Abstract
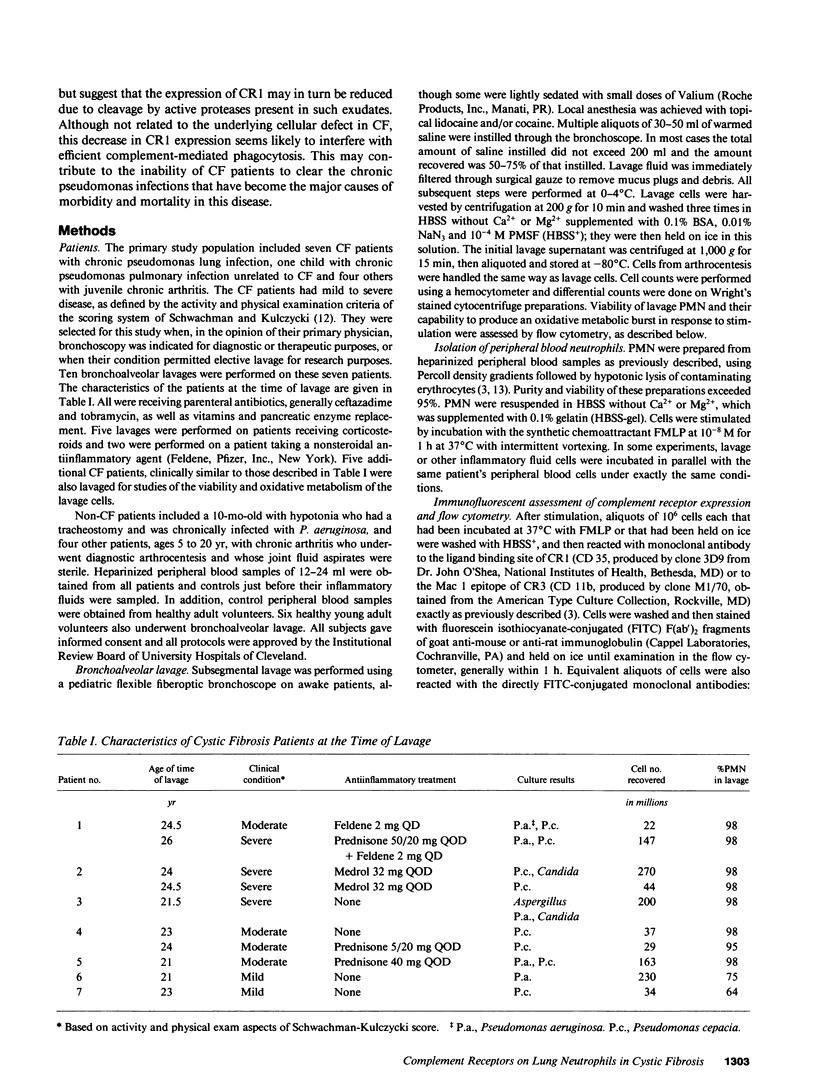
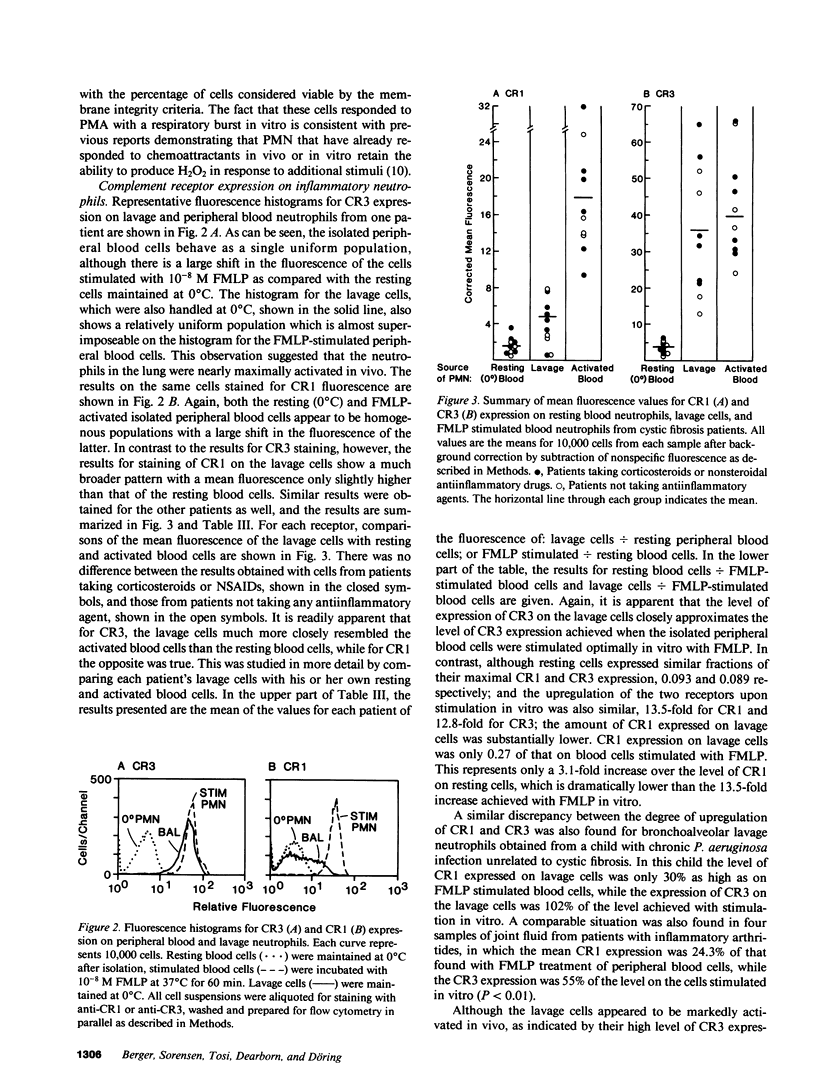
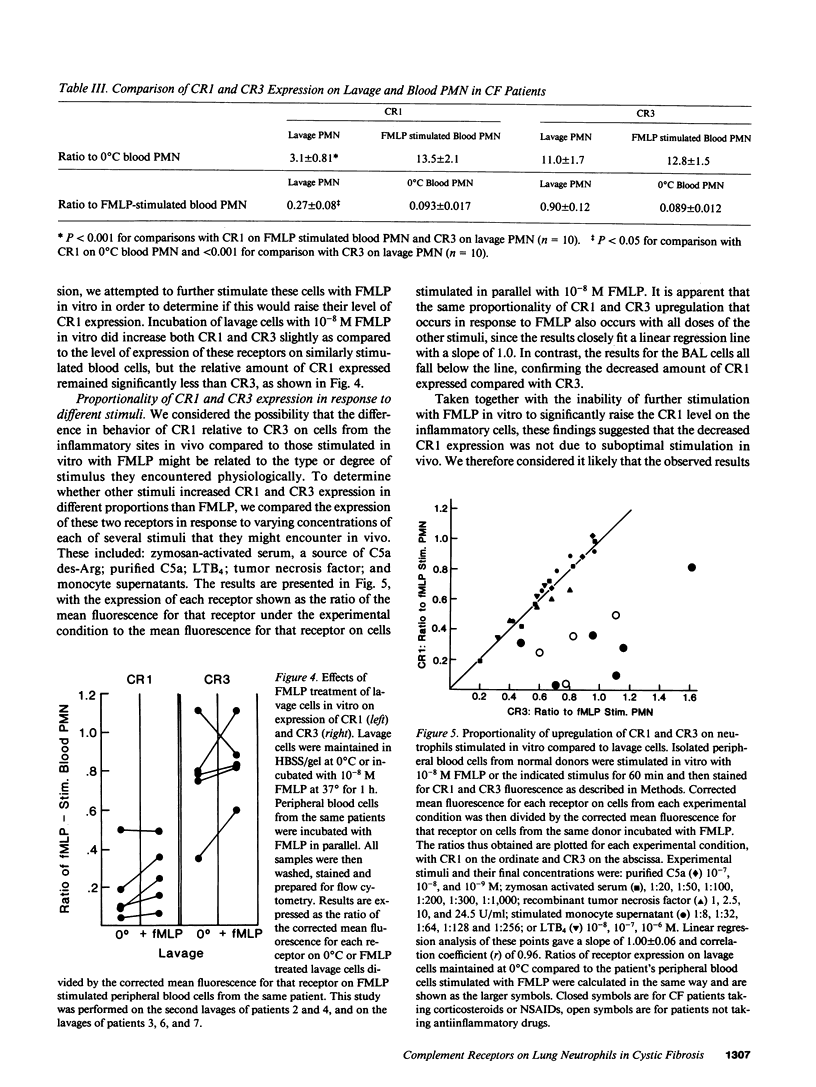
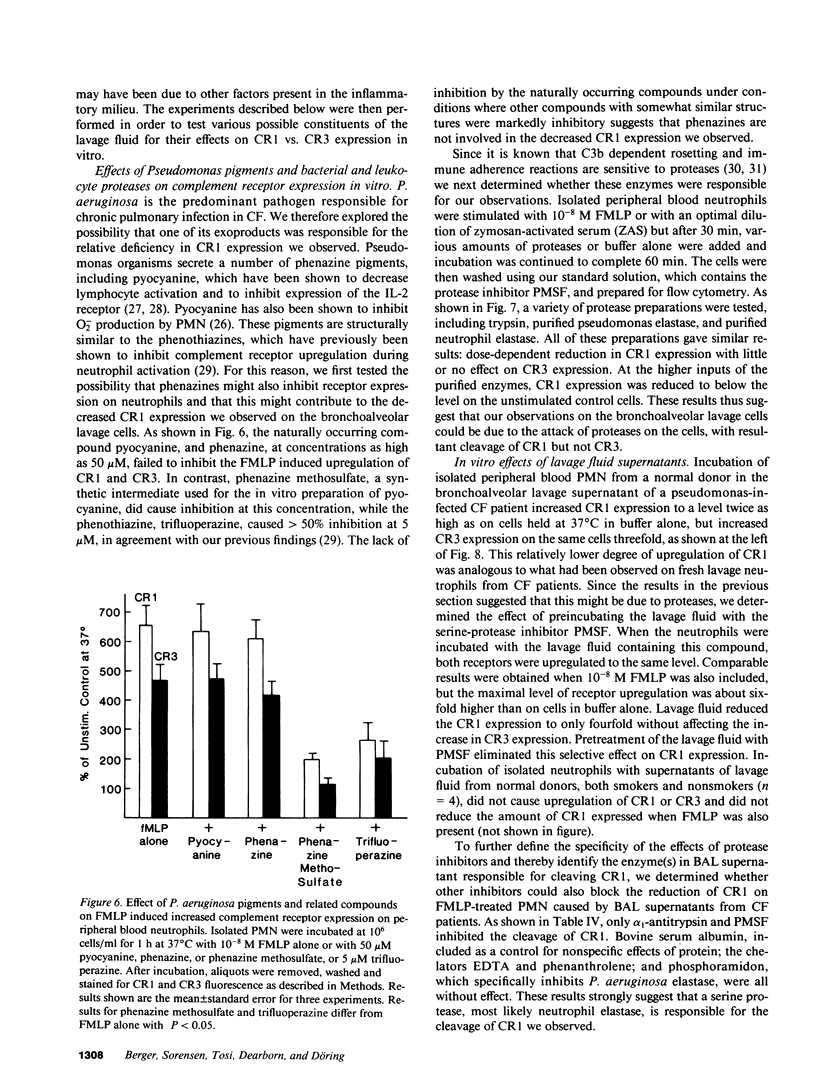
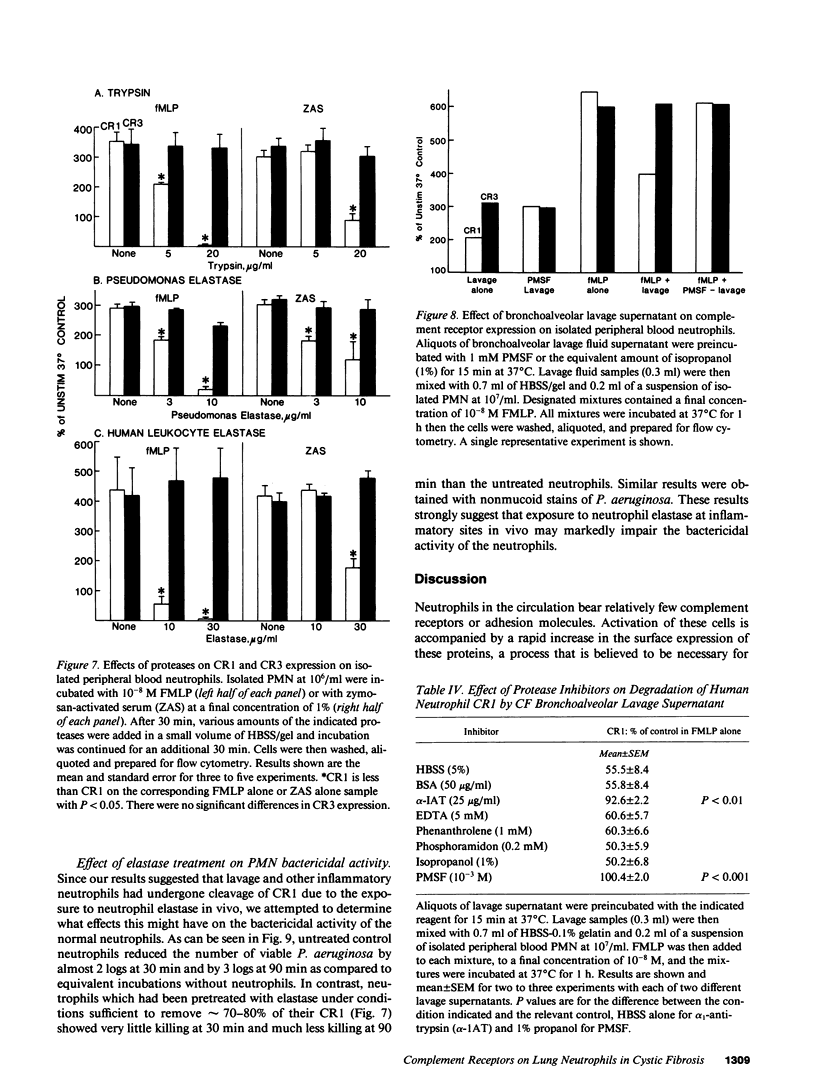
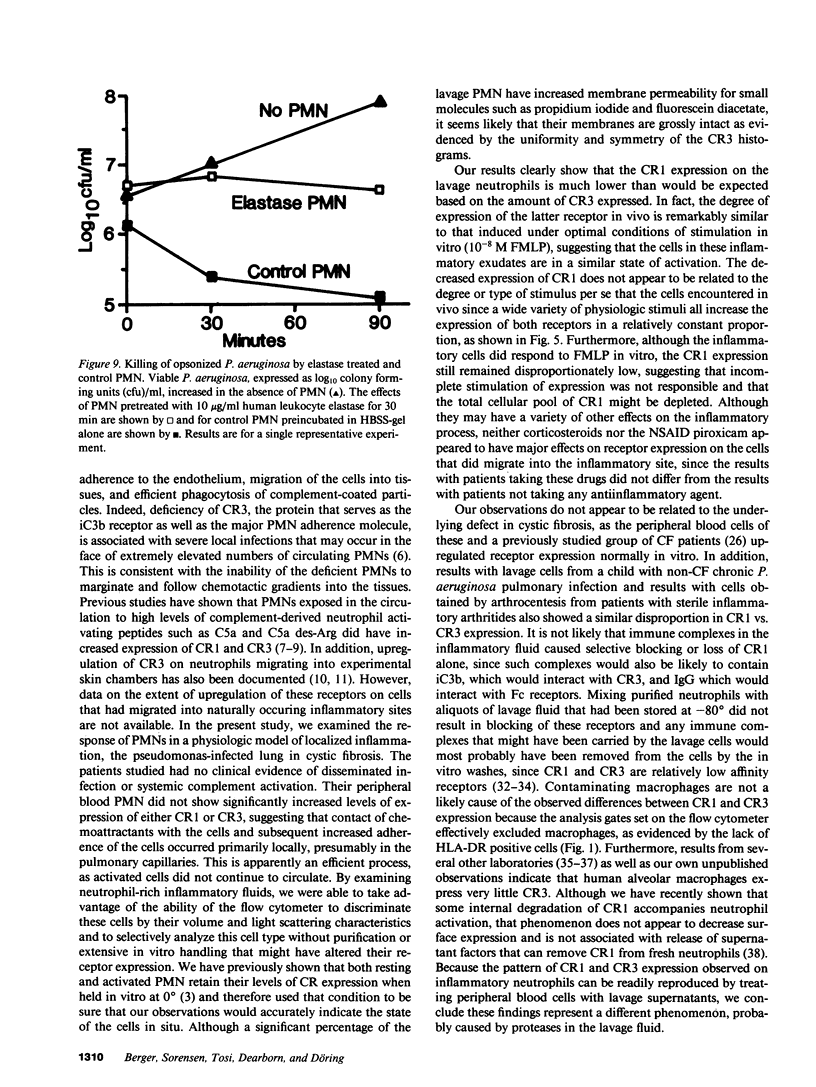
Activation of human neutrophils (PMN) is accompanied by rapid upregulation of CR1, the C3b receptor, and CR3, the iC3b receptor, which also serves as the PMN's major adherence protein. This is necessary for migration and phagocytosis, but the extent of expression of these proteins on PMN at inflammatory sites has not been determined. We used monoclonal antibodies and flow cytometry to assess CR1 and CR3 expression on PMN in bronchoalveolar lavage (BAL) fluid of cystic fibrosis (CF) patients chronically infected with pseudomonas and in sterile joint fluid of arthritis patients. Resting peripheral blood PMN from these patients and normals expressed similar low levels of CR1 and CR3, and the patients' PMN increased CR1 and CR3 expression normally when stimulated in vitro. CR3 expression on CF BAL PMN was 90 +/- 12% of that on the same patient's blood cells stimulated in vitro with FMLP. In contrast, CR1 expression on BAL PMN was only 27 +/- 8% of that on stimulated blood cells. Similar results were obtained for joint PMN. This pattern could be reproduced in vitro by treating FMLP-stimulated blood cells with BAL supernatants or with pseudomonas or PMN elastase. The serine protease inhibitors, PMSF and alpha 1-antitrypsin prevented the lavage supernatant from reducing CR1 expression, while metalloprotease inhibitors had no effect. Treatment of PMN with elastase in vitro decrease their ability to kill opsonized Pseudomonas aeruginosa. These results suggest that PMN at inflammatory sites have maximally upregulated expression of their complement receptors, but that CR1 is then cleaved by proteolysis in situ. Although not related to the basic defect in CF, this may interfere with efficient phagocytosis and contribute to the CF patient's inability to eradicate chronic lung infection.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnaout M. A., Hakim R. M., Todd R. F., 3rd, Dana N., Colten H. R. Increased expression of an adhesion-promoting surface glycoprotein in the granulocytopenia of hemodialysis. N Engl J Med. 1985 Feb 21;312(8):457–462. doi: 10.1056/NEJM198502213120801. [DOI] [PubMed] [Google Scholar]
- Arnaout M. A., Melamed J., Tack B. F., Colten H. R. Characterization of the human complement (c3b) receptor with a fluid phase C3b dimer. J Immunol. 1981 Oct;127(4):1348–1354. [PubMed] [Google Scholar]
- Barrett A. J. Leukocyte elastase. Methods Enzymol. 1981;80(Pt 100):581–588. doi: 10.1016/s0076-6879(81)80046-8. [DOI] [PubMed] [Google Scholar]
- Bass D. A., Parce J. W., Dechatelet L. R., Szejda P., Seeds M. C., Thomas M. Flow cytometric studies of oxidative product formation by neutrophils: a graded response to membrane stimulation. J Immunol. 1983 Apr;130(4):1910–1917. [PubMed] [Google Scholar]
- Berger M., Birx D. L., Wetzler E. M., O'Shea J. J., Brown E. J., Cross A. S. Calcium requirements for increased complement receptor expression during neutrophil activation. J Immunol. 1985 Aug;135(2):1342–1348. [PubMed] [Google Scholar]
- Berger M., Cross A. S. Lymphoblastoid cell supernatants increase expression of C3b receptors on human polymorphonuclear leucocytes: direct binding studies with 125I-C3b. Immunology. 1984 Mar;51(3):431–440. [PMC free article] [PubMed] [Google Scholar]
- Berger M., Gaither I. A., Frank M. M. Complement receptors. Clin Immunol Rev. 1981;1(4):471–545. [PubMed] [Google Scholar]
- Berger M., O'Shea J., Cross A. S., Folks T. M., Chused T. M., Brown E. J., Frank M. M. Human neutrophils increase expression of C3bi as well as C3b receptors upon activation. J Clin Invest. 1984 Nov;74(5):1566–1571. doi: 10.1172/JCI111572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggar W. D., Holmes B., Good R. A. Opsonic defect in patients with cystic fibrosis of the pancreas. Proc Natl Acad Sci U S A. 1971 Aug;68(8):1716–1719. doi: 10.1073/pnas.68.8.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce M. C., Poncz L., Klinger J. D., Stern R. C., Tomashefski J. F., Jr, Dearborn D. G. Biochemical and pathologic evidence for proteolytic destruction of lung connective tissue in cystic fibrosis. Am Rev Respir Dis. 1985 Sep;132(3):529–535. doi: 10.1164/arrd.1985.132.3.529. [DOI] [PubMed] [Google Scholar]
- Craddock P. R., Fehr J., Dalmasso A. P., Brighan K. L., Jacob H. S. Hemodialysis leukopenia. Pulmonary vascular leukostasis resulting from complement activation by dialyzer cellophane membranes. J Clin Invest. 1977 May;59(5):879–888. doi: 10.1172/JCI108710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Döring G., Goldstein W., Botzenhart K., Kharazmi A., Schiøtz P. O., Høiby N., Dasgupta M. Elastase from polymorphonuclear leucocytes: a regulatory enzyme in immune complex disease. Clin Exp Immunol. 1986 Jun;64(3):597–605. [PMC free article] [PubMed] [Google Scholar]
- Ehlenberger A. G., Nussenzweig V. The role of membrane receptors for C3b and C3d in phagocytosis. J Exp Med. 1977 Feb 1;145(2):357–371. doi: 10.1084/jem.145.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon D. T., Collins L. A. Increased expression of C3b receptors on polymorphonuclear leukocytes induced by chemotactic factors and by purification procedures. J Immunol. 1983 Jan;130(1):370–375. [PubMed] [Google Scholar]
- Fearon D. T. Identification of the membrane glycoprotein that is the C3b receptor of the human erythrocyte, polymorphonuclear leukocyte, B lymphocyte, and monocyte. J Exp Med. 1980 Jul 1;152(1):20–30. doi: 10.1084/jem.152.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez H. N., Hugli T. E. Partial characterization of human C5a anaphylatoxin. I. Chemical description of the carbohydrate and polypeptide prtions of human C5a. J Immunol. 1976 Nov;117(5 Pt 1):1688–1694. [PubMed] [Google Scholar]
- Fick R. B., Jr, Naegel G. P., Matthay R. A., Reynolds H. Y. Cystic fibrosis pseudomonas opsonins. Inhibitory nature in an in vitro phagocytic assay. J Clin Invest. 1981 Oct;68(4):899–914. doi: 10.1172/JCI110345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fick R. B., Jr, Naegel G. P., Squier S. U., Wood R. E., Gee J. B., Reynolds H. Y. Proteins of the cystic fibrosis respiratory tract. Fragmented immunoglobulin G opsonic antibody causing defective opsonophagocytosis. J Clin Invest. 1984 Jul;74(1):236–248. doi: 10.1172/JCI111407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freyer D. R., Morganroth M. L., Rogers C. E., Arnaout M. A., Todd R. F., 3rd Modulation of surface CD11/CD18 glycoproteins (Mo1, LFA-1, p150,95) by human mononuclear phagocytes. Clin Immunol Immunopathol. 1988 Feb;46(2):272–283. doi: 10.1016/0090-1229(88)90189-4. [DOI] [PubMed] [Google Scholar]
- Goldstein W., Döring G. Lysosomal enzymes from polymorphonuclear leukocytes and proteinase inhibitors in patients with cystic fibrosis. Am Rev Respir Dis. 1986 Jul;134(1):49–56. doi: 10.1164/arrd.1986.134.1.49. [DOI] [PubMed] [Google Scholar]
- Gordon D. L., Johnson G. M., Hostetter M. K. Characteristics of iC3b binding to human polymorphonuclear leucocytes. Immunology. 1987 Apr;60(4):553–558. [PMC free article] [PubMed] [Google Scholar]
- Harbeck R. J., Hoffman A. A., Redecker S., Biundo T., Kurnick J. The isolation and functional activity of polymorphonuclear leukocytes and lymphocytes separated from whole blood on a single percoll density gradient. Clin Immunol Immunopathol. 1982 Jun;23(3):682–690. doi: 10.1016/0090-1229(82)90331-2. [DOI] [PubMed] [Google Scholar]
- Jackson A. H., Hill S. L., Afford S. C., Stockley R. A. Sputum sol-phase proteins and elastase activity in patients with cystic fibrosis. Eur J Respir Dis. 1984 Feb;65(2):114–124. [PubMed] [Google Scholar]
- Kay A. B., Glass E. J., Salter D. M. Leucoattractants enhance complement receptors on human phagocytic cells. Clin Exp Immunol. 1979 Nov;38(2):294–299. [PMC free article] [PubMed] [Google Scholar]
- Knight M., Hartman P. E., Hartman Z., Young V. M. A new method of preparation of pyocyanin and demonstration of an unusual bacterial sensitivity. Anal Biochem. 1979 May;95(1):19–23. doi: 10.1016/0003-2697(79)90179-9. [DOI] [PubMed] [Google Scholar]
- Lampson L. A., Levy R. Two populations of Ia-like molecules on a human B cell line. J Immunol. 1980 Jul;125(1):293–299. [PubMed] [Google Scholar]
- Lay W. H., Nussenzweig V. Receptors for complement of leukocytes. J Exp Med. 1968 Nov 1;128(5):991–1009. doi: 10.1084/jem.128.5.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Hakim R. M., Fearon D. T. Increased expression of the C3b receptor by neutrophils and complement activation during haemodialysis. Clin Exp Immunol. 1984 Apr;56(1):205–214. [PMC free article] [PubMed] [Google Scholar]
- MANDL I., KELLER S., COHEN B. Microbial elastases. A comparative study. Proc Soc Exp Biol Med. 1962 Apr;109:923–925. doi: 10.3181/00379727-109-27379. [DOI] [PubMed] [Google Scholar]
- Martodam R. R., Baugh R. J., Twumasi D. Y., Liener I. E. A rapid procedure for the large scale purification of elastase and cathepsin G from human sputum. Prep Biochem. 1979;9(1):15–31. doi: 10.1080/00327487908061669. [DOI] [PubMed] [Google Scholar]
- Miller K. M., Dearborn D. G., Sorensen R. U. In vitro effect of synthetic pyocyanine on neutrophil superoxide production. Infect Immun. 1987 Mar;55(3):559–563. doi: 10.1128/iai.55.3.559-563.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller L. J., Bainton D. F., Borregaard N., Springer T. A. Stimulated mobilization of monocyte Mac-1 and p150,95 adhesion proteins from an intracellular vesicular compartment to the cell surface. J Clin Invest. 1987 Aug;80(2):535–544. doi: 10.1172/JCI113102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore F. D., Jr, Davis C., Rodrick M., Mannick J. A., Fearon D. T. Neutrophil activation in thermal injury as assessed by increased expression of complement receptors. N Engl J Med. 1986 Apr 10;314(15):948–953. doi: 10.1056/NEJM198604103141503. [DOI] [PubMed] [Google Scholar]
- Murphey S. A., Root R. K., Schreiber A. D. The role of antibody and complement in phagocytosis by rabbit alveolar macrophages. J Infect Dis. 1979 Dec;140(6):896–903. doi: 10.1093/infdis/140.6.896. [DOI] [PubMed] [Google Scholar]
- Mühlradt P. F., Tsai H., Conradt P. Effects of pyocyanine, a blue pigment from Pseudomonas aeruginosa, on separate steps of T cell activation: interleukin 2 (IL 2) production, IL 2 receptor formation, proliferation and induction of cytolytic activity. Eur J Immunol. 1986 Apr;16(4):434–440. doi: 10.1002/eji.1830160421. [DOI] [PubMed] [Google Scholar]
- Nishino N., Powers J. C. Pseudomonas aeruginosa elastase. Development of a new substrate, inhibitors, and an affinity ligand. J Biol Chem. 1980 Apr 25;255(8):3482–3486. [PubMed] [Google Scholar]
- Nutman J., Berger M., Chase P. A., Dearborn D. G., Miller K. M., Waller R. L., Sorensen R. U. Studies on the mechanism of T cell inhibition by the Pseudomonas aeruginosa phenazine pigment pyocyanine. J Immunol. 1987 May 15;138(10):3481–3487. [PubMed] [Google Scholar]
- O'Shea J. J., Brown E. J., Seligmann B. E., Metcalf J. A., Frank M. M., Gallin J. I. Evidence for distinct intracellular pools of receptors for C3b and C3bi in human neutrophils. J Immunol. 1985 Apr;134(4):2580–2587. [PubMed] [Google Scholar]
- Obernesser H. J., Döring G., Botzenhart K. Extrazelluläre Toxine von Pseudomonas aeruginosa. I. Reinigung und Charakterisierung zweier Exoproteasen. Zentralbl Bakteriol A. 1981 Mar;249(1):76–88. [PubMed] [Google Scholar]
- Phillips J. H., Babcock G. F. NKP-15: a monoclonal antibody reactive against purified human natural killer cells and granulocytes. Immunol Lett. 1983 Mar;6(3):143–149. doi: 10.1016/0165-2478(83)90096-2. [DOI] [PubMed] [Google Scholar]
- Quie P. G., White J. G., Holmes B., Good R. A. In vitro bactericidal capacity of human polymorphonuclear leukocytes: diminished activity in chronic granulomatous disease of childhood. J Clin Invest. 1967 Apr;46(4):668–679. doi: 10.1172/JCI105568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripoche J., Sim R. B. Loss of complement receptor type 1 (CR1) on ageing of erythrocytes. Studies of proteolytic release of the receptor. Biochem J. 1986 May 1;235(3):815–821. doi: 10.1042/bj2350815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHWACHMAN H., KULCZYCKI L. L. Long-term study of one hundred five patients with cystic fibrosis; studies made over a five- to fourteen-year period. AMA J Dis Child. 1958 Jul;96(1):6–15. doi: 10.1001/archpedi.1958.02060060008002. [DOI] [PubMed] [Google Scholar]
- Shalit M., von Allmen C., Atkins P. C., Zweiman B. Increased expression of CR3 (C3bi receptor) on neutrophils in human inflammatory skin reactions. J Clin Immunol. 1987 Nov;7(6):456–462. doi: 10.1007/BF00915055. [DOI] [PubMed] [Google Scholar]
- Suter S., Schaad U. B., Roux L., Nydegger U. E., Waldvogel F. A. Granulocyte neutral proteases and Pseudomonas elastase as possible causes of airway damage in patients with cystic fibrosis. J Infect Dis. 1984 Apr;149(4):523–531. doi: 10.1093/infdis/149.4.523. [DOI] [PubMed] [Google Scholar]
- Thomassen M. J., Boxerbaum B., Demko C. A., Kuchenbrod P. J., Dearborn D. G., Wood R. E. Inhibitory effect of cystic fibrosis serum on pseudomonas phagocytosis by rabbit and human alveolar macrophages. Pediatr Res. 1979 Sep;13(9):1085–1088. doi: 10.1203/00006450-197909000-00030. [DOI] [PubMed] [Google Scholar]
- Todd R. F., 3rd, Arnaout M. A., Rosin R. E., Crowley C. A., Peters W. A., Babior B. M. Subcellular localization of the large subunit of Mo1 (Mo1 alpha; formerly gp 110), a surface glycoprotein associated with neutrophil adhesion. J Clin Invest. 1984 Oct;74(4):1280–1290. doi: 10.1172/JCI111538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosi M. F., Berger M. Functional differences between the 40 kDa and 50 to 70 kDa IgG Fc receptors on human neutrophils revealed by elastase treatment and antireceptor antibodies. J Immunol. 1988 Sep 15;141(6):2097–2103. [PubMed] [Google Scholar]
- Turner J. R., Tartakoff A. M., Berger M. Intracellular degradation of the complement C3b/C4b receptor in the absence of ligand. J Biol Chem. 1988 Apr 5;263(10):4914–4920. [PubMed] [Google Scholar]
- Wallis R. S., Fujiwara H., Ellner J. J. Direct stimulation of monocyte release of interleukin 1 by mycobacterial protein antigens. J Immunol. 1986 Jan;136(1):193–196. [PubMed] [Google Scholar]
- Zimmerli W., Seligmann B., Gallin J. I. Exudation primes human and guinea pig neutrophils for subsequent responsiveness to the chemotactic peptide N-formylmethionylleucylphenylalanine and increases complement component C3bi receptor expression. J Clin Invest. 1986 Mar;77(3):925–933. doi: 10.1172/JCI112391. [DOI] [PMC free article] [PubMed] [Google Scholar]


