Abstract
Currently, the species Bifidobacterium animalis consists of two subspecies, B. animalis subsp. lactis and B. animalis subsp. animalis. Among these two subspecies, B. animalis subsp. lactis is especially important because it is widely used in the manufacture of probiotic dairy products. The application of these microbes in the food industry demands fast, accurate and low cost methods to differentiate between species and strains. Although various genotypic methods have been employed to discriminate between these two subspecies, they are not easily adapted for rapid identification in the industry. The purpose of this study was to evaluate the use of matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS) to differentiate between the two subspecies of B. animalis, and for discrimination at strain level. We identify twenty-three strains of B. animalis at subspecies and strain level by genotypic methods and by proteomics using MALDI-TOF MS. The proteomics identification by MALDI-TOF was nearly identical to that obtained by genotypic identification using comparison of tuf and atpD genes sequences, and single-nucleotide polymorphisms (SNPs), insertions, and deletions (INDELs). We identified four protein markers, L1, L2, A1, and A2, which are useful for discriminating between both subspecies. Proteomics identification using MALDI-TOF MS was therefore an accurate method for discriminating and identifying these bacteria. Given the speed in which this method is achieved (~20 min including sample preparation), MALDI-TOF MS is promising as a tool for rapid discrimination of starter cultures and probiotics.
Keywords: Bifidobacterium animalis subsp. lactis, Bifidobacterium animalis subsp. animalis, MALDI-TOF, mass spectrometry
1. Introduction
Bifidobacteria are natural inhabitants of the human and mammalian gastrointestinal tract (GIT) and have been widely used as probiotics in food products (Sanders et al., 1996, Gomes and Malcata, 1999; Turroni et al., 2009). One bifidobacterial species of considerable importance is Bifidobacterium animalis, which contains the two subspecies, lactis and animalis. Importantly, Bifidobacterium animalis subsp. lactis is commonly used as a probiotic in dairy products and infant foods, in part, due to its tolerance of the stressful conditions of both commercial culture production and the gastrointestinal tract (Meile et al., 1997, Prasad et al., 1998, Masco et al., 2005, 2007, Mättö et al. 2006, Li et al., 2010, Zacarías et al., 2011). To guarantee the quality, safety and correct labelling of products that contain probiotic microorganisms, it is important to quickly and accurately identify the microorganisms added at species and strain level (Saarela et al., 2000).
Traditional identification of bifidobacteria based on phenotypic tools are increasingly being replaced with molecular methods offering a more rapid analysis, higher reproducibility and more accurate results (Matsuki et al., 2002, Ventura et al., 2004). In the last decade, several molecular methods have been proposed to discriminate between the two subspecies of B. animalis, subsp. animalis and subsp. lactis, e.g., ribotyping (Mättö et al., 2004), specific PCR primers Bflact2-Bflact5 (Ventura et al., 2001), comparative sequence analysis of specific genes (Ventura et al., 2003, Masco et al., 2004), and the 16S-23S ITS region (Ventura et al., 2003), multiplex PCR (Ventura et al., 2002), multilocus sequence typing (MLST) (Delétoile et al., 2010), pulsed-field gel electrophoresis (PFGE) (Ventura et al., 2002, Mayer et al., 2007), randomly amplified polymorphic DNA (RAPD) analysis (Mayer et al. 2007, Křížová et al., 2008), protein fingerprinting FAFLP (Ventura et al., 2004), and repetitive element sequences techniques: ERIC-PCR (Ventura et al. 2002), BOX-PCR (Ventura at al., 2004), and REP-PCR (Křížová et al., 2008). While some of these techniques such as PFGE or RAPD-PCR, can differentiate strains to an extent, the high degree of genomic identity among B. animalis subsp. lactis strains, makes strain identification difficult (Mayer et al., 2007, Briczinski et al., 2009). Perhaps the most promising molecular method for differentiation between B. animalis strains is MLST analysis of nine polymorphic genetic loci (Briczinski et al., 2009). Although molecular methods are commonly used to identify bacteria, tools for very rapid identification are needed.
As an alternative to molecular methods, various mass spectrometric strategies have been examined to type bacterial species and strains (Mott et al., 2010, Sědo et al., 2011, Carbonnelle, et al., 2011). Matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS) is of interest to clinical microbiologists, because of its powerful features that allow very rapid bacterial identification (taking roughly ~20 min), including clinically important microbes (Mandrell et al., 2005, Dieckmann et al., 2007, Barbuddhe et al., 2008, Benagli et al., 2011) and food microorganisms (Teramoto et al., 2007, Tanigawa et al., 2010, Albesharat et al., 2011, Angelakis et al. 2011). Moreover, this modality has been successfully applied to biomarker discovery and characterization of various bacterial agents, including bifidobacteria strains (Tanigawa et al., 2010, Sato et al., 2011).
The aim of the present work was to evaluate the accuracy and feasibility of MALDI-TOF MS to differentiate between the two subspecies of B. animalis, and for identification at strain level.
2. Material and Methods
2.1. Strains and growth conditions
A total of 23 Bifidobacterium animalis strains assigned as B. animalis subsp. lactis and B. animalis subsp. animalis were obtained from American Type Culture Collection (ATCC), Japan Collection of Microorganisms (JCM), University of California-Davis Viticulture and Enology Culture Collection (Davis, CA), University of Parma bacteria culture collection (Parma, Italy) or isolated from human faeces (Ruiz-Moyano et al. 2008) or dairy products (Table 1). To isolate B. animalis strains from dairy products, we used dairy products from seven different companies which contain B. animalis as starter culture. One g of each sample was taken aseptically, transferred to a sterile tube, 10-fold diluted with 1% sterile peptone water (Becton Dickinson, Sparks, MD), and homogenized. Serial 10-fold dilutions were prepared with 1% peptone water and inoculated on Raffinose Bifidobacterium agar (RB) prepared according to Hartemink et al. (1996). The plates were incubated 48 h at 37°C in an anaerobic chamber (Coy Laboratory Products, Grass Lake, MI). Colonies with yellow colour and 3-4 mm of diameter were streaked onto RB agar. Finally, pure isolates were grown in deMan, Rogosa, Sharpe (MRS) media (Becton Dickinson, Sparks, MD) supplemented with 0.05% L-cysteine-HCl and stored at −80°C plus 50% glycerol. Prior to the assays all bacteria strains were subcultured twice in MRS broth supplemented with 0.05% L-cysteine-HCl and incubated at 37°C for 18 h in an anaerobic chamber.
Table 1.
List of Bifidobacterium strains used in this study.
| Code | Putative Species | Additional strain informationa | Source | Speciesb |
|---|---|---|---|---|
| S149 | B. animalis subsp. lactis | Ruiz-Moyano et al. 2008 | Human faeces | B. animalis subsp. lactis |
| 252 | B. animalis subsp. lactis | University of Parma bacteria culture collection | Human faeces | B. animalis subsp. lactis |
| S306 | B. animalis subsp. lactis | Ruiz-Moyano et al. 2008 | Human faeces | B. animalis subsp. lactis |
| UCD 316 | B. animalis subsp. lactis | Isolate | Human faeces | B. animalis subsp. lactis |
| JCM 8224 | B. gallicum | ATCC 49850; DSMZ 20093 | Human faeces | B. animalis subsp. lactis |
| S332 | B. animalis subsp. lactis | Ruiz-Moyano et al. 2008 | Human faeces | B. animalis subsp. lactis |
| 358 | B. animalis subsp. lactis | University of Parma bacteria culture collection | Human faeces | B. animalis subsp. lactis |
| DSMZ 10140 | B. animalis subsp. lactis | JCM 10602 | Dairy product | B. animalis subsp. lactis |
| Y450 | B. animalis subsp. lactis | Isolate | Dairy products | B. animalis subsp. lactis |
| Y452 | B. animalis subsp. lactis | Isolate | Dairy products | B. animalis subsp. lactis |
| Y454 | B. animalis subsp. lactis | Isolate | Dairy products | B. animalis subsp. lactis |
| Y456 | B. animalis subsp. lactis | Isolate | Dairy products | B. animalis subsp. lactis |
| Y463 | B. animalis subsp. lactis | Isolate | Dairy products | B. animalis subsp. lactis |
| Y466 | B. animalis subsp. lactis | Isolate | Dairy products | B. animalis subsp. lactis |
| Y470 | B. animalis subsp. lactis | Isolate | Dairy products | B. animalis subsp. lactis |
| 533 | B. animalis subsp. lactis | University of Parma bacteria culture collection | Human faeces | B. animalis subsp. lactis |
| 672 | B. animalis subsp. lactis | University of Parma bacteria culture collection | Human faeces | B. animalis subsp. lactis |
| 892 | B. animalis subsp. lactis | University of Parma bacteria culture collection | Human faeces | B. animalis subsp. lactis |
| ATCC 27536 | B. animalis subsp. animalis | JCM 1253; DSMZ 20105 | Chicken faeces | B. animalis subsp. lactis |
| JCM 7117 | B. animalis subsp. animalis | ATCC 27674 | Rabbit faeces | B. animalis subsp. lactis |
| JCM 1190 | B. animalis subsp. animalis | ATTC 25527; DSMZ 20104 | Rat feces | B. animalis subsp. animalis |
| JCM 11658 | B. animalis subsp. animalis | CGMCC 1.3003 | - | B. animalis subsp. animalis |
| ATCC 27672 | B. animalis subsp. animalis | ATCC 27672 | Rat faeces | B. animalis subsp. animalis |
The original strain numbers are also noted, if known. JCM, Japan Collection of Microorganisms, ATCC, American Type Culture Collection; DSMZ, German Collection of Microorganisms and Cell Cultures; CGMCC, China General Microbiological Culture Collection Center.
Species determined by sequence the genes tuf and atpD.
2.2 Isolation of DNA
To get the genomic DNA 1 ml of each culture was centrifuged for 5 min at 10,000 rpm. The bacterial pellet was resuspended and incubated for 30 min at 37°C with enzymatic lysis buffer (20 mM Tris-Cl pH 8.0, 2 mM sodium EDTA, 1.2% Triton X-100, and 40 mg/ml lysozyme (Sigma, MO)), followed by an incubation with proteinase K and buffer for 30 min at 56°C. After enzymatic lysis was carried out, bacterial DNA was isolated from the samples using the DNeasy tissue kit (Qiagen, Valencia, CA) according to the instructions of the manufacturer. DNA quality and yield was checked using a Nanodrop (Wilmington, DE); the DNA was then stored at −20°C until further use.
2.3 Classification and identification of Bifidobacterium animalis strains by single-nucleotide polymorphisms (SNPs), insertions, and deletions (INDELs)
All strains were confirmed at genus and susbspecies level by using PCR amplification of genes tuf and atpD as described by Ventura and Zink (2003), and Masco et al. (2004), respectively. Moreover, to discriminate the 23 Bifidobacterium animalis at strain level, intragenic regions of ten genes containing SNPs or INDELs (igr 6, igr 8, igr 9, balat_0051, balat_0141, balat_0710, balat_0864, glcU, oxc, and INDEL 2) were selected and amplified based on a previous study by Briczinski et al. (2009). PCR amplifications were performed on an Applied Biosystem 2720m Thermal Cycler (ABI, Mountain View, CA) using the Promega GoTaq Green Master Mix. PCR reactions (50 µL) were prepared according to the manufacturer protocol, and the resulting amplicons separated on a 1% agarose gel, followed by GelRed staining (Phenix Research Products, Candler, NC), and purification using a Qiagen QIAquick PCR Purificaton Kit (Valencia, CA). Sequencing was performed on an ABI 3730 Capillary Electrophoresis Genetic Analyzer using BigDye Terminator chemistries at the University of California Sequencing Center. Sequencing data for all loci was edited using BioEdit 7.0 (http://www.mbio.ncsu.edu/BioEdit/BioEdit.html) and aligned using CLUSTAL W (Thompson et al., 1994). The sequence of the genes tuf and atpD were checked by nucleotide-nucleotide BLAST of the NCBI database (http://blast.ncbi.nlm.nih.gov/Blast.cgi) to identify the strains. With respect to the ten genes which contained SNPs or INDELs, sequences from a strain that matched the sequence from the reference strain (B. animalis subsp. lactis DSMZ 10140) were assigned letter “A”. Each unique sequence from a strain that did not matched with the reference strain was assigned a unique letter. These analyses provide an allelic profile for each strain and clustering of the strains in similar genotypes.
2.4 Sample preparation and identification by MALDI-TOF MS
MALDI-TOF mass spectrometer was performed for the 23 strains. The protocol developed by Bruker Daltonics (Bremen, Germany) was then used for the extraction of proteins, as described below.
A colony from 48 h bacterial culture on MRS agar (3 or 4 mg) was washed in 300 µl of water and additionally in 900 µl of absolute ethanol. Then the tube were centrifuged at 15000 rpm for 2 min, after which the supernatant was discarded, and the rest of ethanol was removed completely. Cells were dissolved by vortexing in 50 µl of 70% formic acid (Sigma, MO) and additionally 50 µl of acetonitrile and vortexing. The mixture was centrifuged at 15,000 rpm for 2 min to pellet the cell debris, and then the supernatant (containing the cell extract) was transferred to a new tube. One µl of the culture extract was spotted on microscout target plates and allowed to air dry, then overlaid with 1µl of matrix solution containing 1.5 mg of α-cyano-4-hydroxynamic acid in 50% acetonitrile with 2.5% trifluoroacetic acid (TFA) in H2O and dried under ambient conditions. Ten spectra for each strain were obtained and each sample was spotted in duplicate onto the MALDI target plate. The mass range was set from 2000 to 12000 m/z in positive linear mode by averaging of 200 laser shots on a MALDI-TOF MS (Microflex LRF mass spectrometer (Bruker Daltonics, Billerica, MA) analyzed by MALDI-bioTyper (MBT) software , Bruker Daltonics, GmBH, Bremen, Germany).
A cluster analysis with the m/z of the peaks list from each sample was carried out with similarity estimates by using the unweighted pair group method with arithmetic average (UPGMA), from which a dendrogram showing the relationships between Bifidobacterium animalis strains was obtained. The analysis was performed by using the NTSYS.PC package, version 2.0 (Rohlf F J. NTSYS.PC. Numerical taxonomy and multivariate analysis system, version 2.0. New York, N.Y.: Applied Biostatistics Inc.; 1993.)
3. Results and Discussion
3.1- Classifications of the strains
All the strains in the study were confirmed as Bifidobacterium animalis subsp. lactis or Bifidobacterium animalis subsp. animalis using subspecies specific primers for the genes tuf and atpD. The sequencing of these two genes has been shown to differentiate between these two subspecies by Ventura and Zink (2003) and Masco et al. (2004). Twenty of the 23 B. animalis strains had the same sequence with both genes, and were identified as subsp. lactis, including all the strains isolated from dairy products. Only three strains, JCM 1190, JCM 11658 and ATCC 27672, were identified as subsp. animalis and showed different sequence for both genes. Moreover, the identification of three strains, B. gallicum JCM 8224, B. animalis subsp. animalis ATCC 27536 and JCM 7117, do not match with the previous identification for these strains (Table 1). However, in the case of ATCC 27536 and JCM 7117 several authors have already classified these strain as subsp. lactis (Ventura and Zink, 2003; Masco et al., 2004; Kwon et al., 2005).
3.2. Identification of Bifidobacterium animalis strains by single-nucleotide polymorphisms (SNPs), insertions, and deletions (INDELs)
The 23 strains were examined by this strain-specific typing method. The analysis based on ten loci previously identified by Briczinski et al. (2009) was able to resolve the 23 strains of B. animalis into 12 different clusters (Table 2). The 20 B. animalis subsp lactis strains clustered into 9 different groups. Of these 9 groups, 5 are possibly comprised of a single strain, and four contain more than one strain. Clusters containing more than one strain may include different strains that are not differentiated with the loci examined in this work, or they may represent different isolates of the same strain. This is not unusual as all but one of the strains in multistrain clusters were isolated from dairy products and human faeces. Other authors have shown significant genetic homogeneity of the strain ATCC 27536 with other B. animalis subsp. lactis used as commercial starter strains using RAPD-PCR and PFGE approaches (Mayer et al. 2007). Interestingly, all 3 strains of B. animalis subsp. animalis gave a unique allelic profile using this method.
Table 2.
Allelic profiles of 23 B. animalis strains analyzed by single-nucleotide polymorphisms (SNPs), insertions, and deletions (INDELs).
| Clusters | Strains | Subspecies | Locus |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| igr6 | igr8 | igr9 | Balat_0051 | Balat_0141 | Balat_0710 | Balat_0864 | glcU | oxc | INDEL2 | |||
| Cluster 1 | DSMZ 10140 | lactis | A | A | A | A | A | A | A | A | A | A |
| Cluster 2 | S149, 252, 892, Y452, Y454 | lactis | A | A | B | B | A | A | A | A | A | A |
| Cluster 3 | S306, Y463, Y466, Y470 | lactis | B | B | B | B | A | A | B | B | B | B |
| Cluster 4 | UCD 316, JCM 8224, ATCC 27536 | lactis | A | A | B | B | A | C | A | A | A | A |
| Cluster 5 | S332 | lactis | A | A | B | B | A | A | A | C | A | A |
| Cluster 6 | 358, 533, 672 | lactis | A | A | B | B | B | A | A | A | A | A |
| Cluster 7 | Y450 | lactis | A | A | B | B | B | B | A | A | A | A |
| Cluster 8 | Y456 | lactis | A | A | A | B | A | A | A | D | A | A |
| Cluster 9 | JCM 7117 | lactis | B | A | B | B | A | B | B | E | A | A |
| Cluster 10 | JCM 1190 | animalis | C | C | C | B | C | D | C | F | C | C |
| Cluster 11 | JCM 11658 | animalis | D | D | D | B | D | E | D | G | D | C |
| Cluster 12 | ATCC 27672 | animalis | E | E | E | B | E | F | E | H | E | D |
In general the diversity between the strains of B. animalis subsp. lactis was low, and only two different sequence types were found for all the loci, except for glcU locus which showed the highest diversity exhibiting 5 different sequence types (Table 2). Three strains, S332, Y456 and JCM 7117, have a specific allele for the glcU locus, these results are in agreement with those determined by Briczinski et al. (2009). On the other hand, all B. animalis subsp. animalis strains have specific alleles for each locus, except for locus Balat_0051, where only two different sequence types were found, so it was the most conservative locus studied between both subspecies.
3.3 Discrimination by MALDI-TOF MS
Previous efforts have shown that MALDI-TOF analysis can detect slight sequence variations in cellular proteins, and can be useful for discriminating strains at the subspecies, or even at strain, level (Teramoto et al., 2007; Mott et al., 2010, Tanigawa et al. 2010; Sato et al., 2011). The 23 strains of B. animalis were profiled by proteomic analysis using the mass of the proteins extracted by the method described above. Sixty-one peaks were monitored in the range from m/z 2,000 to 11,000, a result which is similar to that obtained for clostridia (Mandrell et al., 2005), Listeria (Barbuddhe et al., 2008), mycobacteria (Hettick et al., 2006), Lactococcus lactis (Tanigawa et al., 2010), and other nonfermenting bacteria (Mellmann et al., 2008). Peaks with m/z 3956, 4000, 4047, 4059, 4859 and 4924 were excluded of the analysis as potential [M+2H]2+ ions of the corresponding peaks 7910, 8000, 8090, 8117, 9700 and 9850 ([M+H]+), respectively. The remaining peaks matched from 63.6%–100% (with 35 to 55 peaks matching) among the B. animalis strains. In addition, 11 (20%) and 8 (14.5%) peaks were specific for strains of subspecies animalis and lactis respectively. These results indicate that more than half of the proteins identified by MALDI-TOF MS are the same in both subspecies and reflect their close phylogenetic relationship (Ventura and Zink 2003; Masco et al., 2004). However, more than 30% of the peaks examined have different mass, and thus provide potential markers for subspecies discrimination in B. animalis. Similar results by MALDI-TOF MS were found by Tanigawa et al (2010) and Sato et al. (2011), who could differentiate the subspecies of Lactococcus lactis and Bifidobacterium longum respectively, with the comparison of the mass spectra of ribosomal proteins.
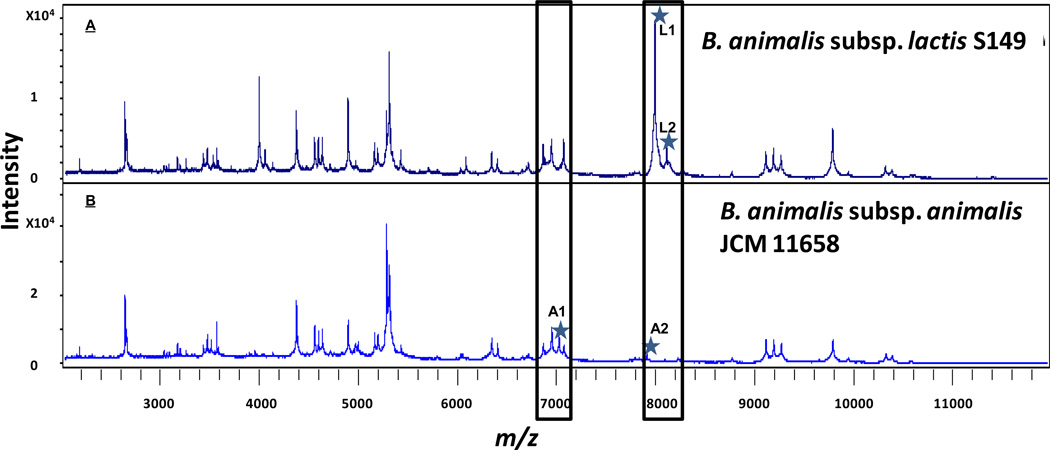
Table 3 summarizes the mass list of the 20 specific peaks found in the 23 B. animalis samples. Four peaks were identified as subspecies type-specific; two in subspecies lactis (L) and two in subspecies animalis (A), and represent potential markers for rapid identification of isolates at the subspecies level. . Figure 1 shows a comparison of two representative spectra of the strains B. animalis subsp lactis S149 (fig. 1a) and B. animalis subsp. animalis JCM 11658 (Fig. 1b) with subspecies potential biomarkers peaks designated as asterisks. Subspecies lactis type-specific biomarkers were observed at masses of 7998 and 8117 m/z (Table 3; L1, L2, respectively), whereas animalis type-specific peaks were noted at 7025 (A1) and 7910 (A2) m/z (Table 3; A1, A2 respectively). The intensity of the biomarker L1 was higher than the other three biomarkers. As a consequence peaks L1 was proved to be effective and reliable biomarker for discrimination of B. animalis subspecies. Using these MALDI-TOF MS criteria, 20 of the 23 strains were identified as B. animalis subsp. lactis and three as B. animalis subsp. animalis. This result corresponds with the identification previously determined using the genes tuf and atpD above (Table 1). Subspecies discrimination of bacteria by MALDI-TOF MS has previously been shown for streptococci (Friedrichs et al., 2007) Salmonellae (Dieckmann et al., 2008) Lactococcus lactis (Tanigawa et al., 2010) and Bifidobacterium longum (Sato et al., 2011).
Table 3.
Masses of the specific peaks observed by MALDI-TOF MS in this study
| Observed (+) or not observed (−) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mass (m/z) [M+H]+ |
Peaksa | Subsp. lactis |
Subsp. animalis |
||||||||||
| Cluster 1 | Cluster 2 | Cluster 3 | Cluster 4 | Cluster 5 | Cluster 6 | cluster 7 | Cluster 8 | Cluster 9 | Cluster 10 | Cluster 11 | Cluster 12 | ||
| 2604 | S | − | − | − | − | − | − | − | − | − | − | − | + |
| 2629 | S | − | − | − | − | − | − | − | − | − | − | − | + |
| 2684 | S | − | − | − | − | − | − | − | − | − | − | − | + |
| 4838 | S | − | − | − | + | − | − | + | − | − | − | − | − |
| 4937 | S | − | − | − | − | − | − | − | − | − | + | + | − |
| 4998 | S | − | − | − | − | − | − | − | − | − | − | + | − |
| 5300 | S | − | − | − | − | − | − | − | − | − | + | − | − |
| 5431 | S | − | + | − | + | + | + | − | − | − | − | + | − |
| 5622 | S | − | − | − | − | + | − | − | − | − | − | − | − |
| 5708 | S | − | − | − | − | + | − | − | + | − | − | − | − |
| 6052 | S | − | − | − | − | − | − | − | − | − | − | − | + |
| 6101 | S | − | − | − | − | − | − | − | − | − | + | − | − |
| 6655 | S | − | − | + | − | − | − | − | − | − | − | − | − |
| 7025 | A | − | − | − | − | − | − | − | − | − | + | + | + |
| 7910 | A | − | − | − | − | − | − | − | − | − | + | + | + |
| 7998 | L | + | + | + | + | + | + | + | + | + | − | − | − |
| 8090 | S | − | − | − | − | − | − | − | − | − | + | − | + |
| 8117 | L | + | + | + | + | + | + | + | + | + | − | − | − |
| 9721 | S | − | − | − | − | − | − | + | − | − | − | − | − |
| 9846 | S | − | − | + | + | − | − | + | − | + | − | − | − |
S, m/z strain specific peak; L (Lactis type), m/z specific for B. animalis subsp. lactis strains; A (Animalis type), m/z specific for B. animalis subsp. animalis strains.
Figure 1.
MALDI-TOF MS spectra of proteins of two Bifidobacterium animalis type strains. (a) B. animalis subsp. lactis S149, and (b) B. animalis subsp. animalis JCM 11658. The relative intensities of ions are shown on the y axis, and the m/z values of the ions are shown on the x axis. The m/z value is the mass to charge ratio. The asterisks indicate specific peaks (biomarkers) for each subspecies. The label L1 and L2 indicate subspecies lactis type-specific biomarkers, and A1 and A2 subspecies animalis type-specific biomarkers.
Eleven peaks in B. animalis subsp. animalis strains, and 8 in B. animalis subsp. lactis strains were strain-specific (Table 3). These differences in the mass of the peaks could be due to variations in the amino acid sequence of the protein(s) and are potentially useful for strain identification. To assess if strain discrimination was reliable, the whole peak list was then processed by UPGMA cluster analysis using a DICE coefficient. Figure 2 shows the resulting phylogentic tree. The samples clearly divided into two major clusters at a similarity of 82,5%. Cluster A contained all the strains of subsp. animalis, whereas cluster L contained the strains of the subsp. lactis. The three strains of subsp. animalis included in the cluster A showed similarity below 92%, and were clearly differentiated at strain level by MALDI-TOF MS. However, the strains of subsp. lactis included in cluster L showed more similar spectra, although it was possible to group the 20 subsp. lactis strains in 8 groups. These results are in agreement with our genotypic identification, which showed a greater diversity in the allelic profiling of the subsp. animalis strains than in subsp. lactis strains. Only the strains identified in cluster 2 and 6 by SNP/INDEL analysis (Table 2) could not be differentiated with MALDI-TOF MS proteomics. In general, however, the proteomics profiling by MALDI-TOF MS demonstrated a significant discriminatory power for classification of B. animalis strains as the MALDI-TOF results produced nearly the same results as the most robust molecular method used to date for identification B. animals strains (Briczinski et al., 2009).
Figure 2.
Phylogenetic tree of B. animalis strains based on protein profiling employing MALDI-TOF MS. Major clusters (A and L) are divided at a similarity of 82,5%. Cluster A contains B. animalis subsp. animalis strains, and cluster L B. animalis subsp. lactis strains.
In conclusion, we describe four potential protein biomarkers, by MALDI-TOF MS that are useful for accurate differentiation of B. animalis strains at the subspecies level. In addition, the MALDI-TOF proteomics analysis enabled differentiation of B. animalis isolates at strain level. Given that the MALDI-TOF MS methods are very fast (up to 20 min including protein extraction and sample preparation) this typing method would appear to be useful to monitor starter culture and probiotic strains (like B. animalis) in various clinical and commercial settings.
Acknowledgments
This work was supported by grants from the University of California Discovery Grant Program, the California Dairy Research Foundation and National Institutes of Health Awards R01HD059127, R21AT006180 and R01HD061923. MALDI-TOF analysis was supported by Brucker Dalctonics. Ruiz-Moyano S. was supported by the Ministry of Education and Science of Spain and University of Extremadura, Spain. We are also grateful to Marco Ventura from the University of Parma for generously providing B. animalis subsp. lactis strains and to Reviewer 1 for insightful comments/recommendations on the interpretation of our MS data.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albesharata R, Ehrmanna MA, Koraklib M, Yazajic S, Vogel RF. Phenotypic and genotypic analyses of lactic acid bacteria in local fermented food, breast milk and faeces of mothers and their babies. Syst. Appl. Microbiol. 2011;34:148–155. doi: 10.1016/j.syapm.2010.12.001. [DOI] [PubMed] [Google Scholar]
- Angelakis E, Million M, Henry M, Raoult D. Rapid and accurate bacterial indentification in probiotics and yoghurts by MALDI-TOF mass spectrometry. J. Food. Sci. 2011;76:568–572. doi: 10.1111/j.1750-3841.2011.02369.x. [DOI] [PubMed] [Google Scholar]
- Barbuddhe SB, Maier T, Schwarz G, Kostrzewa M, Hof H, Domann E, Chakraborty T, Hain T. Rapid identification and typing of Listeria species by matrix-assisted laser desorption ionization-time of flight mass spectrometry. Appl. Environ. Microbiol. 2008;74:5402–5407. doi: 10.1128/AEM.02689-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benagli C, Rossi V, Dolina M, Tonolla M, Petrini O. Matrix-assisted laser desorption ionization-time of flight mass spectrometry for the identification of clinically relevant bacteria. Plos One. 2011;6:e16424. doi: 10.1371/journal.pone.0016424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briczinski EP, Loquasto JR, Barrangou R, Dudley EG, Roberts AM, Roberts RF. Strain-Specific Genotyping of Bifidobacterium animalis subsp. lactis by Using Single-Nucleotide Polymorphisms, Insertions, and Deletions. Appl. Environ. Microbiol. 2009;75:7501–7508. doi: 10.1128/AEM.01430-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbonnelle E, Mesquita C, Bille E, Day N, Dauphin B, Beretti JL, Ferroni A, Gutmann L, Nassif X. MALDI-TOF mass spectrometry tools for bacterial identification in clinical microbiology laboratory. Clin. Biochem. 2011;44:104–109. doi: 10.1016/j.clinbiochem.2010.06.017. [DOI] [PubMed] [Google Scholar]
- Deletoile A, Passet V, Aires J, Chambaud I, Butel MJ, Smokvina T, Brisse S. Species delineation and clonal diversity in four Bifidobacterium species as revealed by multilocus sequencing. Res. Microbiol. 2010;161:82–90. doi: 10.1016/j.resmic.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Dieckmann R, Helmuth R, Erhard M, Malorny B. Rapid classification and identification of salmonellae at the species and subspecies levels by whole-cell matrix-assisted laser desorption ionization-time of flight mass spectrometry. Appl. Environ. Microbiol. 2008;74:7767–7778. doi: 10.1128/AEM.01402-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrichs C, Rodloff AC, Chhatwal GS, Schellenberger W, Eschrich K. Rapid identification of viridans streptococci by mass spectrometric discrimination. J. Clin. Microbiol. 2007;45:2392–2397. doi: 10.1128/JCM.00556-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes AMP, Malcata FX. Bifidobacterium spp and Lactobacillus acidophilus: Biological, biochemical, technological and therapeutical properties relevant for use as probiotics. Trends Food Sci. Technol. 1999;10:139–157. [Google Scholar]
- Hartemink R, Kok BJ, Weenk GH, Rombouts FM. Raffinose-Bifidobacterium (RB) agar, a new selective medium for bifidobacteria. J. Microbiol. Meth. 1996;27:33–43. [Google Scholar]
- Hettick JM, Kashon ML, Slaven JE, Ma Y, Simpson JP, Siegel PD, Mazurek G, Weissman DN. Discrimination of intact mycobacteria at the strain level: a combined MALDI-TOF MS and biostatistical analysis. Proteomics. 2006;6:6416–6425. doi: 10.1002/pmic.200600335. [DOI] [PubMed] [Google Scholar]
- Křížová J, Španová A, Rittich B. RAPD and rep-PCR fingerprinting for characterisation of Bifidobacterium species. Folia Microbiol. 2008;53:99–104. doi: 10.1007/s12223-008-0014-1. [DOI] [PubMed] [Google Scholar]
- Kwon HS, Yang EH, Lee SH, Yeon SW, Kang BH, Kim TY. Rapid identification of potentially probiotic Bifidobacterium species by multiplex PCR using species-specific primers based on the region extending from 16S rRNA through 23S rRNA. FEMS Microbiol. Lett. 2005;250:55–62. doi: 10.1016/j.femsle.2005.06.041. [DOI] [PubMed] [Google Scholar]
- Li Q, Chen Q, Ruan H, Zhu D, He G. Isolation and characterisation of an oxygen, acid and bile resistant Bifidobacterium animalis subsp. lactis Qq08. J. Sci. Food Agri. 2010;90:1340–1346. doi: 10.1002/jsfa.3942. [DOI] [PubMed] [Google Scholar]
- Mandrell RE, Harden LA, Bates A, Miller WG, Haddon WF, Fagerquist CK. Speciation of Campylobacter coli, C. jejuni, C. helveticus, C. lari, C. sputorum, and C. upsaliensis by matrix-assisted laser desorption ionization-time of flight mass spectrometry. Appl. Environ. Microbiol. 2005;71:6292–6307. doi: 10.1128/AEM.71.10.6292-6307.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masco L, Ventura M, Zink R, Huys G, Swings J. Polyphasic taxonomic analysis of Bifidobacterium animalis and Bifidobacterium lactis reveals relatedness at the subspecies level reclassification of Bifidobacterium animalis as Bifidobacterium animalis subsp. animalis subsp. nov. and Bifidobacterium lactis as Bifidobacterium animalis subsp. lactis subsp. nov. Int. J. Syst. Evol. Microbiol. 2004;54:113–143. doi: 10.1099/ijs.0.03011-0. [DOI] [PubMed] [Google Scholar]
- Masco L, Huys G, De Brandt E, Temmerman R, Swings J. Culture-dependent and culture-independent qualitative analysis of probiotic products claimed to contain bifidobacteria. Int. J. Food Microbiol. 2005;102:221–230. doi: 10.1016/j.ijfoodmicro.2004.11.018. [DOI] [PubMed] [Google Scholar]
- Masco L, Crockaert C, Van Hoorde K, Swings J, Huys G. In vitro assessment of gastrointestinal transit tolerance of taxonomic reference strains from human origin and probiotic product isolates of Bifidobacterium. J. Dairy Sci. 2007;90:3572–3578. doi: 10.3168/jds.2006-548. [DOI] [PubMed] [Google Scholar]
- Matsuki T, Watanabe K, Tanaka R. Genus-and species-specific PCR primers for the detection and identification of bifidobacteria. In: Tannock GW, editor. Probiotics and Prebiotics. Where Are We Going? Wymondham: Caister Academic Press; 2002. pp. 85–105. [Google Scholar]
- Mättö J, Malinen E, Suihko ML, Alander M, Palva A, Saarela M. Genetic heterogeneity and functional properties of intestinal bifidobacteria. J. Appl. Microbiol. 2004;97:459–470. doi: 10.1111/j.1365-2672.2004.02340.x. [DOI] [PubMed] [Google Scholar]
- Mättö J, Alakomi HL, Vaari A, Virkajärvi I, Saarela M. Influence of processing conditions on Bifidobacterium animalis subsp. lactis functionality with a special focus on acid tolerance and factors affecting it. Int. Dairy J. 2006;16:1029–1037. [Google Scholar]
- Mayer HK, Amtmann E, Philippi E, Steinegger G, Mayrhofer S, Kneifel W. Molecular discrimination of new isolates of Bifidobacterium animalis subsp. lactis from reference strains and commercial probiotic strains. Int. Dairy J. 2007;17:565–573. [Google Scholar]
- Meile L, Ludwig W, Rueger U, Gut C, Kaufmann P, Dasen G, Wenger S, Teuber M. Bifidobacterium lactis sp. nov, a moderately oxygen tolerant species isolated from fermented milk. System. Appl. Microbiol. 1997;20:57–64. [Google Scholar]
- Mellmann A, Cloud J, Maier T, Keckevoet U, Ramminger I, Iwen P, Dunn J, Hall G, Wilson D, LaSala P, Kostrzewa M, Harmsen D. Evaluation of matrix-assisted laser desorption ionization-time-of-flight mass spectrometry in comparison to 16S rRNA gene sequencing for species identification of nonfermenting bacteria. J. Clin. Microbiol. 2008;46:1946–1954. doi: 10.1128/JCM.00157-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mott MM, Elverley RA, Wyatt SA, Toney DM, Croley TR. Comparison of MALDI-TOF/MS and LC-QTOF/MS methods for the identification of enteric bacteria. Int. J. of Mass Spectrom. 2010;291:24–32. [Google Scholar]
- Prasad J, Gill H, Smart J, Gopal PK. Selection and characterisation of Lactobacillus and Bifidobacterium strains for use as probiotics. Int. Dairy J. 1998;8:993–1002. [Google Scholar]
- Ruiz-Moyano S, Martín A, Benito MJ, Pérez Nevado F, Córdoba MG. Screening of lactic acid bacteria and bifidobacteria for potential probiotic use in Iberian dry fermented sausages. Meat Sci. 2008;80:715–721. doi: 10.1016/j.meatsci.2008.03.011. [DOI] [PubMed] [Google Scholar]
- Saarela M, Mogensen G, Fondén R, Mättö J, Mattila-Sandholm T. Probiotic bacteria: safety, functional and technological properties. J. Biotechnol. 2000;84:197–215. doi: 10.1016/s0168-1656(00)00375-8. [DOI] [PubMed] [Google Scholar]
- Sanders ME, Walker DC, Walker KM, Aoyama K, Klaenhammer TR. Performance of commercial cultures in fluid milk applications. J. Dairy Sci. 1996;79:943–955. doi: 10.3168/jds.S0022-0302(96)76445-7. [DOI] [PubMed] [Google Scholar]
- Sato H, Teramoto K, Ishii Y, Watanabe K, Benno Y. Phylogenetic analysis of Bifidobacterium longum strains based on ribosomal protein profiling by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Syst. Appl. Microbiol. 2011;34:76–80. doi: 10.1016/j.syapm.2010.07.003. [DOI] [PubMed] [Google Scholar]
- Sědo O, Sedláčcek I, Zdráhal Z. Sample preparation methods for MALDI-MS profiling of bacteria. Mass Spectrom. Rev. 2011;30:417–434. doi: 10.1002/mas.20287. [DOI] [PubMed] [Google Scholar]
- Tanigawa K, Kawabata H, Watanabe K. Identification and typing of Lactococcus lactis by matrix-assisted laser desorption ionization-time of flight mass spectrometry. Appl. Environ. Microbiol. 2010;76:4055–4062. doi: 10.1128/AEM.02698-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teramoto K, Sato H, Sun L, Torimura M, Tao H. A simple intact protein analysis by MALDI-MS for characterization of ribosomal proteins of two genome-sequenced lactic acid bacteria and verification of their amino acid sequences. J. Proteome Res. 2007;6:3899–3907. doi: 10.1021/pr070218l. [DOI] [PubMed] [Google Scholar]
- Turroni F, Foroni E, Pizzetti P, Giubellini V, Ribbera A, Merusi P, Cagnasso P, Bizzarri B, de'Angelis GL, Shanahan F, van Sinderen D, Ventura M. Exploring the diversity of the bifidobacterial population in the human intestinal tract. Appl. Environ. Microbiol. 2009;75:1534–1545. doi: 10.1128/AEM.02216-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura M, Reniero R, Zink R. Specific identification and targeted characterization of Bifidobacterium lactis from different environmental isolates by a combined multiplex–PCR approach. Appl. Environ. Microbiol. 2001;67:2760–2765. doi: 10.1128/AEM.67.6.2760-2765.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacarías MF, Binetti A, Laco M, Reinheimer J, Vinderola G. Preliminary technological and potential probiotic chracterisation of bifidobacteria isolated from breast milk for use in dairy products. Int. Dairy J. 2011;21:548–555. [Google Scholar]
- Ventura M, Zink R. Rapid identification, differentiation, and proposed new taxonomic classification of Bifidobacterium lactis. Appl. Environ. Microbiol. 2002;68:6429–6434. doi: 10.1128/AEM.68.12.6429-6434.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura M, Zink R. Comparative sequence analysis of the tuf and recA genes and restriction fragment length polymorphism of the internal transcribed spacer region sequences supply additional tools for discriminating Bifidobacterium lactis from Bifidobacterium animalis. Appl. Environ. Microbiol. 2003;69:7517–7522. doi: 10.1128/AEM.69.12.7517-7522.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura M, van Sinderen D, Fitzgerald GF, Zink R. Insights into the taxonomy, genetics and physiology of bifidobacteria. Anton. van Leeuwen. 2004;86:205–223. doi: 10.1023/B:ANTO.0000047930.11029.ec. [DOI] [PubMed] [Google Scholar]