This issue of the Journal of Gerontology: Medical Sciences highlights new findings on the aging lung and its consequences for health and function (1–6). The aging lung is likely to have experienced frequent and cumulative exposures to environmental toxins, particularly tobacco smoke and respiratory infections, as well as substantial reductions in physiologic capacity (1). As a result, older persons are at an increased risk of developing respiratory impairment, with a wide range of negative consequences that include respiratory symptoms, frailty-related features, disability, and mortality (1). Concurrently, there is an increased prevalence of various pulmonary diseases and cancer in older individuals (2). This issue reviews our current understanding of the diagnostic, epidemiologic, and biologic aspects of the aging lung.
In the first article, Vaz Fragoso and Gill (1) propose that respiratory impairment be defined as an age-adjusted reduction in pulmonary function that is independently associated with adverse outcomes. The rationale for this definition is twofold. First, to more accurately establish an underlying respiratory disease, the reduction in pulmonary function must be distinguished from the reduction that is due to normal aging. Second, to avoid inappropriate and potentially harmful pharmacotherapy, as well as delays in the consideration of other diagnoses, the threshold that establishes an age-adjusted reduction in pulmonary function should be linked to adverse outcomes. Using this approach, the authors review the strong evidence that now exists to support the use of spirometric Z-scores as a basis for defining respiratory impairment in older persons. Alternatively, when spirometry is not available or cannot be adequately performed, respiratory impairment may be established by the peak expiratory flow, also expressed as a Z-score.
In the second article, Akgün, Crothers, and Pisani (2) review the epidemiology of common pulmonary diseases, namely pneumonia, obstructive lung diseases, idiopathic pulmonary fibrosis, and lung cancer, in older populations. In particular, they review the common clinical presentations for these diseases and highlight differences between younger and older age groups. The risk factors, treatment, and mortality associated with these pulmonary diseases are also discussed, including the relationship with comorbidities, respiratory symptoms, and quality of life.
The remaining articles focus on immune-mediated biological mechanisms that potentially underlie the age-related increase in the prevalence of respiratory impairment and pulmonary disease (3–6). Goldstein, for example, reviews the impact of aging on the innate immune response, the first line of defense to pathogen invasion. Based on emerging studies, the author suggests that age-related changes in the innate immune system may contribute to the impaired response to viral infection (3). Of note, from the period 1979 through 2001, persons aged 75 years or more had a 10-fold or greater increase in the rate of influenza-associated hospitalization, relative to any other age group (7). Lee, Shin, and Kang (4) next review the impact of aging on T-cell immunity, as this could lead to an increased vulnerability for developing viral infections, inflammatory diseases (eg, asthma and chronic obstructive lung disease), and malignancies. Based on current evidence, the authors conclude that a range of alterations in T-cell immunity occurs with aging, from cell function to the proportion of T-cell subsets, but that their clinical significance has yet to be elucidated, particularly regarding the aging lung. Jane-Wit and Chun (5) subsequently review the impact of aging on the pulmonary endothelium, as this can lead to infection-related sequelae, a higher prevalence of pulmonary hypertension, and an attenuation of parenchymal healing. Based on current evidence, the authors suggest that the age-related mishandling of intracellular reactive oxygen species, pathologic nitric oxide signaling, and deficient recruitment of stem cell precursors provide a rationale for future therapies that may subvert or even reverse the effects of aging on the respiratory system.
Interestingly, studies by Volkova, Zhang, Shaw, and Lee (6) have offered a novel mechanism whereby age-related dysregulation of intracellular reactive oxygen species may occur. They discovered a previously unappreciated role for the innate immune receptor, Toll-like receptor 4 (TLR4), in maintaining an oxidant–antioxidant balance in the lungs and endothelium. They found that mice deficient in TLR4, the canonical receptor for lipopolysaccharide (a component of specific bacteria), exhibit an accelerated form of age-induced lung enlargement that resembles human emphysema, both histologically and functionally (6). Their animal data are supported by recent human studies. As reviewed by Volkova et al., TLR4 function declines with age, which may in part contribute to “senile emphysema” even in the absence of significant exposures (6). Human studies have also found that chronic smoking leads to decreased TLR4 function in the lung and that the level of TLR4 depression is correlated with the severity of chronic obstructive lung disease (8). The synergistic or additive effects of age and smoking on TLR4 function in susceptible individuals may explain the pathogenesis and temporal characteristics of smoke-induced emphysema, which tends to manifest in the latter decades of life despite cessation of smoke exposure. In addition to TLR4, other innate immune receptors are also affected by age and have an important impact on lung disease.
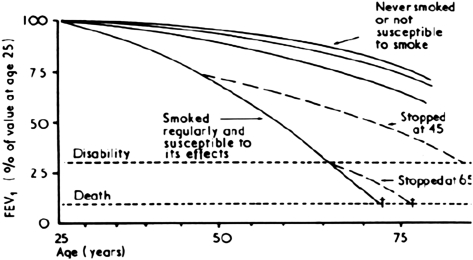
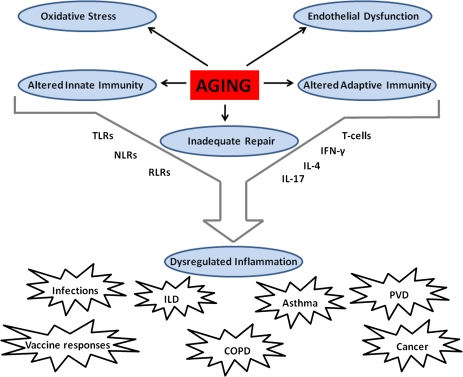
In the spirit of “a picture is worth a thousand words,” the very essence of what characterizes an aging lung is best illustrated in Figures 1 and 2. In the first figure (9), the forced expiratory volume in 1-second—a spirometric measure of pulmonary function—shows a progressive age-related decline across the adult lifespan (top 3 curves), which in vulnerable individuals is substantially accelerated by smoking exposures—a major risk factor for pulmonary disease (bottom curve). In Figure 2, an example is shown of the biology that underlies the increased vulnerability of the aging lung. Specifically, the age-related alterations in innate and adaptive immunity, oxidative stress, endothelial function, and repair processes culminate in dysregulated inflammatory processes and subsequent lung pathology such as infections, airways, interstitial and vascular pulmonary processes, and cancer. Ultimately, it is the interplay of age-related changes in biology and the subsequent responses to environmental exposures that largely define the physiology and clinical presentation of the aging lung.
Figure 1.
The forced expiratory volume in 1-second (FEV1) across the adult lifespan, in adult males. Reproduced with permission from reference (9).
Figure 2.
Proposed biologic mechanisms in the aging lung. TLRs = Toll-like receptors; NLRs = Nod-like receptors; RLRs = Rig-I-like receptors; IFN-γ = Interferon-gamma; IL- = Interleukin; ILD = Interstitial lung disease; COPD = Chronic obstructive lung disease; PVD = Pulmonary vascular disease.
FUNDING
Dr. C.A.V.F. is currently a recipient of career development awards from the Department of Veterans Affairs and the Yale Pepper Center and an R03 award from the National Institute on Aging (R03AG037051). Dr. P.J.L. is funded by R01 HL090660, R01 HL071595, and FAMRI 82384. Dr. P.J.L. was also a recipient of the Clinical and Translational Science Award Grant Number UL1 RR024139 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH roadmap for Medical Research. Dr. C.A.V.F. and Dr. P.J.L. have also received support from the Yale Claude D. Pepper Older Americans Independence Center (P30AG21342).
Acknowledgments
The contents are solely the responsibility of the authors and do not necessarily represent the official view of National Center for Research Resources or National Institutes of Health.
References
- 1.Vaz Fragoso CA, Gill TM. Respiratory impairment and the aging lung: a novel paradigm for assessing pulmonary function. J Gerontol A Biol Sci Med Sci. 2012;67:264–275. doi: 10.1093/gerona/glr198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akgün KA, Crothers K, Pisani M. Epidemiology and management of common pulmonary diseases in older persons. J Gerontol A Biol Sci Med Sci. 2012;67:276–291. doi: 10.1093/gerona/glr251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldstein DR. Role of aging on innate responses to viral infections. J Gerontol A Biol Sci Med Sci. 2012;67:242–246. doi: 10.1093/gerona/glr194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee N, Shin MS, Kang I. T-cell biology in aging, with a focus on lung disease. J Gerontol A Biol Sci Med Sci. 2012;67:254–263. doi: 10.1093/gerona/glr237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jane-Wit D, Chun HJ. Mechanisms of dysfunction in senescent pulmonary endothelium. J Gerontol A Biol Sci Med Sci. 2012;67:236–241. doi: 10.1093/gerona/glr248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Volkova M, Zhang Y, Shaw AC, Lee PJ. Toll-like receptors in the aging lung. J Gerontol A Biol Sci Med Sci. 2012;67:247–253. doi: 10.1093/gerona/glr226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thompson WW, Shay DK, Weintraub E, et al. Influenza-associated hospitalizations in the United States. JAMA. 2004;292:1333–1340. doi: 10.1001/jama.292.11.1333. [DOI] [PubMed] [Google Scholar]
- 8.MacRedmond RE, Greene CM, Dorscheid DR, McElvaney NG, O’Neill SJ. Epithelial expression of TLR4 is modulated in COPD and by steroids, salmeterol and cigarette smoke. Respir Res. 2007;8:84. doi: 10.1186/1465-9921-8-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fletcher C, Peto R. The natural history of chronic airflow obstruction. BMJ. 1977;1:1645–1648. doi: 10.1136/bmj.1.6077.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]