Abstract
In the title compound, C17H18N4OS, a pyrazoline derivative, the pyrazoline ring adopts an envelope conformation with the C atom bonded to the benzene ring as the flap atom. The dihedral angle between the pyridine and benzene rings is 80.50 (6)°. The ethoxyphenyl group is approximately planar, with an r.m.s. deviation of 0.0238 (1) Å for the nine non-H atoms. In the crystal, molecules are linked by N—H⋯O and N—H⋯S hydrogen bonds into a tape along the b axis. Weak C—H⋯N and C—H⋯π interactions are also observed.
Related literature
For bond-length data, see: Allen et al. (1987 ▶). For related literature on ring conformations, see: Cremer & Pople (1975 ▶). For related structures, see: Fun et al. (2012 ▶); Nonthason et al. (2011 ▶). For background to and applications of pyrazoline derivatives, see: Amir et al. (2008 ▶); Gong et al. (2011 ▶); Husain et al. (2008 ▶); Manna & Agrawal (2009 ▶); Özdemir et al. (2007 ▶); Sarkar et al. (2010 ▶). For the stability of the temperature controller used in the data collection, see: Cosier & Glazer (1986 ▶).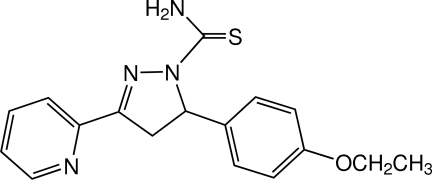
Experimental
Crystal data
C17H18N4OS
M r = 326.42
Monoclinic,
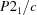
a = 13.4622 (1) Å
b = 9.4175 (1) Å
c = 13.3002 (2) Å
β = 103.146 (1)°
V = 1642.01 (3) Å3
Z = 4
Mo Kα radiation
μ = 0.21 mm−1
T = 100 K
0.34 × 0.22 × 0.20 mm
Data collection
Bruker APEXII CCD area-detector diffractometer
Absorption correction: multi-scan (SADABS; Bruker, 2005 ▶) T min = 0.934, T max = 0.960
23465 measured reflections
4784 independent reflections
3970 reflections with I > 2σ(I)
R int = 0.028
Refinement
R[F 2 > 2σ(F 2)] = 0.040
wR(F 2) = 0.097
S = 1.03
4784 reflections
217 parameters
H atoms treated by a mixture of independent and constrained refinement
Δρmax = 0.45 e Å−3
Δρmin = −0.26 e Å−3
Data collection: APEX2 (Bruker, 2005 ▶); cell refinement: SAINT (Bruker, 2005 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXTL (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXTL; molecular graphics: SHELXTL; software used to prepare material for publication: SHELXTL and PLATON (Spek, 2009 ▶).
Supplementary Material
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S1600536812006642/is5069sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536812006642/is5069Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536812006642/is5069Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
Cg1 and Cg2 are the centroids of the C1–C5/N3 and C9–C14 rings, respectively.
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N4—H1N4⋯S1i | 0.925 (19) | 2.472 (19) | 3.3803 (12) | 167.3 (15) |
| N4—H2N4⋯O1ii | 0.825 (18) | 2.296 (18) | 3.0604 (15) | 154.2 (17) |
| C3—H3A⋯N3iii | 0.95 | 2.51 | 3.4053 (18) | 156 |
| C2—H2A⋯Cg2iii | 0.95 | 2.67 | 3.4401 (14) | 138 |
| C7—H7A⋯Cg1iv | 0.99 | 2.69 | 3.4841 (13) | 138 |
Symmetry codes: (i)  ; (ii)
; (ii)  ; (iii)
; (iii)  ; (iv)
; (iv)  .
.
Acknowledgments
The authors thank the Prince of Songkla Univeristy for financial support through the Crystal Materials Research Unit and the Universiti Sains Malaysia for the Research University grant No. 1001/PFIZIK/811160. PN thanks the Development and Promotion of Science and Technology Talents Project for a fellowship.
supplementary crystallographic information
Comment
Pyrazoline derivatives are of interest in many fields, such as in medicinal chemistry due to their various bioactivities i.e. antidepressant and anticonvulsant (Özdemir et al., 2007), antimicrobial (Manna & Agrawal, 2009), antiamoebic (Husain et al., 2008), analgesic and anti-inflammatory (Amir et al., 2008) properties, as well as being used as fluorescent sensors (Gong et al., 2011) and fluorescent probes (Sarkar et al., 2010). The title pyrazoline derivative (I) was synthesized because we want to modify the structure of heteroaryl chalcone derivative in order to enhance its fluorescence property, by cyclization with thiosemicarbazide. (I) possess fluorescent property as expected which will be reported elsewhere with its closely related compound (Nonthason et al., 2011).
In the molecule of the title pyrazoline derivative (Fig. 1), C17H18N4OS, the pyrazoline ring adopts envelope conformation with the puckered C8 atom having the maximum deviation of 0.1408 (13) Å, and the puckering parameter Q = 0.2236 (13) Å and φ = 77.6 (3)° (Cremer & Pople, 1975). The dihedral angle between the pyridine and benzene ring is 80.50 (6)°. The conformation of the carbothioamide unit with respect to the pyrazoline ring can be indicated by the torsion angles N1–N2–C15–N4 = 1.90 (17)° and N1–N2–C15–S1 = -177.84 (8)°. The ethoxy group is co-planar with its attached benzene ring with the torsion angle C16–O1–C12–C11 = -0.39 (17)° and C12–O1–C16–C17 = 177.61 (11)°, and an r.m.s. deviation of 0.0238 (1) Å for the nine non H atoms (C9–C14/O1/C16/C17). Bond distances of (I) are in normal range (Allen et al., 1987) and comparable with the related structures (Fun et al., 2012; Nonthason et al., 2011)
In the crystal packing (Fig. 2), the molecules are linked by N—H···O, and N—H···S hydrogen bonds into a tape along the b axis. Weak C—H···N and C—H···π interactions are also present (Table 1).
Experimental
The title compound was synthesized by cyclization reaction of E-3-(4-ethoxyphenyl)-1-(pyridin-2-yl)prop-2-en-1-one (0.25 g, 1 mmol) with excess thiosemicarbazide (0.18 g, 2 mmol) in a solution of KOH (0.11 g, 2 mmol) in ethanol (10 ml). The reaction mixture was vigorously stirred and refluxed for 5 h. The pale-yellow solid of the title compound obtained after cooling of the reaction was then filtered off under vacuum. Pale yellow block-shaped single crystals of the title compound suitable for X-ray structure determination were recrystallized from ethanol by slow evaporation of the solvent at room temperature after several days (m.p. 480–481 K).
Refinement
Amide H atoms were located in a difference maps and refined isotropically. The remaining H atoms were positioned geometrically and allowed to ride on their parent atoms, with C—H = 0.95 Å for aromatic, 1.00 Å for CH, 0.99 Å for CH2 and 0.98 Å for CH3 atoms. The Uiso values were constrained to be 1.5Ueq of the carrier atom for methyl H atoms and 1.2Ueq for the remaining H atoms. A rotating group model was used for the methyl groups.
Figures
Fig. 1.
The molecular structure of the title compound, showing 60% probability displacement ellipsoids and the atom-numbering scheme.
Fig. 2.
A packing diagram of the title compound viewed along the c axis. For the sake of clarity, only H atoms involved in the hydrogen bonds were shown. The hydrogen bonds were drawn as dashed lines.
Crystal data
| C17H18N4OS | F(000) = 688 |
| Mr = 326.42 | Dx = 1.320 Mg m−3 |
| Monoclinic, P21/c | Melting point = 480–481 K |
| Hall symbol: -P 2ybc | Mo Kα radiation, λ = 0.71073 Å |
| a = 13.4622 (1) Å | Cell parameters from 4784 reflections |
| b = 9.4175 (1) Å | θ = 1.6–30.0° |
| c = 13.3002 (2) Å | µ = 0.21 mm−1 |
| β = 103.146 (1)° | T = 100 K |
| V = 1642.01 (3) Å3 | Block, pale yellow |
| Z = 4 | 0.34 × 0.22 × 0.20 mm |
Data collection
| Bruker APEXII CCD area-detector diffractometer | 4784 independent reflections |
| Radiation source: sealed tube | 3970 reflections with I > 2σ(I) |
| Graphite monochromator | Rint = 0.028 |
| φ and ω scans | θmax = 30.0°, θmin = 1.6° |
| Absorption correction: multi-scan (SADABS; Bruker, 2005) | h = −18→18 |
| Tmin = 0.934, Tmax = 0.960 | k = −13→11 |
| 23465 measured reflections | l = −18→13 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.040 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.097 | H atoms treated by a mixture of independent and constrained refinement |
| S = 1.03 | w = 1/[σ2(Fo2) + (0.0417P)2 + 0.7316P] where P = (Fo2 + 2Fc2)/3 |
| 4784 reflections | (Δ/σ)max = 0.001 |
| 217 parameters | Δρmax = 0.45 e Å−3 |
| 0 restraints | Δρmin = −0.26 e Å−3 |
Special details
| Experimental. The crystal was placed in the cold stream of an Oxford Cryosystems Cobra open-flow nitrogen cryostat (Cosier & Glazer, 1986) operating at 120.0 (1) K. |
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > 2sigma(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| S1 | 0.59440 (2) | 0.80604 (3) | 1.06339 (3) | 0.01960 (9) | |
| O1 | 0.68026 (7) | 0.21264 (9) | 0.77613 (7) | 0.01893 (19) | |
| N1 | 0.79666 (8) | 0.91705 (11) | 0.90593 (8) | 0.0162 (2) | |
| N2 | 0.74574 (8) | 0.84262 (11) | 0.96929 (8) | 0.0159 (2) | |
| N3 | 1.05784 (8) | 0.87566 (12) | 0.89934 (8) | 0.0186 (2) | |
| N4 | 0.61973 (9) | 1.00933 (12) | 0.93150 (9) | 0.0214 (2) | |
| H1N4 | 0.5594 (14) | 1.0488 (19) | 0.9404 (13) | 0.033 (5)* | |
| H2N4 | 0.6522 (13) | 1.0458 (19) | 0.8925 (14) | 0.032 (5)* | |
| C1 | 1.12851 (10) | 0.92690 (15) | 0.85186 (10) | 0.0216 (3) | |
| H1A | 1.1969 | 0.8950 | 0.8749 | 0.026* | |
| C2 | 1.10730 (10) | 1.02328 (15) | 0.77156 (10) | 0.0228 (3) | |
| H2A | 1.1596 | 1.0559 | 0.7399 | 0.027* | |
| C3 | 1.00762 (10) | 1.07148 (15) | 0.73821 (10) | 0.0211 (3) | |
| H3A | 0.9908 | 1.1381 | 0.6833 | 0.025* | |
| C4 | 0.93292 (9) | 1.02150 (14) | 0.78583 (10) | 0.0187 (2) | |
| H4A | 0.8643 | 1.0528 | 0.7644 | 0.022* | |
| C5 | 0.96155 (9) | 0.92381 (13) | 0.86619 (9) | 0.0159 (2) | |
| C6 | 0.88757 (9) | 0.86558 (13) | 0.92109 (9) | 0.0153 (2) | |
| C7 | 0.90988 (9) | 0.75117 (13) | 1.00217 (9) | 0.0162 (2) | |
| H7A | 0.9520 | 0.7875 | 1.0681 | 0.019* | |
| H7B | 0.9445 | 0.6689 | 0.9789 | 0.019* | |
| C8 | 0.80072 (9) | 0.71273 (13) | 1.01217 (9) | 0.0147 (2) | |
| H8A | 0.7983 | 0.6995 | 1.0862 | 0.018* | |
| C9 | 0.76061 (9) | 0.58201 (13) | 0.94880 (9) | 0.0142 (2) | |
| C10 | 0.68335 (9) | 0.58700 (13) | 0.85977 (9) | 0.0167 (2) | |
| H10A | 0.6505 | 0.6749 | 0.8388 | 0.020* | |
| C11 | 0.65281 (9) | 0.46572 (13) | 0.80034 (10) | 0.0179 (2) | |
| H11A | 0.5992 | 0.4710 | 0.7401 | 0.022* | |
| C12 | 0.70147 (9) | 0.33741 (13) | 0.83010 (9) | 0.0159 (2) | |
| C13 | 0.77894 (10) | 0.32974 (13) | 0.91996 (10) | 0.0184 (2) | |
| H13A | 0.8118 | 0.2418 | 0.9409 | 0.022* | |
| C14 | 0.80746 (9) | 0.45060 (13) | 0.97800 (9) | 0.0180 (2) | |
| H14A | 0.8600 | 0.4447 | 1.0391 | 0.022* | |
| C15 | 0.65479 (9) | 0.89184 (13) | 0.98368 (9) | 0.0159 (2) | |
| C16 | 0.60157 (10) | 0.21507 (15) | 0.68243 (11) | 0.0246 (3) | |
| H16A | 0.6181 | 0.2859 | 0.6337 | 0.029* | |
| H16B | 0.5352 | 0.2406 | 0.6977 | 0.029* | |
| C17 | 0.59640 (12) | 0.06844 (16) | 0.63618 (12) | 0.0306 (3) | |
| H17A | 0.5425 | 0.0655 | 0.5728 | 0.046* | |
| H17B | 0.5814 | −0.0008 | 0.6857 | 0.046* | |
| H17C | 0.6620 | 0.0453 | 0.6200 | 0.046* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| S1 | 0.02002 (15) | 0.01758 (16) | 0.02402 (17) | 0.00250 (12) | 0.01091 (12) | 0.00250 (12) |
| O1 | 0.0221 (4) | 0.0132 (4) | 0.0194 (4) | −0.0009 (3) | 0.0005 (3) | −0.0023 (3) |
| N1 | 0.0165 (5) | 0.0138 (5) | 0.0194 (5) | −0.0013 (4) | 0.0066 (4) | 0.0001 (4) |
| N2 | 0.0172 (5) | 0.0121 (5) | 0.0197 (5) | 0.0011 (4) | 0.0070 (4) | 0.0016 (4) |
| N3 | 0.0160 (5) | 0.0194 (6) | 0.0208 (5) | 0.0001 (4) | 0.0048 (4) | 0.0000 (4) |
| N4 | 0.0191 (5) | 0.0181 (6) | 0.0301 (6) | 0.0044 (4) | 0.0119 (5) | 0.0068 (5) |
| C1 | 0.0161 (6) | 0.0240 (7) | 0.0258 (7) | 0.0004 (5) | 0.0072 (5) | −0.0014 (5) |
| C2 | 0.0230 (6) | 0.0245 (7) | 0.0236 (6) | −0.0037 (5) | 0.0114 (5) | −0.0013 (5) |
| C3 | 0.0248 (6) | 0.0207 (6) | 0.0180 (6) | −0.0025 (5) | 0.0054 (5) | 0.0018 (5) |
| C4 | 0.0179 (6) | 0.0186 (6) | 0.0193 (6) | −0.0007 (5) | 0.0032 (4) | 0.0007 (5) |
| C5 | 0.0165 (5) | 0.0145 (6) | 0.0171 (5) | −0.0017 (4) | 0.0046 (4) | −0.0023 (4) |
| C6 | 0.0158 (5) | 0.0136 (6) | 0.0165 (5) | −0.0012 (4) | 0.0036 (4) | −0.0016 (4) |
| C7 | 0.0158 (5) | 0.0145 (6) | 0.0179 (6) | 0.0005 (4) | 0.0032 (4) | 0.0004 (5) |
| C8 | 0.0159 (5) | 0.0125 (6) | 0.0162 (5) | 0.0012 (4) | 0.0044 (4) | 0.0006 (4) |
| C9 | 0.0143 (5) | 0.0130 (6) | 0.0163 (5) | −0.0002 (4) | 0.0055 (4) | 0.0007 (4) |
| C10 | 0.0152 (5) | 0.0139 (6) | 0.0206 (6) | 0.0023 (4) | 0.0033 (4) | 0.0012 (5) |
| C11 | 0.0158 (5) | 0.0168 (6) | 0.0193 (6) | 0.0013 (5) | 0.0001 (4) | −0.0005 (5) |
| C12 | 0.0165 (5) | 0.0141 (6) | 0.0181 (6) | −0.0021 (4) | 0.0059 (4) | −0.0010 (5) |
| C13 | 0.0209 (6) | 0.0129 (6) | 0.0204 (6) | 0.0020 (5) | 0.0026 (5) | 0.0025 (5) |
| C14 | 0.0207 (6) | 0.0161 (6) | 0.0154 (5) | 0.0008 (5) | 0.0005 (4) | 0.0022 (5) |
| C15 | 0.0161 (5) | 0.0129 (6) | 0.0188 (6) | −0.0004 (4) | 0.0043 (4) | −0.0032 (4) |
| C16 | 0.0219 (6) | 0.0227 (7) | 0.0254 (7) | −0.0008 (5) | −0.0027 (5) | −0.0057 (5) |
| C17 | 0.0315 (7) | 0.0262 (8) | 0.0311 (8) | −0.0015 (6) | 0.0010 (6) | −0.0125 (6) |
Geometric parameters (Å, º)
| S1—C15 | 1.6822 (13) | C7—C8 | 1.5483 (16) |
| O1—C12 | 1.3726 (15) | C7—H7A | 0.9900 |
| O1—C16 | 1.4406 (15) | C7—H7B | 0.9900 |
| N1—C6 | 1.2889 (15) | C8—C9 | 1.5201 (17) |
| N1—N2 | 1.3906 (14) | C8—H8A | 1.0000 |
| N2—C15 | 1.3628 (15) | C9—C10 | 1.3878 (16) |
| N2—C8 | 1.4756 (15) | C9—C14 | 1.4032 (17) |
| N3—C1 | 1.3454 (16) | C10—C11 | 1.3966 (17) |
| N3—C5 | 1.3492 (16) | C10—H10A | 0.9500 |
| N4—C15 | 1.3341 (17) | C11—C12 | 1.3885 (17) |
| N4—H1N4 | 0.925 (18) | C11—H11A | 0.9500 |
| N4—H2N4 | 0.825 (18) | C12—C13 | 1.3979 (17) |
| C1—C2 | 1.3810 (19) | C13—C14 | 1.3799 (18) |
| C1—H1A | 0.9500 | C13—H13A | 0.9500 |
| C2—C3 | 1.3902 (19) | C14—H14A | 0.9500 |
| C2—H2A | 0.9500 | C16—C17 | 1.5069 (19) |
| C3—C4 | 1.3871 (17) | C16—H16A | 0.9900 |
| C3—H3A | 0.9500 | C16—H16B | 0.9900 |
| C4—C5 | 1.3964 (18) | C17—H17A | 0.9800 |
| C4—H4A | 0.9500 | C17—H17B | 0.9800 |
| C5—C6 | 1.4685 (16) | C17—H17C | 0.9800 |
| C6—C7 | 1.5056 (17) | ||
| C12—O1—C16 | 117.61 (10) | N2—C8—H8A | 111.0 |
| C6—N1—N2 | 107.28 (10) | C9—C8—H8A | 111.0 |
| C15—N2—N1 | 119.74 (10) | C7—C8—H8A | 111.0 |
| C15—N2—C8 | 127.98 (10) | C10—C9—C14 | 117.94 (11) |
| N1—N2—C8 | 112.27 (9) | C10—C9—C8 | 123.27 (11) |
| C1—N3—C5 | 117.10 (11) | C14—C9—C8 | 118.69 (10) |
| C15—N4—H1N4 | 118.9 (11) | C9—C10—C11 | 121.48 (11) |
| C15—N4—H2N4 | 119.8 (12) | C9—C10—H10A | 119.3 |
| H1N4—N4—H2N4 | 121.3 (16) | C11—C10—H10A | 119.3 |
| N3—C1—C2 | 123.70 (12) | C12—C11—C10 | 119.43 (11) |
| N3—C1—H1A | 118.2 | C12—C11—H11A | 120.3 |
| C2—C1—H1A | 118.2 | C10—C11—H11A | 120.3 |
| C1—C2—C3 | 118.44 (12) | O1—C12—C11 | 124.56 (11) |
| C1—C2—H2A | 120.8 | O1—C12—C13 | 115.40 (11) |
| C3—C2—H2A | 120.8 | C11—C12—C13 | 120.03 (11) |
| C4—C3—C2 | 119.40 (12) | C14—C13—C12 | 119.62 (11) |
| C4—C3—H3A | 120.3 | C14—C13—H13A | 120.2 |
| C2—C3—H3A | 120.3 | C12—C13—H13A | 120.2 |
| C3—C4—C5 | 118.07 (12) | C13—C14—C9 | 121.48 (11) |
| C3—C4—H4A | 121.0 | C13—C14—H14A | 119.3 |
| C5—C4—H4A | 121.0 | C9—C14—H14A | 119.3 |
| N3—C5—C4 | 123.29 (11) | N4—C15—N2 | 115.59 (11) |
| N3—C5—C6 | 114.95 (11) | N4—C15—S1 | 124.18 (9) |
| C4—C5—C6 | 121.75 (11) | N2—C15—S1 | 120.24 (9) |
| N1—C6—C5 | 120.60 (11) | O1—C16—C17 | 107.17 (11) |
| N1—C6—C7 | 114.13 (10) | O1—C16—H16A | 110.3 |
| C5—C6—C7 | 125.17 (10) | C17—C16—H16A | 110.3 |
| C6—C7—C8 | 100.97 (9) | O1—C16—H16B | 110.3 |
| C6—C7—H7A | 111.6 | C17—C16—H16B | 110.3 |
| C8—C7—H7A | 111.6 | H16A—C16—H16B | 108.5 |
| C6—C7—H7B | 111.6 | C16—C17—H17A | 109.5 |
| C8—C7—H7B | 111.6 | C16—C17—H17B | 109.5 |
| H7A—C7—H7B | 109.4 | H17A—C17—H17B | 109.5 |
| N2—C8—C9 | 111.92 (10) | C16—C17—H17C | 109.5 |
| N2—C8—C7 | 100.10 (9) | H17A—C17—H17C | 109.5 |
| C9—C8—C7 | 111.48 (10) | H17B—C17—H17C | 109.5 |
| C6—N1—N2—C15 | 167.71 (11) | C6—C7—C8—C9 | 97.86 (11) |
| C6—N1—N2—C8 | −13.34 (13) | N2—C8—C9—C10 | 1.69 (16) |
| C5—N3—C1—C2 | −0.8 (2) | C7—C8—C9—C10 | −109.50 (13) |
| N3—C1—C2—C3 | 0.6 (2) | N2—C8—C9—C14 | 178.00 (10) |
| C1—C2—C3—C4 | −0.2 (2) | C7—C8—C9—C14 | 66.81 (14) |
| C2—C3—C4—C5 | 0.03 (19) | C14—C9—C10—C11 | −0.20 (18) |
| C1—N3—C5—C4 | 0.56 (19) | C8—C9—C10—C11 | 176.13 (11) |
| C1—N3—C5—C6 | −179.50 (11) | C9—C10—C11—C12 | −0.76 (18) |
| C3—C4—C5—N3 | −0.21 (19) | C16—O1—C12—C11 | −0.39 (17) |
| C3—C4—C5—C6 | 179.86 (11) | C16—O1—C12—C13 | −179.50 (11) |
| N2—N1—C6—C5 | −179.01 (10) | C10—C11—C12—O1 | −177.82 (11) |
| N2—N1—C6—C7 | −2.46 (14) | C10—C11—C12—C13 | 1.26 (18) |
| N3—C5—C6—N1 | 170.77 (11) | O1—C12—C13—C14 | 178.37 (11) |
| C4—C5—C6—N1 | −9.29 (18) | C11—C12—C13—C14 | −0.79 (18) |
| N3—C5—C6—C7 | −5.37 (17) | C12—C13—C14—C9 | −0.19 (19) |
| C4—C5—C6—C7 | 174.57 (12) | C10—C9—C14—C13 | 0.68 (18) |
| N1—C6—C7—C8 | 15.72 (14) | C8—C9—C14—C13 | −175.83 (11) |
| C5—C6—C7—C8 | −167.92 (11) | N1—N2—C15—N4 | 1.90 (17) |
| C15—N2—C8—C9 | 82.65 (15) | C8—N2—C15—N4 | −176.86 (11) |
| N1—N2—C8—C9 | −96.19 (11) | N1—N2—C15—S1 | −177.84 (8) |
| C15—N2—C8—C7 | −159.15 (12) | C8—N2—C15—S1 | 3.40 (17) |
| N1—N2—C8—C7 | 22.01 (12) | C12—O1—C16—C17 | 177.61 (11) |
| C6—C7—C8—N2 | −20.67 (11) |
Hydrogen-bond geometry (Å, º)
Cg1 and Cg2 are the centroids of the C1–C5/N1 and C9–C14 rings, respectively.
| D—H···A | D—H | H···A | D···A | D—H···A |
| N4—H1N4···S1i | 0.925 (19) | 2.472 (19) | 3.3803 (12) | 167.3 (15) |
| N4—H2N4···O1ii | 0.825 (18) | 2.296 (18) | 3.0604 (15) | 154.2 (17) |
| C3—H3A···N3iii | 0.95 | 2.51 | 3.4053 (18) | 156 |
| C2—H2A···Cg2iii | 0.95 | 2.67 | 3.4401 (14) | 138 |
| C7—H7A···Cg1iv | 0.99 | 2.69 | 3.4841 (13) | 138 |
Symmetry codes: (i) −x+1, −y+2, −z+2; (ii) x, y+1, z; (iii) −x+2, y+1/2, −z+3/2; (iv) −x+2, −y+2, −z+2.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: IS5069).
References
- Allen, F. H., Kennard, O., Watson, D. G., Brammer, L., Orpen, A. G. & Taylor, R. (1987). J. Chem. Soc. Perkin Trans. 2, pp. S1–19.
- Amir, M., Kumar, H. & Khan, S. A. (2008). Bioorg. Med. Chem. Lett. 18, 918–922. [DOI] [PubMed]
- Bruker (2005). APEX2, SAINT and SADABS Bruker AXS Inc., Madison, Wisconsin, USA.
- Cosier, J. & Glazer, A. M. (1986). J. Appl. Cryst. 19, 105–107.
- Cremer, D. & Pople, J. A. (1975). J. Am. Chem. Soc. 97, 1354–1358.
- Fun, H.-K., Suwunwong, T. & Chantrapromma, S. (2012). Acta Cryst E68, o259–o260. [DOI] [PMC free article] [PubMed]
- Gong, Z.-L., Zhao, B.-X., Liu, W.-Y. & Lv, H.-S. (2011). J. Photochem. Photobiol. A, 218, 6–10.
- Husain, K., Abid, M. & Azam, A. (2008). Eur. J. Med. Chem. 43, 393–403. [DOI] [PubMed]
- Manna, K. & Agrawal, Y. K. (2009). Bioorg. Med. Chem. Lett. 19, 2688–2692. [DOI] [PubMed]
- Nonthason, P., Suwunwong, T., Chantrapromma, S. & Fun, H.-K. (2011). Acta Cryst. E67, o3501–o3502. [DOI] [PMC free article] [PubMed]
- Özdemir, Z., Kandilci, H. B., Gümüşel, B., Çaliş, Ü. & Bilgin, A. A. (2007). Eur. J. Med. Chem. 42, 373–379. [DOI] [PubMed]
- Sarkar, A., Mandal, T. K., Rana, D. K., Dhar, S., Chall, S. & Bhattacharya, S. C. (2010). J. Lumin. 130, 2271–2276.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Spek, A. L. (2009). Acta Cryst. D65, 148–155. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S1600536812006642/is5069sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536812006642/is5069Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536812006642/is5069Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report




