Abstract
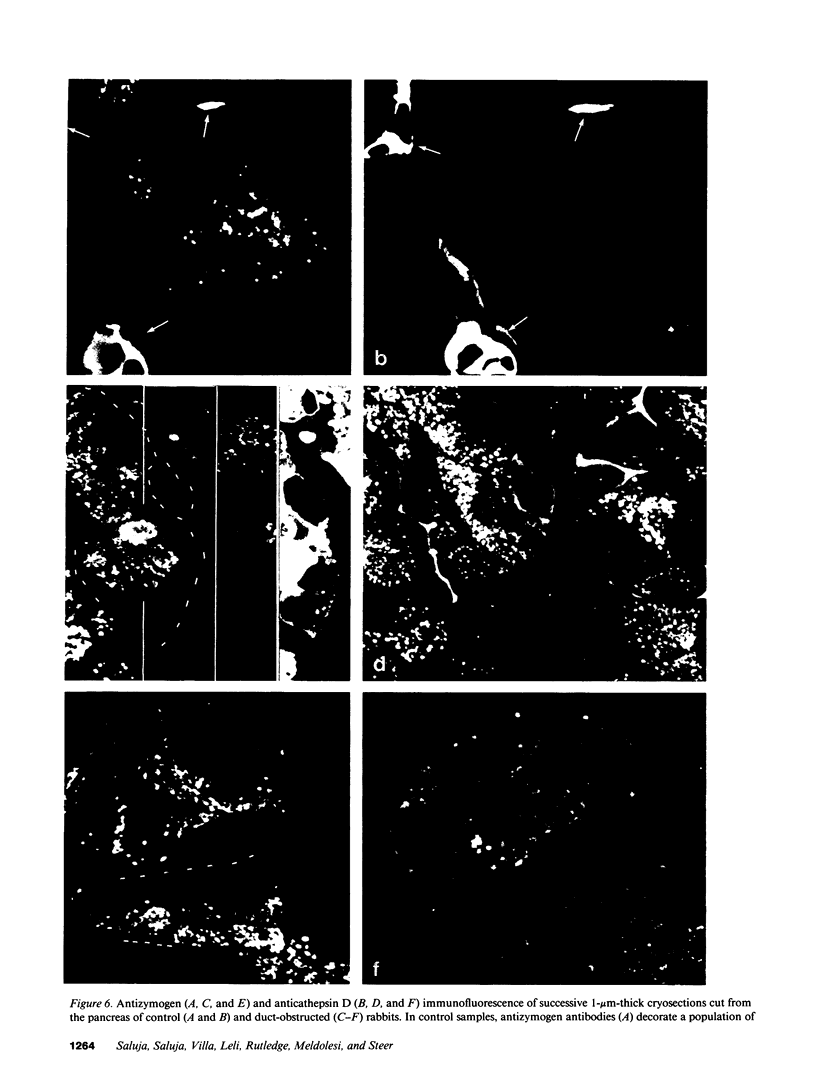
The pancreatic duct of anesthetized rabbits was cannulated and, in some animals, flow of pancreatic exocrine secretions was blocked by raising the cannula to a vertical position. Blockage for 3-7 h caused a rapid and significant rise in serum amylase activity and an increase in amylase activity within the pancreas. The concentration of lysosomal enzymes in the pancreas was not altered but they became redistributed among subcellular fractions and, as a result, an increased amount was recovered in the 1,000-g, 15-min pellet, which was enriched in zymogen granules. Immunofluorescence studies indicated that lysosomal enzymes become localized within organelles which, in size and distribution, resemble zymogen granules. They also contain digestive enzyme zymogens. Blockage of pancreatic secretions also caused lysosomal enzyme-containing organelles to become more fragile and subject to in vitro rupture. These changes noted after short-term pancreatic duct obstruction are remarkably similar to those previously noted to occur during the early stages of diet and secretagogue-induced experimental pancreatitis, observations that have suggested that colocalization of digestive enzyme zymogens and lysosomal hydrolases might result in intracellular digestive enzyme activation and be an important early event in the evolution of those forms of experimental acute pancreatitis.
Full text
PDF
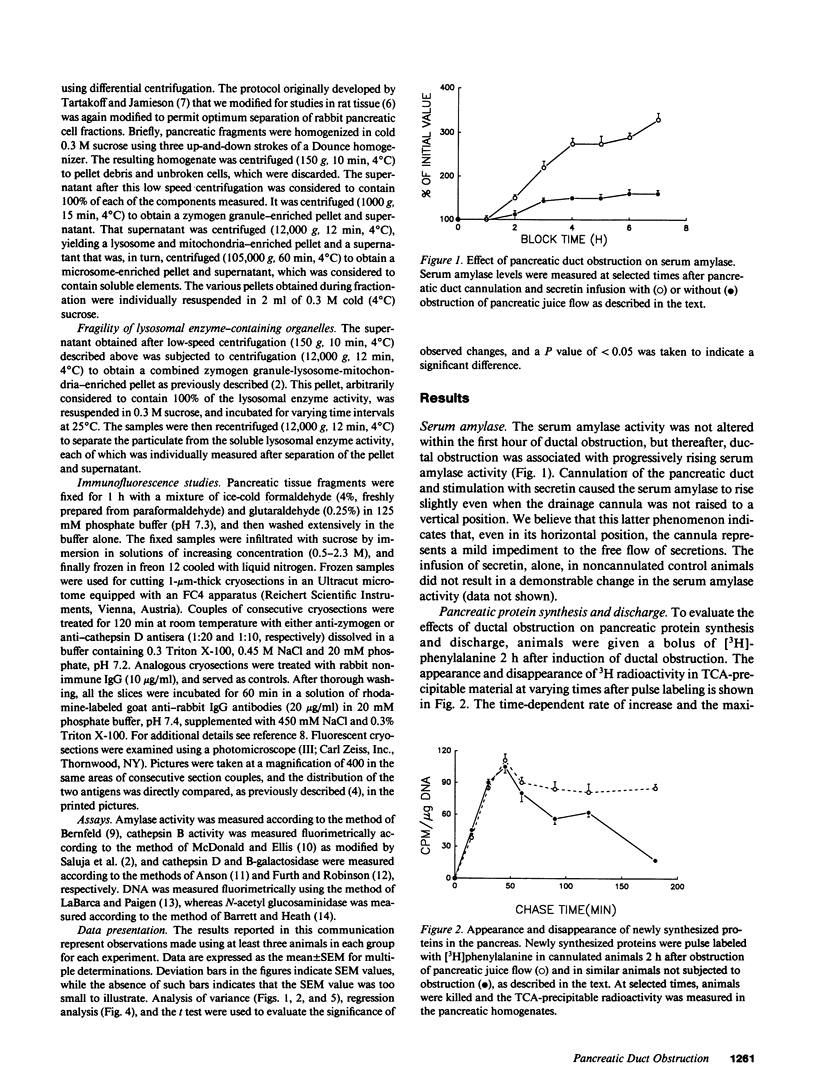
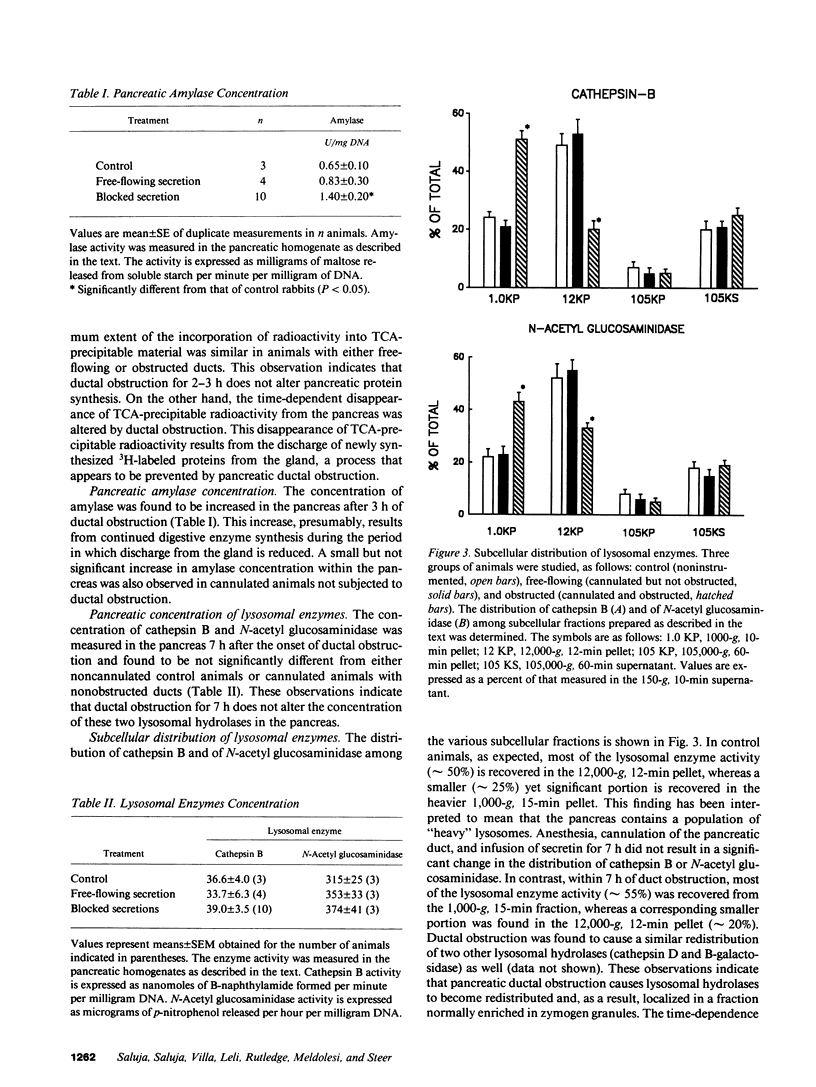
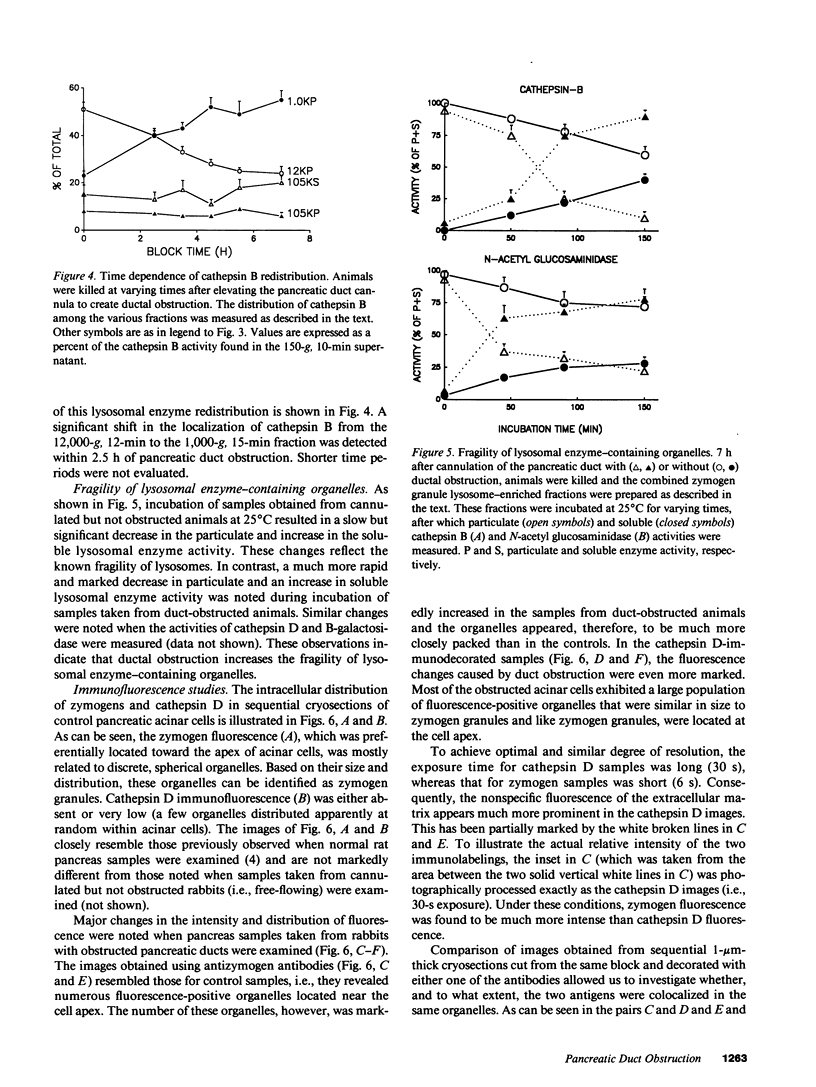


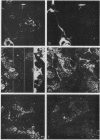
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acosta J. M., Ledesma C. L. Gallstone migration as a cause of acute pancreatitis. N Engl J Med. 1974 Feb 28;290(9):484–487. doi: 10.1056/NEJM197402282900904. [DOI] [PubMed] [Google Scholar]
- Farquhar M. G. Secretion and crinophagy in prolactin cells. Adv Exp Med Biol. 1977;80:37–94. doi: 10.1007/978-1-4615-6675-5_3. [DOI] [PubMed] [Google Scholar]
- Furth A. J., Robinson D. Specificity and multiple forms of beta-galactosidase in the rat. Biochem J. 1965 Oct;97(1):59–66. doi: 10.1042/bj0970059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENBAUM L. M., HIRSHKOWITZ A. Endogenous cathepsin activation of trypsinogen in extracts of dog pancreas. Proc Soc Exp Biol Med. 1961 May;107:74–76. doi: 10.3181/00379727-107-26539. [DOI] [PubMed] [Google Scholar]
- Gilliland L., Steer M. L. Effects of ethionine on digestive enzyme synthesis and discharge by mouse pancreas. Am J Physiol. 1980 Nov;239(5):G418–G426. doi: 10.1152/ajpgi.1980.239.5.G418. [DOI] [PubMed] [Google Scholar]
- Hashimoto S., Bruno B., Lew D. P., Pozzan T., Volpe P., Meldolesi J. Immunocytochemistry of calciosomes in liver and pancreas. J Cell Biol. 1988 Dec;107(6 Pt 2):2523–2531. doi: 10.1083/jcb.107.6.2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike H., Steer M. L., Meldolesi J. Pancreatic effects of ethionine: blockade of exocytosis and appearance of crinophagy and autophagy precede cellular necrosis. Am J Physiol. 1982 Apr;242(4):G297–G307. doi: 10.1152/ajpgi.1982.242.4.G297. [DOI] [PubMed] [Google Scholar]
- Kornfeld S. Trafficking of lysosomal enzymes in normal and disease states. J Clin Invest. 1986 Jan;77(1):1–6. doi: 10.1172/JCI112262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labarca C., Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 1980 Mar 1;102(2):344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- Lampel M., Kern H. F. Acute interstitial pancreatitis in the rat induced by excessive doses of a pancreatic secretagogue. Virchows Arch A Pathol Anat Histol. 1977 Mar 11;373(2):97–117. doi: 10.1007/BF00432156. [DOI] [PubMed] [Google Scholar]
- Lombardi B., Estes L. W., Longnecker D. S. Acute hemorrhagic pancreatitis (massive necrosis) with fat necrosis induced in mice by DL-ethionine fed with a choline-deficient diet. Am J Pathol. 1975 Jun;79(3):465–480. [PMC free article] [PubMed] [Google Scholar]
- McDonald J. K., Ellis S. On the substrate specificity of cathepsins B1 and B2 including a new fluorogenic substrate for cathepsin B1. Life Sci. 1975 Oct 15;17(8):1269–1276. doi: 10.1016/0024-3205(75)90137-x. [DOI] [PubMed] [Google Scholar]
- Saluja A., Hashimoto S., Saluja M., Powers R. E., Meldolesi J., Steer M. L. Subcellular redistribution of lysosomal enzymes during caerulein-induced pancreatitis. Am J Physiol. 1987 Oct;253(4 Pt 1):G508–G516. doi: 10.1152/ajpgi.1987.253.4.G508. [DOI] [PubMed] [Google Scholar]
- Saluja A., Saito I., Saluja M., Houlihan M. J., Powers R. E., Meldolesi J., Steer M. In vivo rat pancreatic acinar cell function during supramaximal stimulation with caerulein. Am J Physiol. 1985 Dec;249(6 Pt 1):G702–G710. doi: 10.1152/ajpgi.1985.249.6.G702. [DOI] [PubMed] [Google Scholar]
- Steer M. L., Meldolesi J., Figarella C. Pancreatitis. The role of lysosomes. Dig Dis Sci. 1984 Oct;29(10):934–938. doi: 10.1007/BF01312483. [DOI] [PubMed] [Google Scholar]
- Steer M. L., Meldolesi J. The cell biology of experimental pancreatitis. N Engl J Med. 1987 Jan 15;316(3):144–150. doi: 10.1056/NEJM198701153160306. [DOI] [PubMed] [Google Scholar]
- Tartakoff A. M., Jamieson J. D. Subcellular fractionation of the pancreas. Methods Enzymol. 1974;31:41–59. doi: 10.1016/0076-6879(74)31006-3. [DOI] [PubMed] [Google Scholar]
- Watanabe O., Baccino F. M., Steer M. L., Meldolesi J. Supramaximal caerulein stimulation and ultrastructure of rat pancreatic acinar cell: early morphological changes during development of experimental pancreatitis. Am J Physiol. 1984 Apr;246(4 Pt 1):G457–G467. doi: 10.1152/ajpgi.1984.246.4.G457. [DOI] [PubMed] [Google Scholar]