Abstract
Hereditary leiomyomatosis and renal cell cancer (HLRCC) is an autosomal dominant syndrome characterized by skin piloleiomyomas, uterine leiomyomas and papillary type 2 renal cancer caused by germline mutations in the fumarate hydratase (FH) gene. Previously, we proposed renal imaging for FH mutation carriers starting at the age of 20 years. However, recently an 18-year-old woman from a Dutch family with HLRCC presented with metastatic renal cancer. We describe the patient and family data, evaluate current evidence on renal cancer risk and surveillance in HLRCC and consider the advantages and disadvantages of starting surveillance for renal cancer in childhood. We also discuss the targeted therapies administered to our patient.
Keywords: Hereditary leiomyomatosis, Papillary renal cancer, Fumarate hydratase
Introduction
In Western Europe the lifetime risk for renal cancer is about 1% for men and 0.5% for women. In The Netherlands, for the period 2008–2009, the mean age at diagnosis of renal cancer was 64 years and 90% of cases were diagnosed after the age of 50 years. Histologically, 74% of these cancers were of the clear cell (or unspecified) subtype, whereas papillary renal cancer was diagnosed in 7% of cases (O. Visser, Netherlands Comprehensive Cancer Centre, personal communication 2011).
Hereditary renal cancer is the common denominator for a heterogeneous group of conditions, which includes Von Hippel-Lindau disease, Birt-Hogg-Dubé syndrome, hereditary papillary renal cancer, hereditary leiomyomatosis and renal cell cancer (HLRCC) and other syndromes. In general, hereditary renal cancer presents at an early age and is often multifocal and/or bilateral. Notably, each condition is characterized by a predominant histological subtype of cancer, for example, Von Hippel-Lindau disease is characterized by multiple, early-onset, clear cell renal tumours [1, 2].
In hereditary leiomyomatosis and renal cell cancer (HLRCC, MIM 605839), also known as multiple uterine and cutaneous leiomyomatosis (MUCL, MIM 150800), patients develop multiple cutaneous piloleiomyomas and women develop, in addition, multiple and often symptomatic uterine leiomyomas. A small subset of cases develops papillary type 2 renal cancer, which often runs an unfavourable clinical course. Other histological types of renal cancer may also occur, in particular collecting duct cancer [3–5]. HLRCC is an autosomal dominant condition caused by heterozygous mutations in the fumarate hydratase (FH) gene [6]. In contrast with other forms of hereditary renal cancer, most renal tumours in HLRCC are unifocal and unilateral. Although renal cancer has been consistently reported in a subset of HLRCC families, the percentages of families in which renal cancer was observed has varied widely, from 2 [7] to 50% [5] of the kindreds studied (see Table 1). The reasons for the different reported prevalences have not been clarified. Although the risk of renal cancer may differ between populations, the reported variation in prevalence may also be due to the method of ascertainment, i.e. via the dermatologist or urologist. No clear genotype-phenotype correlations have been found for HLRCC: as yet no association has been found between the renal cancer phenotype and the type or location of the FH mutation or FH enzyme activity [8, 9]. Vahteristo et al. [10] found no evidence for a genetic modifier for renal cancer risk.
Table 1.
Proportion of HLRCC families with one or more cases of renal cancer
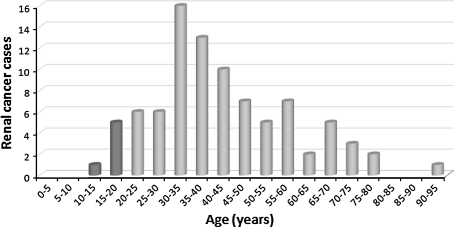
Although early age at onset of renal cancer is common in HLRCC, onset before the age of 20 years is relatively rare. The youngest age at diagnosis was recently reported by Alrashdi et al. [11]: an 11-year-old FH mutation carrier in whom localized asymptomatic renal cancer was detected by presymptomatic renal imaging. This patient is doing well three years after nephrectomy (S. V. Hodgson, personal communication 2011). Among 89 cases reported in the literature, six (7%) were found in individuals younger than 20 years (see Fig. 1). The main characteristics of renal cancer in the early-onset cases that have been reported are summarized in Table 2. Most cases for which relevant data were available presented with metastatic disease.
Fig. 1.
Age distribution of renal cancer in 89 published patients with HLRCC [3, 7, 11, 12, 15–24]
Table 2.
Characteristics of renal cancer cases in HLRCC families diagnosed before the age of 20 years
| Patient no. | Age at diagnosis (years) | Histology | Stage at diagnosis | Outcome | Reference |
|---|---|---|---|---|---|
| 1 | 11 | Papillary type 2 | Stage 1 | No evidence of disease 3 years after diagnosis | [11]; S. V. Hodgson, personal communication 2011 |
| 2 | 16 | Collecting duct | Metastatic | Died at age 18 years | [7] |
| 3 | 17 | Papillary type 2 | Metastatic | Died 15 months after diagnosis | [19] |
| 4 | 17 | Papillary type 2 | Metastatic | Died at age 19 years | [23]; M.H. Tan, personal communication 2011 |
| 5 | 17 | [3] | |||
| 6 | 18 | [3] | |||
| 7 | 18 | Papillary type 2 | Metastatic | Died 8 months after diagnosis | Our case |
Previously, we described the clinical and genetic findings in HLRCC families in The Netherlands [12]. In this cohort of 14 families with FH mutations, we indentified one case of renal cancer papillary type 2 and one Wilms tumour. The association of the latter tumour with HLRCC is uncertain [13]. Based on the literature data and our own findings, in 2010 we advocated intensive renal surveillance from age 20 onward, using ultrasound every 6 months and annual MRI.
Recently, an 18-year-old woman from a Dutch HLRCC family presented with symptomatic metastatic renal cancer. Here we present the patient and family data, review the renal cancer risk in HLRCC, and consider the advantages and disadvantages of intensive surveillance starting in childhood. We also discuss our choices for targeted treatment of metastatic papillary type 2 renal cancer in this case.
Patient and pedigree data
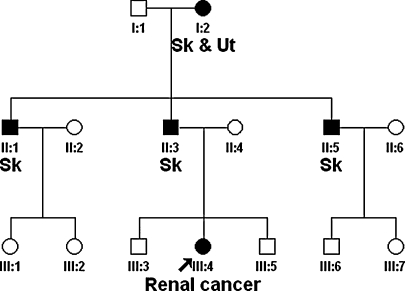
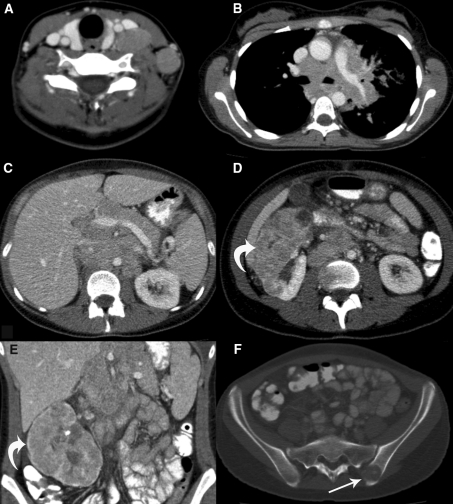
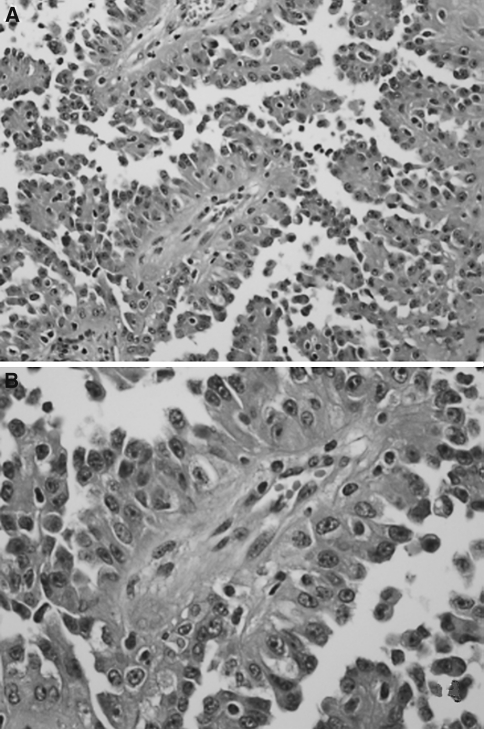
The pedigree of this family is depicted in Fig. 2. A novel pathogenic germline missense FH mutation, c.1002T>G, p.Ser334Arg in exon 7, was detected in this kindred and previously reported by Badeloe et al. [14]. At the time of the original report, the family member we present here (Fig. 2, patient III-4) was 15 years old and apparently unaffected (not clinically evaluated). We advised genetic analysis around age 18. Three years later, the patient was referred with symptoms of fatigue, weight loss and cough. At physical examination enlarged cervical lymph nodes and a large right abdominal mass were palpable. Several small skin lesions on the back were indicative of leiomyomas, but histological examination was not performed. CT scans showed a tumour in the right kidney, extensive cervical, mediastinal, abdominal and retroperitoneal lymphadenopathy, and bone metastases (Fig. 3). No uterine abnormalities were noted. A biopsy taken from a cervical lymph node showed a metastatic carcinoma with a papillary architecture and cells with prominent nucleoli. The histological pattern corresponded with type 2 papillary carcinoma. Focally, there were atypical large nucleoli surrounded by a halo, which according to Merino et al. [3] is a typical finding for HRLCC-associated renal cancer (Fig. 4). Immunohistochemistry showed positive staining for CK pan, CK 8/18, vimentin and CD10. Staining was negative for CK7, CK20, Ber-Ep4, calcitonin, thyroglobulin, EMA, CD15, PR, ER, TTF-1, CEA, P63 and OC125. These immuno-histochemical findings were also compatible with primary renal cell carcinoma, papillary type 2. The patient was shown to be a carrier of the familial FH germline mutation.
Fig. 2.
Pedigree of our HLRCC family. Abbreviations: Sk = skin piloleiomyomas, Ut = uterine leiomyomas
Fig. 3.
CT imaging of the abdomen in the 18-year old patient showing: massive cervical (a) mediastinal (b) and abdominal (c) lymphadenopathy; the large renal cell carcinoma of the right kidney (d and e; arrow); and a bone metastasis in the iliac bone on the left side (f)
Fig. 4.
Histological image of a biopsy taken from a cervical lymph node in the patient showing metastatic carcinoma with a papillary architecture (a 100×) and in the nuclei prominent nucleoli (b 200×) (hematoxylin and eosin)
Initial systemic treatment consisted of sunitinib, a multi-tyrosine kinase inhibitor. After two 6-week cycles stable disease was documented and therapy was continued. However, 5 months after initiation of therapy, progressive disease developed with pleural effusion, treated by drainage and pleurodesis. Second line systemic treatment with temsirolimus, an inhibitor of mammalian target of rapamycin (mTOR), was started, but after eight weekly administrations CT scans revealed progressive disease. Further treatment consisted of symptom control and the patient died 8 months after the diagnosis.
Discussion
The patient described in this report is a member of a previously described kindred with HLRCC, in which only skin and uterine leiomyomas had occurred thus far. The protocol for these families proposed by the Dutch national working group on HLRCC advocates intensive screening for renal cancer from the age of 20 years onward [12]. Other authors have also proposed surveillance starting in the 18–20 year age range [5]. However, Alrashdi et al. [11] advocated surveillance starting in childhood, based on childhood-onset cases. The patient described here presented with symptomatic metastatic papillary type 2 renal cancer at the age of 18 years. This led us to reconsider the arguments in favour of genetic testing and surveillance for FH mutation carriers starting in childhood.
One of the main unresolved issues is the renal cancer risk for FH mutation carriers. The reported proportion of FH mutation-positive families with renal cancer among all the kindreds studied varies widely as summarized in Table 1. We have previously observed only one HLRCC family with one papillary renal cancer case among 14 known FH mutation-positive families [12]. The different prevalence figures reported may reflect various forms of bias, in particular in the ascertainment of the disease in dermatology versus urology clinics, or they might indicate different renal cancer risks for different populations. Thus no reliable renal cancer risk for a FH mutation carrier has yet been determined. The second relevant issue is the age distribution of HLRCC-associated renal cancer. Whereas onset at the age of 30–45 years is common, onset before age 20 is relatively rare, as shown in Table 2 and Fig. 1. Among 89 patients reported with HLRCC-associated renal cancer [3, 7, 11, 12, 15–24], only six cases (7%) were diagnosed before the age of 20 [3, 7, 11, 19, 23]. Finally, the biological behaviour of renal cancer in HLRCC and its implication on surveillance should be considered. HLRCC-associated renal cancer often runs an aggressive clinical course. Long-term follow-up data are available on the initial 19 renal cancer patients with HLRCC diagnosed at the National Institute of Health, USA. In two of these patients the kidney tumours were detected by surveillance. Thirteen patients had advanced disease at presentation (stage III or IV), three of which had distant metastasis. At a median follow-up of 34 months (range 6–141 months) seven patients had died due to disease, three had disease and nine were free of detectable disease at last follow-up. Based on these observations, the authors concluded that renal tumours in HLRCC are significantly more aggressive than in patients with other hereditary tumour syndromes [4]. For Von Hippel –Lindau disease it has been established that surveillance for renal cancer and nephron-sparing treatment for lesions of 3 cm or larger is an effective procedure [25]. For HLRCC-associated tumours that may grow and spread aggressively, early radical treatment by nephrectomy has been proposed. However, in HLRCC it is currently unknown to what extent intensive surveillance leading to early detection and treatment of malignant lesions will lead to a reduction in mortality.
Systemic therapy for metastatic renal cell cancer has evolved rapidly over the last 5 years. Immunotherapy has largely been replaced by molecularly targeted agents that inhibit angiogenesis. These targeted agents were developed after it was recognized that VHL (Von Hippel-Lindau) mutations, present in most clear cell renal cancers, lead to accumulation of HIF (hypoxia inducible factor) and the activation of cellular mechanisms which, in normal conditions, become active as a response to hypoxia. One of these mechanisms is the induction of angiogenesis by production of the pro-angiogenic growth factors VEGF (vascular endothelial growth factor) and PDGF (platelet-derived growth factor). Currently, there is no standard systemic treatment for advanced non-clear cell renal cancer. However, there is evidence that biallelic loss of fumarate hydratase in HLRCC tumors results in VHL-independent HIF accumulation [26, 27]. Therefore, treatment with sunitinib, which blocks downstream effects of HIF activation, was chosen for this patient. For papillary renal cell cancer the reported response rates for sunitinib treatment ranged from 0 to 17% and progression-free survival from 1.6 to 11.9 months [28–30]. Unfortunately, in most of these studies, no distinction was made between type 1 and type 2 papillary renal cell cancer and specific data for HLRCC were lacking. HIF pathway can also be blocked by inhibiting its positive regulator mammalian target of rapamycin (mTOR) with temsirolimus (admistered intravenously). Exploratory subgroup analysis of a 3-arm phase III study comparing temsirolimus, interferon, and temsirolimus plus interferon revealed that patients with papillary renal cell cancer treated with temsirolimus had a better progression-free survival and overall survival than patients treated with interferon [31]. Unfortunately, also for temsirolimus no specific data for HLRCC are available. One HLRCC case with metastatic cancer unsuccessfully treated with temsirolinus plus a glycolytic inhibitor 2-deoxy-d-glucose was reported [24]. The course of disease in our patient was aggressive and the effect of treatment was disappointing. Prospective multicentre studies are necessary to assess the optimal treatment for patients with metastatic renal cancer associated with HLRCC. But it seems unrealistic to reach enough HLRCC cases for such a trail, unless a global network is established. Currently, a phase II study for HLRCC and sporadic papillary renal cell cancer (bevacizumab and erlotinib) (NCT01130519), and single agent phase II studies with foretinib (NCT00726323), sunitinib (NCT00541008) and everolimus (NCT00688753) for papillary renal cell cancer are ongoing in the USA and France.
In summary, the optimal management for HLRCC has not yet been established. Renal cancer risk is increased in this syndrome, and it has been suggested that perhaps renal cancer surveillance could be restricted to families with previous cases of renal cancer or specific FH mutations [7]. However, there is no evidence that family history or the type of FH mutation can predict renal cancer risk. Therefore, current data indicate that surveillance should be aimed at all FH mutation carriers. Apparently, renal cancer in this syndrome may occur between 10 and 20 years of age, and its successful early detection and treatment in an 11-year-old FH mutation carrier has been reported [11]. In general, presymptomatic genetic testing in children is performed if disease and death due to the hereditary condition can be prevented by an early start of preventive measures. The optimal policy for HLRCC has not yet been established. The early-onset cases reported previously and the present case have led us to consider the option of childhood genetic testing for HLRCC families with a pathogenic FH mutation and renal surveillance in mutation carriers. Since renal cancer risk is probably low in childhood some may want to restrict this procedure for families with early-onset renal cancer cases. MRI is clearly the most sensitive option and regular CT scanning is contra-indicated because of radiation dose. Since MRI might be distressing in childhood, we propose renal ultrasound every 6 months performed by an expert radiologist when surveillance is started at or around 10 years of age followed at a later age (16–18 years) by alternating MRI and ultrasound every 6 months. Close monitoring of our cohort of patients and collaboration of the centres studying HLRCC will provide data needed for future protocols on surveillance and treatment.
Acknowledgments
We thank the patient and her family for their permission to publish their data. And we thank Ms. Jacky Senior for providing editorial assistance during preparation of this manuscript.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Linehan WM, Srinivasan R, Schmidt LS. The genetic basis of kidney cancer: a metabolic disease. Nat Rev Urol. 2010;7:277–285. doi: 10.1038/nrurol.2010.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maher ER. Genetics of familial renal cancers. Nephron Exp Nephrol. 2011;118:e21–e26. doi: 10.1159/000320892. [DOI] [PubMed] [Google Scholar]
- 3.Merino MJ, Torres-Cabala C, Pinto P, et al. The morphologic spectrum of kidney tumors in hereditary leiomyomatosis and renal cell carcinoma (HLRCC) syndrome. Am J Surg Pathol. 2007;31:1578–1585. doi: 10.1097/PAS.0b013e31804375b8. [DOI] [PubMed] [Google Scholar]
- 4.Grubb RL, III, Franks ME, Toro J, et al. Hereditary leiomyomatosis and renal cell cancer: a syndrome associated with an aggressive form of inherited renal cancer. J Urol. 2007;177:2074–2080. doi: 10.1016/j.juro.2007.01.155. [DOI] [PubMed] [Google Scholar]
- 5.Lehtonen HJ. Hereditary leiomyomatosis and renal cell cancer: update on clinical and molecular characteristics. Fam Cancer. 2011;10:397–411. doi: 10.1007/s10689-011-9428-z. [DOI] [PubMed] [Google Scholar]
- 6.Tomlinson IPM, Alam NA, Rowan AJ, et al. The multiple leiomyoma consortium. Germline mutations in FH predispose to dominantly inherited uterine fibroids, skin leiomyomata and papillary renal cancer. Nat Genet. 2002;30:406–410. doi: 10.1038/ng849. [DOI] [PubMed] [Google Scholar]
- 7.Alam NA, Barclay E, Rowan AJ, et al. Clinical features of multiple cutaneous and uterine leiomyomatosis: an underdiagnosed tumor syndrome. Arch Dermatol. 2005;141:199–206. doi: 10.1001/archderm.141.2.199. [DOI] [PubMed] [Google Scholar]
- 8.Wei M-H, Toure O, Glenn GM, et al. Novel mutations in FH and expansion of the spectrum of phenotypes expressed in families with hereditary leiomyomatosis and renal cell cancer. J Med Genet. 2006;43:18–27. doi: 10.1136/jmg.2005.033506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bayley JP, Launonen V, Tomlinson IP. The FH mutation database: an online database of fumarate hydratase mutations involved in the MCUL (HLRCC) tumor syndrome and congenital fumarase deficiency. BMC Med Genet. 2008;9:20. doi: 10.1186/1471-2350-9-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vahteristo P, Koski TA, Naatsaari L, et al. No evidence for a genetic modifier for renal cancer risk in HLRCC syndrome. Fam Cancer. 2010;9:245–251. doi: 10.1007/s10689-009-9312-2. [DOI] [PubMed] [Google Scholar]
- 11.Alrashdi I, Levine S, Paterson J, et al. Hereditary leiomyomatosis and renal cell carcinoma: very early diagnosis of renal cancer in a paediatric patient. Fam Cancer. 2010;9:239–243. doi: 10.1007/s10689-009-9306-0. [DOI] [PubMed] [Google Scholar]
- 12.Smit DL, Mensenkamp AR, Badeloe S, et al. Hereditary leiomyomatosis and renal cell cancer in families referred for fumarate hydratase germline mutation analysis. Clin Genet. 2011;79:49–59. doi: 10.1111/j.1399-0004.2010.01486.x. [DOI] [PubMed] [Google Scholar]
- 13.Badeloe S, van Spaendonck-Zwarts KY, van Steensel MA, et al. Wilms tumour as a possible early manifestation of hereditary leiomyomatosis and renal cell cancer? Br J Dermatol. 2009;160:707–709. doi: 10.1111/j.1365-2133.2008.09000.x. [DOI] [PubMed] [Google Scholar]
- 14.Badeloe S, Bladergroen RS, Jonkman MF, et al. Hereditary multiple cutaneous leiomyoma resulting from novel mutations in the fumarate hydratase gene. J Dermatol Sci. 2008;51:139–143. doi: 10.1016/j.jdermsci.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 15.Reed WB, Walker R, Horowitz R. Cutaneous leiomyomata with uterine leiomyomata. Acta Dermat Venereol. 1973;53:409–416. [PubMed] [Google Scholar]
- 16.Chan I, Wong T, Martinez-Mir A, et al. Familial multiple cutaneous and uterine leiomyomas associated with papillary renal cancer. Clin Exp Dermatol. 2005;30:75–78. doi: 10.1111/j.1365-2230.2004.01675.x. [DOI] [PubMed] [Google Scholar]
- 17.Lehtonen HJ, Kiuru M, Ylisaukko-oja SK, et al. Increased risk of cancer in patients with fumarate hydratase germline mutation. J Med Genet. 2006;43:523–526. doi: 10.1136/jmg.2005.036400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lehtonen HJ, Blanco I, Piulats JM, et al. Conventional renal cancer in a patient with fumarate hydratase mutation. Hum Pathol. 2007;38:793–796. doi: 10.1016/j.humpath.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 19.Al Refae M, Wong N, Patenaude F, et al. Hereditary leiomyomatosis and renal cancer: an unusual and aggressive form of hereditary renal carcinoma. Nat Clin Pract Oncol. 2007;4:256–261. doi: 10.1038/ncponc0773. [DOI] [PubMed] [Google Scholar]
- 20.Ghaninejad H, Moeineddin F, Rajaee A, et al. Hereditary leiomyomatosis and renal cell carcinoma syndrome: a case report. Dermatol Online J. 2008;14:16. [PubMed] [Google Scholar]
- 21.Ahvenainen T, Lehtonen HJ, Lehtonen R, et al. Mutation screening of fumarate hydratase by multiplex ligation-dependent probe amplification: detection of exonic deletion in a patient with leiomyomatosis and renal cell cancer. Cancer Genet Cytogenet. 2008;183:83–88. doi: 10.1016/j.cancergencyto.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 22.Rongioletti F, Fausti V, Ferrando B, et al. A novel missense mutation in fumarate hydratase in an Italian patient with a diffuse variant of cutaneous leiomyomatosis (Reed’s syndrome) Dermatology. 2010;221:378–380. doi: 10.1159/000321336. [DOI] [PubMed] [Google Scholar]
- 23.Gardie B, Remenieras A, Kattygnarath D, et al. Novel FH mutations in families with hereditary leiomyomatosis and renal cancer (HLRCC) and patients with isolated type 2 papillary renal cell carcinoma. J Med Genet. 2011;48:226–234. doi: 10.1136/jmg.2010.085068. [DOI] [PubMed] [Google Scholar]
- 24.Yamasaki T, Tran TAT, Oz OK, et al. Exploring a glycolytic inhibitor for the treatment of an FH-deficient type-2 papillary RCC. Nat Rev Urol. 2011;8:165–171. doi: 10.1038/nrurol.2010.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meister M, Choyke P, Anderson C, et al. Radiological evaluation, management, and surveillance of renal masses in Von-Hippel-Lindau disease. Clin Radiol. 2009;64:589–600. doi: 10.1016/j.crad.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 26.Isaacs JS, Jung YJ, Mole DR, et al. HIF overexpression correlates with biallelic loss of fumarate hydratase in renal cancer: novel role of fumarate in regulation of HIF stability. Cancer Cell. 2005;8:143–153. doi: 10.1016/j.ccr.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 27.Selak MA, Armour SM, MacKenzie ED, et al. Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-alpha prolyl hydroxylase. Cancer Cell. 2005;7:77–85. doi: 10.1016/j.ccr.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 28.Choueiri TK, Atkins MB. Targeted therapies: sunitinib in RCC-expanded access equals expanded benefit? Nat Rev Clin Oncol. 2009;6:679–680. doi: 10.1038/nrclinonc.2009.170. [DOI] [PubMed] [Google Scholar]
- 29.Plimack ER, Tannir N, Lin E, Bekele BN, Jonasch E. Patterns of disease progression in metastatic renal cell carcinoma patients treated with antivascular agents and interferon: impact of therapy on recurrence patterns and outcome measures. Cancer. 2009;115:1859–1866. doi: 10.1002/cncr.24211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ravaud A, Bello CL. Exposure-response relationships in patients with metastatic renal cell carcinoma receiving sunitinib: maintaining optimum efficacy in clinical practice. Anticancer Drugs. 2011;22:377–383. doi: 10.1097/CAD.0b013e3283442039. [DOI] [PubMed] [Google Scholar]
- 31.Dutcher JP, de Souza P, McDermott D, et al. Effect of temsirolimus versus interferon-alpha on outcome of patients with advanced renal cell carcinoma of different tumor histologies. Med Oncol. 2009;26:202–209. doi: 10.1007/s12032-009-9177-0. [DOI] [PubMed] [Google Scholar]
- 32.Chuang GS, Martinez-Mir A, Engler DE, et al. Multiple cutaneous and uterine leiomyomata resulting from missense mutations in the fumarate hydratase gene. Clin Exp Dermatol. 2006;31:118–121. doi: 10.1111/j.1365-2230.2005.01977.x. [DOI] [PubMed] [Google Scholar]