Abstract
Purpose
Little is known about the actual involvement of the general practitioner (GP) during the active breast cancer treatment phase. Therefore, this study explored (disease-specific) primary health care use among women undergoing active treatment for breast cancer compared with women without breast cancer.
Methods
A total of 185 women with a first diagnosis of early-stage breast cancer between 1998 and 2007 were identified in the primary care database of the Registration Network Groningen and matched with a reference population of 548 women without breast cancer on birth year and GP.
Results
Since diagnosis, patients with breast cancer had twice as many face-to-face contacts compared with women from the reference population (median 6.0 vs 3.0/year, Mann–Whitney (M-W) test p < 0.001). The median number of drug prescriptions and referrals was also significantly higher among patients than among the reference population (11.0 vs 7.0/year, M-W test p < 0.001 and 1.0 vs 0.0/year, M-W test p < 0.001). More patients than women from the reference population had face-to-face contacts or were prescribed drugs for reasons related to breast cancer and its treatment, including gastrointestinal problems, psychological reasons and endocrine therapy.
Conclusions
During the active breast cancer treatment phase, GPs are involved in the management of treatment-related side effects and psychological symptoms, as well as in the administration of endocrine therapy. Based on the findings of this study, interventions across the primary/secondary interface can be planned to improve quality of life and other outcomes in patients undergoing breast cancer treatment.
Keywords: Breast cancer, Health care utilisation, General practice, Active treatment phase, Primary care database
Introduction
Little is known about the actual involvement of the general practitioner (GP) in care for patients undergoing treatment for breast cancer. Traditionally, GPs have had a major role in early detection of cancer [1–3] and in symptom control during the palliative cancer stage [1, 2]. In Europe and North America, there is a now an explicit recognition that GPs and other primary care physicians should be involved in all stages of cancer care [1–5] and that they are players on the same team as oncologists [6–8].
Surgical treatment and systemic therapies can have a major impact on patients’ lives. For example, patients may experience psychosocial distress and treatment-related side effects that negatively affect their health-related quality of life and adherence to adjuvant therapies [9–11]. A better understanding of the role of the GP during the active breast cancer treatment phase is important for coordination of care across the primary/secondary interface to improve these and other outcomes in patients undergoing breast cancer treatment [2, 3, 5, 12, 13].
A British study showed that women with early breast cancer consulted their GP more often in the year after diagnosis than in the year before diagnosis [14], but primary care consultations were not compared between women with and without breast cancer. In another study among Canadian patients with (breast) cancer, contact rates with GPs and family physicians increased compared to baseline (pre-diagnosis), during the peri-diagnostic and active treatment periods [15]. However, it remains unclear for what reasons patients with breast cancer do consult their primary care physician. The objectives of the current study were (1) to explore (disease-specific) primary health care use among women undergoing treatment for breast cancer compared with women without breast cancer and (2) to identify specific patient characteristics associated with this health care use.
Patients and methods
Design and setting
An analysis of health care use was conducted using the database of the Registration Network Groningen (RNG). This general practice research network was established in 1989 and consists of three group practices with 17 GPs and a dynamic population of approximately 30,000 patients in the city of Groningen and the smaller towns Hoogeveen and Sappemeer in the northern part of the Netherlands. According to the privacy instructions given by the RNG, anonymized patient records were used in data collection and data analysis. No further approval from the Institutional Review Board of the University Medical Centre Groningen was needed because the study was in agreement with the regulations for publication of patient data.
Participants and data collection
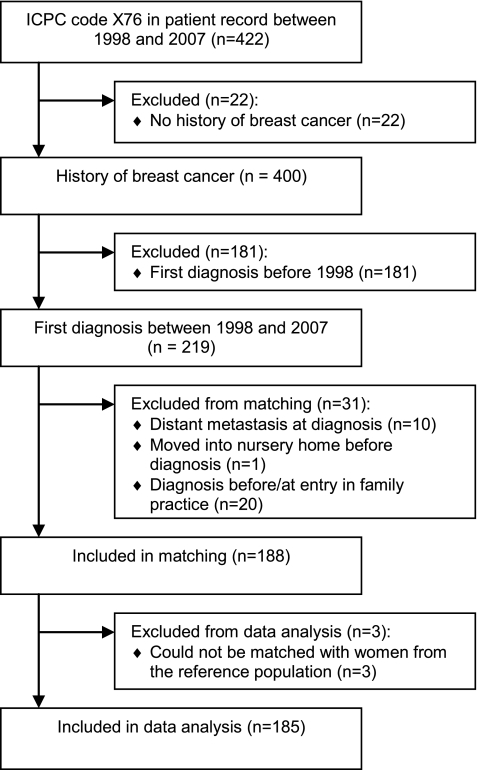
A total of 422 women with a recorded code for female breast malignancy in at least one of five files in their patient record (worksheet, episodes, prescriptions, referrals and problem list) between 1998 and 2007 were identified in the RNG database. Confirmation of breast cancer in history was obtained for 400 patients by going back to participating general practices and examining electronic records, electronic specialist letters and paper records (when available) [16]. Additional information about breast cancer diagnosis, stages, treatments and recurrences was also collected for these patients. Specialist letters were found for 312 patients (78.0%). The first date when the recorded code for female breast malignancy was entered into the patient record was not taken as the date of diagnosis, since the actual diagnosis will be made in the hospital and will usually later be recorded by GPs [17]. In 326 patients (81.5%), date of first recording of breast symptoms/breast cancer in worksheet and/or date of first referral letter was taken as the date of breast cancer diagnosis, while in 74 patients (18.5%), date of first hospital visit, date of specialist letter after first hospital visit or first date of breast cancer surgery was taken as the date of breast cancer diagnosis. The number of patients with a first diagnosis of early-stage breast cancer between 1998 and 2007 and registered with a participating GP at time of diagnosis determined the study size. Patients with a first breast cancer diagnosis before 1998 (n = 181), patients with distant metastasis at diagnosis (n = 10) and patients who were not registered with a participating GP at diagnosis (n = 21) were excluded from the study (Fig. 1).
Fig. 1.
Flow diagram of the identification and inclusion of patients with early-stage breast cancer
A reference population was identified from the entire female population in the RNG database, not including women with a history of breast cancer. Eligible patients (n = 188) were individually matched with three women from the reference population on birth year (±1 year) and GP, to control for age [18] and variation in recording diagnoses and prescribing drugs among GPs. Women from the reference population were eligible if they were registered with the same GP as the corresponding patient at breast cancer diagnosis. Three patients could not be matched and were excluded, leaving 185 patients and 548 women from the reference population for data analysis (Fig. 1). For each of the 180 patients (97.3%), 3 women from the reference population who met the matching criteria could be identified. At least one woman from the reference population was identified for each of the remaining five patients (2.7%). For patients and reference population, data collected by the RNG between 1998 and 2007 were entered into an anonymous database and included patient contacts recorded by participating GPs using the International Classification of Primary Care (ICPC) version 1 [19], prescribed medication automatically classified according to the Anatomical Therapeutical Chemical (ATC) classification [20] and referrals.
Data analysis
To study the active breast cancer treatment phase, we calculated health care utilisation rates in the year since diagnosis compared with these rates in the year before diagnosis. However, utilisation rates in general practice are not only affected by the chosen study period (1998–2007) but also by the time of entry in or departure from general practice, and death. Therefore, 30 patients and 83 women from the reference population have a shorter observation time before diagnosis (<365 days) due to time of entry in general practice. In addition, four patients have a shorter observation time since diagnosis (<365 days) because of recurrent breast cancer (n = 1), departure (n = 3) and death (n = 1). The same is true for 24 women from the reference population, due to departure (n = 10), death (n = 11) and other (unknown) reasons (n = 3). Nevertheless, median observation time is not statistically different between both groups (Table 1). Annual health care utilisation rates were calculated by dividing the number of face-to-face contacts, drug prescriptions and referrals in a period by the observation time in that period (Table 2). Face-to-face contacts included consultations in general practice as well as home visits in the patient’s home, carried out by GPs and other general practice workers. Frequencies of face-to-face contacts, drug prescriptions and referrals per year and per month were calculated (Table 2 and Fig. 2), as well as frequencies of face-to-face contacts and drug prescriptions for specific reasons based on ICPC chapters, ATC chapters and the three to seven digit ATC codes. Frequencies of face-to-face contacts were only divided by subgroups based on ICPC chapters, due to small numbers when using three digits ICPC codes. An exception was made for breast symptoms and breast cancer (Table 3).
Table 1.
Characteristics of women with early-stage breast cancer (n = 185) and women from the reference population (ref. pop.: n = 548)
| Patients | Ref. pop. | p value | |
|---|---|---|---|
| General practice, n (%) | 0.926a | ||
| Groningen | 64 (34.6) | 182 (33.2) | |
| Hoogeveen | 55 (29.7) | 170 (31.0) | |
| Sappemeer | 66 (35.7) | 196 (35.8) | |
| Age at diagnosis, median (range), years | 56.2 (27.4–96.0) | 55.8 (26.8–94.8) | 0.854b |
| T stage, n (%) | |||
| Tis | 15 (8.6) | ||
| T1 | 83 (47.7) | ||
| T2/T3/T4 | 76 (43.7) | ||
| Unknown | 11 | ||
| N stage, n (%) | |||
| N0 | 112 (62.6) | ||
| N+ | 67 (37.4) | ||
| Unknown | 6 | ||
| Surgery, n (%) | |||
| None | 3 (1.6) | ||
| Lumpectomy | 2 (1.1) | ||
| Lumpectomy + radiation therapy | 93 (50.3) | ||
| Mastectomyc | 61 (33.0) | ||
| Mastectomyc + radiation therapy | 26 (14.1) | ||
| Systemic treatment, n (%) | |||
| None | 85 (45.9) | ||
| Chemotherapy | 20 (10.8) | ||
| Endocrine therapy | 36 (19.5) | ||
| Chemotherapy + endocrine therapy | 44 (23.8) | ||
| Early endpoints in year since diagnosis, n (%) | |||
| Recurrent breast cancer | 1 (0.5) | ||
| Departure | 3 (1.6) | 10 (1.8) | |
| Death | 1 (0.5) | 11 (2.0) | |
| Other | 0 (0.0) | 3 (0.5) | |
| Observation time, median (range), days | |||
| Period before diagnosis | 365.0 (4.0–366.0) | 365.0 (4.0–366.0) | 0.716b |
| Period since diagnosis | 365.0 (33.0–366.0) | 365.0 (10.0–366.0) | 0.922b |
aChi-square test
bMann–Whitney test
cIncluding patients treated with lumpectomy followed by mastectomy
Table 2.
Annual health care utilisation rates before and since breast cancer diagnosis as well as frequencies of women with any face-to-face contact, any drug prescription and any referral in both periods among women with breast cancer (n = 185) and women from the reference population (n = 548)
| Annual health care utilisation | ||||
|---|---|---|---|---|
| Before diagnosis | Since diagnosis | |||
| Patients | Ref. pop. | Patients | Ref. pop. | |
| Face-to-face contacts | ||||
| Median (range) | 2.2 (0.0–18.7) | 2.0 (0.0–32.0) | 6.0 (0.0–46.0) | 3.0 (0.0–31.7) |
| Women with any face-to-face contact, n (%) | 149 (80.5) | 428 (78.1) | 184 (99.5) | 428 (78.1) |
| Drug prescriptions | ||||
| Median (range) | 6.0 (0.0–220.0) | 6.0 (0.0–111.0) | 11.0 (0.0–212.0) | 7.0 (0–127.0) |
| Women with any drug prescription, n (%) | 156 (84.3) | 437 (79.7) | 170 (91.9) | 447 (81.6) |
| Referrals | ||||
| Median (range) | 0.0 (0.0–13.8) | 0.0 (0.0–6.9) | 1.0 (0.0–11.1) | 0.0 (0.0–7.9) |
| Women with any referral, n (%) | 53 (28.6) | 157 (28.6) | 165 (89.2) | 164 (29.9) |
All changes in utilisation rates and frequencies within patients are significant (Wilcoxon signed-rank test, p < 0.001 and McNemar Test, p < 0.01), as well as all differences in utilisation rates and frequencies in the year since diagnosis between patients and reference population (Mann–Whitney test, p < 0.001 and Chi-square test, p < 0.01)
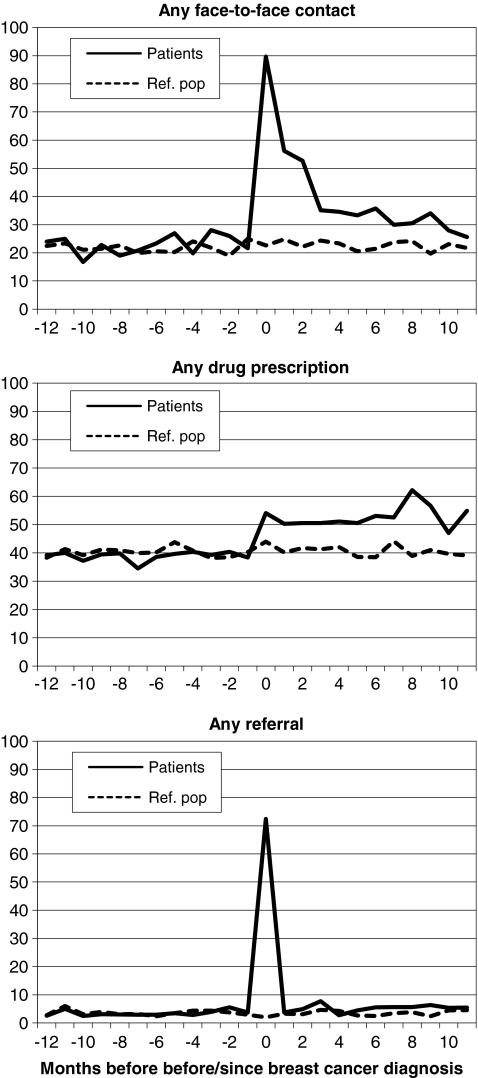
Fig. 2.
Timing of health care use in relation to breast cancer diagnosis among women with breast cancer (n = 185) and women from the reference population (n = 548). Percentage of women with any face-to-face contact, any drug prescription and any referral per month. Note: time 0 is the first month since date of breast cancer diagnosis
Table 3.
Frequencies of women with breast cancer (n = 185) and women from the reference population (ref. pop.: n = 548) with any face-to-face contact and any drug prescription since breast cancer diagnosis for specific reasons
| Since diagnosis | ||
|---|---|---|
| Patients | Ref. pop. | |
| Women with any face-to-face contact by ICPC chapter/code, n (%) | ||
| General and unspecified (A)*** | 62 (33.5) | 78 (14.2) |
| Blood, blood-forming organs and immune mechanism (B)* | 13 (7.0) | 16 (2.9) |
| Digestive (D) | 40 (21.6) | 92 (16.8) |
| Eye (F) | 10 (5.4) | 34 (6.2) |
| Ear (H) | 12 (6.5) | 51 (9.3) |
| Cardiovascular (K) | 40 (21.6) | 142 (25.9) |
| Musculoskeletal (L) | 69 (37.3) | 188 (34.3) |
| Neurological (N) | 20 (10.8) | 60 (10.9) |
| Psychological (P)* | 34 (18.4) | 64 (11.7) |
| Respiratory (R) | 54 (29.2) | 121 (22.1) |
| Skin (S)** | 65 (35.1) | 135 (24.6) |
| Endocrine, metabolic and nutritional (T) | 18 (9.7) | 48 (8.8) |
| Urological (U) | 13 (7.0) | 34 (6.2) |
| Pregnancy, childbearing and family planning (W)a | 1 (0.5) | 12 (2.2) |
| Female genital (X)*** | 177 (95.7) | 99 (18.1) |
| Breast symptoms (X18–X21)*** | 131 (70.8) | 11 (2.0) |
| Breast cancer (X76)*** | 157 (84.9) | 0 (0.0) |
| Social problems (Z) | 12 (6.5) | 37 (6.8) |
| Women with any drug prescription by ATC chapter/code, n (%) | ||
| Alimentary tract and metabolism (A)*** | 94 (50.8) | 165 (30.1) |
| Drugs for functional gastrointestinal disorders (A03)*** | 34 (18.4) | 16 (2.9) |
| Propulsives (A03F)*** | 31 (16.8) | 10 (1.8) |
| Antiemetics and antinauseants (A04)*** | 24 (13.0) | 2 (0.4) |
| Serotonin (5-HT3) antagonists (A04AA)*** | 24 (13.0) | 2 (0.4) |
| Laxatives (A06)*** | 35 (18.9) | 41 (7.5) |
| Osmotically acting laxatives (A06AD)*** | 27 (14.6) | 28 (5.1) |
| Antidiarrheals, intestinal anti-inflammatory/infective agents (A07)*** | 16 (8.6) | 9 (1.6) |
| Blood and blood-forming organs (B) | 31 (16.8) | 77 (14.1) |
| Cardiovascular system (C) | 72 (38.9) | 206 (37.6) |
| Dermatologicals (D)* | 55 (29.7) | 114 (20.8) |
| Antibiotics and chemotherapeutics for dermatological use (D06)** | 12 (6.5) | 13 (2.4) |
| Genito-urinary system and sex hormones (G)** | 17 (9.2) | 94 (17.2) |
| Sex hormones and modulators of the genital system (G03)** | 9 (4.9) | 79 (14.4) |
| Hormonal contraceptives for systemic use (G03A)** | 3 (1.6) | 43 (7.8) |
| Systemic hormonal preparations, excl. sex hormones and insulins (H)* | 32 (17.3) | 60 (10.9) |
| Corticosteroids for systemic use (H02)* | 22 (11.9) | 34 (6.2) |
| Corticosteroids for systemic use, plain (H02A)* | 22 (11.9) | 34 (6.2) |
| Anti-infectives for systemic use (J)** | 98 (53.0) | 211 (38.5) |
| Antibacterials for systemic use (J01)*** | 77 (41.6) | 134 (24.5) |
| Beta-lactam antibacterials, penicillins (J01C)*** | 35 (18.9) | 45 (8.2) |
| Antineoplastic and immunomodulating agents (L)*** | 66 (35.7) | 6 (1.1) |
| Endocrine therapy (L02)*** | 65 (35.1) | 0 (0.0) |
| Hormone antagonists and related agents (L02B)*** | 64 (34.6) | 0 (0.0) |
| Anti-estrogens (L02BA)*** | 53 (28.6) | 0 (0.0) |
| Tamoxifen (L02BA01)*** | 53 (28.6) | 0 (0.0) |
| Musculoskeletal system (M) | 50 (27.0) | 145 (26.5) |
| Nervous system (N)*** | 96 (51.9) | 203 (37.0) |
| Analgesics (N02)** | 47 (25.4) | 80 (14.6) |
| Other analgesics and antipyretics (N02B)** | 31 (16.8) | 50 (9.1) |
| Anilides (N02BE)*** | 31 (16.8) | 45 (8.2) |
| Psycholeptics (N05)*** | 81 (43.8) | 133 (24.3) |
| Anxiolytics (N05B)*** | 62 (33.5) | 90 (16.4) |
| Benzodiazepine derivatives (N05BA)*** | 61 (33.0) | 88 (16.1) |
| Hypnotics and sedatives (N05C)*** | 37 (20.0) | 55 (10.0) |
| Benzodiazepine derivatives (N05CD)*** | 37 (20.0) | 53 (9.7) |
| Antiparasitic products, insecticides and repellents (P)a | 0 (0.0) | 0 (0.0) |
| Respiratory system (R) | 54 (29.2) | 125 (22.8) |
| Sensory organs (S) | 26 (14.1) | 68 (12.4) |
| Various (V)a | 0 (0.0) | 2 (0.4) |
Chi-square test, *p < 0.05, **p < 0.01 and ***p < 0.001
aGroups with an expected count of less than five were not tested with the Chi-square test
Database management was performed in Microsoft Access 2003. For analysis, data were transferred to SPSS version 16. Non-parametric tests and logistic regression models were used due to the skewed distribution of the data. Differences in characteristics of women with breast cancer and women from the reference population were analysed with the Chi-square (χ 2) test and Mann–Whitney (M-W) test (Table 1). The Wilcoxon signed-rank test and McNemar test were conducted to explore change in rates and frequencies within patients (Table 2), while the M-W test and χ 2 test were used to compare differences in rates and frequencies between patients and women from the reference population in the period since diagnosis (Tables 2 and 3). Furthermore, univariate logistic regression analysis was used to evaluate if annual face-to-face contact rates and drug prescription rates in the period before diagnosis and certain patient characteristics were associated with face-to-face contact rates and drug prescription rates in the period since diagnosis. Multivariate logistic regression analysis was then performed to evaluate patient characteristics that were significantly related to drug prescription rates in univariate analysis (p < 0.01). Only the main effects were tested in the model due to the small number of patients (Table 4).
Table 4.
Face-to-face contact rates and drug prescription rates since breast cancer diagnosis by characteristics of women with breast cancer (n = 185)
| Univariate | Multivariate | |||
|---|---|---|---|---|
| ≤ Median | > Median | OR (CI) | OR (CI) | |
| Outcome: annual face-to-face contact rate since diagnosis | n = 100 | n = 85 | ||
| Face-to-face contact rate before diagnosis, n (%) | ||||
| ≤ Median | 61 (61.0) | 32 (37.6) | 1 | |
| > Median | 39 (39.0) | 53 (62.4) | 2.59 (1.43–4.70)** | |
| General practice, n (%) | ||||
| Groningen | 41 (41.0) | 23 (27.1) | 1 | |
| Hoogeveen | 27 (27.0) | 28 (32.9) | 1.85 (0.89–3.86) | |
| Sappemeer | 32 (32.0) | 34 (40.0) | 1.89 (0.94–3.82) | |
| Age at diagnosis, n (%) | ||||
| ≤ Median | 55 (55.0) | 38 (44.7) | 1 | |
| > Median | 45 (45.0) | 47 (55.3) | 1.51 (0.85–2.70) | |
| T stage, n (%) | ||||
| Tis/T1 | 61 (63.5) | 37 (47.2) | 1 | |
| T2/T3/T4 | 35 (36.5) | 41 (52.6) | 1.93 (1.05–3.55)* | |
| N stage, n (%) | ||||
| N0 | 63 (64.3) | 49 (60.5) | 1 | |
| N+ | 35 (35.7) | 32 (39.5) | 1.18 (0.64–2.16) | |
| Surgery, n (%) | ||||
| Lumpectomya | 58 (59.2) | 37 (44.0) | 1 | |
| Mastectomyb | 40 (40.8) | 47 (56.0) | 1.86 (1.03–3.37)* | |
| Chemotherapy, n (%) | ||||
| No | 65 (65.0) | 56 (65.9) | 1 | |
| Yes | 35 (35.0) | 29 (34.1) | 0.96 (0.52–1.77) | |
| Endocrine therapy, n (%) | ||||
| No | 65 (65.0) | 40 (47.1) | 1 | |
| Yes | 35 (35.0) | 45 (52.9) | 2.09 (1.16–3.78)* | |
| Outcome: annual drug prescription rate since diagnosis | n = 95 | n = 90 | ||
| Drug prescription rate before diagnosis, n (%)* | ||||
| ≤ Median | 80 (84.2) | 17 (18.9) | 1 | 1 |
| > Median | 15 (15.8) | 73 (81.1) | 22.90 (10.67–49.14)*** | 23.23 (10.14–53.22)*** |
| General practice, n (%)* | ||||
| Groningen | 41 (43.2) | 23 (25.6) | 1 | 1 |
| Hoogeveen | 21 (22.1) | 34 (37.8) | 2.89 (1.37–6.09)** | 4.09 (1.49–11.24)** |
| Sappemeer | 33 (34.7) | 33 (36.7) | 1.78 (0.88–3.60) | 1.56 (0.60–4.04) |
| Age at diagnosis, n (%)* | ||||
| ≤ Median | 59 (62.1) | 34 (37.8) | 1 | 1 |
| > Median | 36 (37.9) | 56 (62.2) | 2.70 (1.49–4.89)** | 1.50 (0.66–3.40) |
| T stage, n (%) | ||||
| Tis/T1 | 53 (57.0) | 45 (55.6) | 1 | |
| T2/T3/T4 | 40 (43.0) | 36 (44.4) | 1.06 (0.58–1.93) | |
| N stage, n (%) | ||||
| N0 | 58 (61.7) | 54 (63.5) | 1 | |
| N+ | 36 (38.3) | 31 (36.5) | 0.92 (0.50–1.70) | |
| Surgery, n (%) | ||||
| Lumpectomya | 52 (55.3) | 43 (48.9) | 1 | |
| Mastectomyb | 42 (44.7) | 45 (51.1) | 1.36 (0.76–2.44) | |
| Chemotherapy, n (%) | ||||
| No | 58 (61.1) | 63 (70.0) | 1 | |
| Yes | 37 (38.9) | 27 (30.0) | 0.76 (0.36–1.24) | |
| Endocrine therapy, n (%) | ||||
| No | 59 (62.1) | 46 (51.1) | 1 | |
| Yes | 36 (37.9) | 44 (48.9) | 1.57 (0.87–2.81) | |
Univariate analysis, odds ratios (OR) and 95% confidence intervals (95% CI) estimated with multivariate logistic regression analysis
Significant at *p < 0.05, **p < 0.01 and ***p < 0.001
aIncluding patients treated with lumpectomy, with and without radiation therapy
bIncluding patients treated with lumpectomy followed by mastectomy, with and without radiation therapy
Results
Characteristics of women with early-stage breast cancer (n = 185) and women from the reference population (n = 548) are presented in Table 1. There were no significant differences between the two groups in general practice, age at diagnosis and amount of observation time in the periods before and since breast cancer diagnosis. Median age at diagnosis among patients was 56.2 (range 27.4–96.0) years. T stage and N stage were known for 174 patients (94.1%) and 179 patients (96.8%), respectively. Among 112 patients (60.5%), breast cancer was detected without regional lymph nodes involved. A total of 93 patients (53.0%) underwent lumpectomy and radiation therapy, while 100 patients (54.1%) received systemic treatment. One patient presented with recurrent breast cancer (distant metastasis) during the first year since breast cancer diagnosis.
Table 2 gives annual health care utilisation rates in the periods before and since breast cancer diagnosis as well as frequencies of women with any face-to-face contact, any drug prescription and any referral in both periods. Since breast cancer diagnosis, health care utilisation in general practice increased significantly among patients, compared to the period before diagnosis and compared to the reference population. Patients had twice as many face-to-face contacts as women from the reference population (median 6.0 vs 3.0/year, M-W test p < 0.001). The median number of drug prescriptions and referrals was also significantly higher among patients than among the reference population (11.0 vs 7.0/year, M-W test p < 0.001 and 1.0 vs 0.0/year, M-W test p < 0.001). Frequencies of patients with any face-to-face contact, any drug prescription and any referral also increased since diagnosis. With respect to referrals, more patients than women from the reference population were referred to a surgeon [132 (71.4%) vs 15 (2.7%), χ 2 test p < 0.001] and a physical therapist [27 (14.6%) vs 33 (6.0%), χ 2 test p < 0.001] in this period (data not shown in Table 2).
Timing of health care use in relation to breast cancer diagnosis is shown in Fig. 2. The percentage of women with breast cancer with any face-to-face contact, any drug prescription and any referral per month increased since breast cancer diagnosis: e.g. in the first month since diagnosis, 166 patients (89.7%) had any face-to-face contact, 100 patients (54.1%) were prescribed any drugs, and 134 patients (72.4%) were referred at least once. The percentage of patients with any face-to-face contact per month decreased gradually after the first month since diagnosis, while the percentage of women with any drug prescription remained higher among patients than among women from the reference population. In the first month since diagnosis, 126 patients (87.6%) were referred to a surgeon (data not shown in Fig. 2).
Among patients and reference population, 35 (2.6%) and 57 (3.0%) face-to-face contacts, respectively, in the period since diagnosis could not be linked to any ICPC code. Table 3 shows that, compared to the reference population, significantly more patients had face-to-face contacts in the period following diagnosis for general reasons, blood(-forming organs) and immune mechanism, psychological reasons, the skin, breast symptoms and breast cancer. Also, significantly more patients than women from the reference population were prescribed drugs for the alimentary tract, dermatologicals, corticosteroids, anti-infective drugs, endocrine therapy, analgesics and psycholeptics. Less patients than women from the reference population were prescribed drugs for the genito-urinary system and sex hormones.
Patient characteristics associated with face-to-face contact rates and drug prescription rates are summarized in Table 4. In univariate analysis, a higher face-to-face contact rate before diagnosis (OR 2.59, 95% CI 1.43–4.70), a higher T stage (OR 1.93, 95% CI 1.05–3.55), treatment with mastectomy (OR 1.86, 95% CI 1.03–3.37) and treatment with endocrine therapy (OR 2.09, 95% CI 1.16–3.78) were related to a higher face-to-face contact rate since diagnosis. Patient characteristics associated with a higher drug prescription rate since diagnosis were a higher drug prescription rate before diagnosis (OR 22.90, 95% CI 10.67–49.14), general practice (Hoogeveen: OR 2.89, 95% CI 1.37–6.09) and age at diagnosis (OR 2.70, 95% CI 1.49–4.89). Multivariate analysis was not performed for face-to-face contact rates since only a higher face-to-face contact rate before diagnosis reached a lower p value of 0.01. The main patient characteristics associated with higher face-to-face contact rates and drug prescription rates since diagnosis were higher face-to-face contact rates and drug prescription rates before diagnosis (OR 2.59, 95% CI 1.43–4.70 and OR 23.23, 95% CI 10.14–53.22).
Discussion
This is the first study to compare (disease-specific) primary health care use between women undergoing treatment for breast cancer and women without breast cancer and to identify specific patient characteristics associated with primary health care use. In accordance with the two previous studies, contact rates with GPs increased during the active breast cancer period when compared to the pre-diagnostic period [14, 15]. The percentage of women with any face-to-face contact decreased gradually after the first month since diagnosis but remained higher among patients than among the reference population. The increase in the percentage of patients with at least one drug prescription also sustained over time. Furthermore, we found that treatment-related side effects, including nausea and vomiting, constipation, diarrhoea, skin problems, pain and sleep disturbance, were managed in general practice. Severe side effects and co-morbidities are associated with less (frequent) use, reduction in dose and breaks and discontinuation of adjuvant therapies [11, 21–25]. Management of these side effects and co-morbidities is very important for patients with breast cancer to improve their health-related quality of life, to complete adjuvant therapies and to prevent early mortality [2, 9–11]. In our study, women with breast cancer had more contacts with general practice for psychological reasons and more prescriptions for psycholeptics since diagnosis than women without breast cancer. Although psychological symptoms are often unrecognized [12, 26], there is potential for these symptoms to be identified and treated in primary care, especially given the ongoing nature of the patient–doctor relationship and the easy access to primary care [26]. Our study also showed that GPs were involved in the administration of endocrine therapy and that treatment with endocrine therapy is predictive of a higher face-to-face contact rate in general practice in the period since diagnosis. Involvement of Dutch GPs is expected to increase because more and more patients with breast cancer will be treated with endocrine therapy, including treatment with an aromatase inhibitor after tamoxifen.
A major strength of the present study is the use of a primary care database to analyse health care utilisation rates among women undergoing treatment for breast cancer and women without breast cancer. As the actual presentation of health problems was recorded by GPs of the RNG, our study is much less prone to recall or non-response bias than surveys with self-reported data on health care use [17]. Moreover, the RNG database made it possible to identify women with an ICPC code for female breast malignancy (X76) in their patient record during a specific time period and to match them with a reference group within the same population. Although some concerns have been raised about the validity of the data in primary care databases [16], the likelihood that the X76 code was valid in the patient records increased considerably by going back to participating general practices and examining electronic records, electronic specialist letters and paper records in these practices. A matter of concern might be the completeness or sensitivity of the data in primary care databases [16, 17]. In the present study, matching was performed on GP to ensure that inaccuracies in recording and prescribing were evenly distributed among patients and women from the reference population. The possibility of patients diagnosed with breast cancer without the X76 code in their record [27] cannot be ruled out, as the used data pertain to those conditions that were brought to the attention of the GPs and were recorded with ICPC codes [18]. Record linkage with a local cancer registry might be a reliable method to verify the completeness of primary care data [28] given informed consent from patients in both registries. Another limitation might be the small number of patients in the present study. As a result, we were not able to provide a further breakdown of the ICPC diagnostic categories and to perform regression analysis for count data. Future studies should involve larger samples of women with and without breast cancer. Furthermore, our data apply to a relatively small regional population in the Netherlands that is influenced by local policies, habits and health care structures [18]. The GPs who contribute to the database of the RNG might behave differently from other GPs in the Netherlands [16], but we have no reasons to assume this. Therefore, we believe that our results are also relevant for other primary care contexts [18].
Our study provided insight into the actual role of GPs during the active breast cancer treatment phase. GPs are involved in the management of treatment-related side effects and psychological symptoms, as well as in the administration of endocrine therapy. Therefore, good communication across the primary/secondary interface is important to ensure that they have adequate knowledge of breast cancer treatments and feel confident in managing these aspects of their patient’s care [8, 29]. In addition, the respective roles of GPs and oncologists during active treatment of patients with breast cancer should be clearly communicated. A simple guideline might help GPs and other primary care physicians be more effective when providing care for patients undergoing breast cancer treatment [8, 13]. Based on the findings of this study, interventions across the primary/secondary interface can be planned to improve quality of life and other outcomes in patients undergoing breast cancer treatment.
Acknowledgements
No funding was received for this study. The authors thank the Registration Network Groningen for providing data for our study. We also thank the participating GPs and practice assistants for their help during data collection in general practices.
Conflict of interest statement
All authors declare that no conflicts of interests exist.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Summerton N. General practitioners and cancer. BMJ. 2000;320:1090–1091. doi: 10.1136/bmj.320.7242.1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Campbell NC, Macleod U, Weller D. Primary care oncology: essential if high quality cancer care is to be achieved for all. Fam Pract. 2002;19:577–578. doi: 10.1093/fampra/19.6.577. [DOI] [PubMed] [Google Scholar]
- 3.McAvoy BR. General practitioners and cancer control. Med J Aust. 2007;187:115–117. doi: 10.5694/j.1326-5377.2007.tb01156.x. [DOI] [PubMed] [Google Scholar]
- 4.Grunfeld E. Cancer survivorship: a challenge for primary care physicians. Br J Gen Pract. 2005;55:741–742. [PMC free article] [PubMed] [Google Scholar]
- 5.Weller DP, Harris MF. Cancer care: what role for the general practitioner? Med J Aust. 2008;189:59–60. doi: 10.5694/j.1326-5377.2008.tb01915.x. [DOI] [PubMed] [Google Scholar]
- 6.Brotzman GL, Robertson RG. Role of the primary care physician after the diagnosis of cancer. The importance of the team approach. Prim Care. 1998;25:401–406. doi: 10.1016/S0095-4543(05)70072-5. [DOI] [PubMed] [Google Scholar]
- 7.Pruthi S, Brandt KR, Degnim AC, et al. A multidisciplinary approach to the management of breast cancer, part 1: prevention and diagnosis. Mayo Clin Proc. 2007;82:999–1012. doi: 10.4065/82.8.999. [DOI] [PubMed] [Google Scholar]
- 8.Grunfeld E. Primary care physicians and oncologists are players on the same team. J Clin Oncol. 2008;26:2246–2247. doi: 10.1200/JCO.2007.15.7081. [DOI] [PubMed] [Google Scholar]
- 9.Cella D, Fallowfield LJ. Recognition and management of treatment-related side effects for breast cancer patients receiving adjuvant endocrine therapy. Breast Cancer Res Treat. 2008;107:167–180. doi: 10.1007/s10549-007-9548-1. [DOI] [PubMed] [Google Scholar]
- 10.Montazeri A. Health-related quality of life in breast cancer patients: a bibliographic review of the literature from 1974 to 2007. J Exp Clin Cancer Res. 2008;27:32. doi: 10.1186/1756-9966-27-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hadji P. Improving compliance and persistence to adjuvant tamoxifen and aromatase inhibitor therapy. Crit Rev Oncol Hematol. 2010;73:156–166. doi: 10.1016/j.critrevonc.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 12.Pascoe SW, Neal RD, Allgar VL, et al. Psychosocial care for cancer patients in primary care? Recognition of opportunities for cancer care. Fam Pract. 2004;21:437–442. doi: 10.1093/fampra/cmh415. [DOI] [PubMed] [Google Scholar]
- 13.Grunfeld E, Earle CC. The interface between primary and oncology specialty care: treatment through survivorship. J Natl Cancer Inst Monogr. 2010;2010:25–30. doi: 10.1093/jncimonographs/lgq002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Macleod U, Ross S, Twelves C, et al. Primary and secondary care management of women with early breast cancer from affluent and deprived areas: retrospective review of hospital and general practice records. BMJ. 2000;320:1442–1445. doi: 10.1136/bmj.320.7247.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Del Giudice L, Bondy SJ, Chen Z, et al. et al. Physician care of cancer patients. In: Jaakkimainen L, Upshur R, Klein-Geltink JE, et al.et al., editors. Primary care in Ontario: ICES atlas. Toronto: Institute for Clinical Evaluative Sciences; 2006. pp. 161–174. [Google Scholar]
- 16.Lawrenson R, Williams T, Farmer R. Clinical information for research; the use of general practice databases. J Public Health Med. 1999;21:299–304. doi: 10.1093/pubmed/21.3.299. [DOI] [PubMed] [Google Scholar]
- 17.Tate AR, Martin AG, Murray-Thomas T, et al. Determining the date of diagnosis—is it a simple matter? The impact of different approaches to dating diagnosis on estimates of delayed care for ovarian cancer in UK primary care. BMC Med Res Methodol. 2009;9:42. doi: 10.1186/1471-2288-9-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van der Veen WJ, Meyboom-de Jong B, et al. Age and gender. In: Jones R, Britten N, Culpepper L, et al., editors. Oxford textbook of primary medical care. Oxford: Oxford University Press; 2004. pp. 153–161. [Google Scholar]
- 19.Lamberts H, Wood M. ICPC. International Classification of Primary Care. Oxford: Oxford University Press; 1987. [Google Scholar]
- 20.WHO Collaborating Centre for Drug Statistics Methodology . ATC index with DDS. Oslo: World Health Organisation; 1999. [Google Scholar]
- 21.Hawfield A, Lovato J, Covington D, et al. Retrospective study of the effect of comorbidity on use of adjuvant chemotherapy in older women with breast cancer in a tertiary care setting. Crit Rev Oncol Hematol. 2006;59:250–255. doi: 10.1016/j.critrevonc.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 22.Owusu C, Buist DS, Field TS, et al. Predictors of tamoxifen discontinuation among older women with estrogen receptor-positive breast cancer. J Clin Oncol. 2008;26:549–555. doi: 10.1200/JCO.2006.10.1022. [DOI] [PubMed] [Google Scholar]
- 23.Bickell NA, Weidmann J, Fei K, et al. Underuse of breast cancer adjuvant treatment: patient knowledge, beliefs, and medical mistrust. J Clin Oncol. 2009;27:5160–5167. doi: 10.1200/JCO.2009.22.9773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garg P, Rana F, Gupta R, et al. Predictors of toxicity and toxicity profile of adjuvant chemotherapy in elderly breast cancer patients. Breast J. 2009;15:404–408. doi: 10.1111/j.1524-4741.2009.00745.x. [DOI] [PubMed] [Google Scholar]
- 25.van Herk-Sukel MP, van de Poll-Franse LV, Voogd AC, et al. Half of breast cancer patients discontinue tamoxifen and any endocrine treatment before the end of the recommended treatment period of 5 years: a population-based analysis. Breast Cancer Res Treat. 2010;122:843–851. doi: 10.1007/s10549-009-0724-3. [DOI] [PubMed] [Google Scholar]
- 26.Allgar VL, Neal RD, Pascoe SW. Cancer patients consultation patterns in primary care and levels of psychological morbidity: findings from the Health Survey for England. Psychooncology. 2003;12:736–740. doi: 10.1002/pon.692. [DOI] [PubMed] [Google Scholar]
- 27.Pascoe SW, Neal RD, Heywood PL, et al. Identifying patients with a cancer diagnosis using general practice medical records and Cancer Registry data. Fam Pract. 2008;25:215–220. doi: 10.1093/fampra/cmn023. [DOI] [PubMed] [Google Scholar]
- 28.Schouten LJ, Hoppener P, van den Brandt PA, et al. Completeness of cancer registration in Limburg, The Netherlands. Int J Epidemiol. 1993;22:369–376. doi: 10.1093/ije/22.3.369. [DOI] [PubMed] [Google Scholar]
- 29.Jefford M, Baravelli C, Dudgeon P, et al. Tailored chemotherapy information faxed to general practitioners improves confidence in managing adverse effects and satisfaction with shared care: results from a randomized controlled trial. J Clin Oncol. 2008;26:2272–2277. doi: 10.1200/JCO.2007.14.7710. [DOI] [PubMed] [Google Scholar]