Abstract
Background.
Older persons have an increased risk of developing respiratory impairment because the aging lung is likely to have experienced exposures to environmental toxins as well as reductions in physiological capacity.
Methods.
Systematic review of risk factors and measures of pulmonary function that are most often considered when defining respiratory impairment in aging populations.
Results.
Across the adult life span, there are frequent exposures to environmental toxins, including tobacco smoke, respiratory infections, air pollution, and occupational dusts. Concurrently, there are reductions in physiological capacity that may adversely affect ventilatory control, respiratory muscle strength, respiratory mechanics, and gas exchange. Recent work has provided a strong rationale for defining respiratory impairment as an age-adjusted reduction in spirometric measures of pulmonary function that are independently associated with adverse health outcomes. Specifically, establishing respiratory impairment based on spirometric Z-scores has been shown to be strongly associated with respiratory symptoms, frailty, and mortality. Alternatively, respiratory impairment may be defined by the peak expiratory flow, as measured by a peak flow meter. The peak expiratory flow, when expressed as a Z-score, has been shown to be strongly associated with disability and mortality. However, because it has a reduced diagnostic accuracy, peak expiratory flow should only define respiratory impairment when spirometry is not readily available or an older person cannot adequately perform spirometry.
Conclusions.
Aging is associated with an increased risk of developing respiratory impairment, which is best defined by spirometric Z-scores. Alternatively, in selected cases, respiratory impairment may be defined by peak expiratory flow, also expressed as a Z-score.
Keywords: Spirometry, Respiratory, Impairment, Z-scores
The aging lung is likely to have experienced frequent exposures to environmental toxins, particularly tobacco smoke and respiratory infections, as well as substantial reductions in physiological capacity, particularly respiratory mechanics (eg, increased stiffness of the chest wall and decreased elastic recoil of the lung; 1–3). Because of cumulative effects, older persons are at an increased risk of developing respiratory impairment.
To maximize clinical applicability, respiratory impairment is best defined as an age-adjusted reduction in pulmonary function that is independently associated with adverse health outcomes. The rationale for this definition is twofold. First, to more accurately establish an underlying respiratory disease, the reduction in pulmonary function must be distinguished from the reduction that is due to normal aging (1,2). Second, to avoid inappropriate and potentially harmful pharmacotherapy, as well as delays in the consideration of other diagnoses, the threshold that establishes an age-adjusted reduction in pulmonary function should be linked to adverse health outcomes (3–8). This approach is especially relevant in older populations given their high prevalence of multimorbidity and polypharmacy (9–12).
This article reviews the relevant risk factors and measures of pulmonary function that are most often considered when establishing respiratory impairment. Because the focus is on the aging lung, and given the above definition of respiratory impairment, we also review the reduction in physiological capacity that normally occurs across the adult life span (“normal aging”). To enhance readability, common respiratory terminology is summarized in Table 1. Unless otherwise specified, older persons refer to those aged ≥65 years.
Table 1.
Abbreviations and Explanations of Common Respiratory Terminology
| COPD | Chronic obstructive pulmonary disease |
| Spirometry | |
| FVC | Forced vital capacity; the lung volume that is delivered during a forceful and complete exhalation, starting from maximal inspiration |
| FEV1 | Forced expiratory volume in 1 s; the lung volume that is delivered in the first second of an FVC maneuver |
| FEV1/FVC | The ratio of FEV1 to FVC |
| Normal spirometry | Defined by a normal FEV1/FVC and FVC |
| Airflow limitation | Defined by a reduced FEV1/FVC, with severity subsequently staged according to FEV1; includes diseases that lead to airways obstruction, most commonly COPD, asthma, bronchiectasis, and cystic fibrosis |
| Restrictive pattern | Suggested by a normal FEV1/FVC but reduced FVC; includes diseases that adversely affect the chest wall (kyphosis, scoliosis, and ankylosing spondylitis), respiratory muscles (sarcopenia, myasthenia gravis, and diaphragmatic paralysis), pleura (effusions and fibrosis), and interstitium (edema and fibrosis)—among others |
| PEF | Peak expiratory flow; the maximal expiratory flow delivered with maximal force, starting from maximal inspiration (measured by a peak flow meter). Reductions in PEF may indicate airways obstruction, respiratory muscle weakness, and disorders that limit the expansion of the chest wall or poor effort |
| Body plethysmography* | |
| TLC | Total lung capacity; the lung volume after a full inhalation. When reduced, it confirms restrictive lung disease; when increased, it establishes hyperinflation (most often due to airflow limitation) |
| FRC | Functional residual capacity; the lung volume after a normal (passive) exhalation. When increased, it indicates hyperinflation (most often due to airflow limitation) |
| RV | Residual volume; the lung volume after a full exhalation. When increased, it indicates air trapping (most often due to airflow limitation) |
| DLCO | Diffusion capacity for carbon monoxide; evaluates the oxygen transfer capacity of the alveolar– capillary interface. This may be reduced in interstitial lung disease, COPD, and pulmonary hypertension |
| MIP | Maximal inspiratory pressure; reductions indicate respiratory muscle (diaphragmatic) weakness |
| GOLD | Global initiative for obstructive lung disease† |
| ATS/ERS | American thoracic and European respiratory societies† |
| LMS | Lambda–mu–sigma method† |
Notes: *An alternative is gas dilution, but this lacks diagnostic accuracy in chronic obstructive pulmonary disease.
Provide criteria for defining normal spirometry, airflow limitation, and restrictive pattern.
RISK FACTORS
The most frequent risk factors that can lead to respiratory impairment are environmental exposures, including tobacco smoke, respiratory infections, air pollution (indoor and outdoor), and occupational dusts (13–19). The respiratory system is particularly vulnerable because it has the largest interface with the environment—the alveolar surface area is 85 m2 versus the skin at 1.8 m2 (20). In vulnerable individuals, these environmental exposures induce lung inflammation and, in turn, reductions in pulmonary function that may be subsequently associated with adverse outcomes (1,2,13–26).
Older persons have high rates of environmental exposures (21–36). For example, the current generation of older Americans had a prior smoking rate of about 50% in the mid-1960s, which has subsequently decreased to 9% by 2008 (27). On average, in recent cohorts of older persons, the prevalence of ever-smokers and never-smokers is 56% and 44%, respectively (24). A history of nonsmoking, however, does not exclude prior smoking exposure. In 2008, among nonsmoking, Americans who were ≥60 years, 32% had a documented exposure to secondhand smoke, also known as environmental tobacco smoke (28). Exposure to tobacco smoke, including environmental tobacco smoke, is a leading cause of chronic lung disease (eg, chronic obstructive pulmonary disease [COPD]) as well as cardiovascular disease and cancer (27,28).
Respiratory infections are also highly prevalent in older populations. In a large cohort of community-living older persons, 27% reported a history of pneumonia (26). From the period 1979 through 2001, persons aged ≥75 years had a 10-fold or greater increase in the rate of influenza-associated hospitalization relative to any other age-group (29). Outdoor air pollution is another common exposure, with a surrogate measure being urban residence (30–33). In 2009, 23% of older Americans reported as living in a major city (30). Additional exposures include a prior high-risk occupation (eg, freight, stock, and material handlers, or metal and wood workers) and the use of biomass fuel for indoor cooking or heating (17,33,34). For these exposures, prevalence rates in older persons are currently available for nonsmokers only, previously reported at 12% and 18%, respectively (33).
NORMAL AGING
Across the adult life span, there are reductions in physiological capacity, including ventilatory control, respiratory muscle strength, respiratory mechanics, and gas exchange. These age-related changes have two important implications. First, from a clinical perspective, the age-related decline in physiological reserve may increase the vulnerability of developing a respiratory impairment, particularly in response to tobacco smoke or a respiratory infection (13–16,35,36). Second, from a diagnostic perspective, the age-related decline in physiological capacity must be considered before attributing a reduction in pulmonary function to a pathological process (1,2).
Ventilatory Control
Several studies involving healthy older persons have evaluated age-related changes in ventilatory control, as measured by the P100 and minute ventilation (VE) responses to hypoxemia and hypercapnia. The P100 is the inspiratory pressure that is generated at the mouth 100 ms after airway occlusion and is a validated index of central respiratory drive (37). Based on the P100, prior work has shown that healthy persons aged 65–79 years had a ≥50% reduction in the response to hypoxemia and hypercapnia relative to those aged 22–29 years (38). Similarly, prior work based on VE has shown that healthy men aged 64–73 years had a ≥41% reduction in the response to hypoxemia and hypercapnia relative to those aged 22–30 years (39). In another study that included men and women, healthy persons aged 65–76 years had a nearly one-third reduction in the VE response to hypercapnia relative to those aged 21–37 years (40). Nonetheless, other studies have failed to confirm age-related differences in ventilatory control (41–43) or shown instead that the age-related reduction in ventilatory control is due to a decrease in peripheral CO2 sensitivity (44). These conflicting results likely reflect differences in the techniques used to evaluate ventilatory control (44–46) as well as small sample sizes. For example, in contrast to the rebreathing technique (38–42), only two studies used the dynamic end-tidal forcing technique, which regulates more accurately the end-tidal PO2 and PCO2 and, in turn, evaluates more rigorously ventilatory control (43,44). The results of these two studies suggest an age-related reduction in peripheral CO2 sensitivity rather than a decrease in central respiratory drive (43,44). However, these same two studies only evaluated 5 and 11 older participants, respectively, with all but one being male (43,44).
Other investigators have posited that the increased prevalence of central sleep apnea among older persons suggests an age-related adverse-effect on ventilatory control specifically when asleep (47–50). Based on a frequency of ≥2.5 central apneic events per hour of sleep, the prevalence of central sleep apnea is 12.1% for persons aged 65–100 years, but only 1.7% for those aged 45–64 years (47). The age-related increase in central sleep apnea, including its potential role as a sleep-related cause of death, may be due to a reduction in the number of medullary ventral respiratory neurons (48–50). Preliminary work has shown that aging may be associated with a loss of gray matter volume in brain regions that are involved in breathing functions (48–50). Nonetheless, a more likely mechanism for the age-related increase in central sleep apnea is an exaggerated response to CO2 (ie, increased controller gain), including impaired cerebrovascular reactivity, as seen in left ventricular systolic dysfunction (51).
Finally, prior research comparing older persons aged 60–80 years with those aged 20–46 years has shown an age-related reduction in the awareness of methacholine-provoked bronchoconstriction characterized by older persons having less severe respiratory symptoms, despite having greater reductions in lung function (ie, forced expiratory volume in 1 s, FEV1; 52,53). The mechanisms underlying the reduced awareness are unknown but could involve a diminished feedback from peripheral mechanoreceptors or chemoreceptors (44,52).
Respiratory muscles.—
Several large studies have shown that advancing age is independently associated with a reduction in both the maximal inspiratory pressure, a measure of inspiratory muscle strength, and the maximal expiratory pressure, a measure of expiratory muscle strength (54–56). For example, for a man of average height and weight, maximal inspiratory pressure values at age 50 and 80 years are 111 and 70 cm H2O, respectively (56). The age-related reductions in maximal inspiratory pressure and maximal expiratory pressure are likely a consequence of impaired respiratory mechanics (discussed later) and sarcopenia (1,57–59). Sarcopenia refers to the loss of muscle mass and function, potentially due to the reduced muscle protein synthesis, increased muscle proteolysis, motor neuron loss, and/or increased muscle fat content (58).
Respiratory Mechanics
Age-related reductions in physiological capacity are most pronounced in respiratory mechanics. Developmentally, over the course of the adult life span, there is a progressive increase in the rigidity of the chest wall and decrease in the elastic recoil of the lung (1,2,57,60). These age-related changes in respiratory mechanics lead to airflow limitation, defined by a decreased FEV1 and ratio of FEV1 to forced vital capacity (FVC), as well as to air trapping and hyperinflation, defined by an increase in residual volume and functional residual capacity, respectively (1,2,57,60). In addition, because of a loss in supporting elastic tissue, there is an increase in the “closing volume,” defined as the lung volume above which there is premature collapse of small airways—most evident in the gravity-dependent regions of the lung (57,61). The more important effects of these age-related changes include a decline in FEV1 of up to 30 ml/year, an increase in residual volume of about 50% between ages 20 and 70 years, and an increase in the closing volume, such that by age 65 years, it approaches the functional residual capacity (even during normal tidal breathing; 2,57,60,61).
These changes in FEV1, residual volume, and closing volume impose substantial limitations on the aging lung. As the FEV1 declines, the tidal breathing response during exercise is reduced because of expiratory flow limitation and dynamic hyperinflation (57,62). As the residual volume increases, the curvature of the diaphragm is reduced, shifting the length–tension relationship to a shorter length and, in turn, decreasing the force generating capacity of the muscle (1,57,59). As the closing volume increases, the small airways are more likely to collapse prematurely, leading to a reduced ratio of alveolar ventilation to lung perfusion (VA/Q) and, in turn, decreasing oxygenation (57,61,63,64).
Gas Exchange
Gas exchange is most often dependent on an appropriate matching of ventilation with lung perfusion (20). Using measures of ventilation and lung perfusion, prior work has demonstrated an age-related increase in ventilation-perfusion inequality, characterized by a heterogeneous distribution of lung units having high and low VA/Q ratios (65–69). The ventilation–perfusion inequality is associated with changes in the pulmonary circulation (70–72). In a study involving 3,790 participants aged 1–89 years who had normal echocardiograms, the pulmonary arterial systolic pressure rose an average of about 1 mm Hg per decade of age, yielding an upper limit of 40 mm Hg in those older than 50 years (71). This rise in pulmonary arterial systolic pressure has been attributed to an increase in pulmonary vascular resistance (71).
The age-related increase in ventilation–perfusion inequality may coexist with a decrease in the diffusion capacity of the lung for carbon monoxide (DLCO), a measure of the transfer capacity of oxygen across the alveolar–capillary interface (57,73). In a study involving 74 healthy older participants aged 69–104 years and 55 healthy young participants aged 20–40 years, there was a 50% reduction in the DLCO for older persons relative to younger persons (73). The reduction in DLCO may be due to declines in the alveolar surface area and, possibly, in the density of lung capillaries (72,73).
Subtle but important changes in the arterial tension for carbon dioxide (PaCO2) occur across the adult life span (63,64). To maintain the PaCO2 in the normal range, total minute ventilation (VE) must increase with advancing age (62–72). PaCO2 is largely dependent on the VE, which is the sum of alveolar ventilation (VA) and dead space ventilation (VD; 20). In contrast to VD, VA participates in CO2 elimination because it includes areas of the lung that are both adequately ventilated and perfused. As a consequence of the age-related increase in ventilation–perfusion inequality, specifically in lung units having a high VA/Q ratio, normal aging is associated with an increase in VD (62–72). This phenomenon is exacerbated during exercise by a concurrent age-related reduction in cardiac output (62). At peak exercise, for example, VD is 2.5 times higher in an older versus younger person—32 versus 14 L/min, respectively (62). In response to the increase in VD, VE must increase to maintain VA (20). Although a normal PaCO2 is maintained, this age-related increase in ventilatory requirement further reduces the ventilatory reserve of the aging lung.
Subtle but important changes in the arterial tension for oxygen (PaO2) also occur across the adult life span (63,64). In the setting of the age-related increase in ventilation–perfusion inequality, specifically in lung units having a low VA/Q ratio, the PaO2 declines from an average of 100 mm Hg in young adults (18–24 years) to 89 mm Hg in older adults (≥65 years; 61,64,67–69). Nonetheless, O2 saturation is relatively normal with advancing age because the PaO2 levels remain on the flat portion of the O2 dissociation curve (20). Otherwise, the age-related decline in DLCO is likely to contribute minimally to a decrease in PaO2, except perhaps when oxygen consumption is substantially elevated, as during maximal exercise (62,73).
PULMONARY FUNCTION
Because the environmental toxins and aging predominantly impair respiratory mechanics, including airflow limitation and restriction, respiratory impairment is most often evaluated by spirometry and, in select cases, by peak expiratory flow. Moreover, because aging can also adversely affect ventilatory control, respiratory muscle strength, and gas exchange, additional tests of pulmonary function may be required in order to fully evaluate respiratory impairment.
Spirometry
Due to technological advances, spirometry may be conveniently performed using a portable handheld device. In spirometric testing, the individual is instructed to perform a series of forceful and complete exhalation maneuvers, starting from maximal inspiration (74,75). Based on performance guidelines published by the American Thoracic and European Respiratory Societies (ATS/ERS), these breathing maneuvers generate two specific lung volumes, namely the FVC (an untimed lung volume) and FEV1 (a timed lung volume), as defined in Table 1 (74,75).
Diseases that lead to a greater reduction in the timed lung volume than the untimed lung volume include COPD, asthma, bronchiectasis, and cystic fibrosis (76). In this setting, the FEV1/FVC is reduced and defines airflow limitation that is due to airways obstruction (75). Diseases that lead to comparable reductions in the timed and untimed lung volumes include those which affect the chest wall (kyphosis, scoliosis, or ankylosing spondylitis), respiratory muscles (sarcopenia, myasthenia gravis, or diaphragmatic paralysis), pleura (effusions or fibrosis), interstitium (edema, inflammation, or fibrosis) and circulation (pulmonary hypertension)—among others (76). In this setting, the FEV1/FVC is normal but FVC is reduced, suggesting a restrictive pattern (75). Otherwise, normal spirometry is defined by both a normal FEV1/FVC and FVC (75).
Contemporary practice.—
The current standard for establishing spirometric respiratory impairment is based on criteria published by the Global Initiative for Obstructive Lung Disease (GOLD) and a combined task force from the ATS/ERS (13,75). As shown in Table 2, GOLD establishes respiratory impairment based on an FEV1/FVC threshold of 0.70 and an FVC threshold of 80 percent predicted (%Pred), with airflow limitation further staged according to FEV1 thresholds of 80, 50, and 30%Pred (13). Alternatively, the ATS/ERS establishes respiratory impairment based on a threshold for both FEV1/FVC and FVC set at the lower limit of normal (LLN), with airflow limitation further staged according to FEV1 thresholds of 70, 60, 50, and 35%Pred (75). The LLN is calculated by the ATS/ERS as the 5th percentile distribution of reference values (75,77). The %Pred is calculated by both GOLD and the ATS/ERS as follows (13,75): ([measured/predicted] × 100).
Table 2.
Criteria for Establishing Normal Spirometry and Respiratory Impairment (airflow limitation and restrictive pattern)
| Spirometric Criteria |
|||||
| Airflow Limitation | |||||
| Method | Normal | Mild | Moderate | Severe | Restrictive Pattern |
| GOLD | |||||
| FEV1/FVC | ≥0.70 | <0.70 | ≥0.70 | ||
| FEV1 | NA | ≥80%Pred | 50–79%Pred | <50%Pred | NA |
| FVC | ≥80 %Pred | NA | <80%Pred | ||
| ATS/ERS | |||||
| FEV1/FVC | ≥ATS/ERS-LLN | <ATS/ERS-LLN | ≥ATS/ERS-LLN | ||
| FEV1 | NA | ≥70%Pred | 50–69%Pred | <50%Pred | NA |
| FVC | ≥ATS/ERS-LLN | NA | <ATS/ERS-LLN | ||
| LMS | |||||
| FEV1/FVC | ≥5 LMS tile | <5 LMS tile | ≥5 LMS tile | ||
| FEV1 | NA | ≥5 LMS tile | 0.5–4.9 LMS tile | <0.5 LMS tile | NA |
| FVC | ≥5 LMS tile | NA | <5 LMS tile | ||
Notes: ATS/ERS = American thoracic and European respiratory societies; ATS/ERS-LLN = lower limit of normal as calculated by the ATS/ERS (ie, 5th percentile distribution of reference values); FEV1/FVC = ratio of forced expiratory volume in 1 s (FEV1) to forced vital capacity (FVC); GOLD = global initiative for obstructive lung disease; LMS = lambda–Mu–Sigma; LMS tile = percentile distribution of Z-scores (eg, the five LMS tile is the 5th percentile distribution of Z-scores corresponding to the lower limit of normal); %Pred = percent predicted (calculated as [measured/predicted] × 100); NA = not applicable.
The GOLD and ATS/ERS thresholds for establishing spirometric respiratory impairment may not be age appropriate, however, for at least three reasons (2,8,23–26,78,79). First, because normal aging impairs respiratory mechanics, the FEV1/FVC is frequently less than 0.70 in otherwise healthy never-smokers who are ≥65 years (1,2,33,80). Second, because normal aging is associated with greater variability in spirometric performance, there is increasing disparity between the 80%Pred cut point for FVC and the LLN (2,78,79). In addition, the %Pred staging of FEV1 incorrectly assumes that a given cut point is equivalent for all persons, regardless of age, height, sex, and ethnicity (2,78,79). To illustrate the effect of age, at the LLN as calculated by the European Coal and Steel Community prediction equations, a white male of average height has a value for FEV1 of 74%Pred at age 30 years but only 63%Pred at age 70 years (78). Third, the calculation of the LLN, as currently recommended by the ATS/ERS (75,77), is based on multiple regression equations that incorrectly assume a linear relationship between predictor variables (age and height) and spirometric measures as well as incorrectly assuming that reference values are distributed normally and have constant variability across the life span (2). In older populations, for example, multiple regression equations for FEV1/FVC have limited explanatory ability, with R2 values ranging from only 0.01 to 0.15 (81–83).
Alternative approach.—
To address these limitations of contemporary practice, investigators have suggested that spirometric thresholds should be expressed as a Z-score, which converts a raw measurement on a test to a standardized score in units of standard deviations (78,79). More recently (2), a novel method for calculating spirometric Z-scores has been proposed, termed lambda–mu–sigma (LMS). This strategy uses all three elements of the distribution, including the median (mu)—representing how spirometric measures change based on predictor variables (age and height) and the coefficient-of-variation (sigma)—representing the spread of reference values and adjusting for nonuniform dispersion, and skewness (lambda)—representing the departure from normality (2). The LMS-derived Z-score is then calculated as follows (2): [(measured/predicted median)lambda − 1]/(lambda × sigma). The predicted values for median, lambda, and skewness are calculated from LMS equations that are based on four pooled reference samples, with ages ranging from 4 to 80 years (2). Clinically, Z-scores are routinely used to diagnose osteopenia and osteoporosis based on bone mineral density testing, and the LMS method is already widely applied to growth charts (2,84).
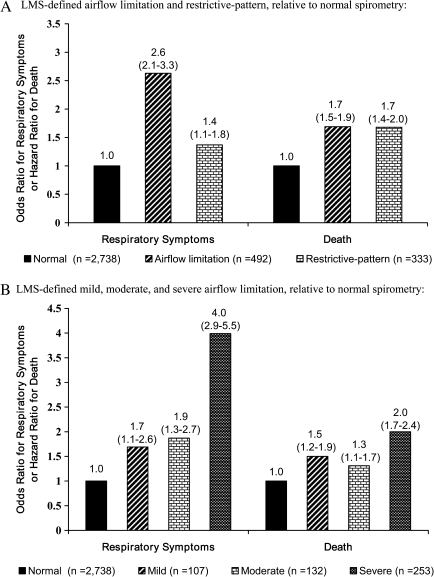
Based on LMS-derived Z-scores, we have proposed that respiratory impairment, including airflow limitation and restrictive pattern, should be defined as shown in Table 2. To assess the clinical validity of this approach, we have evaluated the associations between LMS-defined respiratory impairment and adverse health outcomes, using data from the Cardiovascular Health Study, a longitudinal cohort of community-living older persons that included the age group of 65–80 years (24–26). As shown in Figure 1, the presence and severity of LMS-defined airflow limitation and the presence of LMS-defined restrictive pattern were significantly associated with respiratory symptoms and all-cause mortality, respectively (24,25). Similar associations have since been found for frailty status (Fried phenotype) as well as in middle-aged persons (26,85). Because participants in our analytical samples had high smoking rates and no prior history of asthma, airflow limitation was likely due to COPD. The cause of restrictive pattern could not be ascertained, however, as the requisite diagnostic tests were unavailable (as discussed later under Diagnostic confirmation section).
Figure 1.
Adjusted odds ratios (95% confidence intervals) for respiratory symptoms and adjusted hazard ratios (95% confidence intervals) for death according to LMS (lambda-mu-sigma) criteria—among community-living persons aged 65–80 years. Based on data from the Cardiovascular Health Study extracted from Reference 24 (panel A) and Reference 25 (panel B). (A) LMS-defined airflow limitation and restrictive pattern relative to normal spirometry. (B) LMS-defined mild, moderate, and severe airflow limitation relative to normal spirometry.
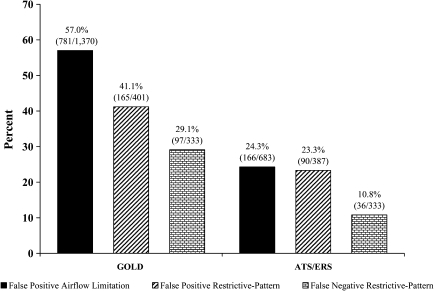
Among older persons, when the LMS approach is considered the reference standard, both airflow limitation and restrictive pattern are commonly misclassified by current spirometric approaches (24). As shown in Figure 2, false-positive and false-negative designations occur frequently in GOLD, with more modest misclassifications seen in the ATS/ERS approach. Both GOLD and the ATS/ERS also misclassify the severity of airflow limitation as shown in Table 3 (25). For example, among the 576 persons classified as having moderate airflow limitation by GOLD, 43 (7.5%) and 71 (12.3%) had mild and severe airflow limitation, respectively, by LMS, while an additional 330 (57.3%) had normal spirometry by LMS.
Figure 2.
Prevalence of false-positive and false-negative designations of global initiative for obstructive lung disease (GOLD)– and American thoracic society/European respiratory society (ATS/ERS)–defined airflow limitation and restrictive pattern, respectively, relative to LMS criteria—among community-living persons aged 65–80 years. A false-positive designation was defined as having a respiratory impairment by GOLD or the ATS/ERS (denominator) but not by lambda-mu-sigma (LMS; numerator), while a false-negative designation was defined as having a respiratory impairment by LMS (denominator) but not by GOLD or ATS/ERS (numerator). There was no false-negative designation for airflow limitation because all participants who had airflow limitation by LMS also had airflow limitation by GOLD and ATS/ERS, respectively. Based on data from the Cardiovascular Health Study extracted from Reference 24.
Table 3.
Cross-Tabulation of Frequency Distributions of Normal Spirometry and Airflow Limitation According to GOLD and the ATS/ERS Criteria within Strata of the LMS Staging System—Among Community-Living Persons Aged 65–80 Years. Based on Data from the Cardiovascular Health Study, Extracted from Reference 25
| (A) GOLD versus LMS* | ||||
| LMS spirometric category‡ | GOLD spirometric category† | |||
| Normal, N = 1,792 | Airflow limitation: FEV1 %Pred | |||
| Mild: ≥80, N = 680 | Moderate: 50–79, N = 576 | Severe: <50, N = 182 | ||
| N (%)§ | ||||
| Normal | 1,792 (100) | 616 (90.6) | 330 (57.3) | 0 |
| Airflow limitation: FEV1 LMS tile | ||||
| Mild: ≥5 | 0 | 64 (9.4) | 43 (7.5) | 0 |
| Moderate: 0.5–4.9 | 0 | 0 | 132 (22.9) | 0 |
| Severe: <0.5 | 0 | 0 | 71 (12.3) | 182 (100) |
| (B) ATS/ERS versus LMS* | ||||
| LMS spirometric category‡ | ATS/ERS spirometric category¶ | |||
| Normal, N = 2,482 | Airflow limitation: FEV1 %Pred | |||
| Mild: ≥70, N = 359 | Moderate: 50–69, N = 207 | Severe: <50, N = 182 | ||
| N (%)§ | ||||
| Normal | 2,482 (100) | 214 (59.6) | 42 (20.3) | 0 |
| Airflow limitation: FEV1 LMS tile | ||||
| Mild: ≥5 | 0 | 107 (29.8) | 0 | 0 |
| Moderate: 0.5–4.9 | 0 | 38 (10.6) | 94 (45.4) | 0 |
| Severe: <0.5 | 0 | 0 | 71 (34.3) | 182 (100) |
Notes: ATS/ERS = American thoracic society/European respiratory society; ATS/ERS-LLN = lower limit of normal; FEV1 = forced expiratory volume in 1 s; %Pred = percent predicted; FVC = forced vital capacity; GOLD = global initiative for obstructive lung disease; LMS = lambda-mu-sigma method; LMS tile = percentile distribution of LMS derived Z-scores.
Concordant spirometric designations are shown by cells with bold values.
Normal spirometry was defined by FEV1/FVC ≥0.70 and FVC ≥80% Pred; airflow limitation by an FEV1/FVC <0.70.
Normal spirometry was defined by FEV1/FVC and FVC, both ≥5 LMS tile; airflow limitation by FEV1/FVC <5 LMS tile.
Column percent.
Normal spirometry was defined by FEV1/FVC and FVC, both ≥ATS/ERS-LLN; airflow limitation by FEV1/FVC < ATS/ERS-LLN.
Because of the potential for misidentifying normal spirometry, it is difficult to directly compare the predictive accuracy of the current and alternative spirometric approaches. Meaning, risk estimates for adverse outcomes may be misleading, if the basis for the direct comparisons is a reference group of misidentified normal spirometry (86,87). We are therefore concerned regarding an often cited report (86) that posits the superior predictive accuracy of the GOLD approach in older persons relative to the ATS/ERS approach (the LMS method had not yet been published). In this report (86), using data from the Cardiovascular Health Study, the GOLD approach identified only 26% of participants as being in the normal spirometry reference group. This arguably represented a “super normal” group that, in turn, likely led to spurious risk estimates when the predictive accuracy of the GOLD approach was compared with that of the ATS/ERS approach. Moreover, in the same report (86), the GOLD approach established an implausibly high rate of respiratory impairment (74%) which, if broadly applied to clinical practice, could result in inappropriate and potentially harmful pharmacotherapy as well as to delays in the consideration of other diagnoses (3–8,80).
Despite the lack of a direct comparison, the evidence supporting the LMS approach as a basis for defining respiratory impairment in aging populations is strong (2,23–26,85). Although currently limited to whites and to persons aged ≤80 years, plans are underway to soon publish LMS equations that will provide Z-scores for other racial and ethnic groups as well as older-aged persons (2,88). Once these additional equations are available, LMS-derived Z-scores will offer the most valid method for establishing respiratory impairment across the life span.
Peak expiratory flow.—
Because valid spirometric measurements cannot be obtained in many older persons, especially those who are physically frail or cognitively impaired, alternative strategies for establishing respiratory impairment are needed (89). One possible strategy is PEF, defined as the maximum flow achieved during expiration delivered with maximal force, starting from maximal inspiration, as assessed by a peak flow meter (90).
PEF is a simple, inexpensive, and readily available measure of pulmonary function. In prior work involving 754 community-living older persons aged ≥70 years, we found that 99.5% completed three PEF readings (21,22). The PEF test was largely performed with good-to-excellent understanding (93%), and the variability in effort was minimal, as evidenced by an intraclass correlation coefficient of 0.92 for the three PEF readings (22).
PEF is most commonly reduced in the setting of airflow limitation, in particular, asthma and COPD (90). Other less common causes of reduced PEF include extrathoracic airway obstruction, respiratory muscle weakness, and disorders that limit the expansion of the chest wall (90). Although PEF is an attractive alternative to spirometry, two limitations warrant comment. First, when establishing respiratory impairment, PEF is less sensitive than spirometry and cannot specifically distinguish airflow limitation from restrictive pattern (90,91). Second, because it requires an initial explosive effort, PEF is much more effort dependent than spirometric measures such as the FEV1 (90,91). Thus, PEF may be reduced simply because of poor effort.
Despite these limitations, when spirometry is not readily available (eg, primary care setting) or when an older person cannot adequately perform spirometry, PEF may be a viable alternative for establishing respiratory impairment. Prior work has shown, for example, that PEF is cross-sectionally associated with health status and physical and cognitive function and is longitudinally associated with cognitive decline, institutionalization, and death (92–98). Until recently, however, diagnostic thresholds for PEF could not be established because suitable reference values did not exist. To establish diagnostic thresholds, we have proposed that PEF should be expressed as a Z-score similar to that described for spirometry (21). However, because LMS equations are not available for PEF, we have instead used Z-scores calculated as a standardized residual (SR; 78,79): [(measured − predicted mean)/(standard deviation of the residuals)]. In this equation, the numerator is the “residual,” whereas the denominator quantifies the spread of the reference data (accounting for variability in age, sex, height, and ethnicity). Hence, an SR-derived Z-score also converts a raw measurement on a test to a standardized score in units of standard deviations (78,79).
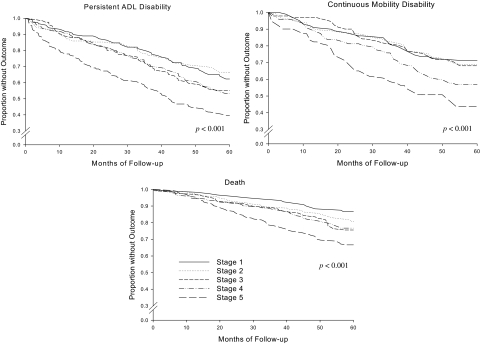
Using data from a large cohort of predominantly white community-living persons aged ≥70 years, we have evaluated the longitudinal association of PEF, expressed as an SR tile, with disability in activities of daily lining, mobility disability, and death (22). Our results showed that the highest cut point for PEF that conferred an increased risk of adverse outcomes occurred at the 10th SR tile. Specifically, at a PEF less than10th SR tile, identifying nearly a quarter of the cohort, hazard ratios adjusted for multiple confounders demonstrated an increased risk of activities of daily lining disability (hazard ratios [95% confidence interval]: 1.8 [1.2–2.6]), mobility disability (1.9 [1.2–3.1]), and death (2.3 [1.3–4.1]). Figure 3 shows the unadjusted Kaplan–Meier curves with PEF staged at five SR tile levels. These results support the use of PEF as a measure of pulmonary function in community-living older persons.
Figure 3.
Kaplan–Meier curves stratified according to peak expiratory flow (PEF) stages, expressed as standardized residual percentiles (SR tiles)—among community-living persons aged ≥70 years. PEF stages 1 (highest PEF values) through 5 (lowest PEF values) were established at an SR tile of 80–100, 50–79, 30–49, 10–29, and <10, respectively. Outcomes were ascertained over the course of 5 years and included persistent disability in one or more of four key activities of daily living (activities of daily lining)—bathing, walking, dressing, or transferring, present for at least two consecutive months; continuous mobility disability—the need for personal assistance or being unable to walk one-fourth of a mile or climb a flight of stairs, present for at least six consecutive months; and death. Reproduced from Reference 22.
To further advance the use of PEF as a tool for evaluating respiratory impairment in older persons, additional work is needed to establish reference equations that are based on more diverse populations in terms of race and ethnicity. In addition, the advantage of calculating Z-scores for PEF based on LMS versus SR should be determined. Last, the use of PEF as a tool for identifying respiratory impairment should be evaluated, specifically among older persons who have respiratory symptoms. Because respiratory symptoms may occur in the setting of normal pulmonary function (23–25,85), PEF could help to initially distinguish pulmonary from nonpulmonary conditions and, as a result, could allow clinicians to better prioritize spirometry versus other nonrespiratory tests (eg, echocardiography). Such an approach may facilitate more prompt and focused care of older persons who have respiratory symptoms (eg, dyspnea), as primary care settings often lack access to high-quality spirometry.
Diagnostic confirmation.—
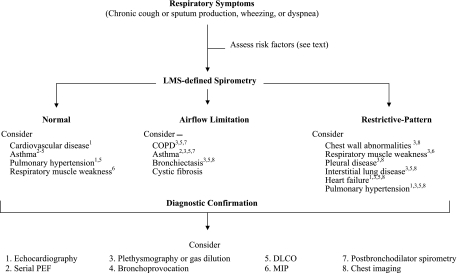
As shown in Figure 4, depending on which diagnosis is suspected clinically, individuals who have respiratory symptoms may require additional tests to further evaluate respiratory impairment. In particular, because its accuracy for identifying restrictive lung disease is limited (99), a spirometric restrictive pattern will require diagnostic confirmation with either a body plethysmography or a gas dilution study (eg, helium dilution or nitrogen washout) to confirm restrictive lung disease (75). Older persons who have normal spirometry but are symptomatic will also require further diagnostic evaluation, potentially including serial PEF measurements, bronchoprovocation, a body plethysmography or gas dilution study, DLCO, maximal inspiratory pressures, or echocardiography (76). In the setting of multimorbidity, several diagnostic tests may be required to identify all the factors contributing to the respiratory impairment. For example, COPD and heart failure frequently coexist in older persons, potentially leading to a mixed airflow limitation and restrictive pattern (76). Last, when the spirometric respiratory impairment is moderate-to-severe, or when sleep disordered breathing and pulmonary hypertension are diagnostic concerns (regardless of spirometric severity), measurements of gas exchange either by pulse oximetry or by arterial blood gas may be required.
Figure 4.
Evaluation of respiratory impairment based on lambda-mu-sigma (LMS)-defined spirometry among persons who have respiratory symptoms. All forms of restrictive pattern require body plethysmography or gas dilution to confirm restrictive lung disease.
CONCLUSIONS
Older persons have an increased risk of developing respiratory impairment because the aging lung is likely to have experienced frequent and cumulative exposures to environmental toxins as well as reductions in physiological capacity. Strong evidence now exists to support the use of spirometric Z-scores to define the presence and severity of respiratory impairment in older persons. Alternatively, when spirometry is not available or cannot be adequately performed, respiratory impairment may be established by PEF, also expressed as a Z-score.
Funding
Pepper Older Americans Independence Center (P30AG21342) and an R03 award from the National Institute on Aging (NIA; R03AG037051) to C.A.V.F. This work was also supported by an NIA Midcareer Investigator Award in Patient-Oriented Research (K24AG021507) to T.M.G.
CONFLICT OF INTEREST
All the authors have no conflicts of interest in regards to this study.
Acknowledgments
This work was supported by career development awards from the Department of Veterans Affairs and the Yale Claude D. Pepper Older Americans Independence Center.
References
- 1.Meyer KC. Aging. Proc Am Thorac Soc. 2005;2:433–439. doi: 10.1513/pats.200508-081JS. [DOI] [PubMed] [Google Scholar]
- 2.Stanojevic S, Wade A, Stocks J, et al. Reference ranges for spirometry across all ages. Am J Respir Crit Care Med. 2008;177:253–260. doi: 10.1164/rccm.200708-1248OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qaseem A, Snow V, Shekelle P, et al. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline from the American college of physicians. Ann Intern Med. 2007;147:633–638. [PubMed] [Google Scholar]
- 4.Singh S, Loke YK, Furberg CD. Inhaled anticholinergics and risk of major adverse cardiovascular events in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. JAMA. 2008;300:1439–1450. doi: 10.1001/jama.300.12.1439. [DOI] [PubMed] [Google Scholar]
- 5.Drummond MB, Dasenbrook EC, Pitz MW, et al. Inhaled corticosteroids in patients with stable chronic obstructive pulmonary disease: a systematic review and meta-analysis. JAMA. 2008;300:2407–2416. doi: 10.1001/jama.2008.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stephenson A, Seitz D, Bell CM, et al. Inhaled anticholinergic drug therapy and the risk of acute urinary retention in chronic obstructive pulmonary disease. Arch Intern Med. 2011;171(10):914–920. doi: 10.1001/archinternmed.2011.170. [DOI] [PubMed] [Google Scholar]
- 7.Taylor DR. The ß-agonist saga and its clinical relevance: on and on it goes. Am J Respir Crit Care Med. 2009;179:976–978. doi: 10.1164/rccm.200901-0055CC. [DOI] [PubMed] [Google Scholar]
- 8.Vaz Fragoso CA, Gill TM. Defining chronic obstructive pulmonary disease in an aging population. J Am Geriatr Soc. 2010;58:2224–2226. doi: 10.1111/j.1532-5415.2010.03128.x. [Editorial] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He W, Sengupta M, Velkoff VA, Debarros KA. U.S. Census Bureau, Current Population Reports, P23-209, 65+ in the United States: 2005, U.S. Washington, DC: Government Printing Office; 2005. [Google Scholar]
- 10.Qato DM, Alexander GC, Conti RM, et al. Use of prescription and over-the-counter medications and dietary supplements among older adults in the United States. JAMA. 2008;300:2867–2878. doi: 10.1001/jama.2008.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fried L, Tangen M, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol Med Sci. 2001;56A:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 12.Karlamangla A, Tinetti M, Guralnik J, et al. Comorbidity in older adults: nosology of impairment, diseases, and conditions. J Gerontol Med Sci. 2007;62A:296–300. doi: 10.1093/gerona/62.3.296. [DOI] [PubMed] [Google Scholar]
- 13.Rabe KF, Suzanne Hurd S, Anzueto A, et al. GOLD executive summary. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;176:532–555. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- 14.Raherison C, Girodet PO. Epidemiology of COPD. Eur Respir Rev. 2009;18(114):213–221. doi: 10.1183/09059180.00003609. [DOI] [PubMed] [Google Scholar]
- 15.Stockley RA, Mannino D, Barnes PJ. Burden and pathogenesis of chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2009;6:524–526. doi: 10.1513/pats.200904-016DS. [DOI] [PubMed] [Google Scholar]
- 16.Donaldson GC, Seemungal TA, Bhowmik A, et al. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax. 2002;57:847–852. doi: 10.1136/thorax.57.10.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chotirmall SH, Watts M, Branagan P, Donegan CF, Moore A, McElvaney NG. Diagnosis and management of asthma in older adults. J Am Geriatr Soc. 2009;57:901–909. doi: 10.1111/j.1532-5415.2009.02216.x. [DOI] [PubMed] [Google Scholar]
- 19.Gibson PG, McDonald VM, Marks GB. Asthma in older adults. Lancet. 2010;376:803–813. doi: 10.1016/S0140-6736(10)61087-2. [DOI] [PubMed] [Google Scholar]
- 20.West JB. Respiratory Physiology: the Essentials. 8th ed. Baltimore, MD: Lippincott Williams and Wilkins; 2008. [Google Scholar]
- 21.Vaz Fragoso CA, Gahbauer E, Van Ness P, Gill T. Reporting peak expiratory flow in older persons. J Gerontol Med Sci. 2007;62A:1147–1151. doi: 10.1093/gerona/62.10.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vaz Fragoso CA, Gahbauer E, Van Ness P, Concato J, Gill T. Peak expiratory flow as a predictor of subsequent disability and death in community-living older persons. J Am Geriatr Soc. 2008;56:1014–1020. doi: 10.1111/j.1532-5415.2008.01687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vaz Fragoso CA, Concato J, McAvay G, et al. The ratio of FEV1 to FVC as a basis for establishing chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2010;181:446–451. doi: 10.1164/rccm.200909-1366OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vaz Fragoso CA, Gill TM, McAvay G, Yaggi HK, Van Ness PH, Concato J. Respiratory impairment and mortality in older persons: a novel spirometric method. J Invest Med. 2011;59(7):1089–1095. doi: 10.231/JIM.0b013e31822bb213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vaz Fragoso CA, Concato J, McAvay G, et al. Staging the severity of chronic obstructive pulmonary disease in older persons based on spirometric Z-scores. J Am Geriatr Soc. 2011;59:1847–1854. doi: 10.1111/j.1532-5415.2011.03596.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vaz Fragoso CA, Enright P, McAvay G, Van Ness PH, Gill TM. Frailty and respiratory impairment in older persons. Am J Med. 2011 doi: 10.1016/j.amjmed.2011.06.024. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention, National Center for Health Statistics. National Health Interview Survey 1965–2008. Analysis for years 1997–2008 by the American Lung Association, Research and Program Services Division. 2011 http://www.lungusa.org/finding-cures/our-research/trend-reports/Tobacco-Trend-Report.pdf. Accessed June 14, 2011. [Google Scholar]
- 28.Kaufmann RB, Babb S, O’Halloran A, Asman K, Bishop E, Tynan M, et al. Vital signs: nonsmokers’ exposure to secondhand smoke—United States, 1999—2008. MMWR. 2010;59(35):1141–1146. [PubMed] [Google Scholar]
- 29.Thompson WW, Shay DK, Weintraub E, et al. Influenza-associated hospitalizations in the United States. JAMA. 2004;292:1333–1340. doi: 10.1001/jama.292.11.1333. [DOI] [PubMed] [Google Scholar]
- 30.U.S. Department of Health and Human Services. A Profile of Older Americans: 2010. http://www.aoa.gov/aoaroot/aging_statistics/Profile/index.aspx. Accessed June 14, 2011. [Google Scholar]
- 31.De Leon SF, Thurston GD, Ito K. Contribution of respiratory disease to non-respiratory mortality associations with air pollution. Am J Respir Crit Care Med. 2003;167:1117–1123. doi: 10.1164/rccm.200205-409OC. [DOI] [PubMed] [Google Scholar]
- 32.Schindler C, Kunzli N, Bongard JP, et al. Short-term variation in air pollution and in average lung function among never-smoker: the Swiss study on air pollution and lung diseases in adults (SALPADIA) Am J Respir Crit Care Med. 2001;163(2):356–361. doi: 10.1164/ajrccm.163.2.9911116. [DOI] [PubMed] [Google Scholar]
- 33.Celli BR, Halbert RJ, Nordyke RJ, Schau B. Airway obstruction in never smokers: results from the third National Health and Nutrition Examination Survey. Am J Med. 2005;118:1364–1372. doi: 10.1016/j.amjmed.2005.06.041. [DOI] [PubMed] [Google Scholar]
- 34.Hnizdo E, Sullivan PA, Bang KM, Wagner G. Association between chronic obstructive pulmonary disease and employment by industry and occupation in the US population: a study of data from the third national health and nutrition examination survey. Am J Epidemiol. 2002;156:738–746. doi: 10.1093/aje/kwf105. [DOI] [PubMed] [Google Scholar]
- 35.Ely EW, Wheeler AP, Thompson BT, et al. Recovery rate and prognosis in older persons who develop acute lung injury and the acute respiratory distress syndrome. Ann Intern Med. 2002;136:25–36. [PubMed] [Google Scholar]
- 36.Fry AM, Shay DK, Holman RC, et al. Trends in hospitalizations for pneumonia among persons aged 65 years or older in the United States, 1988-2002. JAMA. 2005;294(21):2712–2719. doi: 10.1001/jama.294.21.2712. [DOI] [PubMed] [Google Scholar]
- 37.Milic-Emili J, Whitelaw WA, Derenne JPh. Occlusion pressure—a simple measure of the respiratory center’s output. NEJM. 1975:1029–1030. doi: 10.1056/NEJM197511132932006. [DOI] [PubMed] [Google Scholar]
- 38.Peterson DD, Pack AI, Silage DA, Fishman AP. Effects of aging on ventilatory and occlusion pressure responses to hypoxia and hypercapnia. Am Rev Respir Dis. 1981;124:387–391. doi: 10.1164/arrd.1981.124.4.387. [DOI] [PubMed] [Google Scholar]
- 39.Kronenberg RS, Drage CW. Attenuation of the ventilatory and heart rate responses to hypoxia and hypercapnia with aging in normal men. J Clin Invest. 1973;52:1812–1819. doi: 10.1172/JCI107363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brischetto MJ, Millman RP, Peterson DD, et al. Effect of aging on ventilatory response to exercise and CO2. J Appl Physiol Respir Environ Exerc Physiol. 1984;56(5):1143–1150. doi: 10.1152/jappl.1984.56.5.1143. [DOI] [PubMed] [Google Scholar]
- 41.Rubin S, Tack M, Cherniack NS. Effect of aging on respiratory responses to CO2 and inspiratory resistive loads. J Gerontol. 1982;37(3):306–312. doi: 10.1093/geronj/37.3.306. [DOI] [PubMed] [Google Scholar]
- 42.Pokorski VM, Marczak M. Ventilatory response to hypoxia in elderly women. Ann Hum Biol. 2003;30(1):53–64. doi: 10.1080/03014460210162000. [DOI] [PubMed] [Google Scholar]
- 43.Smith WDF, Poulin M, Paterson DH, Cunningham DA. Dynamic ventilatory response to acute isocapnic hypoxia in septuagenarians. Exp Physiol. 2001;86(1):117–126. doi: 10.1113/eph8602006. [DOI] [PubMed] [Google Scholar]
- 44.Poulin MJ, Cunninham DA, Paterson DH, Kowalchuk JM, Smith WDF. Ventilatory sensitivity to CO2 in hyperoxia and hypoxia in older aged humans. J Appl Physiol. 1993;75(5):2209–2216. doi: 10.1152/jappl.1993.75.5.2209. [DOI] [PubMed] [Google Scholar]
- 45.Berkenbosch A, Bovill JG, Dahan A, DeGoede J, Oliever ICW. Ventilatory CO2 sensitivities from Read’s rebreathing method and the steady-state method are not equal. J Physiol. 1989;411:367–377. doi: 10.1113/jphysiol.1989.sp017578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Swanson GD, Bellville W. Step changes in end-tidal CO2: methods and implications. J Appl Physiol. 1975;39:377–385. doi: 10.1152/jappl.1975.39.3.377. [DOI] [PubMed] [Google Scholar]
- 47.Bixler EO, Vgontzas AN, Ten HT, Tyson K, Kales A. Effects of age on sleep apnea in men: I. prevalence and severity. Am J Respir Crit Care Med. 1998;157:144–148. doi: 10.1164/ajrccm.157.1.9706079. [DOI] [PubMed] [Google Scholar]
- 48.McKay LC, Janczewski WA, Feldman JL. Sleep-disordered breathing after targeted ablation of preBotzinger complex neurons. Nature Neurosc. 2005;8:1142–1144. doi: 10.1038/nn1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Celle S, Peyron R, Faillenot I, et al. Undiagnosed sleep-related breathing disorders are associated with focal brainstem atrophy in the elderly. Hum Brain Mapp. 2009;30:2090–2097. doi: 10.1002/hbm.20650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Feldman JL, Del Negro CA. Looking for inspiration: new perspectives on respiratory rhythm. Nature Rev: Neurosci. 2006;7:232–242. doi: 10.1038/nrn1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Garcia-Touchard A, Somers VK, Olson LJ, Caples SM. Central sleep apnea: implications for congestive heart failure. Chest. 2008;133:1495–1504. doi: 10.1378/chest.07-0871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Connolly MJ, Crowley JJ, Charan NB, et al. Reduced subjective awareness of bronchoconstriction provoked by methacholine in elderly asthmatic and normal subjects as measured on a simple awareness scale. Thorax. 1992;47:410–413. doi: 10.1136/thx.47.6.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cuttitta G, Cibella F, Bellia V, et al. Changes in FVC during methacholine-induced bronchoconstriction in elderly patients with asthma: bronchial hyperresponsiveness and aging. Chest. 2001;119:1685–1690. doi: 10.1378/chest.119.6.1685. [DOI] [PubMed] [Google Scholar]
- 54.Chen H-I, Kuo C-S. Relationship between respiratory muscle function and age, sex, and other factors. J Appl Physiol. 1989;66(2):943–948. doi: 10.1152/jappl.1989.66.2.943. [DOI] [PubMed] [Google Scholar]
- 55.Enright PL, Kronmal RA, Manolio TA, et al. Respiratory muscle strength in the elderly; correlates and reference values. Am J Respir Crit Care Med. 1994;149:430–438. doi: 10.1164/ajrccm.149.2.8306041. [DOI] [PubMed] [Google Scholar]
- 56.Sachs MC, Enright PL, Stukovsky KDH, et al. Performance of maximum inspiratory pressure tests and maximum inspiratory pressure reference equations for 4 race/ethnic groups. Respir Care. 2009;54(10):1321–1328. [PMC free article] [PubMed] [Google Scholar]
- 57.Janssens J-P. Aging of the respiratory system: impact on pulmonary function tests and adaptation to exertion. Clin Chest Med. 2005;26:469–484. doi: 10.1016/j.ccm.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 58.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European working group on sarcopenia in older people. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Polkey MI, Hamnega C-H, Hughes PD, et al. Influence of acute lung volume change on contractile properties of human diaphragm. J Appl Physiol. 1998;85(4):1322–1328. doi: 10.1152/jappl.1998.85.4.1322. [DOI] [PubMed] [Google Scholar]
- 60.Fletcher C, Peto R. The natural history of chronic airflow obstruction. BMJ. 1977;1:1645–1648. doi: 10.1136/bmj.1.6077.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.LeBlanc P, Ruff F, Milic-Emili J. Effects of age and body position on “airway closure” in man. J Appl Physiol. 1970;28(4):448–451. doi: 10.1152/jappl.1970.28.4.448. [DOI] [PubMed] [Google Scholar]
- 62.American Thoracic Society, American College of Chest Physicians (ATS/ACCP) Statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med. 2003;167:211–277. doi: 10.1164/rccm.167.2.211. [DOI] [PubMed] [Google Scholar]
- 63.Hardie JA, Vollmer WM, Buist AS, et al. Reference values for arterial blood gases in the elderly. Chest. 2004;125:2053–2060. doi: 10.1378/chest.125.6.2053. [DOI] [PubMed] [Google Scholar]
- 64.Crapo RO, Jensen RL, Hegewald M, Taskin DP. Arterial blood gas reference values for sea level and an altitude of 1,400 meters. Am J Respir Crit Care Med. 1999;160:1525–1531. doi: 10.1164/ajrccm.160.5.9806006. [DOI] [PubMed] [Google Scholar]
- 65.Levin DL, Buxton RB, Spiess JP, et al. Effects of age on pulmonary perfusion heterogeneity measured by magnetic resonance imaging. J Appl Physiol. 2007;102:2064–2070. doi: 10.1152/japplphysiol.00512.2006. [DOI] [PubMed] [Google Scholar]
- 66.Ohlsson J, Middaugh M, Hlastala MP. Reduction of lung perfusion increases VA/Q heterogeneity. J Appl Physiol. 1989;66(5):2423–2430. doi: 10.1152/jappl.1989.66.5.2423. [DOI] [PubMed] [Google Scholar]
- 67.Wagner P, Laravuso R, Uhl R, West J. Continuous distribution of ventilation-perfusion ratios in normal subjects breathing air and 100 per cent O2. J Clin Invest. 1974;54:54–68. doi: 10.1172/JCI107750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Holland J, Milic-Emili J, Macklem PT, Bates DV. Regional distribution of pulmonary ventilation and perfusion in elderly subjects. J Clin Invest. 1968;47:81–92. doi: 10.1172/JCI105717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cardus J, Burgos F, Diaz O, et al. Increase in pulmonary ventilation–perfusion inequality with age in healthy individuals. Am J Respir Crit Care Med. 1997;156:648–653. doi: 10.1164/ajrccm.156.2.9606016. [DOI] [PubMed] [Google Scholar]
- 70.Ersham R, Perruchoud DA, Oberholzer M, et al. Influence of age on pulmonary haemodynamics at rest and during supine exercise. Clin Sci. 1983;65:653–660. doi: 10.1042/cs0650653. [DOI] [PubMed] [Google Scholar]
- 71.McQuillan BM, Picard MH, Leavitt M, Weyman AE. Clinical correlates and reference intervals for pulmonary artery systolic pressure among echocardiographically normal subjects. Circulation. 2001;104:2797–2802. doi: 10.1161/hc4801.100076. [DOI] [PubMed] [Google Scholar]
- 72.Butler C, Kleinerman J. Capillary density: alveolar diameter, a morphometric approach to ventilation and perfusion. Am Rev Respir Dis. 1970;102:886–894. doi: 10.1164/arrd.1970.102.6.886. [DOI] [PubMed] [Google Scholar]
- 73.Guénard H, Marthan R. Pulmonary gas exchange in elderly subjects. Eur Respir J. 1996;9:2573–2577. doi: 10.1183/09031936.96.09122573. [DOI] [PubMed] [Google Scholar]
- 74.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 75.Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26:948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 76.Chan ED, Welsh CH. Geriatric respiratory medicine. Chest. 1998;114:1704–1733. doi: 10.1378/chest.114.6.1704. [DOI] [PubMed] [Google Scholar]
- 77.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 78.Miller MR, Pincock AC. Predicted values: how should we use them? Thorax. 1988;43:265–267. doi: 10.1136/thx.43.4.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Official statement of the European Respiratory Society. Lung volumes and forced ventilatory flows. Eur Respir J. 1993;6(S16):5–40. doi: 10.1183/09041950.005s1693. [DOI] [PubMed] [Google Scholar]
- 80.Vaz Fragoso CA, Concato J, McAvay G, Van Ness PH, Yaggi HK, Gill TM. Chronic obstructive pulmonary disease in older persons: a comparison of two spirometric definitions. Resp Med. 2010;104:1189–1196. doi: 10.1016/j.rmed.2009.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Garcia-Rio F, Pino JM, Dorgham A, Alonso A, Villamor J. Spirometric reference equation for European females and males aged 65–85 years. Eur Respir J. 2004;24:397–405. doi: 10.1183/09031936.04.00088403. [DOI] [PubMed] [Google Scholar]
- 82.Enright PL, Kronmal RA, Higgins M, et al. Spirometry reference values for women and men 65–85 years of age: cardiovascular health study. Am Rev Respir Dis. 1993;147:125–133. doi: 10.1164/ajrccm/147.1.125. [DOI] [PubMed] [Google Scholar]
- 83.Vaz Fragoso CA, Concato J, McAvay G, et al. Defining chronic obstructive pulmonary disease in older persons. Resp Med. 2009;103:1468–1476. doi: 10.1016/j.rmed.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cummings SR, Bates D, Black DM. Clinical use of bone densitometry: scientific review. JAMA. 2002;288:1889–1897. doi: 10.1001/jama.288.15.1889. [DOI] [PubMed] [Google Scholar]
- 85.Vaz Fragoso CA, Gill TM, McAvay G, et al. Evaluating respiratory impairment in middle-aged persons using lambda-mu-sigma derived z-scores. Respir Care. 2011 doi: 10.4187/respcare.01192. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mannino DM, Buist AS, Vollmer WM. Chronic obstructive pulmonary disease in the older adult: what defines abnormal lung function? Thorax. 2007;62:237–241. doi: 10.1136/thx.2006.068379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mannino DM, Diaz-Guzman E. Interpreting lung function data using 80% predicted and fixed thresholds identifies patients at increased risk of mortality. Chest. 2011 doi: 10.1378/chest.11-0797. [DOI] [PubMed] [Google Scholar]
- 88.Quanjer PH, Stanojevic S, Cole T, Stocks J. Lung function in growth and aging. http://www.lungfunction.org/implementingequations.html. Accessed October 19, 2011. [Google Scholar]
- 89.Allen SC, Yeung P. Inability to draw intersecting pentagons as a predictor of unsatisfactory spirometry technique in elderly hospital inpatients. Age Ageing. 2006;35:304–316. doi: 10.1093/ageing/afj090. [DOI] [PubMed] [Google Scholar]
- 90.Quanjer PH, Lebowitz MD, Gregg I, et al. Peak expiratory flow: conclusions and recommendations of a working party of the European respiratory society. Eur Respir J. 1997;10(suppl 24):2S–8S. [PubMed] [Google Scholar]
- 91.Paggiaro PL, Moscato G, Giannini D, et al. Relationship between peak expiratory flow and FEV1. Eur Respir J. 1997;10(suppl 24):39S–41S. [PubMed] [Google Scholar]
- 92.Cook NR, Albert MS, Berkman LF, et al. Interrelationships of peak expiratory flow rate with physical and cognitive function in the elderly: MacArthur foundation studies of aging. J Gerontol Med Sci. 1995;50A:M317–M323. doi: 10.1093/gerona/50a.6.m317. [DOI] [PubMed] [Google Scholar]
- 93.Lan T-Y, Melzer D, Tom BDM, et al. Performance tests and disability: developing an objective index of mobility-related limitation in older populations. J Gerontol Med Sci. 2002;57:M294–M301. doi: 10.1093/gerona/57.5.m294. [DOI] [PubMed] [Google Scholar]
- 94.Schaub RT, Munzberg H, Borchelt M, et al. Ventilatory capacity and risk for dementia. J Gerontol Med Sci. 2000;55A:M677–M683. doi: 10.1093/gerona/55.11.m677. [DOI] [PubMed] [Google Scholar]
- 95.Cook NR, Evans DA, Scherr PA, et al. Peak expiratory flow rate in an elderly population. Am J Epidemiol. 1989;130:66–78. doi: 10.1093/oxfordjournals.aje.a115324. [DOI] [PubMed] [Google Scholar]
- 96.Whitfield KE, Seeman TE, Miles TP, et al. Health indices as predictors of cognition among older African Americans: MacArthur studies of successful aging. Ethnicity Dis. 1997;7:127–136. [PubMed] [Google Scholar]
- 97.McCallum J, Simons LA, Simons J, et al. Patterns and predictors of nursing home placement over 14 years: Dubbo study of elderly Australians. Austr J Ageing. 2005;24:169–173. [Google Scholar]
- 98.Cook NR, Evans DA, Scherr PA, et al. Peak expiratory flow rate and 5-year mortality in an elderly population. Am J Epidemiol. 1991;133:784–794. doi: 10.1093/oxfordjournals.aje.a115957. [DOI] [PubMed] [Google Scholar]
- 99.Glady CA, Aaron SD, Lunau M, Clinch J, Dales R. Spirometry-based algorithm to direct lung function testing in the pulmonary function laboratory. Chest. 2003;123:1939–1946. doi: 10.1378/chest.123.6.1939. [DOI] [PubMed] [Google Scholar]