Abstract
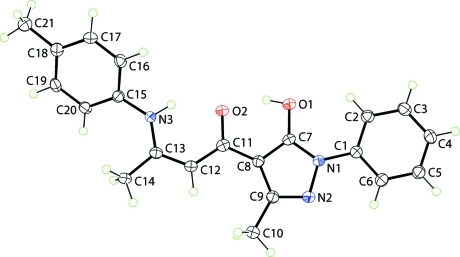
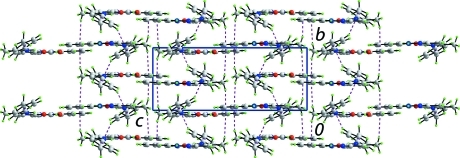
A twist is evident in the title compound, C21H21N3O2, the dihedral angle between the terminal six-membered rings being 29.46 (10)°; the linked five- and six-membered rings are coplanar [1.30 (11)°]. The carbonyl O atom accepts intramolecular hydrogen bonds from the adjacent hydroxy and amine groups. The three-dimensional crystal packing is achieved through C—H⋯π interactions.
Related literature
For background to the synthesis, see: Gelin et al. (1983 ▶); Bendaas et al. (1999 ▶). For the structures of the 4-chloro and 4-methoxy analogues, see: Asiri, Al-Youbi, Alamry et al. (2011 ▶); Asiri, Al-Youbi, Faidallah et al. (2011 ▶).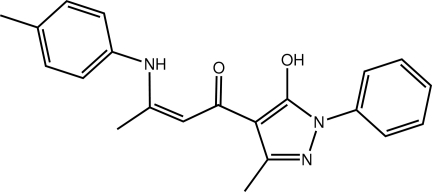
Experimental
Crystal data
C21H21N3O2
M r = 347.41
Monoclinic,
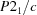
a = 14.9041 (8) Å
b = 6.9222 (4) Å
c = 17.1921 (8) Å
β = 96.190 (5)°
V = 1763.35 (16) Å3
Z = 4
Mo Kα radiation
μ = 0.09 mm−1
T = 100 K
0.40 × 0.02 × 0.02 mm
Data collection
Agilent SuperNova Dual diffractometer with an Atlas detector
Absorption correction: multi-scan (CrysAlis PRO; Agilent, 2011 ▶) T min = 0.967, T max = 0.998
12780 measured reflections
4055 independent reflections
2455 reflections with I > 2σ(I)
R int = 0.076
Refinement
R[F 2 > 2σ(F 2)] = 0.060
wR(F 2) = 0.164
S = 1.02
4055 reflections
246 parameters
2 restraints
H atoms treated by a mixture of independent and constrained refinement
Δρmax = 0.24 e Å−3
Δρmin = −0.29 e Å−3
Data collection: CrysAlis PRO (Agilent, 2011 ▶); cell refinement: CrysAlis PRO; data reduction: CrysAlis PRO; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: ORTEP-3 (Farrugia, 1997 ▶) and DIAMOND (Brandenburg, 2006 ▶); software used to prepare material for publication: publCIF (Westrip, 2010 ▶).
Supplementary Material
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S1600536812006526/hg5179sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536812006526/hg5179Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536812006526/hg5179Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
Cg1 and Cg2 are the centroids of the C1–C6 and C15–C20 rings, respectively.
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| O1—H1⋯O2 | 0.86 (1) | 1.71 (2) | 2.509 (2) | 155 (4) |
| N3—H2⋯O2 | 0.89 (1) | 1.91 (2) | 2.671 (3) | 143 (2) |
| C14—H14A⋯Cg1i | 0.98 | 2.69 | 3.475 (2) | 138 |
| C14—H14C⋯Cg1ii | 0.98 | 2.66 | 3.563 (2) | 153 |
| C17—H17⋯Cg2iii | 0.95 | 2.58 | 3.424 (2) | 148 |
Symmetry codes: (i) 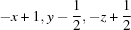 ; (ii)
; (ii) 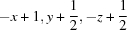 ; (iii)
; (iii) 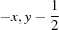 ,
, 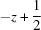 .
.
Acknowledgments
The authors are thankful to the Center of Excellence for Advanced Materials Research and the Chemistry Department of King Abdulaziz University for providing research facilities. We also thank the Ministry of Higher Education (Malaysia) for funding structural studies through the High-Impact Research scheme (UM.C/HIR/MOHE/SC/12).
supplementary crystallographic information
Comment
In connection with recent structural studies (Asiri, Al-Youbi, Alamry et al., 2011; (Asiri, Al-Youbi, Faidallah et al., 2011) of compounds prepared by reactions between pyrazoles and aniline derivatives following literature procedures (Gelin et al., 1983; Bendaas et al., 1999), the title compound, 3-(4-toluidino)-1-(5-hydroxy-3-methyl-1-phenyl-1H-pyrazol-4-yl)but-2-en-1-one (I) was investigated.
While in (I), Fig. 1, the linked five- and six-membered rings are co-planar, forming a dihedral angle of 1.30 (11)°, there is a twist about the N3—C15 bond as seen in the value of the C13—N3—C15—C16 torsion angle of 146.9 (2)°; the dihedral angle between the terminal six-membered rings is 29.46 (10)°. The carbonyl-O2 atom accepts hydrogen bonds from the adjacent hydroxyl- and amine-groups, Table 1. These groups do not participate in intermolecular interactions. Rather, molecules are consolidated in the three-dimensional crystal packing by C—H···π interactions, Fig. 2 and Table 1.
Experimental
A solution of 4-acetoacetyl-5-hydroxy-3-methyl-1-phenylpyrazole (0.005 mol) and p-toluidine (0.005 mol) in ethanol (25 ml) was refluxed for 2 h. The precipitate, obtained from the hot solution, was collected, washed with methanol and recrystallized from its ethanol-benzene solution as yellow needles; M.pt: 419–421 K.
Refinement
Carbon-bound H-atoms were placed in calculated positions [C—H = 0.95 to 0.98 Å, Uiso(H) = 1.2 to 1.5Ueq(C)] and were included in the refinement in the riding model approximation. The N—H and O—H-atoms were located in a difference Fourier map, and were refined with distance restraints of N—H = 0.88±0.01 and O—H = 0.84±0.01 Å, respectively; their Uiso values were refined. Owing to poor agreement, the (3 6 3) reflection was omitted from the final cycles of refinement.
Figures
Fig. 1.
The molecular structure of (I) showing the atom-labelling scheme and displacement ellipsoids at the 50% probability level.
Fig. 2.
A view in projection down the a axis of the unit-cell contents of (I). The C—H···π interactions are shown as purple dashed lines.
Crystal data
| C21H21N3O2 | F(000) = 736 |
| Mr = 347.41 | Dx = 1.309 Mg m−3 |
| Monoclinic, P21/c | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: -P 2ybc | Cell parameters from 2466 reflections |
| a = 14.9041 (8) Å | θ = 2.4–27.5° |
| b = 6.9222 (4) Å | µ = 0.09 mm−1 |
| c = 17.1921 (8) Å | T = 100 K |
| β = 96.190 (5)° | Needle, yellow |
| V = 1763.35 (16) Å3 | 0.40 × 0.02 × 0.02 mm |
| Z = 4 |
Data collection
| Agilent SuperNova Dual diffractometer with an Atlas detector | 4055 independent reflections |
| Radiation source: SuperNova (Mo) X-ray Source | 2455 reflections with I > 2σ(I) |
| Mirror monochromator | Rint = 0.076 |
| Detector resolution: 10.4041 pixels mm-1 | θmax = 27.6°, θmin = 2.4° |
| ω scan | h = −18→19 |
| Absorption correction: multi-scan (CrysAlis PRO; Agilent, 2011) | k = −8→8 |
| Tmin = 0.967, Tmax = 0.998 | l = −22→18 |
| 12780 measured reflections |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.060 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.164 | H atoms treated by a mixture of independent and constrained refinement |
| S = 1.02 | w = 1/[σ2(Fo2) + (0.065P)2 + 0.099P] where P = (Fo2 + 2Fc2)/3 |
| 4055 reflections | (Δ/σ)max = 0.001 |
| 246 parameters | Δρmax = 0.24 e Å−3 |
| 2 restraints | Δρmin = −0.29 e Å−3 |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| O1 | 0.50304 (12) | 0.0677 (2) | 0.39888 (10) | 0.0275 (4) | |
| O2 | 0.37132 (11) | 0.0655 (2) | 0.29485 (9) | 0.0244 (4) | |
| N1 | 0.64285 (13) | 0.0686 (2) | 0.34357 (10) | 0.0192 (4) | |
| N2 | 0.67114 (13) | 0.0711 (2) | 0.26853 (10) | 0.0229 (4) | |
| N3 | 0.23145 (13) | 0.0409 (3) | 0.18426 (11) | 0.0215 (4) | |
| C1 | 0.70881 (15) | 0.0615 (3) | 0.40934 (12) | 0.0186 (5) | |
| C2 | 0.68417 (16) | 0.0546 (3) | 0.48530 (13) | 0.0231 (5) | |
| H2A | 0.6223 | 0.0541 | 0.4941 | 0.028* | |
| C3 | 0.75125 (17) | 0.0486 (3) | 0.54797 (13) | 0.0256 (5) | |
| H3 | 0.7347 | 0.0431 | 0.5998 | 0.031* | |
| C4 | 0.84175 (17) | 0.0503 (3) | 0.53633 (13) | 0.0257 (5) | |
| H4 | 0.8870 | 0.0465 | 0.5797 | 0.031* | |
| C5 | 0.86567 (17) | 0.0577 (3) | 0.46044 (13) | 0.0245 (5) | |
| H5 | 0.9276 | 0.0600 | 0.4519 | 0.029* | |
| C6 | 0.79969 (15) | 0.0618 (3) | 0.39707 (13) | 0.0227 (5) | |
| H6 | 0.8165 | 0.0649 | 0.3453 | 0.027* | |
| C7 | 0.55156 (15) | 0.0701 (3) | 0.33854 (13) | 0.0193 (5) | |
| C8 | 0.51783 (15) | 0.0733 (3) | 0.25960 (12) | 0.0187 (5) | |
| C9 | 0.59647 (16) | 0.0733 (3) | 0.21952 (13) | 0.0215 (5) | |
| C10 | 0.60368 (17) | 0.0740 (3) | 0.13342 (13) | 0.0302 (6) | |
| H10A | 0.6674 | 0.0793 | 0.1243 | 0.045* | |
| H10B | 0.5721 | 0.1871 | 0.1096 | 0.045* | |
| H10C | 0.5763 | −0.0439 | 0.1100 | 0.045* | |
| C11 | 0.42170 (16) | 0.0712 (3) | 0.23786 (13) | 0.0207 (5) | |
| C12 | 0.38378 (16) | 0.0712 (3) | 0.15824 (12) | 0.0205 (5) | |
| H12 | 0.4245 | 0.0831 | 0.1196 | 0.025* | |
| C13 | 0.29317 (16) | 0.0556 (3) | 0.13212 (12) | 0.0200 (5) | |
| C14 | 0.26394 (16) | 0.0493 (3) | 0.04612 (12) | 0.0229 (5) | |
| H14A | 0.2209 | −0.0566 | 0.0348 | 0.034* | |
| H14B | 0.3167 | 0.0283 | 0.0177 | 0.034* | |
| H14C | 0.2351 | 0.1720 | 0.0296 | 0.034* | |
| C15 | 0.13787 (16) | 0.0037 (3) | 0.17314 (13) | 0.0209 (5) | |
| C16 | 0.10125 (15) | −0.1006 (3) | 0.23183 (12) | 0.0225 (5) | |
| H16 | 0.1400 | −0.1484 | 0.2751 | 0.027* | |
| C17 | 0.00953 (16) | −0.1349 (3) | 0.22785 (13) | 0.0231 (5) | |
| H17 | −0.0138 | −0.2033 | 0.2692 | 0.028* | |
| C18 | −0.04959 (16) | −0.0719 (3) | 0.16470 (13) | 0.0227 (5) | |
| C19 | −0.01234 (16) | 0.0307 (3) | 0.10594 (13) | 0.0229 (5) | |
| H19 | −0.0509 | 0.0746 | 0.0618 | 0.028* | |
| C20 | 0.07922 (16) | 0.0702 (3) | 0.11024 (12) | 0.0227 (5) | |
| H20 | 0.1022 | 0.1432 | 0.0700 | 0.027* | |
| C21 | −0.14922 (16) | −0.1052 (3) | 0.16189 (14) | 0.0295 (6) | |
| H21 | −0.1795 | −0.0515 | 0.1132 | 0.044* | |
| H21B | −0.1721 | −0.0416 | 0.2067 | 0.044* | |
| H21C | −0.1612 | −0.2442 | 0.1639 | 0.044* | |
| H1 | 0.4494 (12) | 0.069 (5) | 0.375 (2) | 0.102 (15)* | |
| H2 | 0.2582 (15) | 0.034 (3) | 0.2329 (7) | 0.031 (7)* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| O1 | 0.0212 (10) | 0.0405 (10) | 0.0217 (9) | −0.0003 (8) | 0.0060 (8) | −0.0002 (7) |
| O2 | 0.0207 (9) | 0.0336 (9) | 0.0199 (8) | −0.0008 (7) | 0.0060 (7) | 0.0005 (6) |
| N1 | 0.0186 (10) | 0.0209 (9) | 0.0184 (9) | 0.0007 (7) | 0.0039 (8) | −0.0005 (7) |
| N2 | 0.0219 (11) | 0.0283 (10) | 0.0190 (10) | −0.0012 (8) | 0.0048 (8) | −0.0007 (7) |
| N3 | 0.0180 (11) | 0.0307 (11) | 0.0159 (10) | −0.0003 (8) | 0.0016 (8) | 0.0020 (8) |
| C1 | 0.0204 (12) | 0.0138 (10) | 0.0216 (11) | 0.0004 (9) | 0.0024 (9) | 0.0017 (8) |
| C2 | 0.0235 (13) | 0.0237 (11) | 0.0224 (12) | 0.0006 (10) | 0.0039 (10) | −0.0017 (9) |
| C3 | 0.0288 (14) | 0.0278 (12) | 0.0203 (12) | 0.0009 (10) | 0.0028 (10) | −0.0003 (9) |
| C4 | 0.0277 (14) | 0.0269 (12) | 0.0213 (12) | −0.0019 (10) | −0.0024 (10) | −0.0005 (9) |
| C5 | 0.0224 (13) | 0.0253 (12) | 0.0256 (12) | −0.0007 (10) | 0.0022 (10) | 0.0008 (9) |
| C6 | 0.0218 (13) | 0.0208 (11) | 0.0254 (12) | −0.0010 (9) | 0.0029 (10) | −0.0005 (9) |
| C7 | 0.0182 (12) | 0.0175 (10) | 0.0230 (12) | −0.0001 (9) | 0.0058 (9) | −0.0005 (8) |
| C8 | 0.0184 (12) | 0.0172 (10) | 0.0203 (11) | −0.0008 (9) | 0.0018 (9) | 0.0009 (8) |
| C9 | 0.0218 (13) | 0.0209 (11) | 0.0218 (12) | −0.0001 (9) | 0.0022 (10) | 0.0009 (8) |
| C10 | 0.0248 (14) | 0.0447 (15) | 0.0214 (12) | 0.0012 (11) | 0.0038 (11) | 0.0016 (10) |
| C11 | 0.0219 (13) | 0.0164 (10) | 0.0244 (12) | 0.0002 (9) | 0.0046 (10) | 0.0007 (8) |
| C12 | 0.0216 (12) | 0.0208 (11) | 0.0196 (11) | −0.0004 (9) | 0.0047 (9) | 0.0009 (8) |
| C13 | 0.0230 (13) | 0.0171 (10) | 0.0202 (11) | 0.0002 (9) | 0.0043 (10) | 0.0013 (8) |
| C14 | 0.0249 (13) | 0.0261 (12) | 0.0187 (11) | 0.0002 (10) | 0.0067 (10) | 0.0002 (9) |
| C15 | 0.0195 (13) | 0.0227 (11) | 0.0205 (11) | −0.0008 (9) | 0.0026 (9) | −0.0036 (8) |
| C16 | 0.0218 (13) | 0.0237 (12) | 0.0214 (12) | 0.0037 (9) | 0.0000 (10) | 0.0018 (8) |
| C17 | 0.0250 (13) | 0.0234 (11) | 0.0219 (12) | −0.0010 (10) | 0.0070 (10) | 0.0026 (9) |
| C18 | 0.0226 (13) | 0.0217 (11) | 0.0239 (12) | 0.0032 (9) | 0.0026 (10) | −0.0041 (9) |
| C19 | 0.0232 (13) | 0.0270 (12) | 0.0176 (11) | 0.0038 (9) | −0.0022 (10) | −0.0030 (8) |
| C20 | 0.0247 (13) | 0.0249 (11) | 0.0185 (11) | 0.0027 (10) | 0.0029 (10) | 0.0019 (9) |
| C21 | 0.0260 (14) | 0.0347 (13) | 0.0279 (13) | −0.0015 (11) | 0.0038 (11) | −0.0017 (10) |
Geometric parameters (Å, º)
| O1—C7 | 1.327 (3) | C10—H10A | 0.9800 |
| O1—H1 | 0.858 (10) | C10—H10B | 0.9800 |
| O2—C11 | 1.298 (3) | C10—H10C | 0.9800 |
| N1—C7 | 1.354 (3) | C11—C12 | 1.423 (3) |
| N1—N2 | 1.400 (2) | C12—C13 | 1.381 (3) |
| N1—C1 | 1.417 (3) | C12—H12 | 0.9500 |
| N2—C9 | 1.322 (3) | C13—C14 | 1.496 (3) |
| N3—C13 | 1.356 (3) | C14—H14A | 0.9800 |
| N3—C15 | 1.411 (3) | C14—H14B | 0.9800 |
| N3—H2 | 0.888 (10) | C14—H14C | 0.9800 |
| C1—C6 | 1.393 (3) | C15—C20 | 1.393 (3) |
| C1—C2 | 1.395 (3) | C15—C16 | 1.398 (3) |
| C2—C3 | 1.389 (3) | C16—C17 | 1.382 (3) |
| C2—H2A | 0.9500 | C16—H16 | 0.9500 |
| C3—C4 | 1.385 (3) | C17—C18 | 1.393 (3) |
| C3—H3 | 0.9500 | C17—H17 | 0.9500 |
| C4—C5 | 1.390 (3) | C18—C19 | 1.398 (3) |
| C4—H4 | 0.9500 | C18—C21 | 1.498 (3) |
| C5—C6 | 1.387 (3) | C19—C20 | 1.386 (3) |
| C5—H5 | 0.9500 | C19—H19 | 0.9500 |
| C6—H6 | 0.9500 | C20—H20 | 0.9500 |
| C7—C8 | 1.396 (3) | C21—H21 | 0.9800 |
| C8—C9 | 1.422 (3) | C21—H21B | 0.9800 |
| C8—C11 | 1.441 (3) | C21—H21C | 0.9800 |
| C9—C10 | 1.496 (3) | ||
| C7—O1—H1 | 101 (3) | H10B—C10—H10C | 109.5 |
| C7—N1—N2 | 109.97 (18) | O2—C11—C12 | 121.6 (2) |
| C7—N1—C1 | 131.08 (18) | O2—C11—C8 | 116.39 (19) |
| N2—N1—C1 | 118.94 (18) | C12—C11—C8 | 122.0 (2) |
| C9—N2—N1 | 105.72 (18) | C13—C12—C11 | 125.9 (2) |
| C13—N3—C15 | 130.94 (19) | C13—C12—H12 | 117.1 |
| C13—N3—H2 | 111.0 (16) | C11—C12—H12 | 117.1 |
| C15—N3—H2 | 117.1 (16) | N3—C13—C12 | 120.0 (2) |
| C6—C1—C2 | 120.0 (2) | N3—C13—C14 | 120.3 (2) |
| C6—C1—N1 | 118.77 (19) | C12—C13—C14 | 119.61 (19) |
| C2—C1—N1 | 121.2 (2) | C13—C14—H14A | 109.5 |
| C3—C2—C1 | 119.1 (2) | C13—C14—H14B | 109.5 |
| C3—C2—H2A | 120.4 | H14A—C14—H14B | 109.5 |
| C1—C2—H2A | 120.4 | C13—C14—H14C | 109.5 |
| C4—C3—C2 | 121.2 (2) | H14A—C14—H14C | 109.5 |
| C4—C3—H3 | 119.4 | H14B—C14—H14C | 109.5 |
| C2—C3—H3 | 119.4 | C20—C15—C16 | 118.0 (2) |
| C3—C4—C5 | 119.2 (2) | C20—C15—N3 | 125.0 (2) |
| C3—C4—H4 | 120.4 | C16—C15—N3 | 117.0 (2) |
| C5—C4—H4 | 120.4 | C17—C16—C15 | 120.9 (2) |
| C6—C5—C4 | 120.4 (2) | C17—C16—H16 | 119.5 |
| C6—C5—H5 | 119.8 | C15—C16—H16 | 119.5 |
| C4—C5—H5 | 119.8 | C16—C17—C18 | 121.6 (2) |
| C5—C6—C1 | 120.0 (2) | C16—C17—H17 | 119.2 |
| C5—C6—H6 | 120.0 | C18—C17—H17 | 119.2 |
| C1—C6—H6 | 120.0 | C17—C18—C19 | 117.1 (2) |
| O1—C7—N1 | 125.3 (2) | C17—C18—C21 | 121.2 (2) |
| O1—C7—C8 | 126.2 (2) | C19—C18—C21 | 121.6 (2) |
| N1—C7—C8 | 108.45 (19) | C20—C19—C18 | 121.8 (2) |
| C7—C8—C9 | 104.0 (2) | C20—C19—H19 | 119.1 |
| C7—C8—C11 | 119.7 (2) | C18—C19—H19 | 119.1 |
| C9—C8—C11 | 136.3 (2) | C19—C20—C15 | 120.5 (2) |
| N2—C9—C8 | 111.87 (19) | C19—C20—H20 | 119.7 |
| N2—C9—C10 | 119.0 (2) | C15—C20—H20 | 119.7 |
| C8—C9—C10 | 129.1 (2) | C18—C21—H21 | 109.5 |
| C9—C10—H10A | 109.5 | C18—C21—H21B | 109.5 |
| C9—C10—H10B | 109.5 | H21—C21—H21B | 109.5 |
| H10A—C10—H10B | 109.5 | C18—C21—H21C | 109.5 |
| C9—C10—H10C | 109.5 | H21—C21—H21C | 109.5 |
| H10A—C10—H10C | 109.5 | H21B—C21—H21C | 109.5 |
| C7—N1—N2—C9 | −0.2 (2) | C11—C8—C9—N2 | −178.4 (2) |
| C1—N1—N2—C9 | 178.60 (16) | C7—C8—C9—C10 | 179.3 (2) |
| C7—N1—C1—C6 | −180.0 (2) | C11—C8—C9—C10 | 1.1 (4) |
| N2—N1—C1—C6 | 1.5 (3) | C7—C8—C11—O2 | −0.3 (3) |
| C7—N1—C1—C2 | −0.1 (3) | C9—C8—C11—O2 | 177.8 (2) |
| N2—N1—C1—C2 | −178.62 (17) | C7—C8—C11—C12 | −179.00 (18) |
| C6—C1—C2—C3 | 0.1 (3) | C9—C8—C11—C12 | −1.0 (4) |
| N1—C1—C2—C3 | −179.74 (17) | O2—C11—C12—C13 | −3.5 (3) |
| C1—C2—C3—C4 | 0.4 (3) | C8—C11—C12—C13 | 175.14 (19) |
| C2—C3—C4—C5 | −0.2 (3) | C15—N3—C13—C12 | −172.5 (2) |
| C3—C4—C5—C6 | −0.5 (3) | C15—N3—C13—C14 | 5.7 (3) |
| C4—C5—C6—C1 | 1.0 (3) | C11—C12—C13—N3 | 0.9 (3) |
| C2—C1—C6—C5 | −0.8 (3) | C11—C12—C13—C14 | −177.35 (18) |
| N1—C1—C6—C5 | 179.08 (17) | C13—N3—C15—C20 | −36.1 (3) |
| N2—N1—C7—O1 | 179.73 (18) | C13—N3—C15—C16 | 146.9 (2) |
| C1—N1—C7—O1 | 1.1 (3) | C20—C15—C16—C17 | −0.6 (3) |
| N2—N1—C7—C8 | 0.1 (2) | N3—C15—C16—C17 | 176.58 (19) |
| C1—N1—C7—C8 | −178.55 (18) | C15—C16—C17—C18 | 1.6 (3) |
| O1—C7—C8—C9 | −179.57 (19) | C16—C17—C18—C19 | −1.0 (3) |
| N1—C7—C8—C9 | 0.1 (2) | C16—C17—C18—C21 | −178.35 (19) |
| O1—C7—C8—C11 | −1.0 (3) | C17—C18—C19—C20 | −0.6 (3) |
| N1—C7—C8—C11 | 178.65 (17) | C21—C18—C19—C20 | 176.7 (2) |
| N1—N2—C9—C8 | 0.3 (2) | C18—C19—C20—C15 | 1.7 (3) |
| N1—N2—C9—C10 | −179.30 (17) | C16—C15—C20—C19 | −1.0 (3) |
| C7—C8—C9—N2 | −0.2 (2) | N3—C15—C20—C19 | −177.94 (19) |
Hydrogen-bond geometry (Å, º)
Cg1 and Cg2 are the centroids of the C1–C6 and C15–C20 rings, respectively.
| D—H···A | D—H | H···A | D···A | D—H···A |
| O1—H1···O2 | 0.86 (1) | 1.71 (2) | 2.509 (2) | 155 (4) |
| N3—H2···O2 | 0.89 (1) | 1.91 (2) | 2.671 (3) | 143 (2) |
| C14—H14A···Cg2i | 0.98 | 2.69 | 3.475 (2) | 138 |
| C14—H14C···Cg2ii | 0.98 | 2.66 | 3.563 (2) | 153 |
| C17—H17···Cg3iii | 0.95 | 2.58 | 3.424 (2) | 148 |
Symmetry codes: (i) −x+1, y−1/2, −z+1/2; (ii) −x+1, y+1/2, −z+1/2; (iii) −x, y−1/2, −z+1/2.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: HG5179).
References
- Agilent (2011). CrysAlis PRO Agilent Technologies, Yarnton, Oxfordshire, England.
- Asiri, A. M., Al-Youbi, A. O., Alamry, K. A., Faidallah, H. M., Ng, S. W. & Tiekink, E. R. T. (2011). Acta Cryst. E67, o2157. [DOI] [PMC free article] [PubMed]
- Asiri, A. M., Al-Youbi, A. O., Faidallah, H. M., Ng, S. W. & Tiekink, E. R. T. (2011). Acta Cryst. E67, o2353. [DOI] [PMC free article] [PubMed]
- Bendaas, A., Hamdi, M. & Sellier, N. (1999). J. Heterocycl. Chem. 36, 1291–1294.
- Brandenburg, K. (2006). DIAMOND Crystal Impact GbR, Bonn, Germany.
- Farrugia, L. J. (1997). J. Appl. Cryst. 30, 565.
- Gelin, S., Chantegrel, B. & Nadi, A. I. (1983). J. Org. Chem. 48, 4078–4082.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Westrip, S. P. (2010). J. Appl. Cryst. 43, 920–925.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S1600536812006526/hg5179sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536812006526/hg5179Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536812006526/hg5179Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report