Abstract
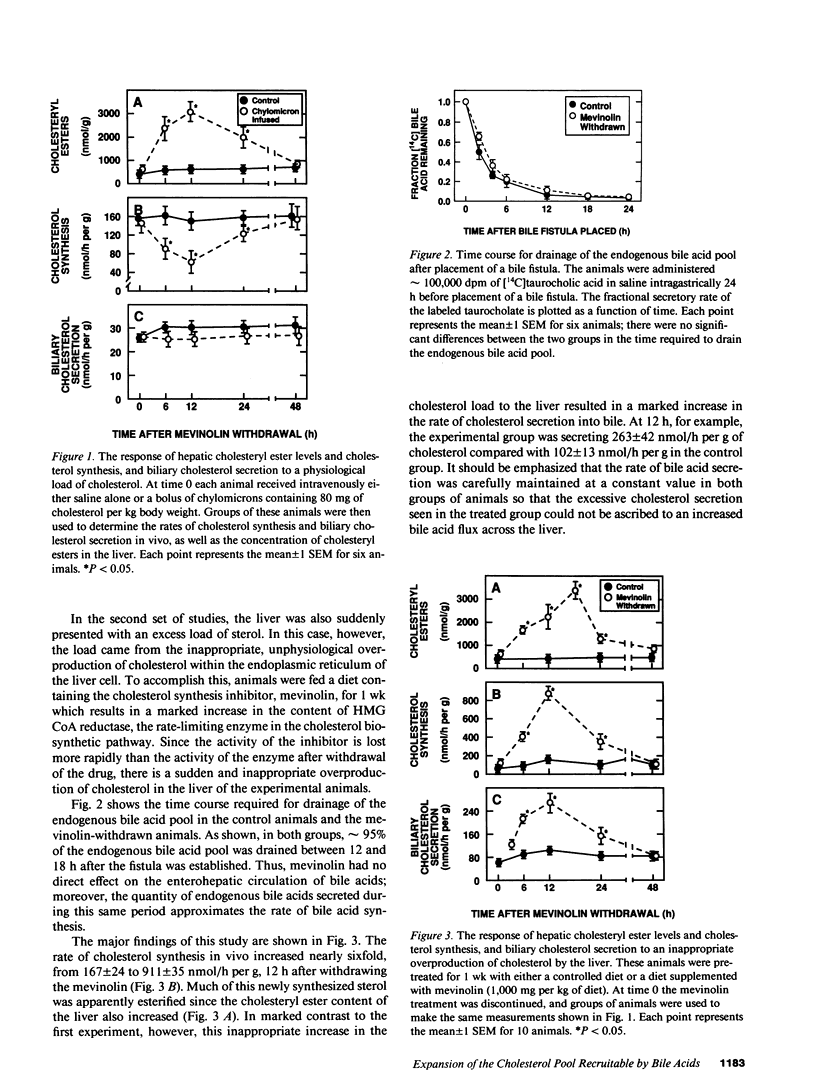
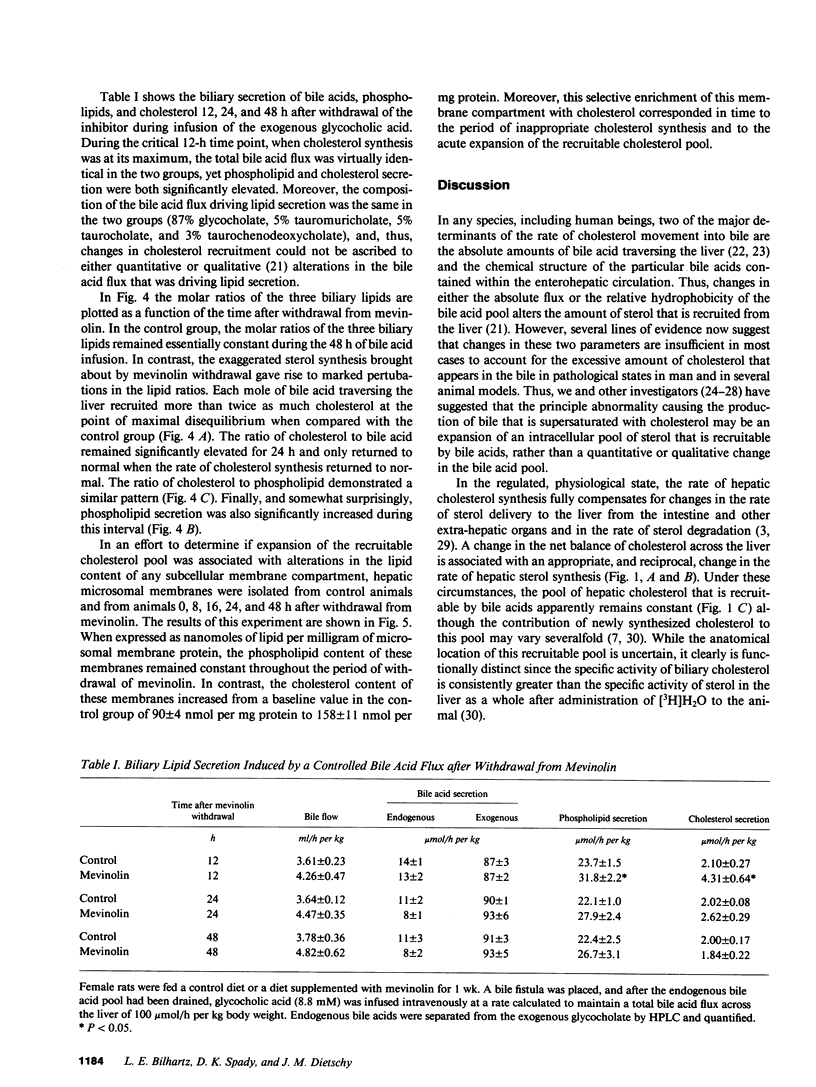
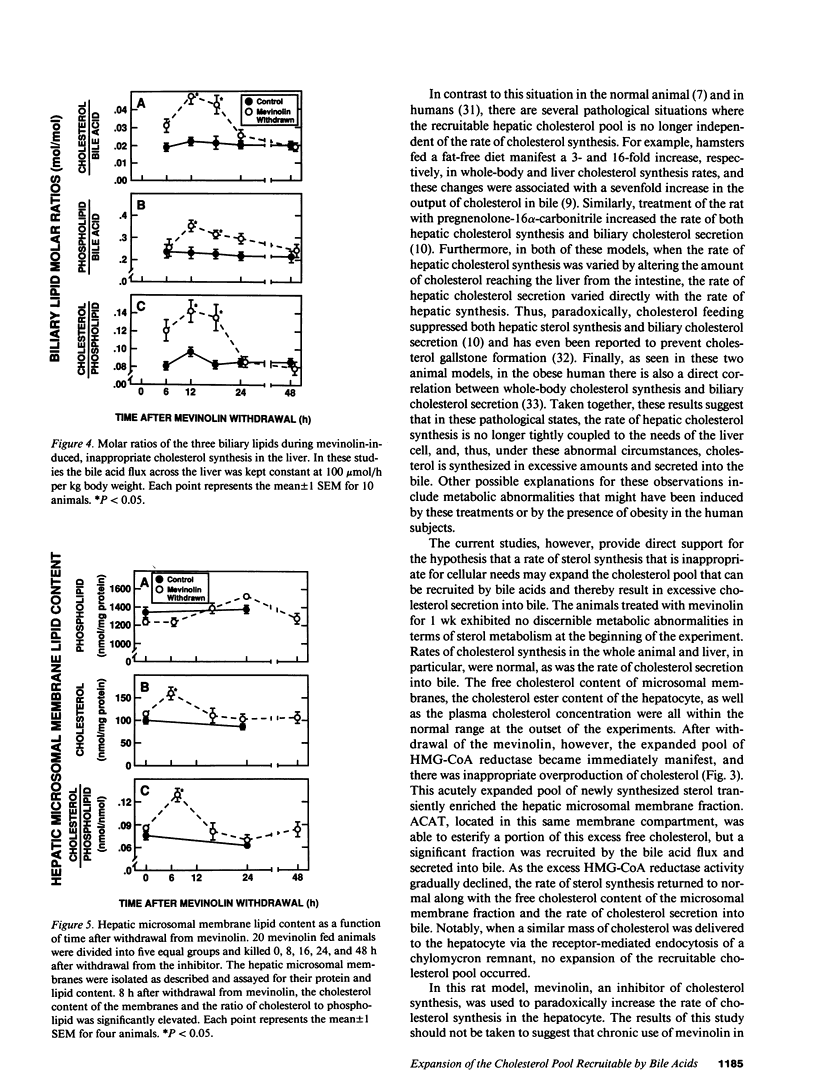
These studies test the hypothesis that a major determinant of excessive biliary cholesterol secretion is a level of hepatic sterol synthesis that is inappropriately high relative to the needs of the liver cell for preserving cholesterol balance. Biliary cholesterol secretion was measured in vivo in two models after loading the hepatocyte with sterol by two different mechanisms. In the first model, cholesterol was delivered physiologically to the liver in chylomicron remnants. This resulted in a sixfold increase in cholesteryl ester content and marked suppression of cholesterol synthesis, but biliary cholesterol secretion remained essentially constant. In the second model, 3-hydroxy-3-methyl-glutaryl CoA reductase levels in the liver were markedly increased by chronic mevinolin (lovastatin) administration. Withdrawal of the inhibitor resulted in a sudden fivefold increase in the rate of sterol synthesis in the liver of the experimental animals that was inappropriately high for cellular needs. This excessive synthesis, in turn, was accompanied by a fivefold increase in the cholesteryl ester content, enrichment of microsomal membranes with cholesterol and, most importantly, by a threefold increase in the rate of biliary sterol secretion. As the rate of sterol synthesis gradually returned to normal over 48 h, the cholesterol ester content, the lipid composition of the microsomal membranes, and rate of cholesterol secretion into bile also returned to baseline values. These results further support the concept of functional compartmentalization of cholesterol in the hepatocyte. Derangements that cause an inappropriately high rate of sterol synthesis in the endoplasmic reticulum may lead to an expansion of that pool of cholesterol that is recruitable by bile acids and, hence, to greater situation of the bile.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen J. M., Dietschy J. M. Relative importance of high and low density lipoproteins in the regulation of cholesterol synthesis in the adrenal gland, ovary, and testis of the rat. J Biol Chem. 1978 Dec 25;253(24):9024–9032. [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Bennion L. J., Grundy S. M. Risk factors for the development of cholelithiasis in man (second of two parts). N Engl J Med. 1978 Nov 30;299(22):1221–1227. doi: 10.1056/NEJM197811302992205. [DOI] [PubMed] [Google Scholar]
- Bilhartz L. E. Cholesterol gallstone disease: the current status of nonsurgical therapy. Am J Med Sci. 1988 Jul;296(1):45–56. doi: 10.1097/00000441-198807000-00009. [DOI] [PubMed] [Google Scholar]
- Bilhartz L. E., Dietschy J. M. Bile salt hydrophobicity influences cholesterol recruitment from rat liver in vivo when cholesterol synthesis and lipoprotein uptake are constant. Gastroenterology. 1988 Sep;95(3):771–779. doi: 10.1016/s0016-5085(88)80027-1. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. A receptor-mediated pathway for cholesterol homeostasis. Science. 1986 Apr 4;232(4746):34–47. doi: 10.1126/science.3513311. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L., Dietschy J. M. Active and inactive forms of 3-hydroxy-3-methylglutaryl coenzyme A reductase in the liver of the rat. Comparison with the rate of cholesterol synthesis in different physiological states. J Biol Chem. 1979 Jun 25;254(12):5144–5149. [PubMed] [Google Scholar]
- Chin D. J., Luskey K. L., Anderson R. G., Faust J. R., Goldstein J. L., Brown M. S. Appearance of crystalloid endoplasmic reticulum in compactin-resistant Chinese hamster cells with a 500-fold increase in 3-hydroxy-3-methylglutaryl-coenzyme A reductase. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1185–1189. doi: 10.1073/pnas.79.4.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dam H., Prange I., Sondergaard E. Alimentary production of gallstones in hamsters. 27. Influence of supplementation of the gallstone producing diet with squalene, cholesterol, certain other sterols, fish oil fatty acid ethyl esters, and modification of the basal diet on gallstone production and levels of cholesterol in serum and liver. Z Ernahrungswiss. 1974 Dec;13(4):208–236. doi: 10.1007/BF02021193. [DOI] [PubMed] [Google Scholar]
- Dietschy J. M., Spady D. K. Measurement of rates of cholesterol synthesis using tritiated water. J Lipid Res. 1984 Dec 15;25(13):1469–1476. [PubMed] [Google Scholar]
- Duane W. C., Hunninghake D. B., Freeman M. L., Pooler P. A., Schlasner L. A., Gebhard R. L. Simvastatin, a competitive inhibitor of HMG-CoA reductase, lowers cholesterol saturation index of gallbladder bile. Hepatology. 1988 Sep-Oct;8(5):1147–1150. doi: 10.1002/hep.1840080531. [DOI] [PubMed] [Google Scholar]
- Grundy S. M., Bilheimer D. W. Inhibition of 3-hydroxy-3-methylglutaryl-CoA reductase by mevinolin in familial hypercholesterolemia heterozygotes: effects on cholesterol balance. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2538–2542. doi: 10.1073/pnas.81.8.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann A. F., Roda A. Physicochemical properties of bile acids and their relationship to biological properties: an overview of the problem. J Lipid Res. 1984 Dec 15;25(13):1477–1489. [PubMed] [Google Scholar]
- Jeske D. J., Dietschy J. M. Regulation of rates of cholesterol synthesis in vivo in the liver and carcass of the rat measured using [3H]water. J Lipid Res. 1980 Mar;21(3):364–376. [PubMed] [Google Scholar]
- Kern F., Jr, Everson G. T. Contraceptive steroids increase cholesterol in bile: mechanisms of action. J Lipid Res. 1987 Jul;28(7):828–839. [PubMed] [Google Scholar]
- Maton P. N., Reuben A., Dowling R. H. Relationship between hepatic cholesterol synthesis and biliary cholesterol secretion in man: hepatic cholesterol synthesis is not a major regulator of biliary lipid secretion. Clin Sci (Lond) 1982 May;62(5):515–519. doi: 10.1042/cs0620515. [DOI] [PubMed] [Google Scholar]
- Nervi F. O., Dietschy J. M. The mechanisms of and the interrelationship between bile acid and chylomicron-mediated regulation of hepatic cholesterol synthesis in the liver of the rat. J Clin Invest. 1978 Apr;61(4):895–909. doi: 10.1172/JCI109015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nervi F., Marinović I., Rigotti A., Ulloa N. Regulation of biliary cholesterol secretion. Functional relationship between the canalicular and sinusoidal cholesterol secretory pathways in the rat. J Clin Invest. 1988 Dec;82(6):1818–1825. doi: 10.1172/JCI113797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins S. J., Fasulo J. M., Collins M. A., Patton G. M. Evidence for separate pathways of transport of newly synthesized and preformed cholesterol into bile. J Biol Chem. 1985 Jun 10;260(11):6511–6513. [PubMed] [Google Scholar]
- Roitelman J., Shechter I. Altered kinetic properties of rat liver 3-hydroxy-3-methylglutaryl coenzyme A reductase following dietary manipulations. J Biol Chem. 1986 Apr 15;261(11):5061–5066. [PubMed] [Google Scholar]
- Sampson W. J., Suffolk R. A., Bowers P., Houghton J. D., Botham K. M., Suckling K. E. The role of acyl-CoA: cholesterol acyltransferase in the metabolism of free cholesterol to cholesteryl esters or bile acids in primary cultures of rat hepatocytes. Biochim Biophys Acta. 1987 Jul 13;920(1):1–8. doi: 10.1016/0005-2760(87)90304-3. [DOI] [PubMed] [Google Scholar]
- Spady D. K., Dietschy J. M. Interaction of dietary cholesterol and triglycerides in the regulation of hepatic low density lipoprotein transport in the hamster. J Clin Invest. 1988 Feb;81(2):300–309. doi: 10.1172/JCI113321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spady D. K., Stange E. F., Bilhartz L. E., Dietschy J. M. Bile acids regulate hepatic low density lipoprotein receptor activity in the hamster by altering cholesterol flux across the liver. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1916–1920. doi: 10.1073/pnas.83.6.1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spady D. K., Turley S. D., Dietschy J. M. Dissociation of hepatic cholesterol synthesis from hepatic low-density lipoprotein uptake and biliary cholesterol saturation in female and male hamsters of different ages. Biochim Biophys Acta. 1983 Oct 11;753(3):381–392. doi: 10.1016/0005-2760(83)90062-0. [DOI] [PubMed] [Google Scholar]
- Stone B. G., Erickson S. K., Craig W. Y., Cooper A. D. Regulation of rat biliary cholesterol secretion by agents that alter intrahepatic cholesterol metabolism. Evidence for a distinct biliary precursor pool. J Clin Invest. 1985 Nov;76(5):1773–1781. doi: 10.1172/JCI112168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turley S. D., Andersen J. M., Dietschy J. M. Rates of sterol synthesis and uptake in the major organs of the rat in vivo. J Lipid Res. 1981 May;22(4):551–569. [PubMed] [Google Scholar]
- Turley S. D., Dietschy J. M. Modulation of the stimulatory effect of pregnenolone-16 alpha-carbonitrile on biliary cholesterol output in the rat by manipulation of the rate of hepatic cholesterol synthesis. Gastroenterology. 1984 Aug;87(2):284–292. [PubMed] [Google Scholar]
- Turley S. D., Dietschy J. M. Regulation of biliary cholesterol output in the rat: dissociation from the rate of hepatic cholesterol synthesis, the size of the hepatic cholesteryl ester pool, and the hepatic uptake of chylomicron cholesterol. J Lipid Res. 1979 Nov;20(8):923–934. [PubMed] [Google Scholar]
- Turley S. D., Dietschy J. M. The contribution of newly synthesized cholesterol to biliary cholesterol in the rat. J Biol Chem. 1981 Mar 10;256(5):2438–2446. [PubMed] [Google Scholar]
- Turley S. D., Spady D. K., Dietschy J. M. Alteration of the degree of biliary cholesterol saturation in the hamster and rat by manipulation of the pools of preformed and newly synthesized cholesterol. Gastroenterology. 1983 Feb;84(2):253–264. [PubMed] [Google Scholar]
- Wagner C. I., Trotman B. W., Soloway R. D. Kinetic analysis of biliary lipid excretion in man and dog. J Clin Invest. 1976 Feb;57(2):473–477. doi: 10.1172/JCI108299. [DOI] [PMC free article] [PubMed] [Google Scholar]



