Abstract
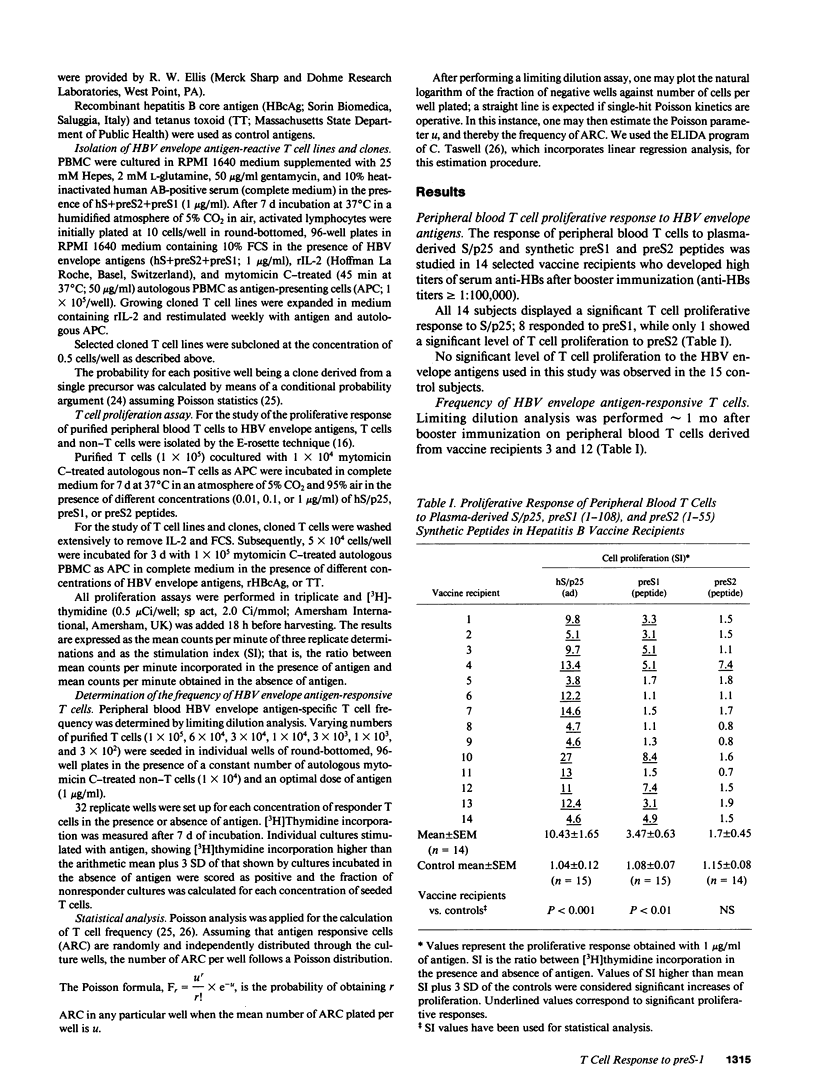
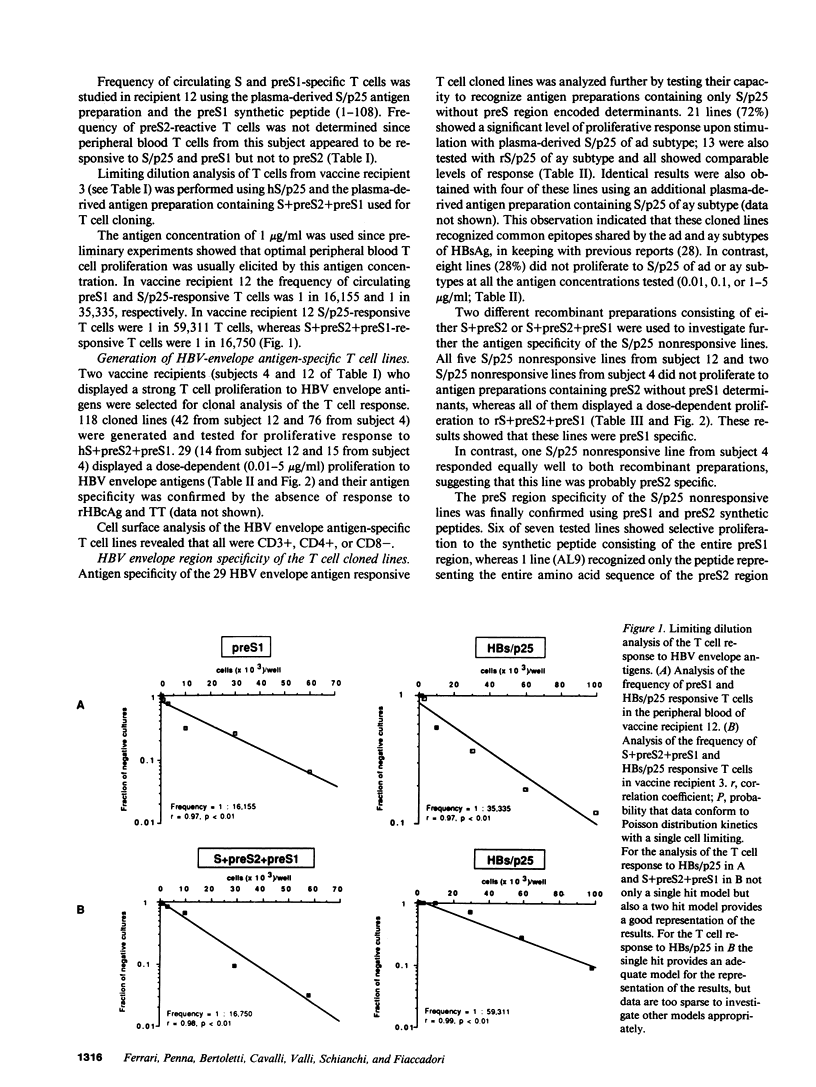
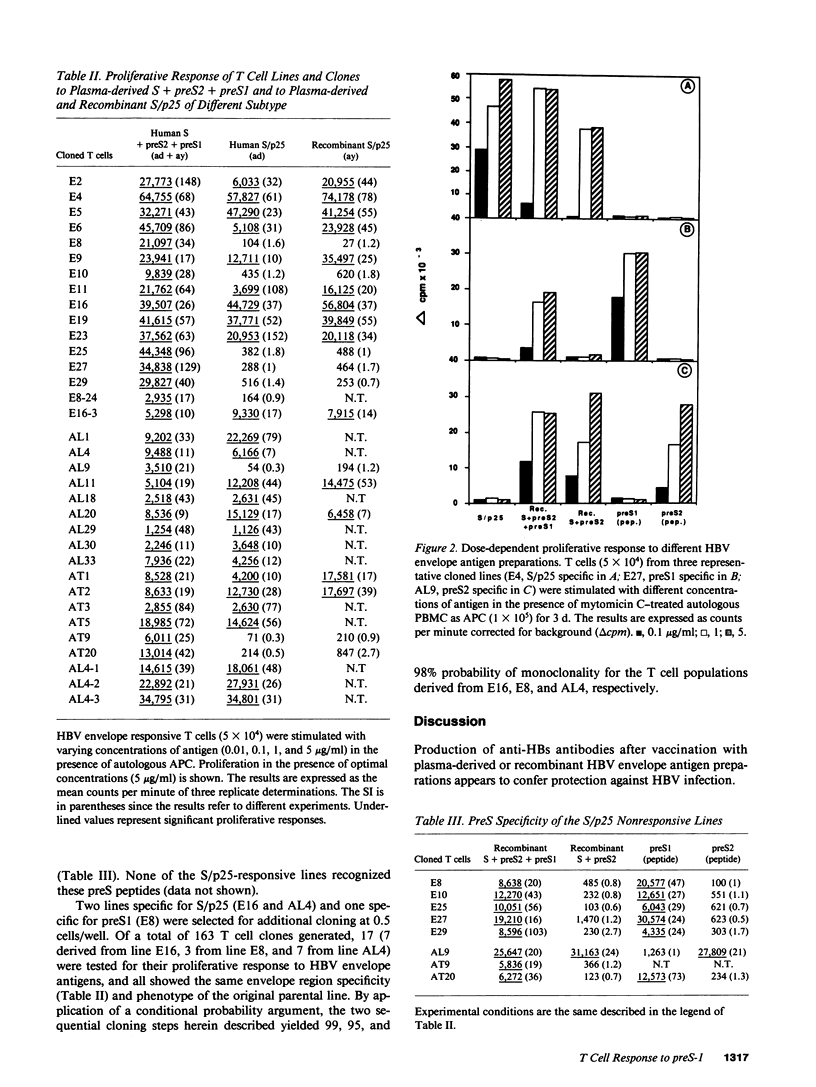
14 hepatitis B vaccine recipients who showed high titers of anti-hepatitis B surface antibodies in serum after booster immunization with a polyvalent hepatitis B surface antigen vaccine that contained trace amounts of hepatitis B virus (HBV) preS1 and preS2 envelope antigens were studied for their in vitro T cell response to these antigens. All 14 subjects displayed a significant proliferative T cell response to the S/p25 envelope region encoded polypeptide; 8 also responded to preS1, while only 1 showed a significant level of T cell proliferation to preS2. Limiting dilution analysis demonstrated that the frequency of preS-specific T cells in two of these vaccine recipients was higher than that of S/p25-specific T cells. T cell cloning was then performed and a total of 29 HBV envelope antigen-reactive CD4+ cloned lines were generated from two preS-responsive vaccines. 21 of these lines were S/p25 specific, 7 preS1 specific, and 1 preS2 specific. Taken together, all these results suggest that the preS1 antigen may function as a strong T cell immunogen in man.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberti A., Cavalletto D., Pontisso P., Chemello L., Tagariello G., Belussi F. Antibody response to pre-S2 and hepatitis B virus induced liver damage. Lancet. 1988 Jun 25;1(8600):1421–1424. doi: 10.1016/s0140-6736(88)92237-4. [DOI] [PubMed] [Google Scholar]
- Alberti A., Pontisso P., Schiavon E., Realdi G. An antibody which precipitates Dane particles in acute hepatitis type B: relation to receptor sites which bind polymerized human serum albumin on virus particles. Hepatology. 1984 Mar-Apr;4(2):220–226. doi: 10.1002/hep.1840040209. [DOI] [PubMed] [Google Scholar]
- Brett S. J., Kingston A. E., Colston M. J. Limiting dilution analysis of the human T cell response to mycobacterial antigens from BCG vaccinated individuals and leprosy patients. Clin Exp Immunol. 1987 Jun;68(3):510–520. [PMC free article] [PubMed] [Google Scholar]
- Budkowska A., Dubreuil P., Capel F., Pillot J. Hepatitis B virus pre-S gene-encoded antigenic specificity and anti-pre-S antibody: relationship between anti-pre-S response and recovery. Hepatology. 1986 May-Jun;6(3):360–368. doi: 10.1002/hep.1840060305. [DOI] [PubMed] [Google Scholar]
- Celis E., Chang T. W. Antibodies to hepatitis B surface antigen potentiate the response of human T lymphocyte clones to the same antigen. Science. 1984 Apr 20;224(4646):297–299. doi: 10.1126/science.6231724. [DOI] [PubMed] [Google Scholar]
- Celis E., Kung P. C., Chang T. W. Hepatitis B virus-reactive human T lymphocyte clones: antigen specificity and helper function for antibody synthesis. J Immunol. 1984 Mar;132(3):1511–1516. [PubMed] [Google Scholar]
- Celis E., Ou D., Otvos L., Jr Recognition of hepatitis B surface antigen by human T lymphocytes. Proliferative and cytotoxic responses to a major antigenic determinant defined by synthetic peptides. J Immunol. 1988 Mar 15;140(6):1808–1815. [PubMed] [Google Scholar]
- Ferrari C., Penna A., Giuberti T., Tong M. J., Ribera E., Fiaccadori F., Chisari F. V. Intrahepatic, nucleocapsid antigen-specific T cells in chronic active hepatitis B. J Immunol. 1987 Sep 15;139(6):2050–2058. [PubMed] [Google Scholar]
- Ferrari C., Penna A., Sansoni P., Giuberti T., Neri T. M., Chisari F. V., Fiaccadori F. Selective sensitization of peripheral blood T lymphocytes to hepatitis B core antigen in patients with chronic active hepatitis type B. Clin Exp Immunol. 1986 Dec;66(3):497–506. [PMC free article] [PubMed] [Google Scholar]
- Gebel H. M., Scott J. R., Parvin C. A., Rodey G. E. In vitro immunization to KLH. II. Limiting dilution analysis of antigen-reactive cells in primary and secondary culture. J Immunol. 1983 Jan;130(1):29–32. [PubMed] [Google Scholar]
- Itoh Y., Takai E., Ohnuma H., Kitajima K., Tsuda F., Machida A., Mishiro S., Nakamura T., Miyakawa Y., Mayumi M. A synthetic peptide vaccine involving the product of the pre-S(2) region of hepatitis B virus DNA: protective efficacy in chimpanzees. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9174–9178. doi: 10.1073/pnas.83.23.9174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y., Shih W. K., Berkower I. Human T cell response to the surface antigen of hepatitis B virus (HBsAg). Endosomal and nonendosomal processing pathways are accessible to both endogenous and exogenous antigen. J Exp Med. 1988 Jul 1;168(1):293–306. doi: 10.1084/jem.168.1.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinkert M. Q., Theilmann L., Pfaff E., Schaller H. Pre-S1 antigens and antibodies early in the course of acute hepatitis B virus infection. J Virol. 1986 May;58(2):522–525. doi: 10.1128/jvi.58.2.522-525.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kniskern P. J., Hagopian A., Burke P., Dunn N., Emini E. A., Miller W. J., Yamazaki S., Ellis R. W. A candidate vaccine for hepatitis B containing the complete viral surface protein. Hepatology. 1988 Jan-Feb;8(1):82–87. doi: 10.1002/hep.1840080117. [DOI] [PubMed] [Google Scholar]
- Koziol J. A., Ferrari C., Chisari F. V. Evaluation of monoclonality of cell lines from sequential dilution assays. J Immunol Methods. 1987 Dec 4;105(1):139–143. doi: 10.1016/0022-1759(87)90424-8. [DOI] [PubMed] [Google Scholar]
- McAleer W. J., Buynak E. B., Maigetter R. Z., Wampler D. E., Miller W. J., Hilleman M. R. Human hepatitis B vaccine from recombinant yeast. Nature. 1984 Jan 12;307(5947):178–180. doi: 10.1038/307178a0. [DOI] [PubMed] [Google Scholar]
- Milich D. R., McLachlan A., Chisari F. V., Kent S. B., Thorton G. B. Immune response to the pre-S(1) region of the hepatitis B surface antigen (HBsAg): a pre-S(1)-specific T cell response can bypass nonresponsiveness to the pre-S(2) and S regions of HBsAg. J Immunol. 1986 Jul 1;137(1):315–322. [PubMed] [Google Scholar]
- Milich D. R., Peterson D. L., Leroux-Roels G. G., Lerner R. A., Chisari F. V. Genetic regulation of the immune response to hepatitis B surface antigen (HBsAg). VI. T cell fine specificity. J Immunol. 1985 Jun;134(6):4203–4211. [PubMed] [Google Scholar]
- Milich D. R., Thornton G. B., Neurath A. R., Kent S. B., Michel M. L., Tiollais P., Chisari F. V. Enhanced immunogenicity of the pre-S region of hepatitis B surface antigen. Science. 1985 Jun 7;228(4704):1195–1199. doi: 10.1126/science.2408336. [DOI] [PubMed] [Google Scholar]
- Neurath A. R., Kent S. B., Strick N. Detection of antiviral antibodies with predetermined specificity using synthetic peptide--beta-lactamase conjugates: application to antibodies specific for the preS region of the hepatitis B virus envelope proteins. J Gen Virol. 1986 Mar;67(Pt 3):453–461. doi: 10.1099/0022-1317-67-3-453. [DOI] [PubMed] [Google Scholar]
- Neurath A. R., Kent S. B., Strick N., Parker K. Identification and chemical synthesis of a host cell receptor binding site on hepatitis B virus. Cell. 1986 Aug 1;46(3):429–436. doi: 10.1016/0092-8674(86)90663-x. [DOI] [PubMed] [Google Scholar]
- Neurath A. R., Kent S. B., Strick N., Stark D., Sproul P. Genetic restriction of immune responsiveness to synthetic peptides corresponding to sequences in the pre-S region of the hepatitis B virus (HBV) envelope gene. J Med Virol. 1985 Oct;17(2):119–125. doi: 10.1002/jmv.1890170204. [DOI] [PubMed] [Google Scholar]
- Neurath A. R., Strick N., Kent S. B., Offensperger W., Wahl S., Christman J. K., Acs G. Enzyme-linked immunoassay of pre-S gene-coded sequences in hepatitis B vaccines. J Virol Methods. 1985 Dec;12(3-4):185–192. doi: 10.1016/0166-0934(85)90128-4. [DOI] [PubMed] [Google Scholar]
- Ohnuma H., Takahashi K., Kishimoto S., Machida A., Imai M., Mishiro S., Usuda S., Oda K., Nakamura T., Miyakawa Y. Large hepatitis B surface antigen polypeptides of Dane particles with the receptor for polymerized human serum albumin. Gastroenterology. 1986 Mar;90(3):695–701. doi: 10.1016/0016-5085(86)91125-x. [DOI] [PubMed] [Google Scholar]
- Okamoto H., Usuda S., Imai M., Tachibana K., Tanaka E., Kumakura T., Itabashi M., Takai E., Tsuda F., Nakamura T. Antibody to the receptor for polymerized human serum albumin in acute and persistent infection with hepatitis B virus. Hepatology. 1986 May-Jun;6(3):354–359. doi: 10.1002/hep.1840060304. [DOI] [PubMed] [Google Scholar]
- Sohnle P. G., Collins-Lech C. Lymphocyte transformation and the number of antigen-responsive cells in humans. J Immunol. 1981 Aug;127(2):612–615. [PubMed] [Google Scholar]
- Steward M. W., Sisley B. M., Stanley C., Brown S. E., Howard C. R. Immunity to hepatitis B: analysis of antibody and cellular responses in recipients of a plasma-derived vaccine using synthetic peptides mimicking S and pre-S regions. Clin Exp Immunol. 1988 Jan;71(1):19–25. [PMC free article] [PubMed] [Google Scholar]
- Vento S., Rondanelli E. G., Ranieri S., O'Brien C. J., Williams R., Eddleston A. L. Prospective study of cellular immunity to hepatitis-B-virus antigens from the early incubation phase of acute hepatitis B. Lancet. 1987 Jul 18;2(8551):119–122. doi: 10.1016/s0140-6736(87)92329-4. [DOI] [PubMed] [Google Scholar]
- van Oers M. H., Pinkster J., Zeijlemaker W. P. Quantification of antigen-reactive cells among human T lymphocytes. Eur J Immunol. 1978 Jul;8(7):477–484. doi: 10.1002/eji.1830080706. [DOI] [PubMed] [Google Scholar]



