described (42).
Scanning and analyses were performed through the Translational Pathology Core Laboratory at UCLA. Hematoxylin and eosin-stained slides were quality assured to be compatible study parameters including normal histology and adequate orientation. Counterstained immunohistochemically stained slides were quality assured for orientation, absence of lymphoid follicles and folding/crush artifacts in areas of analytical interest, in a manner identical to previously published (43). Slides were analyzed using the Ariol SL-50 automated slide scanner (Applied Imaging, San Jose, CA) to quantify the amount of positive staining for each area of interest. A thresholded total area of detected signal for a given immunohistochemical stain was measured vs. slide area. Automated area measurements have been shown to strongly correlate with individual cells counts (43). A 10 % manual count quality assurance was performed to reinforce the linear relationship of cellularity with area detection. Thresholds for each image were applied using the Ariol analytical software based on multiple parameters: red blue green (RGB) algorithm optimized for the detection of DAB chromogen; shape and size restrictions to exclude subcellular and non-cellular structures were included. All analyses were performed with the MultiStain script. Thresholded classifiers were customized for each stain. For assessing cytoplasmic/cell surface staining, the area of positive stain was calculated by applying color thresholds to detect positive brown pixels. Percent of positivity was determined by dividing the total positive stain area (μm2) by the total area analyzed (μm2)×100. The pathologist (G.C.) overseeing the mucosal histology is an experienced GI (gastrointestinal) pathologist with extensive experience in mucosal endocrine and inflammatory cells enumeration (42–44).
Statistical analysis
Comparisons between two groups were analyzed with Mann –Whitney U tests. We used a Bonferroni-adjusted significance level of 0.0083 to account for IBS vs. control comparisons within sex for three types of immunological measures, that is, serum cytokines, colonic cytokines, and neuropeptides/receptors (CRF and NK measures) and cell counts. Spearman ’ s rank correlation test was used where indicated to assess correlations between serum and colonic mucosal cytokine measures. In addition, Spearman’s rank correlation test was used to assess correlations between immune measures and symptoms. Results are presented as mean ± standard error of the mean (s.e.m.). All analyses were performed using the publicly available R statistical software (version 2.11.1) (45).
The study was approved by the UCLA Institutional Review Board and was conducted in accordance with the institutional guidelines regulating human subjects research.
RESULTS
Clinical characteristics
Clinical characteristics of patients and control subjects are shown in Table 1. While the IBS-D and IBS-A/M were evenly distributed among the men, the predominant bowel habit subtype in the women was IBS-C. None of the IBS patients had a history of PI-IBS, which has been defined as the new onset of IBS symptoms immediately following an acute illness characterized by vomiting, diarrhea, or a positive bacterial stool culture (46). Compared with controls, IBS patients within the male and female groups had higher HAD anxiety and depression scores, even though scores were within the normal range. HAD anxiety (P = 0.78) and depression scores (P = 0.55) did not differ significantly between IBS males and females. Menstrual cycle phase and menopausal status were not significantly different between female IBS patients and controls.
Table 1.
Clinical characteristics of study subjects
| Male IBS (n = 19) | Male controls (n = 19) | P value | Female IBS (n = 26) | Female controls (n = 22) | P value | |
|---|---|---|---|---|---|---|
| Age (years ± s.e.m.) | 43.3 ± 2.4 | 42.7 ± 2.5 | 0.86 | 37.7 ± 2.0 | 33.0 ± 1.9 | 0.098 |
| Bowel habits, n (%) | ||||||
| IBS-C | 1 (5%) | N/A | — | 15 (58%) | N/A | — |
| IBS-D | 10 (53%) | N/A | — | 5 (19%) | N/A | — |
| IBS-A/M | 8 (42%) | N/A | — | 6 (23%) | N/A | — |
| Current IBS symptom intensity ratings (0–20) | 9.6 ± 1.3 | N/A | — | 10.3 ± 1.0 | N/A | — |
| HAD scores (0–21) | ||||||
| Anxiety | 7.6 ± 1.3 | 4.4 ± 0.6 | 0.04 | 7.7 ± 0.9 | 3.3 ± 0.7 | < 0.001 |
| Depression | 5.7 ± 1.2 | 1.5 ± 0.5 | 0.005 | 4.4 ± 0.7 | 1.2 ± 0.3 | < 0.001 |
| Current axis I disorder | ||||||
| MDD | 3 (16%) | 0 | 0.09 | 1 (4%) | 0 | 1.0 |
| DD | 4 (21%) | 0 | 0.04 | 0 | 0 | — |
| Menstrual phase (%) | 0.12 | |||||
| Follicular | N/A | N/A | 7 (27%) | 8 (36%) | ||
| Luteal | N/A | N/A | 6 (23%) | 10 (45%) | ||
| Menses | N/A | N/A | 4 (15%) | 3 (14%) | ||
| Undetermined* | N/A | N/A | 3 (12%) | 0 | ||
| Postmenopausal | N/A | N/A | 6 (23%) | 1 (5%) | ||
DD, dysthymic disorder; HAD, Hospital Anxiety and Depression Scale; IBS-C, irritable bowel syndrome with constipation; IBS-D, irritable bowel syndrome with diarrhea; IBS-M, irritable bowel syndrome with mixed pattern; MDD, major depressive disorder; N/A, not applicable.
Premenopausal women with undetermined menstrual cycle phase: 1 was taking Depo-Provera, 1 had the endometrium removed, and 1 had missing data.
Serum and sigmoid colonic mucosal cytokines
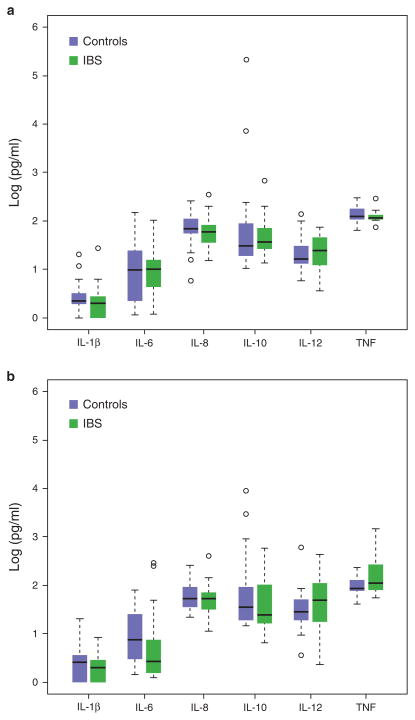
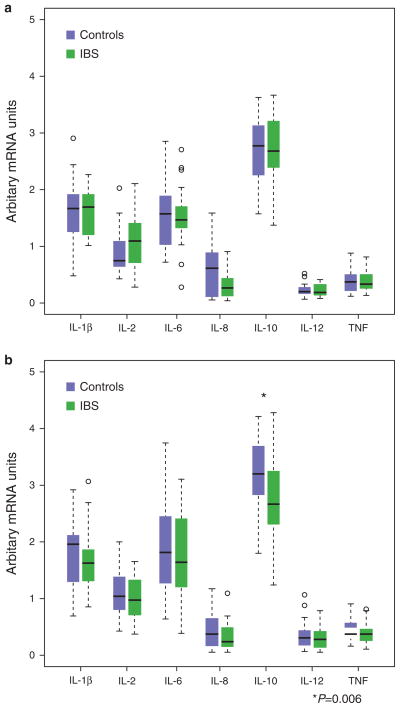
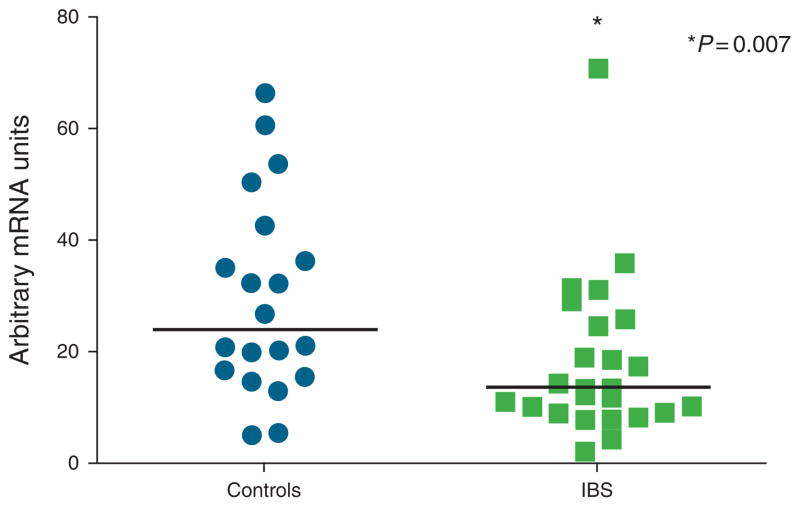
Of the 86 study participants, blood samples for serum cytokine levels could only be collected in 73 participants (17 healthy males, 17 IBS males, 17 healthy females, and 22 IBS females). There were no significant differences in the serum levels of IL-1β, IL-6, IL-8, IL-10, IL-12, and TNF-α between IBS and controls within males and females (Figure 1a, b). With regard to sigmoid colonic mucosal cytokine mRNA expression, IL-1β, IL-6, IL-8, IL-12, and TNF-α mRNA levels (Figure 2a, b) were similar between IBS patients and controls within males and females, and when these groups were combined (P values ranged from 0.052 to 1.0). However, female patients had significantly lower expression of the anti-inflammatory cytokine IL-10 than female controls (18.0 ± 2.9 vs. 29.5 ± 4.0, P = 0.007; Figure 3). This difference remained significant after controlling for menstrual cycle phase. There was a trend for lower IL-10 mRNA expression in IBS vs. controls overall (17.5 ± 2.0 vs. 22.9 ± 2.5, P = 0.073) but no differences within bowel habit subgroups. Colonic IL-10 mRNA levels were not different between the male IBS and control subjects. None of the serum cytokine levels significantly correlated with their respective colonic mucosal mRNA levels.
Figure 1.
Serum cytokine levels in irritable bowel syndrome (IBS) and healthy controls are shown. Modified boxplots of serum cytokine levels (pg/ml) in male and female IBS patients and healthy controls within males (a) and females (b) are shown. The horizontal line indicates the median value and the lower and upper brackets indicate the 25th percentile—1.5 interquartile range (IQR) and 75th percentile + IQR, respectively. The open circles represent values outside of the bracket range and are potential outliers. There were no significant differences between IBS patients and controls. TNF, tumor necrosis factor.
Figure 2.
Colonic mucosal cytokine levels in irritable bowel syndrome (IBS) and healthy controls are shown. Modified boxplots of colonic mucosal cytokine mRNA expression in IBS patients and healthy controls within males (a) and females (b) are shown. The horizontal line indicates the median value and the lower and upper brackets indicate the 25th percentile—1.5 inter-quartile range (IQR) and 75th percentile + IQR, respectively. The open circles represent values outside of the bracket range and are potential outliers. The only significant difference was lower levels of interleukin-10 (IL-10) mRNA in IBS females compared with healthy females (P = 0.006). For IL-12, 92% of samples were available. TNF, tumor necrosis factor.
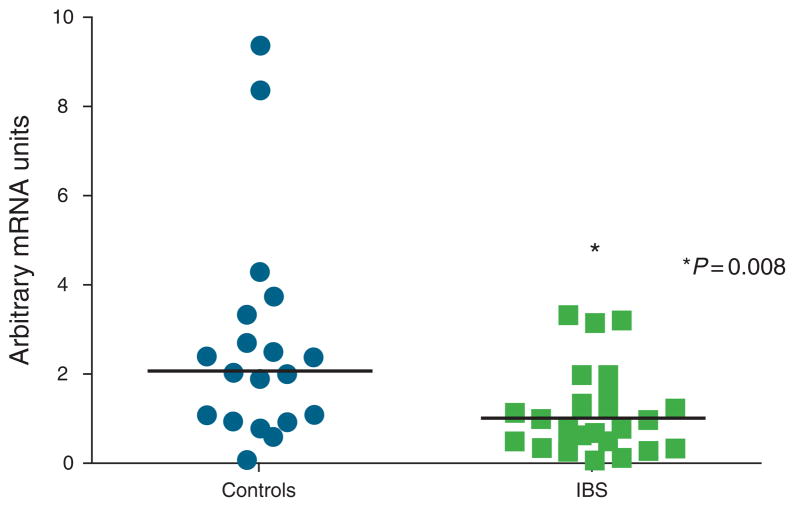
Figure 3.
Individual data for colonic mucosal interleukin-10 (IL-10) mRNA levels in both female controls and irritable bowel syndrome (IBS) patients are shown. IL-10 mRNA levels were significantly lower in IBS patients vs. controls (P = 0.006).
Lower serum IL-10 levels correlated with higher ratings of symptom intensity in the overall group of IBS patients (r = −0.45, P = 0.006). No other immune measures correlated with IBS or psychological symptoms with the exception of a negative correlation between serum IL-12 and HAD depression symptom ratings (r = −0.42, P = 0.007). There were no significant correlations between the immune markers with disease-specific IBS quality of life domains.
Mucosal cell counts
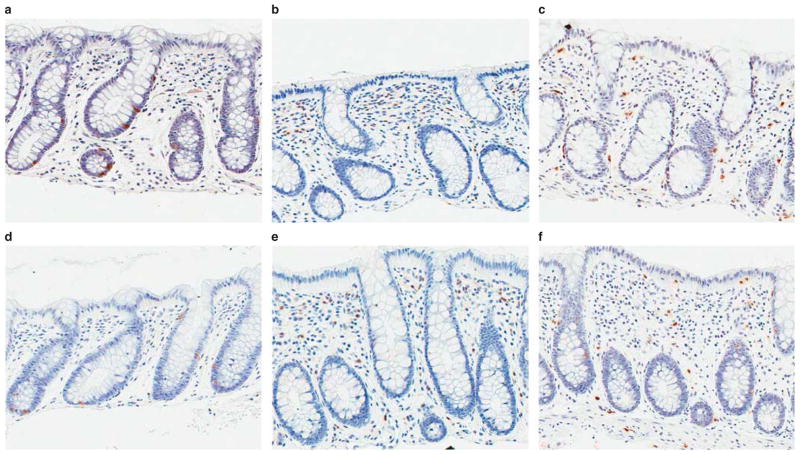
There were no significant disease (IBS vs. controls) or bowel habit subgroup differences for CD3, CD4, and CD8 lymphocyte, or EE, EC, and mast cell counts (P = 0.059–0.892) (Table 2). Representative images of female IBS-D and control colonic mucosa by CD4, mast cell, and EC cells immunohistochemistry are shown in Figure 4.
Table 2.
Cell counts and CRF and NK neuropeptide mRNA expression in sigmoid colonic mucosal tissue in IBS and healthy controls
| Males
|
Females
|
|||||||
|---|---|---|---|---|---|---|---|---|
| N, IBS/controls | IBS (N =19) | Controls (N =19) | P value | N, IBS/controls | IBS (N =26) | Controls (N =22) | P value | |
| Cell count (%, ±s.e.m.)a | ||||||||
|
| ||||||||
| EC cells | 19/19 | 0.072 ± 0.11 | 0.08 ± 0.02 | 0.999 | 26/22 | 0.16 ± 0.05 | 0.09 ± 0.02 | 0.092 |
|
| ||||||||
| EE cells | 19/18 | 0.52 ± 0.12 | 0.45 ± 0.11 | 0.443 | 26/22 | 0.44 ± 0.13 | 0.51 ± 0.09 | 0.114 |
|
| ||||||||
| Mast cells | 19/19 | 0.46 ± 0.14 | 0.33 ± 0.06 | 0.644 | 26/22 | 0.44 ± 0.08 | 0.29 ± 0.03 | 0.211 |
|
| ||||||||
| CD3 lymphocytes | 19/19 | 1.87 ± 0.22 | 1.54 ± 0.27 | 0.172 | 26/22 | 2.83 ± 0.30 | 2.71 ± 0.41 | 0.674 |
|
| ||||||||
| CD4 lymphocytes | 19/19 | 0.33 ± 0.12 | 0.21 ± 0.05 | 0.665 | 26/22 | 0.46 ± 0.10 | 0.35 ± 0.08 | 0.328 |
|
| ||||||||
| CD8 lymphocytes | 19/19 | 0.16 ± 0.031 | 0.16 ± 0.02 | 0.709 | 26/22 | 0.28 ± 0.11 | 0.24 ± 0.08 | 0.814 |
|
| ||||||||
| CRF and NK-related ligands and receptors (% subjects with samples)b | ||||||||
|
| ||||||||
| CRF ligand | 16/19 | 2.76 ± 0.18 | 3.22 ± 0.17 | 0.057 | 25/19 | 2.60 ± 0.15 | 2.92 ± 0.19 | 0.244 |
|
| ||||||||
| CRF2R | 16/19 | 3.17 ± 0.09 | 3.14 ± 0.11 | 0.612 | 25/20 | 2.99 ± 0.12 | 2.89 ± 0.14 | 0.534 |
|
| ||||||||
| Ucn 1 | 16/19 | 3.69 ± 0.06 | 3.72 ± 0.04 | 0.935 | 25/19 | 3.66 ± 0.14 | 3.56 ± 0.18 | 0.462 |
|
| ||||||||
| Ucn 2 | 16/19 | 3.38 ± 0.13 | 3.42 ± 0.07 | 0.883 | 25/19 | 3.29 ± 0.12 | 3.56 ± 0.06 | 0.084 |
|
| ||||||||
| TAC1 | 17/17 | 1.89 ± 1.00 | 0.67 ± 0.16 | 0.540 | 24/19 | 0.64 ± 0.12 | 0.59 ± 0.13 | 0.569 |
|
| ||||||||
| NK-1R | 17/16 | 0.72 ± 0.10 | 0.70 ± 0.08 | 0.709 | 25/19 | 1.15 ± 0.19 | 2.66 ± 0.56 | 0.008c |
|
| ||||||||
| NK-2R | 17/17 | 2.60 ± 0.46 | 2.56 ± 0.83 | 0.496 | 25/20 | 2.39 ± 0.33 | 4.83 ± 0.89 | 0.032 |
CRF, corticotropin releasing factor; EC cells, serotonin containing enterochromaffin cells; EE cells, chromogranin staining enteroendocrine cells; IBS, irritable bowel syndrome; NK-1R, neurokinin 1 receptor; NK-2R, neurokinin 2 receptor; TAC1, tachykinin, precursor 1; Ucn, urocortin.
Cell count measurements: % area of cells stained/total area analyzed.
Values are expressed as arbitrary mRNA units ± s.e.m.
Significant values as determined by Mann–Whitney rank sum analysis with a level of significance set at P < 0.0083.
Figure 4.
Representative immunohistochemistry images of 20× scanned slides. (Top row) (a–c) Colonic mucosa from a healthy control subject stained for serotonin (a), CD4 (b), and mast cell tryptase (c). (Bottom row) (d–f) Colonic mucosa from a patient with IBS-D stained for serotonin (d), CD4 (e), and mast cell tryptase (f). There are no qualitative differences based on immunohistochemically detected enterochromaffin, CD4T, or mast cells between healthy controls and IBS patients.
Sigmoid colonic mucosal mRNA expression of CRF- and NK-related neuropeptides
In some subjects, CRF and NK neuropeptides were non-detectable. In all, 86 % of subjects had undetectable expression levels for CRF1R, 18% for CRF ligand, 1–6% for Ucn 1–2 and NK neuropeptides. Because of the low detection rate for CRF1R, we tried an additional marker set and obtained similar results. As a result, CRF1R was not analyzed due to few samples with detectable levels. NK-1R was expressed in all subjects and mRNA levels were significantly lower in female patients compared with female controls (Mann–Whitney rank sum test P value=0.008) (Table 2; Figure 5). This difference remained significant after controlling for menstrual cycle phase. There was a trend for lower mRNA expression in IBS vs. controls overall (0.97±0.1 vs. 1.8 ±0.3, P=0.054).
Figure 5.
Individual data for colonic mucosal neurokinin (NK)-1R mRNA levels in both female controls and irritable bowel syndrome (IBS) patients are shown. NK-1R expression was significantly lower in IBS patients vs. controls (P = 0.008).
DISCUSSION
In this comprehensive comparison of immune markers from blood and sigmoid colonic tissue samples in well phenotyped male and female IBS patients and healthy controls, the main findings of the study were (i) female patients had significantly lower colonic mucosal IL-10 mRNA levels compared with healthy female controls, but levels did not correlate with IBS symptoms; (ii) lower serum IL-10 levels correlated with higher ratings of symptom intensity in IBS patients; (iii) no group differences in serum cytokine levels were detected; (iv) colonic mucosal NK-1R mRNA expression was significantly lower in female patients compared with healthy females; and (v) no significant differences in the other immune or neuro-endocrine markers were found between patients and controls.
Mucosal or systemic expression of cytokines and mucosal lymphocytes and mast cells in IBS and controls
We did not find increased colonic mucosal expression of pro-inflammatory cytokines but did find lower colonic mucosal mRNA expression of the anti-inflammatory cytokine IL-10 in IBS females compared with healthy females. In addition, serum IL-10 levels negatively correlated with IBS symptom ratings. The anti-inflammatory mediator IL-10 inhibits cytokine synthesis (47) and therefore, decreased IL-10 levels could predispose to increased mucosal cytokine production during infections or other mucosal insults. Our finding is supported by similar reports in the only two previous studies that compared cytokine levels in the sigmoid colonic mucosa in IBS and healthy controls (23,24). Both studies only assessed females and found lower IL-10 mRNA expression in IBS patients than controls and failed to detect increases in pro-inflammatory cytokines despite enrolling IBS females with different bowel habit subtypes (23,24). In one of these studies, cytokine protein analysis in colonic tissue confirmed that pro-inflammatory cytokine levels were similar in IBS and healthy controls, and lower compared to patients with IBD (23). A possible role of reduced IL-10 expression in IBS pathophysiology is also supported by reported findings in 111 IBS patients and 162 healthy controls in which the low producer IL-10 genotype was significantly more prevalent in IBS patients compared with healthy controls (48). In addition, a recent study found that baseline and stimulated levels of IL-10 in the blood were significantly lower in children with IBS vs. healthy children (49). Future studies will need to determine the mechanism involved in the reduction in IL-10 expression, and why this alteration is more prevalent in female patients.
The finding of similar levels of serum cytokines in IBS and controls in our study differs from other reports where elevated levels of pro-inflammatory cytokines in blood or secreted from stimulated peripheral blood mononuclear cells have been reported (12,20,21,50,51). Kindt et al. (52) reported that patients with IBS or other functional GI disorders had a shift toward a Th2 plasma cytokine profile. We measured levels of the Th2 cytokines, IL-6 and IL-10, and neither was altered in the blood but IL-10 mRNA expression was lower in the colon. We did not fully address the Th2 cytokine group in this study, but reduced expression of IL-10 suggests that a shift toward a Th2 pattern may be occurring in the colonic mucosa in IBS. It is also possible that IBS patients have a more “hyperresponsive” immune system to stimuli (e.g., stress, antigens) than healthy controls. Factors that influence the reactivity of the immune system may differ between studies and contribute to the heterogeneity of subject data. Previously reported studies have not measured cytokine levels from the blood and colonic tissue in the same subjects. The lack of correlation of cytokine levels in the blood and colonic tissue suggest that the cytokine levels in the blood in IBS and controls may not be primarily derived from the gut. Increased levels of systemic pro-inflammatory cytokines have been reported in association with psychological and physical stressors, including depression and strenuous exercise (53,54). Cytokines measured in the blood can be derived from multiple sources, including the spleen, liver, heart, gut, and fat tissue and from skeletal muscle (55–57). Acting alone or in conjunction with components of the stress system, cytokines can induce fever, sleepiness, fatigue, loss of appetite, and decreased libido (“sickness behavior ”) (53). Thus, it is not surprising that increased blood levels of the pro-inflammatory cytokines, IL-1β, IL-6, and IL-8, but not IL-10 were found in female IBS patients with extraintestinal comorbidities (fibromyalgia, chronic fatigue syndrome, and premenstrual syndrome) compared with controls (58). In our study, only three of the IBS females had a history of a coexistent functional pain syndrome (fibromyalgia, interstitial cystitis, and myofascial pain condition).
With the exception of lower colonic IL-10 mRNA levels in females with IBS, the lack of differences in cytokine levels between patients and controls is supported by the finding of similar colonic mucosal cellularity, even when comparing within each sex. These results are in apparent contrast to previous studies, which have demonstrated higher numbers of colonic mucosal lymphocytes (3,11,13) and mast cells (2,11,13,15,16) in IBS. However, with respect to mast cell numbers, our findings are congruent with studies by Chadwick et al. (3), Klooker et al. (18), and Cenac et al. (19), which did not detect differences between IBS patients and controls. There may be several reasons to explain these differences, including subject heterogeneity, the location of the tissue collected, and the methodology of determining cell counts. Alternatively, there could be only a small difference in mast cell counts between IBS patients and controls, requiring very large studies or meta-analyses to consistently detect the difference. It is important to note that Klooker et al. (18) found that the mast cell stabilizer antagonist ketotifen reduced IBS symptoms. While this suggests that ketotifen could be exerting its beneficial effect on symptoms by decreasing mast cell activity rather than number, non-specific sedative effects of the drug may also be involved. Studies indicate that increased levels of tryptase and histamine, which are inflammatory mediators released during mast cell degranulation, in colonic mucosal tissue collected from IBS patients activate sensory afferent nerves (6,19) and increase colonic paracellular permeability (59) to a greater degree than that from controls.
Expression of neuropeptide and neuroendocrine markers (CRF, NK, and 5-HT signaling systems) that are associated with mucosal inflammation
This is the first study that has comprehensively measured colonic mucosal mRNA expression of CRF and NK receptors and ligands in IBS patients. NK-1R mRNA levels were significantly lower in female patients compared with controls. Interestingly, chronic water avoidance stress in rats, which induces sustained visceral hyperalgesia, was associated with decreased expression of NK-1R mRNA and protein in the distal colon (60). It is not clear why there is reduced expression of NK-1R in the colon, but it may be related to chronic engagement of stress systems. Cottrell et al. (61) demonstrated that chronic stimulation with substance P induces ubiquitination of the NK-1R, which mediates its degradation and downregulation.
Both central and peripheral NK and CRF signaling system have been characterized, with the central systems being engaged by psychosocial stressors (62), and the peripheral systems being engaged in response to tissue irritation and mucosal inflammation (25,26). In our study, CRF1R mRNA expression was below detection limits in most subjects and therefore could not be adequately compared between IBS and controls. If mucosal inflammation were present in IBS, higher expression of these neuropeptides compared with controls would be expected. Activation of CRF and NK signaling systems in the gut has been associated with activation of a pro-inflammatory response, both in animal models of colitis and in patients with IBD (25,26). CRF and CRF receptor expression is increased at the gene and protein levels in the intestine of animal models of intestinal inflammation (63,64). Furthermore, both CRF-deficient and CRF2R-deficient mice showed reduced intestinal inflammatory responses. Several studies have demonstrated increased CRF, Ucn 1, Ucn 2, and CRF2R expression in the colonic mucosa of IBD patients (28–30), while studies in experimental models of intestinal inflammation show that blockade or genetic deficiency of these receptors are associated with reduced intestinal inflammatory responses (65–68). In the colonic mucosa of IBD patients, increased numbers of CRF-immunoreactive macrophages and EC cells and increased Ucn expression in lamina propria mononuclear cells were demonstrated (26). Similarly, substance P, NK-1R, and NK-2R expression were increased in colonic tissue of patients with IBD in most, but not all, studies (26,31).
In the current study, no differences in EC or EE cell counts were found between patients and controls. While EC and EE cell counts were found to be increased in PI-IBS (8,14,69), EC cells were not increased in unselected IBS patients (14,32,33). Our results are similar to the latter studies, which would be expected since our sample did not contain any PI-IBS patients. These similar findings occurred despite mucosal sampling from the rectum in two of these studies (14,32), small intestine in the other (33), and from the sigmoid colon in our study. Although EC cell hyperplasia has been demonstrated in both animal and human enteric infection studies, it is not a universal response to infection (1).
Taken together, the lack of increased immune cellularity, pro-inflammatory cytokines and mRNA expression in the sigmoid colonic mucosa of CRF- and NK-related neuropeptides in our IBS sample fails to support a significant pro-inflammatory state in non-PI-IBS. However, the possibility that there is an enhanced innate immunity to pathogens as suggested by the recent finding of abnormal toll-like receptor expression in the colonic mucosa of IBS (17) is intriguing and requires further study.
Sex differences
The lack of findings in IBS males further supports the role of sex differences in IBS (70). In previous studies, the gender distribution differed between control and IBS groups with most of the IBS patients being female. Enrolling similar numbers of males and females is important because sex differences in lymphocytes and mast cells exist in IBS (11). It is possible that these sex differences contribute to the fact that female sex is an independent risk factor of developing PI-IBS (10). These findings can in part be due to estrogen’s influence on pro-inflammatory and anti-inflammatory pathways (71,72).
Our study has limitations. Our colonic mucosal findings are limited to the sigmoid colon and it is possible that there are regional differences, such as more proximal regions of the colon or the small bowel. While cytokine mRNA expression was measured in the colonic mucosa, there was no measurement of colonic cytokine protein levels. Although EC cell counts were performed, we did not measure mucosal 5-HT content or SERT expression. The latter has been shown to be decreased in IBD in the setting of normal (non-severe UC) or decreased (severe UC) EC cell counts (32). In our study, colonic mucosal mast cell and EC cell counts were similar in IBS and controls, but we did not perform functional assays of mediators (e.g., tryptase) released from cultured colonic biopsies on activation of afferent nerves as has been previously described in IBS (5,6). In addition, measurements of the expression of neuropeptides and their respective receptors do not take into account if they are expressed on the cell surface or have been internalized. Finally, the heterogeneity of IBS patients and the disease complexity may result in small effect sizes that require hundreds to thousands of patients to detect reliably. Thus, although our patients have been carefully phenotyped and our study is comparable in size to other reported studies addressing this question, additional much larger studies are needed to confirm our results.
In summary, we have demonstrated lower IL-10 mRNA expression in the colonic mucosa in IBS females compared with healthy females, suggesting a possible pathogenic role of IL-10 in IBS. However, the lack of significant findings in all other immune-related measures in a relatively large number of IBS patients compared with controls questions the hypothesis that colonic mucosal inflammation has a primary role in the pathogenesis of non-PI-IBS. Further studies are needed to confirm these results.
Study Highlights.
WHAT IS CURRENT KNOWLEDGE
Irritable bowel syndrome (IBS) is a multifactorial, heterogenous condition.
Increased colonic mucosal cellularity has been reported in post-infectious IBS (PI-IBS) and with less consistency in non-PI-IBS.
Elevated basal and stimulated cytokine levels in the blood have been reported in some but not all IBS studies.
No study has measured cytokine levels in the blood and colonic mucosa in the same IBS patients.
Sex differences have been reported in IBS, including colonic mucosal mast cell counts.
WHAT IS NEW HERE
Interleukin-10 (IL-10) mRNA expression in the sigmoid colon mucosa is decreased in female irritable bowel syndrome (IBS) patients compared with female controls, but there is no difference in males. This is a potential biomarker for IBS.
Neurokinin (NK)-1 receptor expression in the sigmoid colon mucosa is decreased in female IBS patients compared with female controls, but there is no difference in males.
Corticotropin releasing factor 1 receptor (CRF1R) mRNA is expressed at levels below usual detection limits in most non-post-infectious IBS (npn-PI-IBS) patients and healthy controls.
Cytokine levels in the blood do not correlate to their respective expression levels in the sigmoid colon mucosa in IBS.
Levels of cytokines and CRF- and NK-related neuropeptides and cell counts are not significantly elevated in non-PI-IBS and thus, do not support that colonic mucosal inflammation consistently has a primary role in these patients.
Acknowledgments
Financial support: This study was supported by NIH Grants P50 DK64539, P01 DK33506, DK047343, and Prometheus Laboratories. Prometheus Laboratories’ support was limited to measurement of serum cytokines. The remainder of the work was independent of sponsor support.
Footnotes
CONFLICT OF INTEREST
Guarantor of the article: Lin Chang, MD.
Potential competing interests: Hua Gong and Sharat Singh are employees of Prometheus Laboratories.
Specific author contributions: Principal Investigator, funding, concept and design, performed medical history and physical examinations, performed sigmoidoscopy with biopsies, analysis and interpretation of data, and manuscript preparation: Lin Chang; data analysis and manuscript preparation: Mopelola Adeyemo; colonic mucosal cytokine, CRF, and tachykinin expression measurements: Iordanis Karagiannidis; supervision of colonic mucosal experiments, concept and design, and manuscript review: Charalabos Pothoulakis; data analysis and manuscript review: Elizabeth J. Videlock; colonic mucosal cytokine expression measurements: Collin Bowe; colonic mucosal CRF1R expression measurements: Pu-Qing Yuan; statistical analysis, manuscript preparation, and review: Wendy Shih and Angela P. Presson; study coordinator and data acquisition: Arlene Licudine; supervised cell count measurements in colonic tissue: Galen Cortina; serum cytokine measurements: Hua Gong and Sharat Singh; psychological screening and interview, SCID: Minou Mayer; supervision of CRF1R expression experiments and manuscript review: Yvette Tache; concept and design and manuscript review: Emeran A. Mayer.
References
- 1.Spiller R, Garsed K. Postinfectious irritable bowel syndrome. Gastroenterology. 2009;136:1979–88. doi: 10.1053/j.gastro.2009.02.074. [DOI] [PubMed] [Google Scholar]
- 2.Barbara G, Stanghellini V, De Giorgio R, et al. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology. 2004;126:693–702. doi: 10.1053/j.gastro.2003.11.055. [DOI] [PubMed] [Google Scholar]
- 3.Chadwick VS, Chen W, Shu D, et al. Activation of the mucosal immune system in irritable bowel syndrome. Gastroenterology. 2002;122:1778–83. doi: 10.1053/gast.2002.33579. [DOI] [PubMed] [Google Scholar]
- 4.Goral V, Kucukoner M, Buyukbayram H. Mast cells count and serum cytokine levels in patients with irritable bowel syndrome. Hepato-Gastroenterology. 2010;57:751–4. [PubMed] [Google Scholar]
- 5.Barbara G, Wang B, Stanghellini V, et al. Mast cell-dependent excitation of visceral-nociceptive sensory neurons in irritable bowel syndrome. Gastroenterology. 2007;132:26–37. doi: 10.1053/j.gastro.2006.11.039. [DOI] [PubMed] [Google Scholar]
- 6.Buhner S, Li Q, Vignali S, et al. Activation of human enteric neurons by supernatants of colonic biopsy specimens from patients with irritable bowel syndrome. Gastroenterology. 2009;137:1425–34. doi: 10.1053/j.gastro.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 7.Khan WI, Collins SM. Gut motor function: immunological control in enteric infection and inflammation. Clin Exp Immunol. 2006;143:389–97. doi: 10.1111/j.1365-2249.2005.02979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dunlop SP, Jenkins D, Neal KR, et al. Relative importance of enterochromaffin cell hyperplasia, anxiety, and depression in postinfectious IBS. Gastroenterology. 2003;125:1651–9. doi: 10.1053/j.gastro.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 9.Gwee KA, Collins SM, Read NW, et al. Increased rectal mucosal expression of interleukin 1B in recently acquired post-infectious irritable bowel syndrome. Gut. 2003;52:523–6. doi: 10.1136/gut.52.4.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marshall JK, Thabane M, Garg AX, et al. Incidence and epidemiology of irritable bowel syndrome after a large waterborne outbreak of bacterial dysentery. Gastroenterology. 2006;131:445–50. doi: 10.1053/j.gastro.2006.05.053. quiz 660. [DOI] [PubMed] [Google Scholar]
- 11.Cremon C, Gargano L, Morselli-Labate AM, et al. Mucosal immune activation in irritable bowel syndrome: gender-dependence and association with digestive symptoms. Am J Gastroenterol. 2009;104:392–400. doi: 10.1038/ajg.2008.94. [DOI] [PubMed] [Google Scholar]
- 12.Ohman L, Isaksson S, Lindmark AC, et al. T-cell activation in patients with irritable bowel syndrome. Am J Gastroenterol. 2009;104:1205–12. doi: 10.1038/ajg.2009.116. [DOI] [PubMed] [Google Scholar]
- 13.Akbar A, Yiangou Y, Facer P, et al. Increased capsaicin receptor TRPV1-expressing sensory fibres in irritable bowel syndrome and their correlation with abdominal pain. Gut. 2008;57:923–9. doi: 10.1136/gut.2007.138982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee KJ, Kim YB, Kim JH, et al. The alteration of enterochromaffin cell, mast cell, and lamina propria T lymphocyte numbers in irritable bowel syndrome and its relationship with psychological factors. J Gastroenterol Hepatol. 2008;23:1689–94. doi: 10.1111/j.1440-1746.2008.05574.x. [DOI] [PubMed] [Google Scholar]
- 15.Coeffer M, Gloro R, Boukhettala N, et al. Increased proteasome-mediated degradation of occludin in irritable bowel syndrome. Am J Gastroenterol. 2010;105:1181–8. doi: 10.1038/ajg.2009.700. [DOI] [PubMed] [Google Scholar]
- 16.Cremon C, Carini G, Wang B, et al. Intestinal serotonin release, sensory neuron activation, and abdominal pain in irritable bowel syndrome. Am J Gastroenterol. 2011;106:1290–8. doi: 10.1038/ajg.2011.86. [DOI] [PubMed] [Google Scholar]
- 17.Brint EK, MacSharry J, Fanning A, et al. Differential expression of toll-like receptors in patients with irritable bowel syndrome. Am J Gastroenterol. 2011;106:329–36. doi: 10.1038/ajg.2010.438. [DOI] [PubMed] [Google Scholar]
- 18.Klooker TK, Braak B, Koopman KE, et al. The mast cell stabiliser ketotifen decreases visceral hypersensitivity and improves intestinal symptoms in patients with irritable bowel syndrome. Gut. 2010;59:1213–21. doi: 10.1136/gut.2010.213108. [DOI] [PubMed] [Google Scholar]
- 19.Cenac N, Andrews CN, Holzhausen M, et al. Role for protease activity in visceral pain in irritable bowel syndrome. J Clin Invest. 2007;117:636–47. doi: 10.1172/JCI29255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liebregts T, Adam B, Bredack C, et al. Immune activation in patients with irritable bowel syndrome. Gastroenterology. 2007;132:913–20. doi: 10.1053/j.gastro.2007.01.046. [DOI] [PubMed] [Google Scholar]
- 21.Dinan TG, Quigley EM, Ahmed SM, et al. Hypothalmic-pituitary-gut axis dysregulation in irritable bowel syndrome: plasma cytokines as a potential marker? Gastroenterology. 2006;130:304–11. doi: 10.1053/j.gastro.2005.11.033. [DOI] [PubMed] [Google Scholar]
- 22.McKernan DP, Gaszner G, Quigley EM, et al. Altered peripheral toll-like receptor responses in the irritable bowel syndrome. Aliment Pharmacol Ther. 2011;33:1045–52. doi: 10.1111/j.1365-2036.2011.04624.x. [DOI] [PubMed] [Google Scholar]
- 23.Macsharry J, O’Mahony L, Fanning A, et al. Mucosal cytokine imbalance in irritable bowel syndrome. Scand J Gastroenterol. 2008;43:1467–76. doi: 10.1080/00365520802276127. [DOI] [PubMed] [Google Scholar]
- 24.Chang L, Sundaresh S, Elliott J, et al. Dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis in irritable bowel syndrome. Neurogastroenterol Motil. 2009;21:149–59. doi: 10.1111/j.1365-2982.2008.01171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kiank C, Tache Y, Larauche M. Stress-related modulation of inflammation in experimental models of bowel disease and post-infectious irritable bowel syndrome: role of corticotropin-releasing factor receptors. Brain Behav Immun. 2010;24:41–8. doi: 10.1016/j.bbi.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gross KJ, Pothoulakis C. Role of neuropeptides in inflammatory bowel disease. Inflamm Bowel Dis. 2007;13:918–32. doi: 10.1002/ibd.20129. [DOI] [PubMed] [Google Scholar]
- 27.Bischoff SC, Mailer R, Pabst O, et al. Role of serotonin in intestinal inflammation: knockout of serotonin reuptake transporter exacerbates 2,4,6-trinitrobenzene sulfonic acid colitis in mice. Am J Physiol. 2009;296:G685–95. doi: 10.1152/ajpgi.90685.2008. [DOI] [PubMed] [Google Scholar]
- 28.Moss AC, Anton P, Savidge T, et al. Urocortin II mediates pro-inflammatory effects in human colonocytes via corticotropin-releasing hormone receptor 2alpha. Gut. 2007;56:1210–7. doi: 10.1136/gut.2006.110668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kawahito Y, Sano H, Mukai S, et al. Corticotropin releasing hormone in colonic mucosa in patients with ulcerative colitis. Gut. 1995;37:544–51. doi: 10.1136/gut.37.4.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saruta M, Takahashi K, Suzuki T, et al. Urocortin 1 in colonic mucosa in patients with ulcerative colitis. J Clin Endocrinol Metab. 2004;89:5352–61. doi: 10.1210/jc.2004-0195. [DOI] [PubMed] [Google Scholar]
- 31.Renzi D, Pellegrini B, Tonelli F, et al. Substance P (neurokinin-1) and neurokinin-A (neurokinin-2) receptor gene and protein expression in the healthy and inflamed human intestine. Am J Pathol. 2000;157:1511–22. doi: 10.1016/S0002-9440(10)64789-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coates MD, Mahoney CR, Linden DR, et al. Molecular defects in mucosal serotonin content and decreased serotonin reuptake transporter in ulcerative colitis and irritable bowel syndrome. Gastroenterology. 2004;126:1657–64. doi: 10.1053/j.gastro.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 33.Wang SH, Dong L, Luo JY, et al. Decreased expression of serotonin in the jejunum and increased numbers of mast cells in the terminal ileum in patients with irritable bowel syndrome. World J Gastroenterol. 2007;13:6041–7. doi: 10.3748/wjg.v13.45.6041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Agarwal S, Busse PJ. Innate and adaptive immunosenescence. Ann Allergy Asthma Immunol. 2010;104:183–90. doi: 10.1016/j.anai.2009.11.009. quiz 190–192. [DOI] [PubMed] [Google Scholar]
- 35.Thompson WG, Longstreth GF, Drossman DA, et al. Functional bowel disorders and functional abdominal pain. Gut. 1999;45:II43–7. doi: 10.1136/gut.45.2008.ii43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Munakata J, Naliboff B, Harraf F, et al. Repetitive sigmoid stimulation induces rectal hyperalgesia in patients with irritable bowel syndrome. Gastroenterology. 1997;112:55–63. doi: 10.1016/s0016-5085(97)70219-1. [DOI] [PubMed] [Google Scholar]
- 37.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 38.Longstreth GF, Bolus R, Naliboff B, et al. Impact of irritable bowel syndrome on patients’ lives: development and psychometric documentation of a disease-specific measure for use in clinical trials. Eur J Gastroenterol Hepatol. 2005;17:411–20. doi: 10.1097/00042737-200504000-00004. [DOI] [PubMed] [Google Scholar]
- 39.Spitzer RL, Williams JBW, Gibbon M, et al. Structured clinical interview for DSM-III-R. American Psychiatric Press; Arlington, VA: 1990. [Google Scholar]
- 40.Im E, Choi YJ, Pothoulakis C, et al. Bacillus polyfermenticus ameliorates colonic inflammation by promoting cytoprotective effects in colitic mice. J Nutr. 2009;139:1848–54. doi: 10.3945/jn.109.108613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Karagiannides I, Kokkotou E, Tansky M, et al. Induction of colitis causes inflammatory responses in fat depots: evidence for substance P pathways in human mesenteric preadipocytes. Proc Natl Acad Sci USA. 2006;103:5207–12. doi: 10.1073/pnas.0600821103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cortina G, Smart CN, Farmer DG, et al. Enteroendocrine cell dysgenesis and malabsorption, a histopathologic and immunohistochemical characterization. Hum Pathol. 2007;38:570–80. doi: 10.1016/j.humpath.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 43.Ohsie S, Gerney G, Gui D, et al. A paucity of colonic enteroendocrine and/or enterochromaffin cells characterizes a subset of patients with chronic unexplained diarrhea/malabsorption. Hum Pathol. 2009;40:1006–14. doi: 10.1016/j.humpath.2008.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McGowan I, Elliott J, Cortina G, et al. Characterization of baseline intestinal mucosal indices of injury and inflammation in men for use in rectal microbicide trials (HIV Prevention Trials Network-056) J Acquir Immune Defic Syndr. 2007;46:417–25. doi: 10.1097/QAI.0b013e318156ef16. [DOI] [PubMed] [Google Scholar]
- 45.R Development Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2011. http://www.R-project.org/ [Google Scholar]
- 46.Dunlop SP, Jenkins D, Spiller RC. Distinctive clinical, psychological, and histological features of postinfective irritable bowel syndrome. Am J Gastroenterol. 2003;98:1578–83. doi: 10.1111/j.1572-0241.2003.07542.x. [DOI] [PubMed] [Google Scholar]
- 47.Macdonald TT, Monteleone G. Immunity, inflammation, and allergy in the gut. Science. 2005;307:1920–5. doi: 10.1126/science.1106442. [DOI] [PubMed] [Google Scholar]
- 48.van der Veek PP, van den Berg M, de Kroon YE, et al. Role of tumor necrosis factor-alpha and interleukin-10 gene polymorphisms in irritable bowel syndrome. Am J Gastroenterol. 2005;100:2510–6. doi: 10.1111/j.1572-0241.2005.00257.x. [DOI] [PubMed] [Google Scholar]
- 49.Hua MC, Lai MW, Kuo ML, et al. Decreased interleukin-10 secretion by peripheral blood mononuclear cells in children with irritable bowel syndrome. J Pediatr Gastroenterol Nutr. 2011;52:376–81. doi: 10.1097/MPG.0b013e3181fd9816. [DOI] [PubMed] [Google Scholar]
- 50.Dinan TG, Clarke G, Quigley EM, et al. Enhanced cholinergic-mediated increase in the pro-inflammatory cytokine IL-6 in irritable bowel syndrome: role of muscarinic receptors. Am J Gastroenterol. 2008;103:2570–6. doi: 10.1111/j.1572-0241.2008.01871.x. [DOI] [PubMed] [Google Scholar]
- 51.O’Mahony L, McCarthy J, Kelly P, et al. Lactobacillus and bifidobacterium in irritable bowel syndrome: symptom responses and relationship to cytokine profiles. Gastroenterology. 2005;128:541–51. doi: 10.1053/j.gastro.2004.11.050. [DOI] [PubMed] [Google Scholar]
- 52.Kindt S, Van Oudenhove L, Broekaert D, et al. Immune dysfunction in patients with functional gastrointestinal disorders. Neurogastroenterol Motil. 2009;21:389–98. doi: 10.1111/j.1365-2982.2008.01220.x. [DOI] [PubMed] [Google Scholar]
- 53.Elenkov IJ, Chrousos GP. Stress, cytokine patterns and susceptibility to disease. Baillieres Best Practice and Research. Clin Endocrinol Metab. 1999;13:583–95. doi: 10.1053/beem.1999.0045. [DOI] [PubMed] [Google Scholar]
- 54.Zhou D, Kusnecov AW, Shurin MR, et al. Exposure to physical and psychological stressors elevates plasma interleukin 6: relationship to the activation of hypothalamic-pituitary-adrenal axis. Endocrinology. 1993;133:2523–30. doi: 10.1210/endo.133.6.8243274. [DOI] [PubMed] [Google Scholar]
- 55.Pedersen BK, Steensberg A, Fischer C, et al. Exercise and cytokines with particular focus on muscle-derived IL-6. Exerc Immunol Rev. 2001;7:18–31. [PubMed] [Google Scholar]
- 56.Tracey KJ. Fat meets the cholinergic antiinflammatory pathway. J Exp Med. 2005;202:1017–21. doi: 10.1084/jem.20051760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tracey KJ. The inflammatory reflex. Nature. 2002;420:853–9. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- 58.Scully P, McKernan DP, Keohane J, et al. Plasma cytokine profiles in females with irritable bowel syndrome and extra-intestinal co-morbidity. Am J Gastroenterol. 2010;105:2235–43. doi: 10.1038/ajg.2010.159. [DOI] [PubMed] [Google Scholar]
- 59.Gecse K, Roka R, Ferrier L, et al. Increased faecal serine protease activity in diarrhoeic IBS patients: a colonic lumenal factor impairing colonic permeability and sensitivity. Gut. 2008;57:591–9. doi: 10.1136/gut.2007.140210. [DOI] [PubMed] [Google Scholar]
- 60.Bradesi S, Kokkotou E, Simeonidis S, et al. The role of neurokinin 1 receptors in the maintenance of visceral hyperalgesia induced by repeated stress in rats. Gastroenterology. 2006;130:1729–42. doi: 10.1053/j.gastro.2006.01.037. [DOI] [PubMed] [Google Scholar]
- 61.Cottrell GS, Padilla B, Pikios S, et al. Ubiquitin-dependent down-regulation of the neurokinin-1 receptor. J Biol Chem. 2006;281:27773–83. doi: 10.1074/jbc.M603369200. [DOI] [PubMed] [Google Scholar]
- 62.Martinez V, Taché Y. CRF1 receptors as a therapeutic target for irritable bowel syndrome. Curr Pharm Des. 2006;12:4071–88. doi: 10.2174/138161206778743637. [DOI] [PubMed] [Google Scholar]
- 63.van Tol EA, Petrusz P, Lund PK, et al. Local production of corticotropin releasing hormone is increased in experimental intestinal inflammation in rats. Gut. 1996;39:385–92. doi: 10.1136/gut.39.3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Koon HW, Zhao D, Zhan Y, et al. Substance P mediates antiapoptotic responses in human colonocytes by Akt activation. Proc Natl Acad Sci USA. 2007;104:2013–8. doi: 10.1073/pnas.0610664104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gay J, Kokkotou E, O’Brien M, et al. Corticotropin-releasing hormone deficiency is associated with reduced local inflammation in a mouse model of experimental colitis. Endocrinology. 2008;149:3403–9. doi: 10.1210/en.2007-1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kokkotou E, Torres D, Moss AC, et al. Corticotropin-releasing hormone receptor 2 deficient mice have reduced intestinal inflammatory responses. J Immunol. 2006;177:3355–61. doi: 10.4049/jimmunol.177.5.3355. [DOI] [PubMed] [Google Scholar]
- 67.Anton PM, Gay J, Mykoniatis A, et al. Corticotropin-releasing hormone (CRH) requirement in Clostridium difficile toxin A-mediated intestinal inflammation. Proc Natl Acad Sci USA. 2004;101:8503–8. doi: 10.1073/pnas.0402693101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wlk M, Wang CC, Venihaki M, et al. Corticotropin-releasing hormone antagonists possess anti-inflammatory effects in the mouse ileum. Gastroenterology. 2002;123:505–15. doi: 10.1053/gast.2002.34783. [DOI] [PubMed] [Google Scholar]
- 69.Spiller RC, Jenkins D, Thornley JP, et al. Increased rectal mucosal enteroendocrine cells, T-lymphocytes, and increased gut permeability following acute Campylobacter enteritis and in post-dysenteric irritable bowel syndrome. Gut. 2000;47:804–11. doi: 10.1136/gut.47.6.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chang L, Toner BB, Fukudo S, et al. Gender, age, society, culture, and the patient’s perspective in the functional gastrointestinal disorders. Gastroenterology. 2006;130:1435–46. doi: 10.1053/j.gastro.2005.09.071. [DOI] [PubMed] [Google Scholar]
- 71.Moss-Morris R, Spence M. To “lump” or to “split” the functional somatic syndromes: can infectious and emotional risk factors differentiate between the onset of chronic fatigue syndrome and irritable bowel syndrome? Psychosom Med. 2006;68:463–9. doi: 10.1097/01.psy.0000221384.07521.05. [DOI] [PubMed] [Google Scholar]
- 72.Straub RH. The complex role of estrogens in inflammation. Endocr Rev. 2007;28:521–74. doi: 10.1210/er.2007-0001. [DOI] [PubMed] [Google Scholar]