Abstract
Pulmonary disease prevalence increases with age and contributes to morbidity and mortality in older patients. Dyspnea in older patients is often ascribed to multiple etiologies such as medical comorbidities and deconditioning. Common pulmonary disorders are frequently overlooked as contributors to dyspnea in older patients. In addition to negative impacts on morbidity and mortality, quality of life is reduced in older patients with uncontrolled, undertreated pulmonary symptoms. The purpose of this review is to discuss the epidemiology of common pulmonary diseases, namely pneumonia, chronic obstructive pulmonary disease, asthma, lung cancer, and idiopathic pulmonary fibrosis in older patients. We will review common clinical presentations for these diseases and highlight differences between younger and older patients. We will also briefly discuss risk factors, treatment, and mortality associated with these diseases. Finally, we will address the relationship between comorbidities, pulmonary symptoms, and quality of life in older patients with pulmonary diseases.
Keywords: Pulmonary diseases, Older persons, Dyspnea, Comorbidity, Quality of life
Although the prevalence of lung diseases has been increasing as the population ages, it is still underestimated in older persons (1–4). Dyspnea and cough are common symptoms of pulmonary diseases in older patients but are also associated with nonpulmonary comorbid conditions such as heart failure, anemia, deconditioning, and muscle weakness. We will review the incidence and prevalence of pneumonia, chronic obstructive pulmonary disease (COPD), asthma, lung cancer, and idiopathic pulmonary fibrosis (IPF) in older patients. We will then focus on specific treatment considerations in older persons as they pertain to survival, functional status, and quality of life with reference to current professional guidelines.
PNEUMONIA
Epidemiology
Community acquired pneumonia (CAP) is responsible for 350,000–620,000 hospitalizations annually in older patients (5). The incidence of CAP is 14 cases/1,000 person-years; this incidence is at least doubled in nursing home residents (6,7). Aspiration pneumonia is two to three times as prevalent in nursing home residents but is often underdiagnosed (8–10). In addition to bacterial pneumonia, viral pneumonia is also responsible for a significant proportion of hospitalizations in older patients each year (11).
Clinical Presentation and Evaluation
Older patients with CAP commonly present with tachypnea, delirium, and constitutional symptoms such as failure to thrive, malaise, and falls rather than fever, cough, and purulent sputum typically associated with bacterial pneumonia (7,8,12–15). Aspiration pneumonia in patients with baseline neurological deficits can be especially challenging to diagnose due to its slow, indolent course, low-grade fevers, and notable absence of rigors (16,17). Viral pneumonia may present with new dyspnea and bronchospasm (18). Between difficulties in obtaining adequate sputum samples and nonspecific symptoms, pneumonia diagnosis is often delayed in older patients (12,13).
Streptococcus pneumoniae is the most common cause of CAP in older patients (8,10), but polymicrobial infections are not uncommon (11,19). Different pathogens are responsible for pneumonia in residents of nursing homes, which are commonly classified with health care–associated pneumonia. Staphylococcus aureus is the most common isolate from nursing home residents (11). Anaerobic organisms may play an important role in aspiration pneumonia. Reactivation of pulmonary tuberculosis should also be considered in older patients with pneumonia (8).
Risk Factors
Age is a risk factor for pneumonia, regardless of whether patients are home dwelling or institutionalized (20–23). Influenza infection is a risk factor for bacterial pneumonia due to bacterial colonization and overgrowth through direct damage to airway epithelial cells and impaired mucociliary clearance (24,25). In addition, there are virus-specific factors such as viral neuraminidase production that may increase host susceptibility to secondary bacterial infection (26).
Impaired host defenses may also increase risk of secondary bacterial pneumonia, including in older persons (27–29). Common comorbidities in older patients including heart failure, liver disease, and underlying lung disease are risk factors for pneumonia (28–32). Comorbid diseases leading to dysphagia and gastroesophageal reflux disease put older patients at increased risk of aspiration pneumonia. Male gender and diabetes are additional risk factors for aspiration pneumonia (33).
Treatment
Treatment of CAP and aspiration pneumonia in older patients should follow the Infectious Diseases Society of America/American Thoracic Society guidelines (34). Age is an important part of several different scores used to calculate pneumonia severity such as the Pneumonia Severity Index which has been validated and used to predict outcomes and need for hospitalization in patients with CAP (35). Drug-resistant pathogens need to be treated in health care–associated pneumonia and hospital-acquired pneumonia (36).
Outcome
Pneumonia-related mortality increases with age (5,37). Older patients who recover from pneumonia have higher mortality rates than younger patients for several years following their pneumonia (10,15,30,38). Similar to outcomes in younger patients, severity of disease and organ failure are the strongest predictors of mortality in older persons (34,35). Comorbid disease and functional status are also significant predictors for readmission and mortality in older patients with pneumonia (38–40). Male gender may also be a risk factor for pneumonia-related deaths (5) (Table 1).
Table 1.
Summary of Community Acquired Pneumonia
| Epidemiology | Incidence of 14 cases/1,000 person-years* |
| Clinical presentation and evaluation | Tachypnea, dyspnea, bronchospasm |
| Failure to thrive, malaise, delirium | |
| Fever, cough, purulent sputum | |
| +/− infiltrate on chest x-ray | |
| Leukocytosis | |
| Risk factors | Age |
| Influenza infection | |
| Comorbid conditions | |
| Treatment | Antibiotics according to IDSA/ATS guidelines |
| Determinants of outcome and health-related quality of life | Severity of acute infection\ |
| Comorbidities | |
| Functional status prior to disease | |
| Possible link between male gender and mortality |
Notes: ATS = American Thoracic Society ; IDSA = Infectious Diseases Society of America.
Incidence doubled in nursing home residents.
CHRONIC OBSTRUCTIVE PULMONARY DISEASE
Epidemiology
COPD is the fourth leading cause of death in the United States (41) and is associated with aging (42–44) (Figure 1). At least 10% of persons aged 65 years and older in the United States are diagnosed with COPD (45). Internationally, the prevalence of COPD has been estimated between 5% and 16% in patients aged 40 years and older, depending on the country (46–53). These numbers likely underestimate the prevalence of COPD due to underdiagnosis and underutilization of pulmonary function tests (PFTs) (1–3,45,48,52–60).
Figure 1.
Prevalence of COPD by age group in the United States. Data from National Health Interview Survey, 2000 (45).
Due to the nature of COPD, most studies can only estimate disease prevalence. However, a large Dutch cohort of nearly 8,000 participants found the incidence rate (IR) of COPD to be 9.2/1,000 person-years in patients greater than or equal to 55 years old, with increasing incidence through ages 75–79 years (61) (Figure 1). Overall, the IR was higher in men than women (14.4/1,000 person-years vs 6.2/1,000 person-years, respectively).
Clinical Presentation and Evaluation
Pulmonary symptoms of COPD are nonspecific and include cough, chronic sputum production, wheeze, and dyspnea. Chronic cough is the best single symptom to predict airway obstruction in smokers more than 60 years old (42). COPD should be considered in all patients with a history of exposure to cigarette smoke or occupational pollutants with chronic cough, sputum production, or dyspnea (62).
Comorbidities affect more than 80% of older patients with COPD (54,63–67). Older patients may attribute their dyspnea to these other comorbid diseases (including congestive heart failure, hypertension, and neurological deficits after stroke) or to muscle weakness, deconditioning, or physiological symptoms related to aging (63,65). Older patients with COPD may use different language to describe dyspnea compared with older patients without COPD (68). Patients with COPD commonly use words like “terrifying,” “frightening,” “helpless,” “depressed,” and “awful” when describing dyspnea symptoms (68). Perhaps related to different symptomatic experiences of dyspnea, anxiety and depression are highly prevalent in older patients with advanced COPD (69–71).
PFTs are the gold standard for diagnosing COPD. Although the majority of patients can perform the test, hearing impairment, cognitive impairment, and comorbid diseases may affect older patients’ ability to complete PFTs (72,73). There is a dose–response relationship between severity of COPD (Global Initiative for Chronic Obstructive Lung Disease stage IV) and dyspnea symptoms (74). Hypoxemia and hypercapnia should not be considered physiological, unavoidable consequences of aging.
Lung function decreases with age, even among individuals without lung disease and this complicates PFT interpretation in older persons. Based on Global Initiative for Chronic Obstructive Lung Disease definitions of COPD, 35% of healthy, asymptomatic, never smokers aged 70 years and older have stage 1 COPD (75). In order to avoid excessive diagnosis of COPD in asymptomatic older persons, adjustments to current diagnostic thresholds and consideration of alternative PFT measurements have been proposed (76–78). In addition, American Thoracic Society and European Respiratory Society guidelines recommend using below the 5th percentile of the normal distribution of forced expiratory volume in one second/vital capacity as the cutoff for COPD diagnosis rather than a fixed ratio of 70% (79).
Risk Factors
Although age is a risk factor for developing COPD (42,80), tobacco smoke is the number one risk factor, regardless of age (62). Current smoking and greater than or equal to 20 pack-year smoking history are particularly strong risk factors for COPD (61). Environmental exposure to biomass smoke is also a risk factor for COPD, especially in developing countries (47,81–84). Certain occupational exposures are risk factors for COPD (84). Alpha-1 antitrypsin gene mutations contribute to 5% of patients with COPD (85). Prior tuberculosis infection has been associated with irreversible airway obstruction (48,53). Male sex may also be a risk factor for COPD although this is not consistently found (61).
Treatment
Treatment of COPD in older persons should follow the Global Initiative for Chronic Obstructive Lung Disease guidelines (41). There are no age-specific recommendations for treatment of COPD. Pharmacological therapies include inhaled beta-agonists, anticholinergics, and corticosteroids, which are prescribed alone or in combination. Treatment of COPD should follow a stepwise approach in symptomatic patients. Long-acting inhalers are recommended for patients with more advanced disease (86–90). Sustained-release theophylline is another treatment option in older patients with COPD although inhaled bronchodilators are preferred for treatment (91). The phosphodiesterase-4 inhibitor roflumilast has been shown to decrease exacerbations in randomized clinical trials (92,93). In addition, roflumilast may improve lung function when used with long-acting beta-agonists or anticholinergic inhalers (93). Although no pharmacological therapies have been shown to definitively improve lung function, pharmacotherapy may reduce symptoms and exacerbations, and improve exercise tolerance and health-related quality of life (HRQOL) (94–96).
There are toxicities associated with COPD medications (Table 2). Beta-agonists can cause tremors and tachycardia, and chronic or excessive use can result in drug tolerance. Long-acting beta-agonists should be used with caution in older persons also because of possible increased systemic inflammation and increased risk of cardiovascular events (97). Concerns exist over the cardiovascular safety profile of anticholinergic inhalers used to treat COPD (98,99). However, in a 4-year randomized controlled trial of 6,000 COPD patients, tiotropium was associated with decreased mortality, particularly from cardiac diseases (100,101).
Table 2.
Side Effects and Treatment Toxicities of Medications Commonly Prescribed for Pulmonary Diseases in Older Patients
| Side Effect/Toxicity | |
| COPD/asthma | |
| Inhaled steroids | Oropharyngeal candidiasis |
| Adrenal suppression | |
| Reduced bone mineral density (possible osteoporosis and hip fracture) | |
| Cataracts | |
| Glaucoma | |
| Possible increased risk of bacterial pneumonia and tuberculosis | |
| Inhaled beta-agonist | Tremor |
| Peripheral arterial dilatation | |
| Reflex tachycardia | |
| Hypokalemia | |
| Theophylline | Tachycardia |
| Narrow therapeutic window | |
| Inhaled anticholinergic | Possible increase in cardiovascular events |
| Lung cancer | |
| Concurrent chemoradiotherapy (cisplatin/etoposide or cisplatin/vinblastine + thoracic irradiation) | Esophagitis |
| Neutropenia | |
| Anemia | |
| Thrombocytopenia | |
| Pneumonitis | |
| Acute kidney injury/nephrotoxicity | |
| Platinum-based doublet therapy (paclitaxel/carboplatin; carboplatin/gemcitabine) | Hematological toxicity |
| Neutropenia/neutropenic fever/pneumonia | |
| Anemia | |
| Thrombocytopenia | |
| Infection | |
| Anaphylaxis, immune hypersensitivity reaction | |
| Pulmonary fibrosis | |
| Mucositis | |
| Nausea/vomiting | |
| Myalgia/arthralgia | |
| Neuromotor/neurosensory toxicity | |
| Bevacizumab (in addition to platinum-based doublet therapy) | Neutropenia/neutropenic fever |
| Gastrointestinal bleeding (especially increased with age) | |
| Hemoptysis/pulmonary hemorrhage | |
| Other severe/near-fatal bleeding events including epistaxis | |
| Deep venous thrombosis | |
| Cardiovascular events | |
| Stroke/transient ischemic attacks | |
| Myocardial infarction/angina | |
| Congestive heart failure | |
| Hypertension, including encephalopathy from hypertension | |
| Alopecia, rash | |
| Impaired wound healing | |
| Hyponatremia | |
| Weight loss | |
| Infusion reactions | |
| Single-agent therapy (vinorelbine or gemcitabine or docetaxel) | Hematological toxicities |
Note: COPD = chronic obstructive pulmonary disease.
Inhaled corticosteroids (ICS) may have a role in COPD treatment when used in combination with inhaled bronchodilators. In a retrospective cohort study of Medicare beneficiaries, health care costs due to emergency room visits and hospitalizations were decreased in older patients receiving combination fluticasone propionate/salmeterol inhalers compared to anticholinergic-only inhalers (102). Similar beneficial results were found in commercially insured COPD patients aged 40 years or older (103). Recent studies of older Veterans found less frequent use of mechanical ventilation and lower mortality in COPD patients with pneumonia who were treated with ICS (104,105).
ICS use is associated with significant side effects, particularly in older patients. Oropharyngeal candidiasis may result from local drug deposition. Systemic side effects are possible due to systemic absorption from the lungs and delivery of drug to the gastrointestinal tract. This can result in loss of bone density and may contribute to osteoporosis and fracture risk (106–111). ICS use can also cause adrenal suppression, especially with high-dose fluticasone (112–114). Older patients on high-dose ICS may have an increased risk for development of glaucoma and cataracts as well (115–117). Some studies have shown an increased risk of pneumonia in patients with COPD on ICS although this is not a consistent finding, and in some studies, ICS was associated with a decreased risk of pneumonia as cited earlier (118–120). ICS have been associated with an increased risk of tuberculosis (119–121). Although the risks are significant, reviewing proper inhaler technique and the use of spacer devices for some patients can minimize systemic absorption of ICS and oral candidiasis (122–125).
Theophylline is used infrequently due to its narrow therapeutic window requiring regular monitoring of serum concentrations to minimize risk of side effects including tachycardia, cardiac arrhythmias, and seizures. In one study of older patients on theophylline, there was no correlation between adverse events and serum levels in patients who reported adverse events (91).
Pulmonary rehabilitation has been studied in older patients with COPD (126). Pulmonary rehabilitation improves physical activity and ability to perform activities of daily living, with sustained benefits in older patients with COPD (126–128). In the absence of formal pulmonary rehabilitation programs, self-administered strength training may be beneficial in older COPD patients. In a small study of older, home-dwelling men with COPD, heavy resistance training improved muscle size, strength, functional performance, and self-reported health perception (129).
Older patients with acute exacerbations of chronic obstructive pulmonary disease (AECOPD) may require augmented inhaler regimens with long-acting bronchodilators, inhaled or systemic corticosteroids, and antibiotics, especially in patients with more severe exacerbations (122,130–141). Antibiotics are underprescribed in older patients with AECOPD (142). In one Dutch study, only half of patients with AECOPD aged 65 years or older received antibiotics (142). Patients with comorbid diseases such as diabetes and heart failure were more likely to receive antibiotics during AECOPD (142).
Medication nonadherence is common in COPD (143). Older patients, even in the absence of COPD, may not have enough strength to generate the negative inspiratory force required to use inhaled medications (144,145). Weakness and inappropriate technique can lead to undertreatment of COPD in older patients who are otherwise adherent to their medications (146). Alternative modes of delivery such as transdermal drug delivery may provide alternative options for older patients with COPD (147). Metered-dose or dry powder inhaler technique should be reassessed regularly.
Cost is an additional barrier to COPD medication compliance in older patients. In a recent study of Medicare beneficiaries, 31% of patients did not use inhalers due to cost (148). Risk of cost-related nonadherence was greatest for patients who paid more than $20 per month out-of-pocket (148).
Outcomes
Comorbidities are responsible for a significant proportion of deaths in older patients with COPD, particularly cardiovascular disease and lung cancer (149–151). CAP and AECOPDs are associated with higher short- and long-term mortality and negatively impact HRQOL (152). Higher forced expiratory volume in 1 second (FEV1) and better 6-minute walk distance are associated with improved survival (153).
The impact of COPD on functional status and HRQOL is dependent on comorbidities and severity of airway obstruction (54,63–65). In older patients with mild COPD, age and non-COPD comorbidities appear to have the greatest impact on physical independence and mortality (154). In older patients with stable COPD, the ability to perform activities of daily living and overall emotional state most affect HRQOL rather than degree of obstructive lung disease on PFTs (155). Although COPD is associated with significant morbidity and mortality, several treatments are available to assist older patients maintain or improve their functional status and HRQOL (Table 3).
Table 3.
Summary of Chronic Obstructive Pulmonary Disease
| Epidemiology | Incidence of 9.2/1,000 person-years in patients ≥55 years old |
| Increases with age through 75–79 years old | |
| Higher incidence in men | |
| Prevalence of at least 10% in patients ≥65 years old in the United States | |
| Prevalence of 5%–16% in patients ≥40 years old internationally | |
| Clinical presentation and evaluation | Cigarette smoking history |
| Occupational exposures | |
| Cough, wheeze, dyspnea | |
| Chronic sputum production | |
| Pulmonary function tests if able | |
| May be better to avoid using fixed ratio of FEV1/FVC to minimize over diagnosis | |
| Risk factors | Age |
| Alpha-1 antitrypsin deficiency | |
| Tobacco smoke: current, >20 pack-year history | |
| Biomass smoke exposure, occupational exposure | |
| Treatment | Inhaled beta-agonists* |
| Inhaled anticholinergics* | |
| Inhaled and systemic corticosteroids* | |
| Theophylline | |
| Roflumilast | |
| Pulmonary rehabilitation | |
| Antibiotics for exacerbations | |
| Determinants of outcome and health-related quality of life | FEV1(positive association) |
| 6-Minute walk distance (positive association) | |
| Comorbidities (negative association) | |
| Severity of airway obstruction (negative association) | |
| Ability to perform activities of daily living | |
| Emotional state |
Notes: FEV1 = forced expiratory volume in 1 s; FVC = forced vital capacity
Assure proper inhaler technique.
ASTHMA
Epidemiology
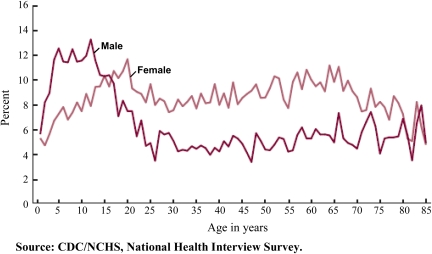
Asthma has a bimodal distribution with increased diagnosis in patients aged 50–65 years (156,157) (Figure 2). Asthma prevalence in patients greater than or equal to 65 years old is estimated between 4% and 8% (59,158–160). In a large cohort of older patients with asthma, more than 50% were diagnosed after the age of 50 years and 23% were diagnosed after 65 years (161). Asthma is underdiagnosed in older persons, particularly in highly functional older patients even when their PFTs demonstrate reversible airway obstruction (162,163).
Figure 2.
Prevalence of current asthma among men and women 1–85 years, by age: United States, annual average 2001–2009 (157).
Clinical Presentation and Evaluation
Older patients with asthma may present with wheeze, morning phlegm, chest tightness, shortness of breath at rest, chronic cough, and symptoms that are often worse at night (164–167). Older persons with asthma have a varying duration of symptoms on presentation (168). Patients with late-onset asthma have a higher forced expiratory volume in 1 second than life-long asthmatics (168,169), but patients with long-standing disease may be less sensitive to their asthma symptoms and more likely to have fixed obstruction (158,167).
Thirty percent of older patients referred for PFT evaluation have reversible airway obstruction, particularly male patients and nonsmokers (170). Patients with more severe obstruction have greater bronchodilator responses compared to patients with mild disease (170). In older patients with normal baseline spirometry in whom asthma is being considered, methacholine challenge testing can be safely used (164,171).
Asthma can be challenging to differentiate from COPD in older patients, particularly in those with physical disability (163) (Table 4). In one study of asthma in older patients, nearly 20% of patients were erroneously diagnosed with COPD (163). Older asthmatic patients have more bronchodilator response (159,172,173), are more likely to be atopic, and have more eosinophils in serum and sputum than patients with COPD (159,172,173). Heavy smoking history, hyperinflation, hypoxemia, and diffusion impairment are more commonly seen in patients with COPD (159,172,173).
Table 4.
Comparison of Clinical Features of Asthma and COPD in the Elderly Adults
| Asthma | COPD | |
| Increases with age | ++ | ++ |
| Early onset (childhood diagnosis) | +++ | – |
| Late onset (mid-40s) | ++ | +++ |
| Identifiable triggers | +++ | – |
| Atopy present | +++ | – |
| Diagnosis preceded by upper respiratory infection | +++ | – |
| Cigarette smoking | +/− | +++ |
| Reversible airflow on PFTs | +++/− | +/− |
| Episodic wheezing | +++ | ++ |
| Daily symptoms | + | +++ |
| Cough | +++ | +++ |
| Dyspnea | ++ | ++ |
| Chest tightness | +++ | ++/− |
| Nocturnal symptoms | ++ | – |
| Blood and sputum eosinophilia | ++ | – |
| Impaired carbon monoxide diffusion | + | +++ |
Note: COPD = chronic obstructive pulmonary disease; PFT = pulmonary function tests.
Risk Factors
Comorbid diseases and obesity are risk factors for asthma in older patients (174–176). Gastroesophageal reflux disease can also complicate asthma symptoms in older persons (177,178). Several medications commonly used in older persons are associated with asthma and include beta-blockers, nonsteroidal anti-inflammatory medications, and postmenopausal hormone replacement therapy (179–181).
Treatment
Asthma in older persons should be managed according to the recommendations made by the National Asthma Education and Prevention Program. The pharmacotherapeutic options are mostly the same as those used for managing COPD with the same side effects and toxicities (see Table 2). However, ICS are first-line treatment for patients with moderate or severe persistent asthma. In addition, antileukotriene agents may provide symptomatic improvement in older asthmatics (182).
ICS may be underutilized in older asthmatics (158,159). In nonsmoking, older asthmatics in the Cardiovascular Health Study, less than half of patients were using ICS or systemic corticosteroids and 39% of patients received no asthma medications at all (159). Combination ICS and long-acting beta-agonist inhalers in older patients with asthma may decrease hospitalizations rates and mortality compared to either treatment alone (118). However, as reviewed for treatment of COPD, there are concerns over the cardiovascular safety profile of long-acting beta-agonists (97).
Treatment adherence is low in older patients with asthma (183). In addition to mechanical limitations, comorbid diseases and cognitive impairment negatively affect adherence to asthma regimens in older patients (183). Depression can also decrease adherence to asthma medications (159,184). Nonpharmacological management including asthma-focused telephone interviews, symptom and medication diaries, and assessment of drug usage may improve adherence (183,185).
Outcome
Older patients with asthma die from their disease more than younger patients and account for 50% of asthma deaths (45,186,187). As with COPD, comorbidity contributes to increased mortality in older asthmatic patients. Although more respiratory symptoms are associated with higher mortality even in the absence of lung disease, lower HRQOL has not been associated with mortality in older persons with asthma (188). Forced expiratory volume in 1 second declines more in older asthmatics compared with nonasthmatics, particularly in those with longer duration of disease (167,189). Studies have shown significant HRQOL impairment in older asthmatic patients (159) (Table 5).
Table 5.
Summary of Asthma
| Epidemiology | Prevalence of 4%–8% of patients ≥65 years old |
| Clinical presentation and evaluation | Wheeze, chest tightness |
| Shortness of breath at rest | |
| Chronic cough | |
| Symptoms worse at night | |
| PFTs with reversible airflow obstruction | |
| Methacholine challenge | |
| Risk factors | Comorbidities |
| Obesity | |
| Medications: beta-blockers, nonsteroidal anti-inflammatory drugs, hormone replacement | |
| Treatment | Pharmacological |
| Inhaled beta-agonists | |
| Inhaled and systemic corticosteroids | |
| Inhaled anticholinergics | |
| Anti-leukotriene agents | |
| Nonpharmacological | |
| Symptom diaries | |
| Telephone interviews regarding symptoms and inhaler use | |
| Peak flow meters | |
| Determinants of outcome and health-related quality of life | Older patients account for 50% of asthma-related deaths |
| Frequent impairment in health-related quality of life |
Note: PFT = pulmonary function tests.
LUNG CANCER
Epidemiology
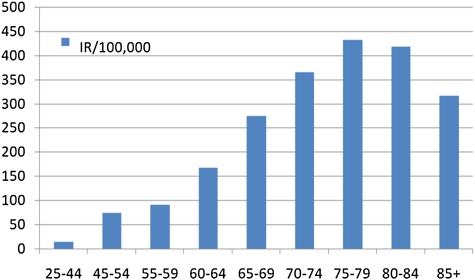
Lung cancer increases with age, particularly after age 60. The median age of patients diagnosed with lung cancer is 70 years old (190). Lung cancer incidence peaks in the 75- to 79-year-old age group (IR = 9.5, age 40–44; IR = 167.8, age 60–64; IR peak = 431.8/100,000) (191) (Figure 3).
Figure 3.
Incidence rates of lung cancer by age group. Data from United States, 2007 SEER database (191).
Non–small cell lung cancer (NSCLC) makes up 85% of lung cancer cases. Lung cancer can metastasize to liver, bone, brain, and adrenal glands (192,193) and more than half of patients diagnosed with NSCLC present with metastatic disease (194). Small cell lung cancer accounts for 10%–15% of lung cancer. Twenty to 40% of patients present with bony metastases, especially in small cell cancer (195,196). Histology and staging of lung cancer have important prognostic and treatment implications.
Clinical Presentation and Evaluation
Symptoms of lung cancer are nonspecific. Symptoms include cough, shortness of breath, chest pain, and hoarseness (197–199). Hemoptysis is another common but nonspecific symptom of lung cancer (197,198,200).
Older persons with lung cancer may present with symptomatic metastatic disease. Patients commonly present with painful bony metastases (195,196). Central nervous system symptoms can be caused by mass effect resulting in headache, visual changes, and seizures. Paraneoplastic syndromes can cause lethargy, weakness, and electrolyte disorders from hypercalcemia; syndrome of inappropriate antidiuretic hormone secretion; Lambert–Eaton myasthenic syndrome; Cushing’s syndrome; hypertrophic osteoarthropathy; and dermatomyositis/polymyositis. Liver and adrenal metastases, while common in advanced disease, rarely cause symptoms (201,202).
Risk Factors
Cigarette smoking is the number one risk factor for lung cancer although 10%–15% of patients with lung cancer are nonsmokers (203,204). Smoking cessation decreases risk of lung cancer, even among patients who smoke into their sixth decade (205). Female gender is a risk factor for adenocarcinoma in never smokers (206).
Radon exposure, history of radiation therapy, secondhand smoke, and other environmental exposures are additional risk factors for lung cancer. COPD, pulmonary fibrosis, and HIV infection also increase the risk of developing lung cancer (207,208). Family history may increase risk of lung cancer.
Treatment
Lung cancer in older patients should be treated according to the American College of Chest Physicians guidelines (209). A multidisciplinary approach including thoracic surgeons, medical oncologists, radiation oncologists, and pulmonologists is recommended in considering treatment options for patients with lung cancer. The guidelines specifically state that “patients with lung cancer not be denied lung resection surgery on the grounds of age alone (209).”
Comprehensive geriatric evaluation is recommended when considering treatment options for older patients with lung cancer (210–212). In addition to pulmonary function and assessment of prevalent comorbidities, focus should be on performance status and cognitive function (210–212). Dementia and limitations in performing activities of daily living are important risk factors for postoperative complications in older patients receiving surgical treatment of lung cancer (210). There are a number of clinical tools available to assess for cognitive or functional impairment in older patients (210–214). Although there is no specific recommendation for which tool to use in older patients with lung cancer, the Barthel Index, and Mini-Mental State Examination have been used in prior studies (210,211,215,216).
Despite the American College of Chest Physicians statement on age, older patients with lung cancer receive less definitive staging and treatment than younger patients (217–222). This may be related to increased perioperative mortality in older patients (211,223–227). However, the burden of comorbidity may not have been completely considered in these studies (224,225,227). Recent studies accounting for comorbidities found no difference in older patients who undergo surgical resection compared with younger patients (228–230) and disease recurrence is no different in older compared with younger patients with early-stage NSCLC (231).
Patients undergoing chemotherapy or radiation therapy for lung cancer often have medical comorbidities (208). Chemotherapy and radiation therapy are associated with a number of toxicities in older patients (232; see Table 2) and prevalent comorbidities may contribute to treatment toxicity (233). Modification of chemotherapy dose and scheduling is possible to allow safe chemotherapy administration (234), and there is a growing body of evidence to suggest that older patients tolerate a variety of chemotherapy agents for both NSCLC and small cell lung cancer (221,235–238).
Outcome
Lung cancer is responsible for more cancer-related deaths than breast, prostate, and colon cancer combined (239). Lung cancer mortality increases with age. Patients aged 65 years and older account for 80% of lung cancer deaths and 20% of lung cancer deaths are in patients aged 80 years and older (240).
Older patients with early-stage NSCLC who undergo surgery have similar postoperative survival to younger patients (241). Patients with metastatic NSCLC have had modest improvements in survival over the last three decades with chemotherapy (240), and this has been observed in older patients as well (242). The effects of chemotherapy on HRQOL in older patients are complicated with some areas of symptomatic improvement but also decrements in HRQOL due to drug toxicity (243) (Table 6).
Table 6.
Summary of Lung Cancer
| Epidemiology | Incidence increases with age |
| Peak incidence of 431.8/100,000 person years in 75- to79-year-old age group | |
| Clinical presentation and evaluation | Non–small cell lung cancer most common |
| Frequently present with metastatic disease | |
| Cough, shortness of breath | |
| Hemoptysis | |
| Chest pain, hoarseness | |
| Constitutional symptoms: fatigue, weight loss | |
| Paraneoplastic syndromes | |
| Imaging | |
| Staging: clinical and pathological if able | |
| Risk factors | Cigarette smoking |
| Radon exposure History of radiation exposure | |
| COPD | |
| Pulmonary fibrosis | |
| HIV | |
| Female gender for adenocarcinoma | |
| Treatment | American College of Chest Physicians guidelines after staging |
| Multidisciplinary approach | |
| Determinants of outcome and health-related quality of life | Depends on stage, treatment, comorbidities, functional status |
Note: COPD = chronic obstructive pulmonary disease.
IDIOPATHIC PULMONARY FIBROSIS
Epidemiology
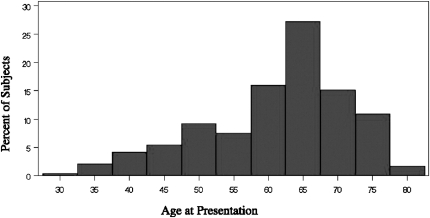
IPF is a relatively rare, progressive fibrotic lung disease of unclear etiology that primarily affects older patients. Prevalence and incidence increase with age, particularly after the sixth decade (Figure 4) (244,245). Overall, the prevalence of IPF in the United States is estimated around 42.7/100,000 but is likely an underestimate (246,247). Incidence is estimated at 10.7 and 7.4 per 100,000 men and women, respectively (246).
Figure 4.
Onset of idiopathic pulmonary fibrosis (IPF) usually occurs between the ages of 50 and 70 years, but the mean age in this population of patients with IPF was 61.4 years (n = 238, age range 27–79 years). Data from cohort of patients enrolled between 1982 and 1996 in the Specialized Center of Research Study at the National Jewish Medical and Research Center. Reproduced with permission from the American College of Chest Physicians (246).
Clinical Presentation and Evaluation
Dyspnea and dry cough are common presenting symptoms of IPF and dry rales can be present on physical examination. Cormorbidites, including diabetes, coronary artery disease, obstructive sleep apnea, steroid-related osteopenia, and sarcopenia, are common in IPF (246,248). Older patients with advanced IPF are at increased risk of pulmonary hypertension (249–251). Patients with both IPF and pulmonary hypertension have significantly higher mortality (252–255).
A restrictive ventilatory defect with diffusion impairment is seen on PFTs. Interstitial pulmonary infiltrates are seen on chest x-ray and CT scan with honeycombing and traction bronchiectasis in more advanced IPF. Lung biopsy may not be necessary if an experienced pulmonologist or radiologist in interstitial lung disease and IPF can make a confident diagnosis based on clinical and radiographic information (1,256).
Risk Factors
Smoking is the main identifiable risk factor for IPF although the pathophysiology of IPF is still uncertain (244,245). Familial variants have been identified (257). Chronic aspiration may be a risk factor (1,258). Gastroesophageal reflux disease is common in patients with IPF and may also be a risk factor for both disease development and progression (259–262). Acute and chronic viral infections might contribute to development and progression of IPF. There is some data implicating Ebstein–Barr virus in IPF, but the specific role of viruses has not been fully elucidated (263–266).
Treatment
According to the most recent American Thoracic Society/European Respiratory Society consensus statement, there is no proven treatment that is effective for IPF (258). Combination therapy with prednisone, azathioprine, and N-acetylcysteine may slow decline in forced vital capacity compared with prednisone and azathioprine alone, but the clinical significance of these findings is not clear (267,268). In multinational, double-blind, placebo-controlled trials, pirfenidone has shown promising results in reducing rate of decline in lung function and may even improve vital capacity in patients with mild–moderate disease (269–275). Early studies have also suggested pirfenidone may decrease acute IPF exacerbations (269). Optimizing comorbid diseases, supplemental oxygen therapy, and pulmonary rehabilitation may help preserve and perhaps improve functional status (246,276).
Lung transplant remains the only available treatment for patients with end-stage IPF. Ideal timing for transplant in IPF has not been determined. Posttransplant survival is lower in older patients, and although age more than 65 years had been a relative contradiction in the past (277), this age group has seen the greatest increase in lung transplantation in recent years (Figure 5) (277–279).
Figure 5.
Percentage of lung transplant recipients age 65 years and older in the United States from 1999 to 2008 (278).
Outcome
Outcomes for older patients with IPF are quite poor. Only 20%–30% of patients survive 5 years after diagnosis (280,281). Older age at presentation is associated with shorter survival times. In addition, heavy tobacco use, lower body mass index, greater gas exchange impairment, more abnormal radiographic findings, and development of other pulmonary diseases are also associated with worse survival (280,281).
Comorbidities significantly affect outcomes in older patients with IPF (246,282). Consideration of performance status, cognitive function, and IPF severity together may be more indicative of prognosis and outcomes than any one of these measures alone (282). Furthermore, given the high mortality, functional status may serve as a valuable measure of response to therapy and prognosis (283,284) (Table 7).
Table 7.
Summary of IPF
| Epidemiology | Incidence and prevalence increase with age but likely underestimated |
| Incidence of 10.7/100,000 men, 7.4/100,000 women | |
| Overall prevalence of 42.7/100,000 | |
| Clinical presentation and evaluation | Dyspnea, dry cough |
| PFTs with restriction | |
| Chest x-ray | |
| Chest CT | |
| May not require lung biopsy | |
| Risk factors | Smoking |
| Genetic | |
| Treatment* | Supplemental oxygen |
| Pulmonary rehabilitation | |
| Prednisone, azathioprine, N-acetylcysteine | |
| Pirfenidone | |
| Lung transplant | |
| Determinants of outcome and health-related quality of life | 20%–30% survival 5 years after diagnosis |
| Older age at presentation, heavy tobacco use, lower body mass index, greater gas exchange impairment, more radiographic abnormalities, and other pulmonary diseases associated with worse survival | |
| Importance of performance status and cognitive function in addition to disease severity on outcomes |
Notes: IPF = idiopathic pulmonary fibrosis; PFT = pulmonary function tests.
No effective therapy proven to treat IPF to date although some promising results with pirfenidone.
CONCLUSIONS
Pulmonary diseases are common in older patients. Presenting symptoms are nonspecific and overlap with each other as well as with nonpulmonary diseases. Although some decline in pulmonary function is associated with aging, clinically significant pulmonary diseases need to be considered in symptomatic patients. Cigarette smoking is the strongest risk factor for nearly all pulmonary diseases in older patients. Age and comorbidity often affect patients’ abilities to respond to, adhere to, and tolerate treatment of lung diseases. Older persons with lung diseases should receive routine vaccinations. Easy-to-administer, effective treatments for lung diseases should undergo rigorous evaluation in randomized, controlled trials with aging patients.
FUNDING
This work was supported by the Association of Specialty Physicians and CHEST Foundation of the American College of Chest Physicians T. Franklin Williams Award to K.M.A. and the National Institutes of Health, National Heart, Lung, and Blood Institute (R01 HL090342 to K.C.). The work for this report was supported, in part, by the Yale Claude D. Pepper Older Americans Independence Center (P30AG21342).
References
- 1.Janssens JP, Herrmann F, MacGee W, Michel JP. Cause of death in older patients with anatomo-pathological evidence of chronic bronchitis or emphysema: a case-control study based on autopsy findings. J Am Geriatr Soc. 2001;49(5):571–576. doi: 10.1046/j.1532-5415.2001.49116.x. [DOI] [PubMed] [Google Scholar]
- 2.Lundback B, Lindberg A, Lindstrom M, et al. Not 15 but 50% of smokers develop COPD?—Report from the Obstructive Lung Disease in Northern Sweden Studies. Respir Med. 2003;97(2):115–122. doi: 10.1053/rmed.2003.1446. [DOI] [PubMed] [Google Scholar]
- 3.Malik A, Saltoun CA, Yarnold PR, Grammer LC. Prevalence of obstructive airways disease in the disadvantaged elderly of Chicago. Allergy Asthma Proc. 2004;25(3):169–173. [PubMed] [Google Scholar]
- 4.Meyer KC. Aging. Proc Am Thorac Soc. 2005;2(5):433–439. doi: 10.1513/pats.200508-081JS. [DOI] [PubMed] [Google Scholar]
- 5.Kaplan V, Angus DC, Griffin MF, Clermont G, Scott WR, Linde-Zwirble WT. Hospitalized community-acquired pneumonia in the elderly: age- and sex-related patterns of care and outcome in the United States. Am J Respir Crit Care Med. 2002;165(6):766–772. doi: 10.1164/ajrccm.165.6.2103038. [DOI] [PubMed] [Google Scholar]
- 6.Marrie TJ. Pneumonia in the elderly. Curr Opin Pulm Med. 1996;2(3):192–197. doi: 10.1097/00063198-199605000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Donowitz GR, Cox HL. Bacterial community-acquired pneumonia in older patients. Clin Geriatr Med. 2007;23(3):515–534. doi: 10.1016/j.cger.2007.03.006. vi. [DOI] [PubMed] [Google Scholar]
- 8.Marrie TJ. Community-acquired pneumonia in the elderly. Clin Infect Dis. 2000;31(4):1066–1078. doi: 10.1086/318124. [DOI] [PubMed] [Google Scholar]
- 9.Reza SM, Huang JQ, Marrie TJ. Differences in the features of aspiration pneumonia according to site of acquisition: community or continuing care facility. J Am Geriatr Soc. 2006;54(2):296–302. doi: 10.1111/j.1532-5415.2005.00608.x. [DOI] [PubMed] [Google Scholar]
- 10.Fernandez-Sabe N, Carratala J, Roson B, et al. Community-acquired pneumonia in very elderly patients: causative organisms, clinical characteristics, and outcomes. Medicine (Baltimore) 2003;82(3):159–169. doi: 10.1097/01.md.0000076005.64510.87. [DOI] [PubMed] [Google Scholar]
- 11.El-Solh AA, Sikka P, Ramadan F, Davies J. Etiology of severe pneumonia in the very elderly. Am J Respir Crit Care Med. 2001;163(3 Pt 1):645–651. doi: 10.1164/ajrccm.163.3.2005075. [DOI] [PubMed] [Google Scholar]
- 12.Waterer GW, Kessler LA, Wunderink RG. Delayed administration of antibiotics and atypical presentation in community-acquired pneumonia. Chest. 2006;130(1):11–15. doi: 10.1378/chest.130.1.11. [DOI] [PubMed] [Google Scholar]
- 13.Metersky ML, Sweeney TA, Getzow MB, Siddiqui F, Nsa W, Bratzler DW. Antibiotic timing and diagnostic uncertainty in Medicare patients with pneumonia: is it reasonable to expect all patients to receive antibiotics within 4 hours? Chest. 2006;130(1):16–21. doi: 10.1378/chest.130.1.16. [DOI] [PubMed] [Google Scholar]
- 14.Riquelme R, Torres A, el-Ebiary M, et al. Community-acquired pneumonia in the elderly. Clinical and nutritional aspects. Am J Respir Crit Care Med. 1997;156(6):1908–1914. doi: 10.1164/ajrccm.156.6.9702005. [DOI] [PubMed] [Google Scholar]
- 15.Metlay JP, Schulz R, Li YH, et al. Influence of age on symptoms at presentation in patients with community-acquired pneumonia. Arch Intern Med. 1997;157(13):1453–1459. [PubMed] [Google Scholar]
- 16.Bartlett JG. Anaerobic bacterial pneumonitis. Am Rev Respir Dis. 1979;119(1):19–23. doi: 10.1164/arrd.1979.119.1.19. [DOI] [PubMed] [Google Scholar]
- 17.Bartlett JG. Anaerobic bacterial infections of the lung and pleural space. Clin Infect Dis. 1993;16(suppl 4):S248–S255. doi: 10.1093/clinids/16.supplement_4.s248. [DOI] [PubMed] [Google Scholar]
- 18.Falsey AR, Erdman D, Anderson LJ, Walsh EE. Human metapneumovirus infections in young and elderly adults. J Infect Dis. 2003;187(5):785–790. doi: 10.1086/367901. [DOI] [PubMed] [Google Scholar]
- 19.Mandell LA, Bartlett JG, Dowell SF, File TM, Jr, Musher DM, Whitney C. Update of practice guidelines for the management of community-acquired pneumonia in immunocompetent adults. Clin Infect Dis. 2003;37(11):1405–1433. doi: 10.1086/380488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Roux A, Cavalcanti M, Marcos MA, et al. Impact of alcohol abuse in the etiology and severity of community-acquired pneumonia. Chest. 2006;129(5):1219–1225. doi: 10.1378/chest.129.5.1219. [DOI] [PubMed] [Google Scholar]
- 21.Nuorti JP, Butler JC, Farley MM, et al. Cigarette smoking and invasive pneumococcal disease. Active Bacterial Core Surveillance Team. N Engl J Med. 2000;342(10):681–689. doi: 10.1056/NEJM200003093421002. [DOI] [PubMed] [Google Scholar]
- 22.Talbot TR, Hartert TV, Mitchel E, et al. Asthma as a risk factor for invasive pneumococcal disease. N Engl J Med. 2005;352(20):2082–2090. doi: 10.1056/NEJMoa044113. [DOI] [PubMed] [Google Scholar]
- 23.Kupronis BA, Richards CL, Whitney CG. Invasive pneumococcal disease in older adults residing in long-term care facilities and in the community. J Am Geriatr Soc. 2003;51(11):1520–1525. doi: 10.1046/j.1532-5415.2003.51501.x. [DOI] [PubMed] [Google Scholar]
- 24.Pittet LA, Hall-Stoodley L, Rutkowski MR, Harmsen AG. Influenza virus infection decreases tracheal mucociliary velocity and clearance of Streptococcus pneumoniae. Am J Respir Cell Mol Biol. 2010;42(4):450–460. doi: 10.1165/rcmb.2007-0417OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Plotkowski MC, Puchelle E, Beck G, Jacquot J, Hannoun C. Adherence of type I Streptococcus pneumoniae to tracheal epithelium of mice infected with influenza A/PR8 virus. Am Rev Respir Dis. 1986;134(5):1040–1044. doi: 10.1164/arrd.1986.134.5.1040. [DOI] [PubMed] [Google Scholar]
- 26.Peltola VT, Murti KG, McCullers JA. Influenza virus neuraminidase contributes to secondary bacterial pneumonia. J Infect Dis. 2005;192(2):249–257. doi: 10.1086/430954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rothberg MB, Haessler SD, Brown RB. Complications of viral influenza. Am J Med. 2008;121(4):258–264. doi: 10.1016/j.amjmed.2007.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mallia P, Johnston SL. Influenza infection and COPD. Int J Chron Obstruct Pulmon Dis. 2007;2(1):55–64. doi: 10.2147/copd.2007.2.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fein AM. Pneumonia in the elderly: overview of diagnostic and therapeutic approaches. Clin Infect Dis. 1999;28(4):726–729. doi: 10.1086/515218. [DOI] [PubMed] [Google Scholar]
- 30.Fry AM, Shay DK, Holman RC, Curns AT, Anderson LJ. Trends in hospitalizations for pneumonia among persons aged 65 years or older in the United States, 1988–2002. JAMA. 2005;294(21):2712–2719. doi: 10.1001/jama.294.21.2712. [DOI] [PubMed] [Google Scholar]
- 31.Sethi S. Bacterial pneumonia. Managing a deadly complication of influenza in older adults with comorbid disease. Geriatrics. 2002;57(3):56–61. [PubMed] [Google Scholar]
- 32.Ruiz M, Ewig S, Marcos MA, et al. Etiology of community-acquired pneumonia: impact of age, comorbidity, and severity. Am J Respir Crit Care Med. 1999;160(2):397–405. doi: 10.1164/ajrccm.160.2.9808045. [DOI] [PubMed] [Google Scholar]
- 33.van der Maarel-Wierink CD, Vanobbergen JN, Bronkhorst EM, Schols JM, de Baat C. Risk factors for aspiration pneumonia in frail older people: a systematic literature review. J Am Med Dir Assoc. 2011;12(5):344–354. doi: 10.1016/j.jamda.2010.12.099. [DOI] [PubMed] [Google Scholar]
- 34.Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(suppl 2):S27–S72. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fine MJ, Auble TE, Yealy DM, et al. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med. 1997;336(4):243–250. doi: 10.1056/NEJM199701233360402. [DOI] [PubMed] [Google Scholar]
- 36.Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171(4):388–416. doi: 10.1164/rccm.200405-644ST. [DOI] [PubMed] [Google Scholar]
- 37.Sligl WI, Majumdar SR. How important is age in defining the prognosis of patients with community-acquired pneumonia? Curr Opin Infect Dis. 2011;24(2):142–147. doi: 10.1097/QCO.0b013e328343b6f8. [DOI] [PubMed] [Google Scholar]
- 38.Sligl WI, Eurich DT, Marrie TJ, Majumdar SR. Only severely limited, premorbid functional status is associated with short- and long-term mortality in patients with pneumonia who are critically ill: a prospective observational study. Chest. 2011;139(1):88–94. doi: 10.1378/chest.10-1054. [DOI] [PubMed] [Google Scholar]
- 39.Jasti H, Mortensen EM, Obrosky DS, Kapoor WN, Fine MJ. Causes and risk factors for rehospitalization of patients hospitalized with community-acquired pneumonia. Clin Infect Dis. 2008;46(4):550–556. doi: 10.1086/526526. [DOI] [PubMed] [Google Scholar]
- 40.Conte HA, Chen YT, Mehal W, Scinto JD, Quagliarello VJ. A prognostic rule for elderly patients admitted with community-acquired pneumonia. Am J Med. 1999;106(1):20–28. doi: 10.1016/s0002-9343(98)00369-6. [DOI] [PubMed] [Google Scholar]
- 41.Rabe KF, Hurd S, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176(6):532–555. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- 42.van Schayck CP, Loozen JM, Wagena E, Akkermans RP, Wesseling GJ. Detecting patients at a high risk of developing chronic obstructive pulmonary disease in general practice: cross sectional case finding study. BMJ. 2002;324(7350):1370. doi: 10.1136/bmj.324.7350.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lindberg A, Eriksson B, Larsson LG, Ronmark E, Sandstrom T, Lundback B. Seven-year cumulative incidence of COPD in an age-stratified general population sample. Chest. 2006;129(4):879–885. doi: 10.1378/chest.129.4.879. [DOI] [PubMed] [Google Scholar]
- 44.Lindberg A, Jonsson AC, Ronmark E, Lundgren R, Larsson LG, Lundback B. Ten-year cumulative incidence of COPD and risk factors for incident disease in a symptomatic cohort. Chest. 2005;127(5):1544–1552. doi: 10.1378/chest.127.5.1544. [DOI] [PubMed] [Google Scholar]
- 45.Mannino DM, Homa DM, Akinbami LJ, Ford ES, Redd SC. Chronic obstructive pulmonary disease surveillance—United States, 1971–2000. MMWR Surveill Summ. 2002;51(6):1–16. [PubMed] [Google Scholar]
- 46.Buist AS, McBurnie MA, Vollmer WM, et al. International variation in the prevalence of COPD (the BOLD Study): a population-based prevalence study. Lancet. 2007;370(9589):741–750. doi: 10.1016/S0140-6736(07)61377-4. [DOI] [PubMed] [Google Scholar]
- 47.Buist AS, Vollmer WM, McBurnie MA. Worldwide burden of COPD in high- and low-income countries. Part I. The burden of obstructive lung disease (BOLD) initiative. Int J Tuberc Lung Dis. 2008;12(7):703–708. [PubMed] [Google Scholar]
- 48.Danielsson P, Olafsdóttir SI, Benediktsdottir B, Gislason T, Janson C. The prevalence of COPD in Uppsala, Sweden—the Burden of Obstructive Lung Disease (BOLD) study: cross-sectional population-based study. Clin Respir J. doi: 10.1111/j.1752-699X.2011.00257.x. [published online ahead of print June 8, 2011] [published online ahead of print June 8, 2011]. doi:10.1111/j.1752-699X. 2011.00257.x. [DOI] [PubMed] [Google Scholar]
- 49.Menezes AM, Perez-Padilla R, Jardim JR, et al. Chronic obstructive pulmonary disease in five Latin American cities (the PLATINO study): a prevalence study. Lancet. 2005;366(9500):1875–1881. doi: 10.1016/S0140-6736(05)67632-5. [DOI] [PubMed] [Google Scholar]
- 50.Menezes AM, Jardim JR, Perez-Padilla R, et al. Prevalence of chronic obstructive pulmonary disease and associated factors: the PLATINO Study in Sao Paulo, Brazil. Cad Saude Publica. 2005;21(5):1565–1573. doi: 10.1590/s0102-311x2005000500030. [DOI] [PubMed] [Google Scholar]
- 51.Menezes AM, Perez-Padilla R, Hallal PC, et al. Worldwide burden of COPD in high- and low-income countries. Part II. Burden of chronic obstructive lung disease in Latin America: the PLATINO study. Int J Tuberc Lung Dis. 2008;12(7):709–712. [PubMed] [Google Scholar]
- 52.Schirnhofer L, Lamprecht B, Vollmer WM, et al. COPD prevalence in Salzburg, Austria: results from the Burden of Obstructive Lung Disease (BOLD) Study. Chest. 2007;131(1):29–36. doi: 10.1378/chest.06-0365. [DOI] [PubMed] [Google Scholar]
- 53.Talamo C, de Oca MM, Halbert R, et al. Diagnostic labeling of COPD in five Latin American cities. Chest. 2007;131(1):60–67. doi: 10.1378/chest.06-1149. [DOI] [PubMed] [Google Scholar]
- 54.Barr RG, Celli BR, Mannino DM, et al. Comorbidities, patient knowledge, and disease management in a national sample of patients with COPD. Am J Med. 2009;122(4):348–355. doi: 10.1016/j.amjmed.2008.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chapman KR, Tashkin DP, Pye DJ. Gender bias in the diagnosis of COPD. Chest. 2001;119(6):1691–1695. doi: 10.1378/chest.119.6.1691. [DOI] [PubMed] [Google Scholar]
- 56.Lindberg A, Bjerg A, Ronmark E, Larsson LG, Lundback B. Prevalence and underdiagnosis of COPD by disease severity and the attributable fraction of smoking Report from the Obstructive Lung Disease in Northern Sweden Studies. Respir Med. 2006;100(2):264–272. doi: 10.1016/j.rmed.2005.04.029. [DOI] [PubMed] [Google Scholar]
- 57.Lindstrom M, Jonsson E, Larsson K, Lundback B. Underdiagnosis of chronic obstructive pulmonary disease in Northern Sweden. Int J Tuberc Lung Dis. 2002;6(1):76–84. [PubMed] [Google Scholar]
- 58.Nascimento OA, Camelier A, Rosa FW, Menezes AM, Perez-Padilla R, Jardim JR. Chronic obstructive pulmonary disease is underdiagnosed and undertreated in Sao Paulo (Brazil): results of the PLATINO study. Braz J Med Biol Res. 2007;40(7):887–895. doi: 10.1590/s0100-879x2006005000133. [DOI] [PubMed] [Google Scholar]
- 59.Renwick DS, Connolly MJ. Prevalence and treatment of chronic airways obstruction in adults over the age of 45. Thorax. 1996;51(2):164–168. doi: 10.1136/thx.51.2.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mannino DM, Gagnon RC, Petty TL, Lydick E. Obstructive lung disease and low lung function in adults in the United States: data from the National Health and Nutrition Examination Survey, 1988–1994. Arch Intern Med. 2000;160(11):1683–1689. doi: 10.1001/archinte.160.11.1683. [DOI] [PubMed] [Google Scholar]
- 61.van Durme YM, Verhamme KM, Stijnen T, et al. Prevalence, incidence, and lifetime risk for the development of COPD in the elderly: the Rotterdam study. Chest. 2009;135(2):368–377. doi: 10.1378/chest.08-0684. [DOI] [PubMed] [Google Scholar]
- 62.Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med. 2001;163(5):1256–1276. doi: 10.1164/ajrccm.163.5.2101039. [DOI] [PubMed] [Google Scholar]
- 63.Padeletti M, Jelic S, LeJemtel TH. Coexistent chronic obstructive pulmonary disease and heart failure in the elderly. Int J Cardiol. 2008;125(2):209–215. doi: 10.1016/j.ijcard.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 64.Yohannes AM, Baldwin RC, Connolly M. Mortality predictors in disabling chronic obstructive pulmonary disease in old age. Age Ageing. 2002;31(2):137–140. doi: 10.1093/ageing/31.2.137. [DOI] [PubMed] [Google Scholar]
- 65.Yeo J, Karimova G, Bansal S. Co-morbidity in older patients with COPD–its impact on health service utilisation and quality of life, a community study. Age Ageing. 2006;35(1):33–37. doi: 10.1093/ageing/afj002. [DOI] [PubMed] [Google Scholar]
- 66.Corsonello A, Pedone C, Battaglia S, Paglino G, Bellia V, Incalzi RA. C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) as inflammation markers in elderly patients with stable chronic obstructive pulmonary disease (COPD) Arch Gerontol Geriatr. 2011;53(2):190–195. doi: 10.1016/j.archger.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 67.Katsura H, Kida K. A comparison of bone mineral density in elderly female patients with COPD and bronchial asthma. Chest. 2002;122(6):1949–1955. doi: 10.1378/chest.122.6.1949. [DOI] [PubMed] [Google Scholar]
- 68.Williams M, Cafarella P, Olds T, Petkov J, Frith P. The language of breathlessness differentiates between patients with COPD and age-matched adults. Chest. 2008;134(3):489–496. doi: 10.1378/chest.07-2916. [DOI] [PubMed] [Google Scholar]
- 69.Yohannes AM, Roomi J, Baldwin RC, Connolly MJ. Depression in elderly outpatients with disabling chronic obstructive pulmonary disease. Age Ageing. 1998;27(2):155–160. doi: 10.1093/ageing/27.2.155. [DOI] [PubMed] [Google Scholar]
- 70.Yohannes AM, Baldwin RC, Connolly MJ. Prevalence of sub-threshold depression in elderly patients with chronic obstructive pulmonary disease. Int J Geriatr Psychiatry. 2003;18(5):412–416. doi: 10.1002/gps.851. [DOI] [PubMed] [Google Scholar]
- 71.Yohannes AM, Willgoss TG, Baldwin RC, Connolly MJ. Depression and anxiety in chronic heart failure and chronic obstructive pulmonary disease: prevalence, relevance, clinical implications and management principles. Int J Geriatr Psychiatry. 2010;25(12):1209–1221. doi: 10.1002/gps.2463. [DOI] [PubMed] [Google Scholar]
- 72.Pezzoli L, Giardini G, Consonni S, et al. Quality of spirometric performance in older people. Age Ageing. 2003;32(1):43–46. doi: 10.1093/ageing/32.1.43. [DOI] [PubMed] [Google Scholar]
- 73.Sherman CB, Kern D, Richardson ER, Hubert M, Fogel BS. Cognitive function and spirometry performance in the elderly. Am Rev Respir Dis. 1993;148(1):123–126. doi: 10.1164/ajrccm/148.1.123. [DOI] [PubMed] [Google Scholar]
- 74.Vaes AW, Wouters EF, Franssen FM, et al. Task-related oxygen uptake during domestic activities of daily life in patients with COPD and healthy elderly subjects. Chest. 2011;140(4):970–979. doi: 10.1378/chest.10-3005. [DOI] [PubMed] [Google Scholar]
- 75.Hardie JA, Buist AS, Vollmer WM, Ellingsen I, Bakke PS, Morkve O. Risk of over-diagnosis of COPD in asymptomatic elderly never-smokers. Eur Respir J. 2002;20(5):1117–1122. doi: 10.1183/09031936.02.00023202. [DOI] [PubMed] [Google Scholar]
- 76.Medbo A, Melbye H. Lung function testing in the elderly—can we still use FEV1/FVC<70% as a criterion of COPD? Respir Med. 2007;101(6):1097–1105. doi: 10.1016/j.rmed.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 77.Vaz Fragoso CA, Gill TM, McAvay G, Van Ness PH, Yaggi H, Concato J. Use of lambda-mu-sigma-derived Z score for evaluating respiratory impairment in middle-aged persons. Respir Care. 2011;56(11):1771–1777. doi: 10.4187/respcare.01192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fragoso CA, Gahbauer EA, Van Ness PH, Concato J, Gill TM. Peak expiratory flow as a predictor of subsequent disability and death in community-living older persons. J Am Geriatr Soc. 2008;56(6):1014–1020. doi: 10.1111/j.1532-5415.2008.01687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26(5):948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 80.Yawn BP, Mapel DW, Mannino DM, et al. Development of the Lung Function Questionnaire (LFQ) to identify airflow obstruction. Int J Chron Obstruct Pulmon Dis. 2010;5:1–10. [PMC free article] [PubMed] [Google Scholar]
- 81.Dennis RJ, Maldonado D, Norman S, Baena E, Martinez G. Woodsmoke exposure and risk for obstructive airways disease among women. Chest. 1996;109(1):115–119. doi: 10.1378/chest.109.1.115. [DOI] [PubMed] [Google Scholar]
- 82.Perez-Padilla R, Regalado J, Vedal S, et al. Exposure to biomass smoke and chronic airway disease in Mexican women. A case-control study. Am J Respir Crit Care Med. 1996;154(3 Pt 1):701–706. doi: 10.1164/ajrccm.154.3.8810608. [DOI] [PubMed] [Google Scholar]
- 83.Zock JP, Sunyer J, Kogevinas M, Kromhout H, Burney P, Anto JM. Occupation, chronic bronchitis, and lung function in young adults. An international study. Am J Respir Crit Care Med. 2001;163(7):1572–1577. doi: 10.1164/ajrccm.163.7.2004195. [DOI] [PubMed] [Google Scholar]
- 84.Eisner MD, Anthonisen N, Coultas D, et al. An official American Thoracic Society public policy statement: Novel risk factors and the global burden of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2010;182(5):693–718. doi: 10.1164/rccm.200811-1757ST. [DOI] [PubMed] [Google Scholar]
- 85.Silverman EK, Speizer FE. Risk factors for the development of chronic obstructive pulmonary disease. Med Clin North Am. 1996;80(3):501–522. doi: 10.1016/s0025-7125(05)70451-x. [DOI] [PubMed] [Google Scholar]
- 86.Ramirez-Venegas A, Ward J, Lentine T, Mahler DA. Salmeterol reduces dyspnea and improves lung function in patients with COPD. Chest. 1997;112(2):336–340. doi: 10.1378/chest.112.2.336. [DOI] [PubMed] [Google Scholar]
- 87.Calverley PM, Anderson JA, Celli B, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356(8):775–789. doi: 10.1056/NEJMoa063070. [DOI] [PubMed] [Google Scholar]
- 88.Cazzola M, Donner CF. Long-acting beta2 agonists in the management of stable chronic obstructive pulmonary disease. Drugs. 2000;60(2):307–320. doi: 10.2165/00003495-200060020-00005. [DOI] [PubMed] [Google Scholar]
- 89.Stockley RA, Whitehead PJ, Williams MK. Improved outcomes in patients with chronic obstructive pulmonary disease treated with salmeterol compared with placebo/usual therapy: results of a meta-analysis. Respir Res. 2006;7:147. doi: 10.1186/1465-9921-7-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.van Noord JA, Aumann JL, Janssens E, et al. Effects of tiotropium with and without formoterol on airflow obstruction and resting hyperinflation in patients with COPD. Chest. 2006;129(3):509–517. doi: 10.1378/chest.129.3.509. [DOI] [PubMed] [Google Scholar]
- 91.Ohta K, Fukuchi Y, Grouse L, et al. A prospective clinical study of theophylline safety in 3810 elderly with asthma or COPD. Respir Med. 2004;98(10):1016–1024. doi: 10.1016/j.rmed.2004.02.020. [DOI] [PubMed] [Google Scholar]
- 92.Calverley PM, Rabe KF, Goehring UM, Kristiansen S, Fabbri LM, Martinez FJ. Roflumilast in symptomatic chronic obstructive pulmonary disease: two randomised clinical trials. Lancet. 2009;374(9691):685–694. doi: 10.1016/S0140-6736(09)61255-1. [DOI] [PubMed] [Google Scholar]
- 93.Fabbri LM, Calverley PM, Izquierdo-Alonso JL, et al. Roflumilast in moderate-to-severe chronic obstructive pulmonary disease treated with longacting bronchodilators: two randomised clinical trials. Lancet. 2009;374(9691):695–703. doi: 10.1016/S0140-6736(09)61252-6. [DOI] [PubMed] [Google Scholar]
- 94.Ram FS, Jones PW, Castro AA, et al. Oral theophylline for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2002;(4):CD003902. doi: 10.1002/14651858.CD003902. doi:10.1002/14651858.CD003902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Man SF, McAlister FA, Anthonisen NR, Sin DD. Contemporary management of chronic obstructive pulmonary disease: clinical applications. JAMA. 2003;290(17):2313–2316. doi: 10.1001/jama.290.17.2313. [DOI] [PubMed] [Google Scholar]
- 96.Sin DD, McAlister FA, Man SF, Anthonisen NR. Contemporary management of chronic obstructive pulmonary disease: scientific review. JAMA. 2003;290(17):2301–2312. doi: 10.1001/jama.290.17.2301. [DOI] [PubMed] [Google Scholar]
- 97.Salpeter SR, Ormiston TM, Salpeter EE. Cardiovascular effects of beta-agonists in patients with asthma and COPD: a meta-analysis. Chest. 2004;125(6):2309–2321. doi: 10.1378/chest.125.6.2309. [DOI] [PubMed] [Google Scholar]
- 98.Singh S, Loke YK, Furberg CD. Inhaled anticholinergics and risk of major adverse cardiovascular events in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. JAMA. 2008;300(12):1439–1450. doi: 10.1001/jama.300.12.1439. [DOI] [PubMed] [Google Scholar]
- 99.Lee TA, Pickard AS, Au DH, Bartle B, Weiss KB. Risk for death associated with medications for recently diagnosed chronic obstructive pulmonary disease. Ann Intern Med. 2008;149(6):380–390. doi: 10.7326/0003-4819-149-6-200809160-00004. [DOI] [PubMed] [Google Scholar]
- 100.Celli B, Decramer M, Kesten S, Liu D, Mehra S, Tashkin DP. Mortality in the 4-year trial of tiotropium (UPLIFT) in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2009;180(10):948–955. doi: 10.1164/rccm.200906-0876OC. [DOI] [PubMed] [Google Scholar]
- 101.Celli B, Decramer M, Leimer I, Vogel U, Kesten S, Tashkin DP. Cardiovascular safety of tiotropium in patients with COPD. Chest. 2010;137(1):20–30. doi: 10.1378/chest.09-0011. [DOI] [PubMed] [Google Scholar]
- 102.Dalal AA, Petersen H, Simoni-Wastila L, Blanchette CM. Healthcare costs associated with initial maintenance therapy with fluticasone propionate 250 mug/salmeterol 50 mug combination versus anticholinergic bronchodilators in elderly US Medicare-eligible beneficiaries with COPD. J Med Econ. 2009;12(4):339–347. doi: 10.3111/13696990903369135. [DOI] [PubMed] [Google Scholar]
- 103.Dalal AA, Shah M, D’Souza AO, Mapel DW. COPD-related healthcare utilization and costs after discharge from a hospitalization or emergency department visit on a regimen of fluticasone propionate-salmeterol combination versus other maintenance therapies. Am J Manag Care. 2011;17(3):e55–e65. [PubMed] [Google Scholar]
- 104.Malo de MR, Mortensen EM, Restrepo MI, Copeland LA, Pugh MJ, Anzueto A. Inhaled corticosteroid use is associated with lower mortality for subjects with COPD and hospitalised with pneumonia. Eur Respir J. 2010;36(4):751–757. doi: 10.1183/09031936.00077509. [DOI] [PubMed] [Google Scholar]
- 105.Chen D, Restrepo MI, Fine MJ, et al. Observational study of inhaled corticosteroids on outcomes for COPD patients with pneumonia. Am J Respir Crit Care Med. 2011;184(3):312–316. doi: 10.1164/rccm.201012-2070OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fujita K, Kasayama S, Hashimoto J, et al. Inhaled corticosteroids reduce bone mineral density in early postmenopausal but not premenopausal asthmatic women. J Bone Miner Res. 2001;16(4):782–787. doi: 10.1359/jbmr.2001.16.4.782. [DOI] [PubMed] [Google Scholar]
- 107.Hubbard R, Tattersfield A. Inhaled corticosteroids, bone mineral density and fracture in older people. Drugs Aging. 2004;21(10):631–638. doi: 10.2165/00002512-200421100-00002. [DOI] [PubMed] [Google Scholar]
- 108.Hubbard R, Tattersfield A, Smith C, West J, Smeeth L, Fletcher A. Use of inhaled corticosteroids and the risk of fracture. Chest. 2006;130(4):1082–1088. doi: 10.1378/chest.130.4.1082. [DOI] [PubMed] [Google Scholar]
- 109.Hubbard RB, Smith CJ, Smeeth L, Harrison TW, Tattersfield AE. Inhaled corticosteroids and hip fracture: a population-based case-control study. Am J Respir Crit Care Med. 2002;166(12 Pt 1):1563–1566. doi: 10.1164/rccm.200206-606OC. [DOI] [PubMed] [Google Scholar]
- 110.Israel E, Banerjee TR, Fitzmaurice GM, Kotlov TV, LaHive K, LeBoff MS. Effects of inhaled glucocorticoids on bone density in premenopausal women. N Engl J Med. 2001;345(13):941–947. doi: 10.1056/NEJMoa002304. [DOI] [PubMed] [Google Scholar]
- 111.The Lung Health Study Research Group. Effect of inhaled triamcinolone on the decline in pulmonary function in chronic obstructive pulmonary disease. N Engl J Med. 2000;343(26):1902–1909. doi: 10.1056/NEJM200012283432601. [DOI] [PubMed] [Google Scholar]
- 112.Casale TB, Nelson HS, Stricker WE, Raff H, Newman KB. Suppression of hypothalamic-pituitary-adrenal axis activity with inhaled flunisolide and fluticasone propionate in adult asthma patients. Ann Allergy Asthma Immunol. 2001;87(5):379–385. doi: 10.1016/S1081-1206(10)62918-3. [DOI] [PubMed] [Google Scholar]
- 113.Lipworth BJ. Systemic adverse effects of inhaled corticosteroid therapy: a systematic review and meta-analysis. Arch Intern Med. 1999;159(9):941–955. doi: 10.1001/archinte.159.9.941. [DOI] [PubMed] [Google Scholar]
- 114.White M, Crisalida T, Li H, Economides A, Kaliner M. Effects of long-term inhaled corticosteroids on adrenal function in patients with asthma. Ann Allergy Asthma Immunol. 2006;96(3):437–444. doi: 10.1016/S1081-1206(10)60911-8. [DOI] [PubMed] [Google Scholar]
- 115.Cumming RG, Mitchell P, Leeder SR. Use of inhaled corticosteroids and the risk of cataracts. N Engl J Med. 1997;337(1):8–14. doi: 10.1056/NEJM199707033370102. [DOI] [PubMed] [Google Scholar]
- 116.Garbe E, LeLorier J, Boivin JF, Suissa S. Inhaled and nasal glucocorticoids and the risks of ocular hypertension or open-angle glaucoma. JAMA. 1997;277(9):722–727. [PubMed] [Google Scholar]
- 117.Garbe E, Suissa S, LeLorier J. Association of inhaled corticosteroid use with cataract extraction in elderly patients. JAMA. 1998;280(6):539–543. doi: 10.1001/jama.280.6.539. [DOI] [PubMed] [Google Scholar]
- 118.Schmier JK, Halpern MT, Jones ML. Effects of inhaled corticosteroids on mortality and hospitalisation in elderly asthma and chronic obstructive pulmonary disease patients: appraising the evidence. Drugs Aging. 2005;22(9):717–729. doi: 10.2165/00002512-200522090-00001. [DOI] [PubMed] [Google Scholar]
- 119.O’Byrne PM, Pedersen S, Carlsson LG, et al. Risks of pneumonia in patients with asthma taking inhaled corticosteroids. Am J Respir Crit Care Med. 2011;183(5):589–595. doi: 10.1164/rccm.201005-0694OC. [DOI] [PubMed] [Google Scholar]
- 120.Singh S, Amin AV, Loke YK. Long-term use of inhaled corticosteroids and the risk of pneumonia in chronic obstructive pulmonary disease: a meta-analysis. Arch Intern Med. 2009;169(3):219–229. doi: 10.1001/archinternmed.2008.550. [DOI] [PubMed] [Google Scholar]
- 121.Brassard P, Suissa S, Kezouh A, Ernst P. Inhaled corticosteroids and risk of tuberculosis in patients with respiratory diseases. Am J Respir Crit Care Med. 2011;183(5):675–678. doi: 10.1164/rccm.201007-1099OC. [DOI] [PubMed] [Google Scholar]
- 122.Demirkan K, Tolley E, Mastin T, Soberman J, Burbeck J, Self T. Salmeterol administration by metered-dose inhaler alone vs metered-dose inhaler plus valved holding chamber. Chest. 2000;117(5):1314–1318. doi: 10.1378/chest.117.5.1314. [DOI] [PubMed] [Google Scholar]
- 123.Lavorini F, Fontana GA. Targeting drugs to the airways: the role of spacer devices. Expert Opin Drug Deliv. 2009;6(1):91–102. doi: 10.1517/17425240802637862. [DOI] [PubMed] [Google Scholar]
- 124.Thorsson L, Geller D. Factors guiding the choice of delivery device for inhaled corticosteroids in the long-term management of stable asthma and COPD: focus on budesonide. Respir Med. 2005;99(7):836–849. doi: 10.1016/j.rmed.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 125.Toogood JH, Baskerville J, Jennings B, Lefcoe NM, Johansson SA. Use of spacers to facilitate inhaled corticosteroid treatment of asthma. Am Rev Respir Dis. 1984;129(5):723–729. doi: 10.1164/arrd.1984.129.5.723. [DOI] [PubMed] [Google Scholar]
- 126.Couser JI, Jr, Guthmann R, Hamadeh MA, Kane CS. Pulmonary rehabilitation improves exercise capacity in older elderly patients with COPD. Chest. 1995;107(3):730–734. doi: 10.1378/chest.107.3.730. [DOI] [PubMed] [Google Scholar]
- 127.Sewell L, Singh SJ, Williams JE, Collier R, Morgan MD. Can individualized rehabilitation improve functional independence in elderly patients with COPD? Chest. 2005;128(3):1194–1200. doi: 10.1378/chest.128.3.1194. [DOI] [PubMed] [Google Scholar]
- 128.Katsura H, Kanemaru A, Yamada K, Motegi T, Wakabayashi R, Kida K. Long-term effectiveness of an inpatient pulmonary rehabilitation program for elderly COPD patients: comparison between young-elderly and old-elderly groups. Respirology. 2004;9(2):230–236. doi: 10.1111/j.1440-1843.2004.00561.x. [DOI] [PubMed] [Google Scholar]
- 129.Kongsgaard M, Backer V, Jorgensen K, Kjaer M, Beyer N. Heavy resistance training increases muscle size, strength and physical function in elderly male COPD-patients—a pilot study. Respir Med. 2004;98(10):1000–1007. doi: 10.1016/j.rmed.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 130.Anthonisen NR, Manfreda J, Warren CP, Hershfield ES, Harding GK, Nelson NA. Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Ann Intern Med. 1987;106(2):196–204. doi: 10.7326/0003-4819-106-2-196. [DOI] [PubMed] [Google Scholar]
- 131.Davies L, Angus RM, Calverley PM. Oral corticosteroids in patients admitted to hospital with exacerbations of chronic obstructive pulmonary disease: a prospective randomised controlled trial. Lancet. 1999;354(9177):456–460. doi: 10.1016/s0140-6736(98)11326-0. [DOI] [PubMed] [Google Scholar]
- 132.Fagon JY, Chastre J. Severe exacerbations of COPD patients: the role of pulmonary infections. Semin Respir Infect. 1996;11(2):109–118. [PubMed] [Google Scholar]
- 133.Maltais F, Ostinelli J, Bourbeau J, et al. Comparison of nebulized budesonide and oral prednisolone with placebo in the treatment of acute exacerbations of chronic obstructive pulmonary disease: a randomized controlled trial. Am J Respir Crit Care Med. 2002;165(5):698–703. doi: 10.1164/ajrccm.165.5.2109093. [DOI] [PubMed] [Google Scholar]
- 134.Nouira S, Marghli S, Belghith M, Besbes L, Elatrous S, Abroug F. Once daily oral ofloxacin in chronic obstructive pulmonary disease exacerbation requiring mechanical ventilation: a randomised placebo-controlled trial. Lancet. 2001;358(9298):2020–2025. doi: 10.1016/S0140-6736(01)07097-0. [DOI] [PubMed] [Google Scholar]
- 135.Patel A, Wilson R. Newer fluoroquinolones in the treatment of acute exacerbations of COPD. Int J Chron Obstruct Pulmon Dis. 2006;1(3):243–250. doi: 10.2147/copd.2006.1.3.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Saint S, Bent S, Vittinghoff E, Grady D. Antibiotics in chronic obstructive pulmonary disease exacerbations. A meta-analysis. JAMA. 1995;273(12):957–960. [PubMed] [Google Scholar]
- 137.Thompson WH, Nielson CP, Carvalho P, Charan NB, Crowley JJ. Controlled trial of oral prednisone in outpatients with acute COPD exacerbation. Am J Respir Crit Care Med. 1996;154(2 Pt 1):407–412. doi: 10.1164/ajrccm.154.2.8756814. [DOI] [PubMed] [Google Scholar]
- 138.Wilson R, Allegra L, Huchon G, et al. Short-term and long-term outcomes of moxifloxacin compared to standard antibiotic treatment in acute exacerbations of chronic bronchitis. Chest. 2004;125(3):953–964. doi: 10.1378/chest.125.3.953. [DOI] [PubMed] [Google Scholar]
- 139.Wilson R, Jones P, Schaberg T, Arvis P, Duprat-Lomon I, Sagnier PP. Antibiotic treatment and factors influencing short and long term outcomes of acute exacerbations of chronic bronchitis. Thorax. 2006;61(4):337–342. doi: 10.1136/thx.2005.045930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wilson R. Short course of antibiotic treatment in acute exacerbations of COPD. Thorax. 2008;63(5):390–392. doi: 10.1136/thx.2007.092932. [DOI] [PubMed] [Google Scholar]
- 141.Ram FS, Rodriguez-Roisin R, Granados-Navarrete A, Garcia-Aymerich J, Barnes NC. WITHDRAWN: antibiotics for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2011;(1):CD004403. doi: 10.1002/14651858.CD004403.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Bont J, Hak E, Birkhoff CE, Hoes AW, Verheij TJ. Is co-morbidity taken into account in the antibiotic management of elderly patients with acute bronchitis and COPD exacerbations? Fam Pract. 2007;24(4):317–322. doi: 10.1093/fampra/cmm023. [DOI] [PubMed] [Google Scholar]
- 143.Ramsey SD. Suboptimal medical therapy in COPD: exploring the causes and consequences. Chest. 2000;117(2 suppl):33S–37S. doi: 10.1378/chest.117.2_suppl.33s. [DOI] [PubMed] [Google Scholar]
- 144.Jarvis S, Ind PW, Shiner RJ. Inhaled therapy in elderly COPD patients; time for re-evaluation? Age Ageing. 2007;36(2):213–218. doi: 10.1093/ageing/afl174. [DOI] [PubMed] [Google Scholar]
- 145.Janssens W, VandenBrande P, Hardeman E, et al. Inspiratory flow rates at different levels of resistance in elderly COPD patients. Eur Respir J. 2008;31(1):78–83. doi: 10.1183/09031936.00024807. [DOI] [PubMed] [Google Scholar]
- 146.Quinet P, Young CA, Heritier F. The use of dry powder inhaler devices by elderly patients suffering from chronic obstructive pulmonary disease. Ann Phys Rehabil Med. 2010;53(2):69–76. doi: 10.1016/j.rehab.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 147.Sugawara T, Nanjo Y, Yamazaki M, et al. Comparison of adherence and efficacy between inhaled salmeterol and transdermal tulobuterol patch in elderly patients with chronic obstructive pulmonary disorder. J Am Geriatr Soc. 2009;57(5):919–920. doi: 10.1111/j.1532-5415.2009.02230.x. [DOI] [PubMed] [Google Scholar]
- 148.Castaldi PJ, Rogers WH, Safran DG, Wilson IB. Inhaler costs and medication nonadherence among seniors with chronic pulmonary disease. Chest. 2010;138(3):614–620. doi: 10.1378/chest.09-3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Anecchino C, Rossi E, Fanizza C, De RM, Tognoni G, Romero M. Prevalence of chronic obstructive pulmonary disease and pattern of comorbidities in a general population. Int J Chron Obstruct Pulmon Dis. 2007;2(4):567–574. [PMC free article] [PubMed] [Google Scholar]
- 150.Mannino DM, Watt G, Hole D, et al. The natural history of chronic obstructive pulmonary disease. Eur Respir J. 2006;27(3):627–643. doi: 10.1183/09031936.06.00024605. [DOI] [PubMed] [Google Scholar]
- 151.Chatila WM, Thomashow BM, Minai OA, Criner GJ, Make BJ. Comorbidities in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2008;5(4):549–555. doi: 10.1513/pats.200709-148ET. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Andersson I, Johansson K, Larsson S, Pehrsson K. Long-term oxygen therapy and quality of life in elderly patients hospitalised due to severe exacerbation of COPD. A 1 year follow-up study. Respir Med. 2002;96(11):944–949. doi: 10.1053/rmed.2002.1370. [DOI] [PubMed] [Google Scholar]
- 153.Incalzi RA, Pedone C, Scarlata S, et al. Correlates of mortality in elderly COPD patients: focus on health-related quality of life. Respirology. 2009;14(1):98–104. doi: 10.1111/j.1440-1843.2008.01441.x. [DOI] [PubMed] [Google Scholar]
- 154.Pedone C, Scarlata S, Sorino C, Forastiere F, Bellia V, Incalzi RA. Does mild COPD affect prognosis in the elderly? BMC Pulm Med. 2010;10:35. doi: 10.1186/1471-2466-10-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yohannes AM, Roomi J, Waters K, Connolly MJ. Quality of life in elderly patients with COPD: measurement and predictive factors. Respir Med. 1998;92(10):1231–1236. doi: 10.1016/s0954-6111(98)90426-7. [DOI] [PubMed] [Google Scholar]
- 156.Bauer BA, Reed CE, Yunginger JW, Wollan PC, Silverstein MD. Incidence and outcomes of asthma in the elderly. A population-based study in Rochester, Minnesota. Chest. 1997;111(2):303–310. doi: 10.1378/chest.111.2.303. [DOI] [PubMed] [Google Scholar]
- 157.Akinbami LJ, Moorman JE, Liu X. Asthma prevalence, health care use, and mortality: United States, 2005–2009. National health statistics reports. 2011 [PubMed] [Google Scholar]
- 158.Parameswaran K, Hildreth AJ, Chadha D, Keaney NP, Taylor IK, Bansal SK. Asthma in the elderly: underperceived, underdiagnosed and undertreated; a community survey. Respir Med. 1998;92(3):573–577. doi: 10.1016/s0954-6111(98)90311-0. [DOI] [PubMed] [Google Scholar]
- 159.Enright PL, McClelland RL, Newman AB, Gottlieb DJ, Lebowitz MD. Underdiagnosis and undertreatment of asthma in the elderly. Cardiovascular Health Study Research Group. Chest. 1999;116(3):603–613. doi: 10.1378/chest.116.3.603. [DOI] [PubMed] [Google Scholar]
- 160.Renwick DS, Connolly MJ. Impact of obstructive airways disease on quality of life in older adults. Thorax. 1996;51(5):520–525. doi: 10.1136/thx.51.5.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zureik M, Orehek J. Diagnosis and severity of asthma in the elderly: results of a large survey in 1,485 asthmatics recruited by lung specialists. Respiration. 2002;69(3):223–228. doi: 10.1159/000063624. [DOI] [PubMed] [Google Scholar]
- 162.Papi A, Corbetta L, Fabbri LM. What can we learn from late-onset and occupational asthma? Clin Exp Allergy. 1998;28(() suppl 5:174–180. doi: 10.1046/j.1365-2222.1998.028s5174.x. [DOI] [PubMed] [Google Scholar]
- 163.Bellia V, Battaglia S, Catalano F, et al. Aging and disability affect misdiagnosis of COPD in elderly asthmatics: the SARA study. Chest. 2003;123(4):1066–1072. doi: 10.1378/chest.123.4.1066. [DOI] [PubMed] [Google Scholar]
- 164.Cuttitta G, Cibella F, Bellia V, et al. Changes in FVC during methacholine-induced bronchoconstriction in elderly patients with asthma: bronchial hyperresponsiveness and aging. Chest. 2001;119(6):1685–1690. doi: 10.1378/chest.119.6.1685. [DOI] [PubMed] [Google Scholar]
- 165.Dow L, Coggon D, Osmond C, Holgate ST. A population survey of respiratory symptoms in the elderly. Eur Respir J. 1991;4(3):267–272. [PubMed] [Google Scholar]
- 166.Quadrelli SA, Roncoroni A. Features of asthma in the elderly. J Asthma. 2001;38(5):377–389. doi: 10.1081/jas-100000259. [DOI] [PubMed] [Google Scholar]
- 167.Weiner P, Magadle R, Waizman J, Weiner M, Rabner M, Zamir D. Characteristics of asthma in the elderly. Eur Respir J. 1998;12(3):564–568. doi: 10.1183/09031936.98.12030564. [DOI] [PubMed] [Google Scholar]
- 168.Cassino C, Berger KI, Goldring RM, et al. Duration of asthma and physiologic outcomes in elderly nonsmokers. Am J Respir Crit Care Med. 2000;162(4 Pt 1):1423–1428. doi: 10.1164/ajrccm.162.4.9912140. [DOI] [PubMed] [Google Scholar]
- 169.Braman SS, Kaemmerlen JT, Davis SM. Asthma in the elderly. A comparison between patients with recently acquired and long-standing disease. Am Rev Respir Dis. 1991;143(2):336–340. doi: 10.1164/ajrccm/143.2.336. [DOI] [PubMed] [Google Scholar]
- 170.Chang JT, Moran MB, Cugell DW, Webster JR., Jr COPD in the elderly. A reversible cause of functional impairment. Chest. 1995;108(3):736–740. doi: 10.1378/chest.108.3.736. [DOI] [PubMed] [Google Scholar]
- 171.Parker AL. Aging does not affect beta-agonist responsiveness after methacholine-induced bronchoconstriction. J Am Geriatr Soc. 2004;52(3):388–392. doi: 10.1111/j.1532-5415.2004.52110.x. [DOI] [PubMed] [Google Scholar]
- 172.Sin BA, Akkoca O, Saryal S, Oner F, Misirligil Z. Differences between asthma and COPD in the elderly. J Investig Allergol Clin Immunol. 2006;16(1):44–50. [PubMed] [Google Scholar]
- 173.Di LG, Mansueto P, Ditta V, et al. Similarity and differences in elderly patients with fixed airflow obstruction by asthma and by chronic obstructive pulmonary disease. Respir Med. 2008;102(2):232–238. doi: 10.1016/j.rmed.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 174.Barr RG, Somers SC, Speizer FE, Camargo CA., Jr Patient factors and medication guideline adherence among older women with asthma. Arch Intern Med. 2002;162(15):1761–1768. doi: 10.1001/archinte.162.15.1761. [DOI] [PubMed] [Google Scholar]
- 175.Luder E, Ehrlich RI, Lou WY, Melnik TA, Kattan M. Body mass index and the risk of asthma in adults. Respir Med. 2004;98(1):29–37. doi: 10.1016/j.rmed.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 176.Ford ES. The epidemiology of obesity and asthma. J Allergy Clin Immunol. 2005;115(5):897–909. doi: 10.1016/j.jaci.2004.11.050. [DOI] [PubMed] [Google Scholar]
- 177.Raiha I, Impivaara O, Seppala M, Knuts LR, Sourander L. Determinants of symptoms suggestive of gastroesophageal reflux disease in the elderly. Scand J Gastroenterol. 1993;28(11):1011–1014. doi: 10.3109/00365529309098301. [DOI] [PubMed] [Google Scholar]
- 178.Raiha I, Hietanen E, Sourander L. Symptoms of gastro-oesophageal reflux disease in elderly people. Age Ageing. 1991;20(5):365–370. doi: 10.1093/ageing/20.5.365. [DOI] [PubMed] [Google Scholar]
- 179.Brooks TW, Creekmore FM, Young DC, Asche CV, Oberg B, Samuelson WM. Rates of hospitalizations and emergency department visits in patients with asthma and chronic obstructive pulmonary disease taking beta-blockers. Pharmacotherapy. 2007;27(5):684–690. doi: 10.1592/phco.27.5.684. [DOI] [PubMed] [Google Scholar]
- 180.Kos-Kudla B, Ostrowska Z, Marek B, et al. Hormone replacement therapy in postmenopausal asthmatic women. J Clin Pharm Ther. 2000;25(6):461–466. doi: 10.1046/j.1365-2710.2000.00310.x. [DOI] [PubMed] [Google Scholar]
- 181.Carlson CL, Cushman M, Enright PL, Cauley JA, Newman AB. Hormone replacement therapy is associated with higher FEV1 in elderly women. Am J Respir Crit Care Med. 2001;163(2):423–428. doi: 10.1164/ajrccm.163.2.2003040. [DOI] [PubMed] [Google Scholar]
- 182.Korenblat PE, Kemp JP, Scherger JE, Minkwitz MC, Mezzanotte W. Effect of age on response to zafirlukast in patients with asthma in the Accolate Clinical Experience and Pharmacoepidemiology Trial (ACCEPT) Ann Allergy Asthma Immunol. 2000;84(2):217–225. doi: 10.1016/S1081-1206(10)62759-7. [DOI] [PubMed] [Google Scholar]
- 183.Bozek A, Jarzab J. Adherence to asthma therapy in elderly patients. J Asthma. 2010;47(2):162–165. doi: 10.3109/02770900903497204. [DOI] [PubMed] [Google Scholar]
- 184.Ng TP, Chiam PC, Kua EH. Mental disorders and asthma in the elderly: a population-based study. Int J Geriatr Psychiatry. 2007;22(7):668–674. doi: 10.1002/gps.1728. [DOI] [PubMed] [Google Scholar]
- 185.Patel RR, Saltoun CA, Grammer LC. Improving asthma care for the elderly: a randomized controlled trial using a simple telephone intervention. J Asthma. 2009;46(1):30–35. doi: 10.1080/02770900802460563. [DOI] [PubMed] [Google Scholar]
- 186.Moorman JE, Mannino DM. Increasing U.S. asthma mortality rates: who is really dying? J Asthma. 2001;38(1):65–71. doi: 10.1081/jas-100000023. [DOI] [PubMed] [Google Scholar]
- 187.Slavin RG. The elderly asthmatic patient. Allergy Asthma Proc. 2004;25(6):371–373. [PubMed] [Google Scholar]
- 188.Leander M, Janson C, Uddenfeldt M, Cronqvist A, Rask-Andersen A. Associations between mortality, asthma, and health-related quality of life in an elderly cohort of Swedes. J Asthma. 2010;47(6):627–632. doi: 10.3109/02770901003617402. [DOI] [PubMed] [Google Scholar]
- 189.Lange P, Parner J, Vestbo J, Schnohr P, Jensen GA. 15-year follow-up study of ventilatory function in adults with asthma. N Engl J Med. 1998;339(17):1194–1200. doi: 10.1056/NEJM199810223391703. [DOI] [PubMed] [Google Scholar]
- 190.O’Rourke MA, Feussner JR, Feigl P, Laszlo J. Age trends of lung cancer stage at diagnosis. Implications for lung cancer screening in the elderly. JAMA. 1987;258(7):921–926. [PubMed] [Google Scholar]
- 191. SEER Database. http://seer.cancer.gov/csr/1975_2008/browse_csr.php?section=15&page=sect_15_table.10.html. Accessed January 18, 2012.
- 192.Hillers TK, Sauve MD, Guyatt GH. Analysis of published studies on the detection of extrathoracic metastases in patients presumed to have operable non-small cell lung cancer. Thorax. 1994;49(1):14–19. doi: 10.1136/thx.49.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Stenbygaard LE, Sorensen JB, Olsen JE. Metastatic pattern at autopsy in non-resectable adenocarcinoma of the lung—a study from a cohort of 259 consecutive patients treated with chemotherapy. Acta Oncol. 1997;36(3):301–306. doi: 10.3109/02841869709001267. [DOI] [PubMed] [Google Scholar]
- 194.Cetin K, Ettinger DS, Hei YJ, O’Malley CD. Survival by histologic subtype in stage IV nonsmall cell lung cancer based on data from the Surveillance, Epidemiology and End Results Program. Clin Epidemiol. 2011;3:139–148. doi: 10.2147/CLEP.S17191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Schumacher T, Brink I, Mix M, et al. FDG-PET imaging for the staging and follow-up of small cell lung cancer. Eur J Nucl Med. 2001;28(4):483–488. doi: 10.1007/s002590100474. [DOI] [PubMed] [Google Scholar]
- 196.Toloza EM, Harpole L, McCrory DC. Noninvasive staging of non-small cell lung cancer: a review of the current evidence. Chest. 2003;123(1 suppl):137S–146S. doi: 10.1378/chest.123.1_suppl.137s. [DOI] [PubMed] [Google Scholar]
- 197.Chute CG, Greenberg ER, Baron J, Korson R, Baker J, Yates J. Presenting conditions of 1539 population-based lung cancer patients by cell type and stage in New Hampshire and Vermont. Cancer. 1985;56(8):2107–2111. doi: 10.1002/1097-0142(19851015)56:8<2107::aid-cncr2820560837>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 198.Hyde L, Hyde CI. Clinical manifestations of lung cancer. Chest. 1974;65(3):299–306. doi: 10.1378/chest.65.3.299. [DOI] [PubMed] [Google Scholar]
- 199.Kuo CW, Chen YM, Chao JY, Tsai CM, Perng RP. Non-small cell lung cancer in very young and very old patients. Chest. 2000;117(2):354–357. doi: 10.1378/chest.117.2.354. [DOI] [PubMed] [Google Scholar]
- 200.Colice GL. Detecting lung cancer as a cause of hemoptysis in patients with a normal chest radiograph: bronchoscopy vs CT. Chest. 1997;111(4):877–884. doi: 10.1378/chest.111.4.877. [DOI] [PubMed] [Google Scholar]
- 201.Oliver TW, Jr, Bernardino ME, Miller JI, Mansour K, Greene D, Davis WA. Isolated adrenal masses in nonsmall-cell bronchogenic carcinoma. Radiology. 1984;153(1):217–218. doi: 10.1148/radiology.153.1.6473783. [DOI] [PubMed] [Google Scholar]
- 202.Pagani JJ. Normal adrenal glands in small cell lung carcinoma: CT-guided biopsy. AJR Am J Roentgenol. 1983;140(5):949–951. doi: 10.2214/ajr.140.5.949. [DOI] [PubMed] [Google Scholar]
- 203.Bach PB, Kattan MW, Thornquist MD, et al. Variations in lung cancer risk among smokers. J Natl Cancer Inst. 2003;95(6):470–478. doi: 10.1093/jnci/95.6.470. [DOI] [PubMed] [Google Scholar]
- 204.Woloshin S, Schwartz LM, Welch HG. Risk charts: putting cancer in context. J Natl Cancer Inst. 2002;94(11):799–804. doi: 10.1093/jnci/94.11.799. [DOI] [PubMed] [Google Scholar]
- 205.Peto R, Darby S, Deo H, Silcocks P, Whitley E, Doll R. Smoking, smoking cessation, and lung cancer in the UK since 1950: combination of national statistics with two case-control studies. BMJ. 2000;321(7257):323–329. doi: 10.1136/bmj.321.7257.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Wakelee HA, Chang ET, Gomez SL, et al. Lung cancer incidence in never smokers. J Clin Oncol. 2007;25(5):472–478. doi: 10.1200/JCO.2006.07.2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Alberg AJ, Samet JM. Epidemiology of lung cancer. Chest. 2003;123(1 suppl):21S–49S. doi: 10.1378/chest.123.1_suppl.21s. [DOI] [PubMed] [Google Scholar]
- 208.Young RP, Hopkins RJ, Christmas T, Black PN, Metcalf P, Gamble GD. COPD prevalence is increased in lung cancer, independent of age, sex and smoking history. Eur Respir J. 2009;34(2):380–386. doi: 10.1183/09031936.00144208. [DOI] [PubMed] [Google Scholar]
- 209.Alberts WM. Diagnosis and management of lung cancer executive summary: ACCP evidence-based clinical practice guidelines (2nd edition) Chest. 2007;132(3 suppl):1S–19S. doi: 10.1378/chest.07-1860. [DOI] [PubMed] [Google Scholar]
- 210.Fukuse T, Satoda N, Hijiya K, Fujinaga T. Importance of a comprehensive geriatric assessment in prediction of complications following thoracic surgery in elderly patients. Chest. 2005;127(3):886–891. doi: 10.1378/chest.127.3.886. [DOI] [PubMed] [Google Scholar]
- 211.Repetto L, Fratino L, Audisio RA, et al. Comprehensive geriatric assessment adds information to Eastern Cooperative Oncology Group performance status in elderly cancer patients: an Italian Group for Geriatric Oncology Study. J Clin Oncol. 2002;20(2):494–502. doi: 10.1200/JCO.2002.20.2.494. [DOI] [PubMed] [Google Scholar]
- 212.Zagonel V. Importance of a comprehensive geriatric assessment in older cancer patients. Eur J Cancer. 2001;37(suppl 7):S229–S233. doi: 10.1016/s0959-8049(01)80024-1. [DOI] [PubMed] [Google Scholar]
- 213.Holsinger T, Deveau J, Boustani M, Williams JW., Jr Does this patient have dementia? JAMA. 2007;297(21):2391–2404. doi: 10.1001/jama.297.21.2391. [DOI] [PubMed] [Google Scholar]
- 214.Sainsbury A, Seebass G, Bansal A, Young JB. Reliability of the Barthel Index when used with older people. Age Ageing. 2005;34(3):228–232. doi: 10.1093/ageing/afi063. [DOI] [PubMed] [Google Scholar]
- 215.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 216.Mahoney FI, Barthel DW. Functional evaluation: the Barthel Index. Md State Med J. 1965;14:61–65. [PubMed] [Google Scholar]
- 217.Chan ED, Welsh CH. Geriatric respiratory medicine. Chest. 1998;114(6):1704–1733. doi: 10.1378/chest.114.6.1704. [DOI] [PubMed] [Google Scholar]
- 218.Guadagnoli E, Weitberg A, Mor V, Silliman RA, Glicksman AS, Cummings FJ. The influence of patient age on the diagnosis and treatment of lung and colorectal cancer. Arch Intern Med. 1990;150(7):1485–1490. [PubMed] [Google Scholar]
- 219.Johnson DH. Small cell lung cancer in the elderly patient. Semin Oncol. 1997;24(4):484–491. [PubMed] [Google Scholar]
- 220.Provencio M, Camps C, Alberola V, et al. Lung cancer and treatment in elderly patients: the Achilles Study. Lung Cancer. 2009;66(1):103–106. doi: 10.1016/j.lungcan.2008.12.021. [DOI] [PubMed] [Google Scholar]
- 221.Davidoff AJ, Tang M, Seal B, Edelman MJ. Chemotherapy and survival benefit in elderly patients with advanced non-small-cell lung cancer. J Clin Oncol. 2010;28(13):2191–2197. doi: 10.1200/JCO.2009.25.4052. [DOI] [PubMed] [Google Scholar]
- 222.Ramsey SD, Howlader N, Etzioni RD, Donato B. Chemotherapy use, outcomes, and costs for older persons with advanced non-small-cell lung cancer: evidence from surveillance, epidemiology and end results-Medicare. J Clin Oncol. 2004;22(24):4971–4978. doi: 10.1200/JCO.2004.05.031. [DOI] [PubMed] [Google Scholar]
- 223.Berry MF, Hanna J, Tong BC, et al. Risk factors for morbidity after lobectomy for lung cancer in elderly patients. Ann Thorac Surg. 2009;88(4):1093–1099. doi: 10.1016/j.athoracsur.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 224.Damhuis RA, Schutte PR. Resection rates and postoperative mortality in 7,899 patients with lung cancer. Eur Respir J. 1996;9(1):7–10. doi: 10.1183/09031936.96.09010007. [DOI] [PubMed] [Google Scholar]
- 225.Jazieh AR, Hussain M, Howington JA, et al. Prognostic factors in patients with surgically resected stages I and II non-small cell lung cancer. Ann Thorac Surg. 2000;70(4):1168–1171. doi: 10.1016/s0003-4975(00)01529-0. [DOI] [PubMed] [Google Scholar]
- 226.Mery CM, Pappas AN, Bueno R, et al. Similar long-term survival of elderly patients with non-small cell lung cancer treated with lobectomy or wedge resection within the surveillance, epidemiology, and end results database. Chest. 2005;128(1):237–245. doi: 10.1378/chest.128.1.237. [DOI] [PubMed] [Google Scholar]
- 227.van Rens MT, de la Riviere AB, Elbers HR, van den Bosch JM. Prognostic assessment of 2,361 patients who underwent pulmonary resection for non-small cell lung cancer, stage I, II, and IIIA. Chest. 2000;117(2):374–379. doi: 10.1378/chest.117.2.374. [DOI] [PubMed] [Google Scholar]
- 228.Chambers A, Routledge T, Pilling J, Scarci M. In elderly patients with lung cancer is resection justified in terms of morbidity, mortality and residual quality of life? Interact Cardiovasc Thorac Surg. 2010;10(6):1015–1021. doi: 10.1510/icvts.2010.233189. [DOI] [PubMed] [Google Scholar]
- 229.Sawada S, Komori E, Nogami N, et al. Advanced age is not correlated with either short-term or long-term postoperative results in lung cancer patients in good clinical condition. Chest. 2005;128(3):1557–1563. doi: 10.1378/chest.128.3.1557. [DOI] [PubMed] [Google Scholar]
- 230.Cerfolio RJ, Bryant AS. Survival and outcomes of pulmonary resection for non-small cell lung cancer in the elderly: a nested case-control study. Ann Thorac Surg. 2006;82(2):424–429. doi: 10.1016/j.athoracsur.2006.02.085. [DOI] [PubMed] [Google Scholar]
- 231.Goodgame B, Viswanathan A, Zoole J, et al. Risk of recurrence of resected stage I non-small cell lung cancer in elderly patients as compared with younger patients. J Thorac Oncol. 2009;4(11):1370–1374. doi: 10.1097/JTO.0b013e3181b6bc1b. [DOI] [PubMed] [Google Scholar]
- 232.Hardy D, Cormier JN, Xing Y, Liu CC, Xia R, Du XL. Chemotherapy-associated toxicity in a large cohort of elderly patients with non-small cell lung cancer. J Thorac Oncol. 2010;5(1):90–98. doi: 10.1097/JTO.0b013e3181c0a128. [DOI] [PubMed] [Google Scholar]
- 233.Chrischilles EA, Pendergast JF, Kahn KL, et al. Adverse events among the elderly receiving chemotherapy for advanced non-small-cell lung cancer. J Clin Oncol. 2010;28(4):620–627. doi: 10.1200/JCO.2009.23.8485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Ngeow J, Leong SS, Gao F, et al. Impact of comorbidities on clinical outcomes in non-small cell lung cancer patients who are elderly and/or have poor performance status. Crit Rev Oncol Hematol. 2010;76(1):53–60. doi: 10.1016/j.critrevonc.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 235.Leighl NB, Zatloukal P, Mezger J, et al. Efficacy and safety of bevacizumab-based therapy in elderly patients with advanced or recurrent nonsquamous non-small cell lung cancer in the phase III BO17704 study (AVAiL) J Thorac Oncol. 2010;5(12):1970–1976. doi: 10.1097/JTO.0b013e3181f49c22. [DOI] [PubMed] [Google Scholar]
- 236.Pezzuolo D, Pennucci MC, Mambrini A, et al. Low dose fractionated cisplatin plus gemcitabine for elderly patients with advanced non small cell lung cancer: a retrospective analysis. J Chemother. 2010;22(4):275–279. doi: 10.1179/joc.2010.22.4.275. [DOI] [PubMed] [Google Scholar]
- 237.Meriggi F, Zaniboni A. Epidermal growth factor receptor tyrosine kinase inhibitors for elderly patients with advanced non-small cell lung cancer. Curr Gerontol Geriatr Res. 2010:348174. doi: 10.1155/2010/348174. 2010; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Igawa S, Ryuge S, Fukui T, et al. Amrubicin for treating elderly and poor-risk patients with small-cell lung cancer. Int J Clin Oncol. 2010;15(5):447–452. doi: 10.1007/s10147-010-0085-2. [DOI] [PubMed] [Google Scholar]
- 239.Alberg AJ, Ford JG, Samet JM. Epidemiology of lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition) Chest. 2007;132(3 suppl):29S–55S. doi: 10.1378/chest.07-1347. [DOI] [PubMed] [Google Scholar]
- 240.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 241.Coate LE, Massey C, Hope A, et al. Treatment of the elderly when cure is the goal: the influence of age on treatment selection and efficacy for stage III non-small cell lung cancer. J Thorac Oncol. 2011;6(3):537–544. doi: 10.1097/JTO.0b013e31820b8b9b. [DOI] [PubMed] [Google Scholar]
- 242.Yau T, Ashley S, Popat S, et al. Time and chemotherapy treatment trends in the treatment of elderly patients (age ≥ 70 years) with small cell lung cancer. Br J Cancer. 2006;94(1):18–21. doi: 10.1038/sj.bjc.6602888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Gridelli C. The ELVIS trial: a phase III study of single-agent vinorelbine as first-line treatment in elderly patients with advanced non-small cell lung cancer. Elderly Lung Cancer Vinorelbine Italian Study. Oncologist. 2001;6(suppl 1):4–7. doi: 10.1634/theoncologist.6-suppl_1-4. [DOI] [PubMed] [Google Scholar]
- 244.Kim DS, Collard HR, King TE., Jr Classification and natural history of the idiopathic interstitial pneumonias. Proc Am Thorac Soc. 2006;3(4):285–292. doi: 10.1513/pats.200601-005TK. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Myers JL, Katzenstein AL. Beyond a consensus classification for idiopathic interstitial pneumonias: progress and controversies. Histopathology. 2009;54(1):90–103. doi: 10.1111/j.1365-2559.2008.03173.x. [DOI] [PubMed] [Google Scholar]
- 246.Castriotta RJ, Eldadah BA, Foster WM, et al. Workshop on idiopathic pulmonary fibrosis in older adults. Chest. 2010;138(3):693–703. doi: 10.1378/chest.09-3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Raghu G, Weycker D, Edelsberg J, Bradford WZ, Oster G. Incidence and prevalence of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2006;174(7):810–816. doi: 10.1164/rccm.200602-163OC. [DOI] [PubMed] [Google Scholar]
- 248.Araki T, Katsura H, Sawabe M, Kida K. A clinical study of idiopathic pulmonary fibrosis based on autopsy studies in elderly patients. Intern Med. 2003;42(6):483–489. doi: 10.2169/internalmedicine.42.483. [DOI] [PubMed] [Google Scholar]
- 249.Lettieri CJ, Nathan SD, Barnett SD, Ahmad S, Shorr AF. Prevalence and outcomes of pulmonary arterial hypertension in advanced idiopathic pulmonary fibrosis. Chest. 2006;129(3):746–752. doi: 10.1378/chest.129.3.746. [DOI] [PubMed] [Google Scholar]
- 250.Nathan SD, Shlobin OA, Ahmad S, et al. Serial development of pulmonary hypertension in patients with idiopathic pulmonary fibrosis. Respiration. 2008;76(3):288–294. doi: 10.1159/000114246. [DOI] [PubMed] [Google Scholar]
- 251.Zisman DA, Karlamangla AS, Ross DJ, et al. High-resolution chest CT findings do not predict the presence of pulmonary hypertension in advanced idiopathic pulmonary fibrosis. Chest. 2007;132(3):773–779. doi: 10.1378/chest.07-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Cottin V, Nunes H, Brillet PY, et al. Combined pulmonary fibrosis and emphysema: a distinct underrecognised entity. Eur Respir J. 2005;26(4):586–593. doi: 10.1183/09031936.05.00021005. [DOI] [PubMed] [Google Scholar]
- 253.Cottin V, Le PJ, Prevot G, et al. Pulmonary hypertension in patients with combined pulmonary fibrosis and emphysema syndrome. Eur Respir J. 2010;35(1):105–111. doi: 10.1183/09031936.00038709. [DOI] [PubMed] [Google Scholar]
- 254.Nadrous HF, Pellikka PA, Krowka MJ, et al. Pulmonary hypertension in patients with idiopathic pulmonary fibrosis. Chest. 2005;128(4):2393–2399. doi: 10.1378/chest.128.4.2393. [DOI] [PubMed] [Google Scholar]
- 255.Mejia M, Carrillo G, Rojas-Serrano J, et al. Idiopathic pulmonary fibrosis and emphysema: decreased survival associated with severe pulmonary arterial hypertension. Chest. 2009;136(1):10–15. doi: 10.1378/chest.08-2306. [DOI] [PubMed] [Google Scholar]
- 256.Hunninghake GW, Zimmerman MB, Schwartz DA, et al. Utility of a lung biopsy for the diagnosis of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2001;164(2):193–196. doi: 10.1164/ajrccm.164.2.2101090. [DOI] [PubMed] [Google Scholar]
- 257.Steele MP, Speer MC, Loyd JE, et al. Clinical and pathologic features of familial interstitial pneumonia. Am J Respir Crit Care Med. 2005;172(9):1146–1152. doi: 10.1164/rccm.200408-1104OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.American Thoracic Society. Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. American Thoracic Society (ATS), and the European Respiratory Society (ERS) Am J Respir Crit Care Med. 2000;161(2 Pt 1):646–664. doi: 10.1164/ajrccm.161.2.ats3-00. [DOI] [PubMed] [Google Scholar]
- 259.Gribbin J, Hubbard R, Smith C. Role of diabetes mellitus and gastro-oesophageal reflux in the aetiology of idiopathic pulmonary fibrosis. Respir Med. 2009;103(6):927–931. doi: 10.1016/j.rmed.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 260.Raghu G, Freudenberger TD, Yang S, et al. High prevalence of abnormal acid gastro-oesophageal reflux in idiopathic pulmonary fibrosis. Eur Respir J. 2006;27(1):136–142. doi: 10.1183/09031936.06.00037005. [DOI] [PubMed] [Google Scholar]
- 261.Raghu G, Yang ST, Spada C, Hayes J, Pellegrini CA. Sole treatment of acid gastroesophageal reflux in idiopathic pulmonary fibrosis: a case series. Chest. 2006;129(3):794–800. doi: 10.1378/chest.129.3.794. [DOI] [PubMed] [Google Scholar]
- 262.Linden PA, Gilbert RJ, Yeap BY, et al. Laparoscopic fundoplication in patients with end-stage lung disease awaiting transplantation. J Thorac Cardiovasc Surg. 2006;131(2):438–446. doi: 10.1016/j.jtcvs.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 263.Kelly BG, Lok SS, Hasleton PS, Egan JJ, Stewart JP. A rearranged form of Epstein-Barr virus DNA is associated with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2002;166(4):510–513. doi: 10.1164/rccm.2103058. [DOI] [PubMed] [Google Scholar]
- 264.Tang YW, Johnson J, Cruz-Gervis R, et al. Increased detection of herpesvirus DNA in idiopathic pulmonary fibrosis. Chest. 2001;120(1 suppl):74S–75S. doi: 10.1378/chest.120.1_suppl.s74-a. [DOI] [PubMed] [Google Scholar]
- 265.Tang YW, Johnson JE, Browning PJ, et al. Herpesvirus DNA is consistently detected in lungs of patients with idiopathic pulmonary fibrosis. J Clin Microbiol. 2003;41(6):2633–2640. doi: 10.1128/JCM.41.6.2633-2640.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Tsukamoto K, Hayakawa H, Sato A, Chida K, Nakamura H, Miura K. Involvement of Epstein-Barr virus latent membrane protein 1 in disease progression in patients with idiopathic pulmonary fibrosis. Thorax. 2000;55(11):958–961. doi: 10.1136/thorax.55.11.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Demedts M, Behr J, Buhl R, et al. High-dose acetylcysteine in idiopathic pulmonary fibrosis. N Engl J Med. 2005;353(21):2229–2242. doi: 10.1056/NEJMoa042976. [DOI] [PubMed] [Google Scholar]
- 268.Felton VM, Borok Z, Willis BC. N-acetylcysteine inhibits alveolar epithelial-mesenchymal transition. Am J Physiol Lung Cell Mol Physiol. 2009;297(5):L805–L812. doi: 10.1152/ajplung.00009.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Azuma A, Nukiwa T, Tsuboi E, et al. Double-blind, placebo-controlled trial of pirfenidone in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2005;171(9):1040–1047. doi: 10.1164/rccm.200404-571OC. [DOI] [PubMed] [Google Scholar]
- 270.Azuma A. Pirfenidone: antifibrotic agent for idiopathic pulmonary fibrosis. Expert Rev Respir Med. 2010;4(3):301–310. doi: 10.1586/ers.10.32. [DOI] [PubMed] [Google Scholar]
- 271.Azuma A, Taguchi Y, Ogura T, et al. Exploratory analysis of a phase III trial of pirfenidone identifies a subpopulation of patients with idiopathic pulmonary fibrosis as benefiting from treatment. Respir Res. 2011;12:143. doi: 10.1186/1465-9921-12-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Costabel U. Emerging potential treatments: new hope for idiopathic pulmonary fibrosis patients? Eur Respir Rev. 2011;20(121):201–207. doi: 10.1183/09059180.00002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Noble PW, Albera C, Bradford WZ, et al. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomised trials. Lancet. 2011;377(9779):1760–1769. doi: 10.1016/S0140-6736(11)60405-4. [DOI] [PubMed] [Google Scholar]
- 274.Richeldi L, du Bois RM. Pirfenidone in idiopathic pulmonary fibrosis: the CAPACITY program. Expert Rev Respir Med. 2011;5(4):473–481. doi: 10.1586/ers.11.52. [DOI] [PubMed] [Google Scholar]
- 275.Taniguchi H, Ebina M, Kondoh Y, et al. Pirfenidone in idiopathic pulmonary fibrosis. Eur Respir J. 2010;35(4):821–829. doi: 10.1183/09031936.00005209. [DOI] [PubMed] [Google Scholar]
- 276.Ferreira A, Garvey C, Connors GL, et al. Pulmonary rehabilitation in interstitial lung disease: benefits and predictors of response. Chest. 2009;135(2):442–447. doi: 10.1378/chest.08-1458. [DOI] [PubMed] [Google Scholar]
- 277.Meyer DM, Edwards LB, Torres F, Jessen ME, Novick RJ. Impact of recipient age and procedure type on survival after lung transplantation for pulmonary fibrosis. Ann Thorac Surg. 2005;79(3):950–957. doi: 10.1016/j.athoracsur.2004.08.076. [DOI] [PubMed] [Google Scholar]
- 278.OPTN/SRTR. Transplant Recipient Characteristics, 1999–2008. 2011. Recipients of Deceased Donor Lungs. http://optn.transplant.hrsa.gov/ar2009/1204_age_lu.htm. Accessed January 18, 2012. [Google Scholar]
- 279.Weiss ES, Merlo CA, Shah AS. Impact of advanced age in lung transplantation: an analysis of United Network for Organ Sharing data. J Am Coll Surg. 2009;208(3):400–409. doi: 10.1016/j.jamcollsurg.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 280.Schwartz DA, Helmers RA, Galvin JR, et al. Determinants of survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 1994;149(2 Pt 1):450–454. doi: 10.1164/ajrccm.149.2.8306044. [DOI] [PubMed] [Google Scholar]
- 281.Schwartz DA, Van Fossen DS, Davis CS, et al. Determinants of progression in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 1994;149(2 Pt 1):444–449. doi: 10.1164/ajrccm.149.2.8306043. [DOI] [PubMed] [Google Scholar]
- 282.Lee SJ, Lindquist K, Segal MR, Covinsky KE. Development and validation of a prognostic index for 4-year mortality in older adults. JAMA. 2006;295(7):801–808. doi: 10.1001/jama.295.7.801. [DOI] [PubMed] [Google Scholar]
- 283.Boyd CM, Landefeld CS, Counsell SR, et al. Recovery of activities of daily living in older adults after hospitalization for acute medical illness. J Am Geriatr Soc. 2008;56(12):2171–2179. doi: 10.1111/j.1532-5415.2008.02023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Covinsky KE, Palmer RM, Fortinsky RH, et al. Loss of independence in activities of daily living in older adults hospitalized with medical illnesses: increased vulnerability with age. J Am Geriatr Soc. 2003;51(4):451–458. doi: 10.1046/j.1532-5415.2003.51152.x. [DOI] [PubMed] [Google Scholar]