Abstract
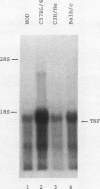
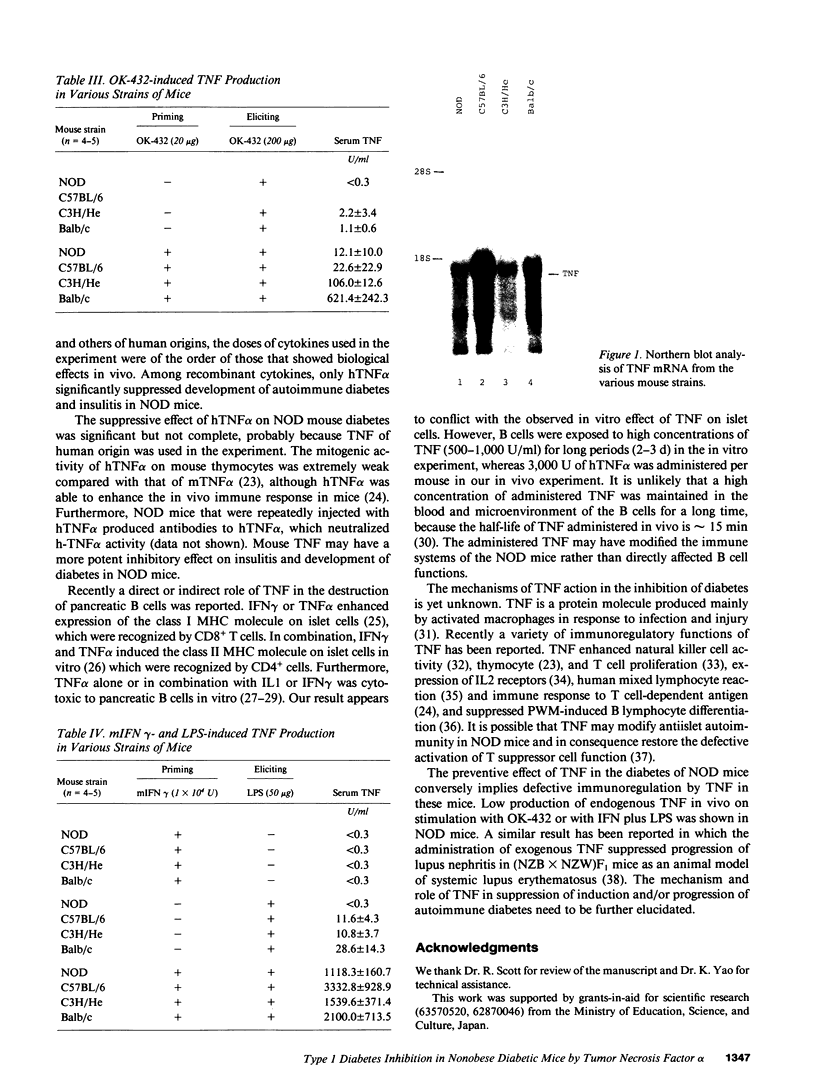
We previously reported that administration of a streptococcal preparation (OK-432) inhibited insulitis and development of autoimmune diabetes in nonobese diabetic (NOD) mice and BB rats as animals models of insulin-dependent diabetes mellitus. In this study, we screened various cytokines that could be induced by OK-432 in vivo, for their preventive effect against diabetes in NOD mice. Among recombinant mouse IFN gamma, human IL1 alpha, human IL2, mouse granulocyte-macrophage colony-stimulating factor and human TNF alpha, only human TNF alpha suppressed insulitis and significantly (P less than 0.001) inhibited development of diabetes. NOD mice were the lowest producers of the mRNA of TNF and serum TNF on stimulation with OK-432 or with IFN gamma plus LPS, compared with C57BL/6, C3H/He, and Balb/c mice. The results imply a role for low productivity of TNF in the pathogenesis of autoimmune diabetes in NOD mice.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bendelac A., Carnaud C., Boitard C., Bach J. F. Syngeneic transfer of autoimmune diabetes from diabetic NOD mice to healthy neonates. Requirement for both L3T4+ and Lyt-2+ T cells. J Exp Med. 1987 Oct 1;166(4):823–832. doi: 10.1084/jem.166.4.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutler B., Cerami A. Cachectin: more than a tumor necrosis factor. N Engl J Med. 1987 Feb 12;316(7):379–385. doi: 10.1056/NEJM198702123160705. [DOI] [PubMed] [Google Scholar]
- Blick M., Sherwin S. A., Rosenblum M., Gutterman J. Phase I study of recombinant tumor necrosis factor in cancer patients. Cancer Res. 1987 Jun 1;47(11):2986–2989. [PubMed] [Google Scholar]
- Campbell I. L., Iscaro A., Harrison L. C. IFN-gamma and tumor necrosis factor-alpha. Cytotoxicity to murine islets of Langerhans. J Immunol. 1988 Oct 1;141(7):2325–2329. [PubMed] [Google Scholar]
- Campbell I. L., Oxbrow L., West J., Harrison L. C. Regulation of MHC protein expression in pancreatic beta-cells by interferon-gamma and tumor necrosis factor-alpha. Mol Endocrinol. 1988 Feb;2(2):101–107. doi: 10.1210/mend-2-2-101. [DOI] [PubMed] [Google Scholar]
- Charlton B., Bacelj A., Mandel T. E. Administration of silica particles or anti-Lyt2 antibody prevents beta-cell destruction in NOD mice given cyclophosphamide. Diabetes. 1988 Jul;37(7):930–935. doi: 10.2337/diab.37.7.930. [DOI] [PubMed] [Google Scholar]
- Ghiara P., Boraschi D., Nencioni L., Ghezzi P., Tagliabue A. Enhancement of in vivo immune response by tumor necrosis factor. J Immunol. 1987 Dec 1;139(11):3676–3679. [PubMed] [Google Scholar]
- Harada M. Immune disturbance and pathogenesis of non-obese diabetes-prone (NOD) mice. Exp Clin Endocrinol. 1987 Aug;89(3):251–258. doi: 10.1055/s-0029-1210647. [DOI] [PubMed] [Google Scholar]
- Hattori M., Buse J. B., Jackson R. A., Glimcher L., Dorf M. E., Minami M., Makino S., Moriwaki K., Kuzuya H., Imura H. The NOD mouse: recessive diabetogenic gene in the major histocompatibility complex. Science. 1986 Feb 14;231(4739):733–735. doi: 10.1126/science.3003909. [DOI] [PubMed] [Google Scholar]
- Ikehara S., Ohtsuki H., Good R. A., Asamoto H., Nakamura T., Sekita K., Muso E., Tochino Y., Ida T., Kuzuya H. Prevention of type I diabetes in nonobese diabetic mice by allogenic bone marrow transplantation. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7743–7747. doi: 10.1073/pnas.82.22.7743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob C. O., McDevitt H. O. Tumour necrosis factor-alpha in murine autoimmune 'lupus' nephritis. Nature. 1988 Jan 28;331(6154):356–358. doi: 10.1038/331356a0. [DOI] [PubMed] [Google Scholar]
- Kashiwa H., Wright S. C., Bonavida B. Regulation of B cell maturation and differentiation. I. Suppression of pokeweed mitogen-induced B cell differentiation by tumor necrosis factor (TNF). J Immunol. 1987 Mar 1;138(5):1383–1390. [PubMed] [Google Scholar]
- Koike T., Itoh Y., Ishii T., Ito I., Takabayashi K., Maruyama N., Tomioka H., Yoshida S. Preventive effect of monoclonal anti-L3T4 antibody on development of diabetes in NOD mice. Diabetes. 1987 Apr;36(4):539–541. doi: 10.2337/diab.36.4.539. [DOI] [PubMed] [Google Scholar]
- Krangel M. S. Unusual RNA splicing generates a secreted form of HLA-A2 in a mutagenized B lymphoblastoid cell line. EMBO J. 1985 May;4(5):1205–1210. doi: 10.1002/j.1460-2075.1985.tb03761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. C., Truneh A., Smith M. F., Jr, Tsang K. Y. Induction of interleukin 2 receptor (TAC) by tumor necrosis factor in YT cells. J Immunol. 1987 Sep 15;139(6):1935–1938. [PubMed] [Google Scholar]
- Makino S., Kunimoto K., Muraoka Y., Mizushima Y., Katagiri K., Tochino Y. Breeding of a non-obese, diabetic strain of mice. Jikken Dobutsu. 1980 Jan;29(1):1–13. doi: 10.1538/expanim1978.29.1_1. [DOI] [PubMed] [Google Scholar]
- Mandrup-Poulsen T., Bendtzen K., Dinarello C. A., Nerup J. Human tumor necrosis factor potentiates human interleukin 1-mediated rat pancreatic beta-cell cytotoxicity. J Immunol. 1987 Dec 15;139(12):4077–4082. [PubMed] [Google Scholar]
- Miller B. J., Appel M. C., O'Neil J. J., Wicker L. S. Both the Lyt-2+ and L3T4+ T cell subsets are required for the transfer of diabetes in nonobese diabetic mice. J Immunol. 1988 Jan 1;140(1):52–58. [PubMed] [Google Scholar]
- Mori Y., Suko M., Okudaira H., Matsuba I., Tsuruoka A., Sasaki A., Yokoyama H., Tanase T., Shida T., Nishimura M. Preventive effects of cyclosporin on diabetes in NOD mice. Diabetologia. 1986 Apr;29(4):244–247. doi: 10.1007/BF00454884. [DOI] [PubMed] [Google Scholar]
- Nishimoto H., Kikutani H., Yamamura K., Kishimoto T. Prevention of autoimmune insulitis by expression of I-E molecules in NOD mice. 1987 Jul 30-Aug 5Nature. 328(6129):432–434. doi: 10.1038/328432a0. [DOI] [PubMed] [Google Scholar]
- Ostensen M. E., Thiele D. L., Lipsky P. E. Tumor necrosis factor-alpha enhances cytolytic activity of human natural killer cells. J Immunol. 1987 Jun 15;138(12):4185–4191. [PubMed] [Google Scholar]
- Prochazka M., Leiter E. H., Serreze D. V., Coleman D. L. Three recessive loci required for insulin-dependent diabetes in nonobese diabetic mice. Science. 1987 Jul 17;237(4812):286–289. doi: 10.1126/science.2885918. [DOI] [PubMed] [Google Scholar]
- Pujol-Borrell R., Todd I., Doshi M., Bottazzo G. F., Sutton R., Gray D., Adolf G. R., Feldmann M. HLA class II induction in human islet cells by interferon-gamma plus tumour necrosis factor or lymphotoxin. Nature. 1987 Mar 19;326(6110):304–306. doi: 10.1038/326304a0. [DOI] [PubMed] [Google Scholar]
- Pukel C., Baquerizo H., Rabinovitch A. Destruction of rat islet cell monolayers by cytokines. Synergistic interactions of interferon-gamma, tumor necrosis factor, lymphotoxin, and interleukin 1. Diabetes. 1988 Jan;37(1):133–136. doi: 10.2337/diab.37.1.133. [DOI] [PubMed] [Google Scholar]
- Ranges G. E., Zlotnik A., Espevik T., Dinarello C. A., Cerami A., Palladino M. A., Jr Tumor necrosis factor alpha/cachectin is a growth factor for thymocytes. Synergistic interactions with other cytokines. J Exp Med. 1988 Apr 1;167(4):1472–1478. doi: 10.1084/jem.167.4.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh J., Shintani S., Oya K., Tanaka S., Nobunaga T., Toyota T., Goto Y. Treatment with streptococcal preparation (OK-432) suppresses anti-islet autoimmunity and prevents diabetes in BB rats. Diabetes. 1988 Sep;37(9):1188–1194. doi: 10.2337/diab.37.9.1188. [DOI] [PubMed] [Google Scholar]
- Satoh M., Shimada Y., Inagawa H., Minagawa H., Kajikawa T., Oshima H., Abe S., Yamazaki M., Mizuno D. Priming effect of interferons and interleukin 2 on endogenous production of tumor necrosis factor in mice. Jpn J Cancer Res. 1986 Apr;77(4):342–344. [PubMed] [Google Scholar]
- Scheurich P., Thoma B., Ucer U., Pfizenmaier K. Immunoregulatory activity of recombinant human tumor necrosis factor (TNF)-alpha: induction of TNF receptors on human T cells and TNF-alpha-mediated enhancement of T cell responses. J Immunol. 1987 Mar 15;138(6):1786–1790. [PubMed] [Google Scholar]
- Serreze D. V., Leiter E. H. Defective activation of T suppressor cell function in nonobese diabetic mice. Potential relation to cytokine deficiencies. J Immunol. 1988 Jun 1;140(11):3801–3807. [PubMed] [Google Scholar]
- Shalaby M. R., Espevik T., Rice G. C., Ammann A. J., Figari I. S., Ranges G. E., Palladino M. A., Jr The involvement of human tumor necrosis factors-alpha and -beta in the mixed lymphocyte reaction. J Immunol. 1988 Jul 15;141(2):499–503. [PubMed] [Google Scholar]
- Shirai T., Shimizu N., Shiojiri S., Horiguchi S., Ito H. Cloning and expression in Escherichia coli of the gene for mouse tumor necrosis factor. DNA. 1988 Apr;7(3):193–201. doi: 10.1089/dna.1988.7.193. [DOI] [PubMed] [Google Scholar]
- Talmadge J. E., Herberman R. B. The preclinical screening laboratory: evaluation of immunomodulatory and therapeutic properties of biological response modifiers. Cancer Treat Rep. 1986 Jan;70(1):171–182. [PubMed] [Google Scholar]
- Toyota T., Satoh J., Oya K., Shintani S., Okano T. Streptococcal preparation (OK-432) inhibits development of type I diabetes in NOD mice. Diabetes. 1986 Apr;35(4):496–499. doi: 10.2337/diab.35.4.496. [DOI] [PubMed] [Google Scholar]
- Wakasugi H., Kasahara T., Minato N., Hamuro J., Miyata M., Morioka Y. In vitro potentiation of human natural killer cell activity by a streptococcal preparation, OK-432: interferon and interleukin-2 participation in the stimulation with OK-432. J Natl Cancer Inst. 1982 Oct;69(4):807–812. [PubMed] [Google Scholar]
- Wang Y., Hao L., Gill R. G., Lafferty K. J. Autoimmune diabetes in NOD mouse is L3T4 T-lymphocyte dependent. Diabetes. 1987 Apr;36(4):535–538. doi: 10.2337/diab.36.4.535. [DOI] [PubMed] [Google Scholar]
- Wicker L. S., Miller B. J., Coker L. Z., McNally S. E., Scott S., Mullen Y., Appel M. C. Genetic control of diabetes and insulitis in the nonobese diabetic (NOD) mouse. J Exp Med. 1987 Jun 1;165(6):1639–1654. doi: 10.1084/jem.165.6.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto A., Nagamuta M., Usami H., Sugawara Y., Watanabe N., Niitsu Y., Urushizaki I. Release of tumor necrosis factor (TNF) into mouse peritoneal fluids by OK-432, a streptococcal preparation. Immunopharmacology. 1986 Apr;11(2):79–86. doi: 10.1016/0162-3109(86)90027-5. [DOI] [PubMed] [Google Scholar]