Abstract
The large majority of patients with the autoimmune disease myasthenia gravis characteristically have detectable antibodies against the acetylcholine receptor (AChR). We used synthetic peptides to identify antibodies in sera of myasthenia gravis patients reactive with the human acetylcholine receptor (HuAChR) alpha-subunit, residues 160-167. Affinity purification of these antibodies, using the HuAChR alpha-subunit 157-170 peptide immobilized on thiopropyl-Sepharose, yielded IgG antibodies that bound to the native AChR and inhibited the binding of alpha-bungarotoxin to the receptor. The HuAChR alpha-subunit 160-167 peptide demonstrated specific immunological cross-reactivity with a shared homologous domain on herpes simplex virus glycoprotein D, residues 286-293, by both binding and inhibition studies. Thus, HuAChR alpha-subunit, residues 160-167, elicits antibodies in myasthenic patients that binds to the native AChR protein and is capable of eliciting a biologic effect. Immunologic cross-reactivity of this "self" epitope with herpes simplex virus suggest that this virus may be associated with the initiation of some cases of myasthenia.
Full text
PDF
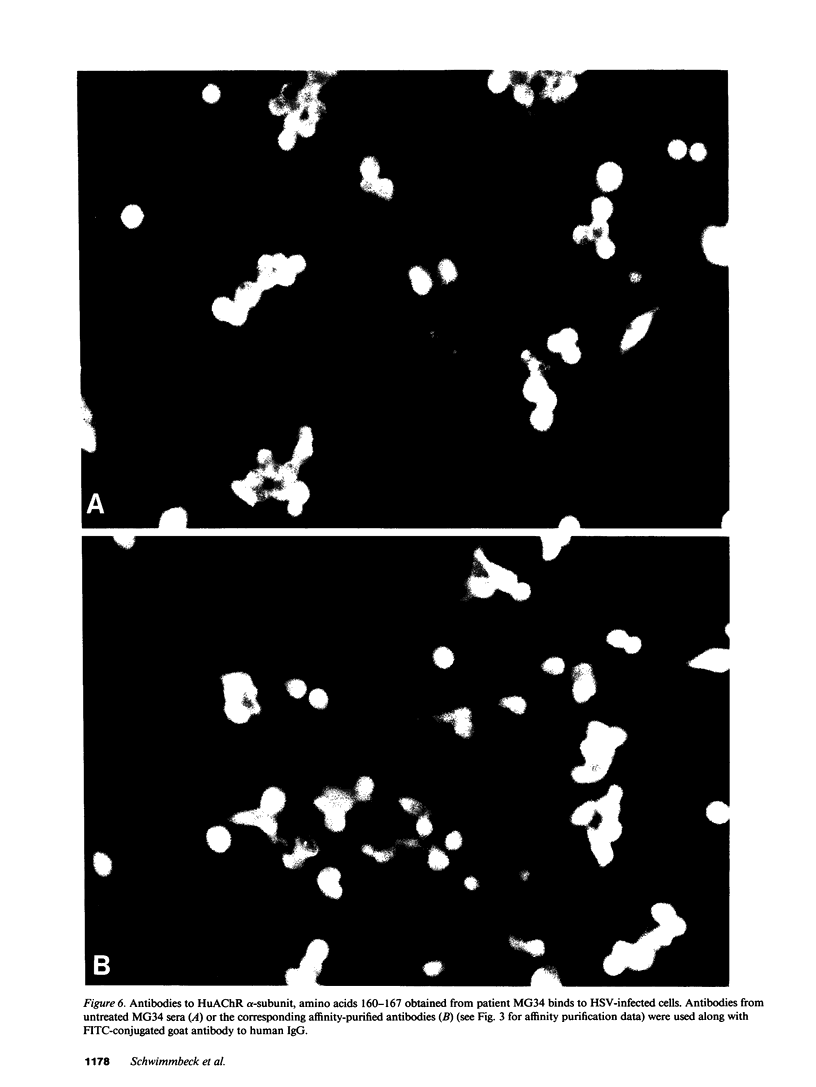


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoki T., Drachman D. B., Asher D. M., Gibbs C. J., Jr, Bahmanyar S., Wolinsky J. S. Attempts to implicate viruses in myasthenia gravis. Neurology. 1985 Feb;35(2):185–192. doi: 10.1212/wnl.35.2.185. [DOI] [PubMed] [Google Scholar]
- Atassi M. Z., Mulac-Jericevic B., Yokoi T., Manshouri T. Localization of the functional sites on the alpha chain of acetylcholine receptor. Fed Proc. 1987 Jun;46(8):2538–2547. [PubMed] [Google Scholar]
- Barkas T., Mauron A., Roth B., Alliod C., Tzartos S. J., Ballivet M. Mapping the main immunogenic region and toxin-binding site of the nicotinic acetylcholine receptor. Science. 1987 Jan 2;235(4784):77–80. doi: 10.1126/science.2432658. [DOI] [PubMed] [Google Scholar]
- Bray J. J., Drachman D. B. Binding affinities of anti-acetylcholine receptor autoantibodies in myasthenia gravis. J Immunol. 1982 Jan;128(1):105–110. [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- Dale J. B., Beachey E. H. Epitopes of streptococcal M proteins shared with cardiac myosin. J Exp Med. 1985 Aug 1;162(2):583–591. doi: 10.1084/jem.162.2.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta S. K., Schwartz R. S. Editorial: Infectious (?) myasthenia. N Engl J Med. 1974 Dec 12;291(24):1304–1305. doi: 10.1056/NEJM197412122912411. [DOI] [PubMed] [Google Scholar]
- DeLisi C., Berzofsky J. A. T-cell antigenic sites tend to be amphipathic structures. Proc Natl Acad Sci U S A. 1985 Oct;82(20):7048–7052. doi: 10.1073/pnas.82.20.7048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drachman D. B., Adams R. N., Josifek L. F., Self S. G. Functional activities of autoantibodies to acetylcholine receptors and the clinical severity of myasthenia gravis. N Engl J Med. 1982 Sep 23;307(13):769–775. doi: 10.1056/NEJM198209233071301. [DOI] [PubMed] [Google Scholar]
- Drachman D. B. Myasthenia gravis (second of two parts). N Engl J Med. 1978 Jan 26;298(4):186–193. doi: 10.1056/NEJM197801262980404. [DOI] [PubMed] [Google Scholar]
- Dyrberg T., Oldstone M. B. Peptides as antigens. Importance of orientation. J Exp Med. 1986 Oct 1;164(4):1344–1349. doi: 10.1084/jem.164.4.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyrberg T., Oldstone M. B. Peptides as probes to study molecular mimicry and virus-induced autoimmunity. Curr Top Microbiol Immunol. 1986;130:25–37. doi: 10.1007/978-3-642-71440-5_3. [DOI] [PubMed] [Google Scholar]
- Fuchs S., Neumann D., Safran A., Souroujon M., Barchan D., Fridkin M., Gershoni J. M., Mantegazza R., Pizzighella S. Synthetic peptides and their antibodies in the analysis of the acetylcholine receptor. Ann N Y Acad Sci. 1987;505:256–271. doi: 10.1111/j.1749-6632.1987.tb51295.x. [DOI] [PubMed] [Google Scholar]
- Fujinami R. S., Oldstone M. B. Amino acid homology between the encephalitogenic site of myelin basic protein and virus: mechanism for autoimmunity. Science. 1985 Nov 29;230(4729):1043–1045. doi: 10.1126/science.2414848. [DOI] [PubMed] [Google Scholar]
- Gnann J. W., Jr, Schwimmbeck P. L., Nelson J. A., Truax A. B., Oldstone M. B. Diagnosis of AIDS by using a 12-amino acid peptide representing an immunodominant epitope of the human immunodeficiency virus. J Infect Dis. 1987 Aug;156(2):261–267. doi: 10.1093/infdis/156.2.261. [DOI] [PubMed] [Google Scholar]
- Goldstein L. C., Corey L., McDougall J. K., Tolentino E., Nowinski R. C. Monoclonal antibodies to herpes simplex viruses: use in antigenic typing and rapid diagnosis. J Infect Dis. 1983 May;147(5):829–837. doi: 10.1093/infdis/147.5.829. [DOI] [PubMed] [Google Scholar]
- Kagnoff M. F., Austin R. K., Hubert J. J., Bernardin J. E., Kasarda D. D. Possible role for a human adenovirus in the pathogenesis of celiac disease. J Exp Med. 1984 Nov 1;160(5):1544–1557. doi: 10.1084/jem.160.5.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klavinskis L. S., Willcox H. N., Richmond J. E., Newsom-Davis J. Attempted isolation of viruses from myasthenia gravis thymus. J Neuroimmunol. 1986 Jun;11(4):287–299. doi: 10.1016/0165-5728(86)90082-2. [DOI] [PubMed] [Google Scholar]
- Korn I. L., Abramsky O. Myasthenia gravis following viral infection. Eur Neurol. 1981;20(6):435–49U. doi: 10.1159/000115275. [DOI] [PubMed] [Google Scholar]
- Krisher K., Cunningham M. W. Myosin: a link between streptococci and heart. Science. 1985 Jan 25;227(4685):413–415. doi: 10.1126/science.2578225. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lindstrom J., Shelton D., Fujii Y. Myasthenia gravis. Adv Immunol. 1988;42:233–284. doi: 10.1016/s0065-2776(08)60847-0. [DOI] [PubMed] [Google Scholar]
- Liu F. T., Zinnecker M., Hamaoka T., Katz D. H. New procedures for preparation and isolation of conjugates of proteins and a synthetic copolymer of D-amino acids and immunochemical characterization of such conjugates. Biochemistry. 1979 Feb 20;18(4):690–693. doi: 10.1021/bi00571a022. [DOI] [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- McCarthy M. P., Earnest J. P., Young E. F., Choe S., Stroud R. M. The molecular neurobiology of the acetylcholine receptor. Annu Rev Neurosci. 1986;9:383–413. doi: 10.1146/annurev.ne.09.030186.002123. [DOI] [PubMed] [Google Scholar]
- Merrifield R. B. Solid-phase peptide synthesis. Adv Enzymol Relat Areas Mol Biol. 1969;32:221–296. doi: 10.1002/9780470122778.ch6. [DOI] [PubMed] [Google Scholar]
- Mulac-Jericevic B., Atassi M. Z. Segment alpha 182-198 of Torpedo californica acetylcholine receptor contains second toxin-binding region and binds anti-receptor antibodies. FEBS Lett. 1986 Apr 7;199(1):68–74. doi: 10.1016/0014-5793(86)81225-x. [DOI] [PubMed] [Google Scholar]
- Neu N., Rose N. R., Beisel K. W., Herskowitz A., Gurri-Glass G., Craig S. W. Cardiac myosin induces myocarditis in genetically predisposed mice. J Immunol. 1987 Dec 1;139(11):3630–3636. [PubMed] [Google Scholar]
- Neumann D., Barchan D., Safran A., Gershoni J. M., Fuchs S. Mapping of the alpha-bungarotoxin binding site within the alpha subunit of the acetylcholine receptor. Proc Natl Acad Sci U S A. 1986 May;83(9):3008–3011. doi: 10.1073/pnas.83.9.3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda M., Furutani Y., Takahashi H., Toyosato M., Tanabe T., Shimizu S., Kikyotani S., Kayano T., Hirose T., Inayama S. Cloning and sequence analysis of calf cDNA and human genomic DNA encoding alpha-subunit precursor of muscle acetylcholine receptor. 1983 Oct 27-Nov 2Nature. 305(5937):818–823. doi: 10.1038/305818a0. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B. Molecular mimicry and autoimmune disease. Cell. 1987 Sep 11;50(6):819–820. doi: 10.1016/0092-8674(87)90507-1. [DOI] [PubMed] [Google Scholar]
- Ralston S., Sarin V., Thanh H. L., Rivier J., Fox J. L., Lindstrom J. Synthetic peptides used to locate the alpha-bungarotoxin binding site and immunogenic regions on alpha subunits of the nicotinic acetylcholine receptor. Biochemistry. 1987 Jun 16;26(12):3261–3266. doi: 10.1021/bi00386a004. [DOI] [PubMed] [Google Scholar]
- Ratnam M., Nguyen D. L., Rivier J., Sargent P. B., Lindstrom J. Transmembrane topography of nicotinic acetylcholine receptor: immunochemical tests contradict theoretical predictions based on hydrophobicity profiles. Biochemistry. 1986 May 6;25(9):2633–2643. doi: 10.1021/bi00357a052. [DOI] [PubMed] [Google Scholar]
- Rice G. P., Schrier R. D., Oldstone M. B. Cytomegalovirus infects human lymphocytes and monocytes: virus expression is restricted to immediate-early gene products. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6134–6138. doi: 10.1073/pnas.81.19.6134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwimmbeck P. L., Yu D. T., Oldstone M. B. Autoantibodies to HLA B27 in the sera of HLA B27 patients with ankylosing spondylitis and Reiter's syndrome. Molecular mimicry with Klebsiella pneumoniae as potential mechanism of autoimmune disease. J Exp Med. 1987 Jul 1;166(1):173–181. doi: 10.1084/jem.166.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefansson K., Dieperink M. E., Richman D. P., Gomez C. M., Marton L. S. Sharing of antigenic determinants between the nicotinic acetylcholine receptor and proteins in Escherichia coli, Proteus vulgaris, and Klebsiella pneumoniae. Possible role in the pathogenesis of myasthenia gravis. N Engl J Med. 1985 Jan 24;312(4):221–225. doi: 10.1056/NEJM198501243120407. [DOI] [PubMed] [Google Scholar]
- Tzartos S. J., Lindstrom J. M. Monoclonal antibodies used to probe acetylcholine receptor structure: localization of the main immunogenic region and detection of similarities between subunits. Proc Natl Acad Sci U S A. 1980 Feb;77(2):755–759. doi: 10.1073/pnas.77.2.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson I. A., Niman H. L., Houghten R. A., Cherenson A. R., Connolly M. L., Lerner R. A. The structure of an antigenic determinant in a protein. Cell. 1984 Jul;37(3):767–778. doi: 10.1016/0092-8674(84)90412-4. [DOI] [PubMed] [Google Scholar]