Abstract
Objective
Neuroimaging is an essential component of the acute stroke evaluation. MRI is more accurate than CT for the diagnosis of stroke, but is more costly and time consuming. We sought to describe changes in MRI utilization from 1999–2008.
Methods
We performed a serial cross-sectional study with time trends of neuroimaging in patients with a primary ICD-9-CM discharge diagnosis of stroke admitted through the Emergency Department in the State Inpatient Databases (SID) from 10 states. MRI utilization was measured by Healthcare Cost and Utilization Project (HCUP) criteria. Data were included for states from 1999–2008 where MRI utilization could be identified.
Results
624,842 patients were hospitalized for stroke in the period of interest. MRI utilization increased in all states. Overall, MRI absolute utilization increased 38 percentage points, and relative utilization increased 235% (28% of strokes in 1999 to 66% in 2008). Over the same interval, CT utilization changed little (92% in 1999 to 95% in 2008). MRI use varied widely by state. In 2008, MRI utilization ranged from a low of 55% of strokes in Oregon to a high of 79% in Arizona. Diagnostic imaging was the fastest growing component of total hospital costs (213% increase from 1999–2007).
Interpretation
MRI utilization during stroke hospitalization increased substantially with wide geographic variation. Rather than replacing CT, MRI is supplementing it. Consequently, neuroimaging has been the fastest growing component of hospitalization cost in stroke. Recent neuroimaging practices in stroke are not standardized and may represent an opportunity to improve the efficiency of stroke care.
Introduction
Neuroimaging is an essential component of acute stroke evaluation. Although MRI has several clear advantages compared to CT in stroke diagnosis, its role in acute stroke management has yet to be completely elucidated. Compared to CT, MRI is a more accurate test for acute ischemia1–5 and comparably accurate for the detection of intracerebral hemorrhage (ICH).6 Additional advantages of MRI include the improved ability to detect subtle hemorrhagic changes,7, 8 to identify stroke location,9 and in some cases to clarify stroke mechanism.10–12
Despite these advantages, downsides to MRI do exist. These include longer scan times and higher costs per scan compared to CT. In addition, there are few data demonstrating that MRI findings lead to changes in patient management versus CT alone.13 Further, no data yet demonstrate improved outcomes in stroke patients undergoing MRI compared with stroke patients who do not undergo MRI.
In this study, we sought to describe trends in MRI use over the 10-year period of 1999 to 2008 as a means of determining its changing role in routine stroke care and to assess for opportunities to optimize its use. We hypothesized that MRI utilization would increase concordant with overall trends in diagnostic imaging utilization.14 We further hypothesized that geographic variation in MRI utilization would exist because evidence-based guidelines did not preferentially recommend CT or MRI for stroke evaluation.15–17 Finally, given prominent societal concerns about health care costs, we sought to characterize costs of MRI and diagnostic imaging during stroke hospitalization. Understanding these trends is essential to the development and implementation of standardized and efficient neuroimaging strategies in stroke.
Methods
Dataset and Study Population
We examined all patients with a discharge diagnosis of stroke in a select group of states from 1999 through 2008. States were chosen to incorporate geographic, temporal and racial variability within the constraints of data availability, consistent participation in SID, data cost, and the ability to identify MRIs. Our specific study population consisted of discharge data from: Washington (1999–2008), New York (1999–2008), Arizona (1999–2008), Florida (1999–2005), Nebraska (2004–2008), Massachusetts (2004–2008), North Carolina (2004–2008), Iowa (2008), Wisconsin (2008), New Jersey (2008) and Oregon (2008).
This study analyzed hospital discharges from State Inpatient Databases (SID) developed as part of the Healthcare Cost and Utilization Project (HCUP), funded by the Agency for Healthcare Research and Quality (AHRQ).18 The SID consists of abstracts for all acute care discharges within a state for a given year. Discharge abstracts consist of basic demographics, payer information, hospitalization characteristics, charges, primary diagnoses and procedural utilization.
We included all discharges for subjects over 18 years of age in whom the primary discharge diagnosis was ischemic stroke, ICD-9-CM (433.x1, 434.x1, 436)19 admitted through the emergency department. This approach has been previously validated and found to have a positive predictive value of 88% and sensitivity of 74%.20
The University of Michigan Institutional Review Board (IRBMED) determined this study to be exempt from formal review as it relied on publicly available data.
Categorizing Comorbidities
To understand how patient-level variables may influence which patients undergo MRI, we characterized secondary diagnoses in our population of stroke patients. Vascular risk factor and potential stroke mimic diagnoses were characterized using HCUP single-level Clinical Classification System21 definitions: hypertension (CCS 98,99), diabetes (49,50), hyperlipidemia (53), epilepsy (83), migraine(84). A stroke modified Charlson co-morbidity index was created for each patient using previously described methods.22, 23
Measuring Neuroimaging exposure
We measured MRI and CT utilization using the criteria for the Healthcare Cost and Utilization Project’s (HCUP) Magnetic Resonance Technology Utilization and CT Scan flags respectively.24 These flags define whether a patient underwent any CT or MRI imaging study during their hospitalization and are primarily defined by Uniform Billing (UB-92) revenue codes. UB-92 revenue codes are standardized codes which are automatically generated by hospital billing systems and reflect charges from specific revenue centers.25 HCUP has internally validated revenue codes for the identification of specific utilization groups, including MRI and CT.26 This approach was used instead of a less reliable ICD-9-CM procedure code-alone identification strategy because ICD-9-CM strategies are unreliable for procedures that do not change Diagnosis Related Group (DRG) assignment25 and stroke DRGs are not impacted by whether patients undergo neuroimaging.
Statistical Analysis
To summarize the characteristics of the population demographic information, stroke risk factors, and neuroimaging utilization were reported with percentages or means and standard deviations. Comparisons between those receiving MRI and not receiving MRI were made using t-tests and chi-square tests for continuous and categorical variables respectively. To describe trends in neuroimaging utilization we divided the number of patients receiving a neuroimaging study by the total number of stroke cases identified, stratifying by state and year. Significance of trends was assessed using one-sample t-tests of change in imaging utilization per year to evaluate the null hypothesis that no change in utilization occurred. To account for imbalance in potentially relevant variables, adjusted estimates of MRI utilization by state and over time were generated using multivariate logistic regression accounting for clustering at the hospital level. In both analyses, we adjusted for age, gender, vascular risk factors (hypertension, diabetes, hyperlipidemia, atrial fibrillation), Charlson comorbidity index and insurance status (Medicare vs. Medicaid vs. Private Insurance vs. Other Insurance). Results were reported as mean posterior probabilities with covariates set at population means.
To estimate the availability of MRI and impact of MRI availability on utilization, hospital-level MRI utilization patterns were analyzed. To minimize the impact of miscoded data, a hospital was defined as having established MRI access if 3 or more stroke patients received MRIs at that facility in a given year. This definition will likely underestimate actual MRI access as some hospitals may have the capacity to obtain MRIs and not opt to use that capacity. We then calculated the annual proportion of stroke patients presenting to hospitals with established MRI access.
Next, we sought to quantify geographic variation at the state and Hospital Referral Region (HRR) levels by calculating aggregate MRI utilization rates at both levels by year. HRRs represent discrete geographic regions defined by regional referral patterns for tertiary medical care defined by the presence of at least one hospital that performs major cardiovascular and neurosurgical procedures.27–29 To measure annual utilization, we aggregated patient-level records at the Hospital Referral Region (HRR) level, calculated utilization over HRR, ranked the HRRs by utilization rate and then calculated utilization by HRR quintile.
Finally, we estimated inflation-adjusted costs for the total hospitalization, UB-92 cost categories, MRI and CT. Total hospital charges were abstracted directly from the SID discharge record. All charges within a given UB-92 cost center, identified by revenue code, were summed to derive charges per cost center. MRI and CT costs were calculated by summing costs associated with UB-92 revenue codes 610–619 and 0350–0359 respectively. This approach combines all CT and MRI-related costs into single categories and consequently is not able to distinguish between the costs of repeat imaging studies and of combined imaging studies such as multi-modal imaging. To account for local variation in charges, HCUP cost-to-charge ratios were applied to hospital charges to estimate hospital costs.30 As a consequence, we were able to estimate actual costs of care as opposed to the amount charged for care. Because we were interested in overall expenditure trends, costs were inflation adjusted to 2008 dollars using the GDP price index.31 For clarity of reporting, state data was aggregated into two separate time cohorts based on the availability of longitudinal data. Cohort 1 consists of data from New York, Florida, Arizona and Washington from 1999–2007 and cohort 2 consists of data from New York, Arizona, Washington, North Carolina, and Massachusetts from 2004–2008. We report costs for the five largest cost centers. All costs were summarized as means and standard deviations. All data analyses were performed with Stata version 11.1 (Stata Corp, College Station, Texas).
Results
Study Population
Over the 10-year study period (1999–2008), 624,842 primary ischemic stroke hospitalizations were identified. (Table 1) Mean age was 73 ± 14 years and 54% were female. Cardiovascular risk factors were common. Most patients (70%) were insured by Medicare.
Table 1.
Study Population Baseline Characteristics
| MRI (n=310,768) | no MRI (n=314,074) | All Subjects (n=624,842) | |
|---|---|---|---|
| Demographics | |||
| Age mean(sd) | 70 (14) | 76 (13) | 73 (14) |
| Female | 52% | 57% | 54% |
| Race/Ethnicity | |||
| White | 60% | 61% | 61% |
| Black | 7% | 7% | 7% |
| Hispanic | 14% | 12% | 13% |
| Insurance | |||
| Medicare | 64% | 75% | 70% |
| Medicaid | 8% | 6% | 7% |
| Private Insurance | 22% | 14% | 18% |
| Other Insurance | 6% | 4% | 5% |
| Comorbidities | |||
| Hypertension | 72% | 67% | 69% |
| Hyperlipidemia | 32% | 19% | 25% |
| Diabetes | 30% | 28% | 29% |
| Coronary Artery Disease | 21% | 27% | 24% |
| Atrial Fibrillation | 16% | 27% | 21% |
| Modified Charlson, mean (sd) | 3.8 (2) | 4.5 (1.9) | 4.1 (1.9) |
MRI was performed in 50% of the total population and CT in 95%. On average, patients who underwent MRI were younger (70 years vs. 76 years, p < 0.001), more likely to have private insurance (22% vs. 15%, p < 0.001) and had a greater burden of vascular risk factors compared with patients who did not undergo MRI. A history of atrial fibrillation was less common in patients who had an MRI (27% vs. 16%, p < 0.001)
Temporal Trends in Diagnostic imaging
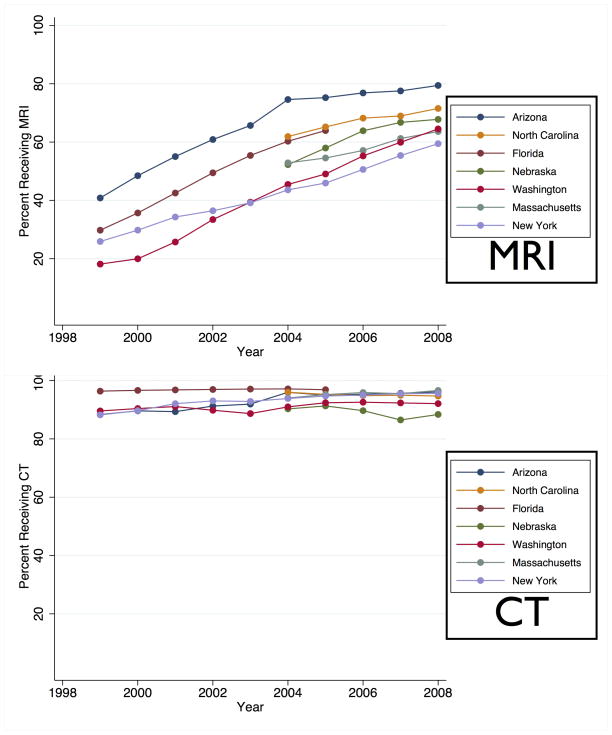
From 1999 to 2008, absolute MRI utilization increased 38 percentage points, a relative increase of 235% (28% in 1999 to 66% in 2008, p = 0.003 for trend, Figure 1). Of patients receiving an MRI, 95% also received a CT. Overall CT utilization did not change during the same interval (92% in 1999 to 95% in 2008, p = 0.34 for trend). After adjusting for the changing age, sex, vascular risk factors, comorbidities and insurance status of the population, each year was associated with a 4.0% increase the probability of receiving MRI in the logistic regression model, (OR 1.20, 95% CI 1.18–1.22).
Figure 1.
Utilization Trends in Diagnostic Imaging for Stroke
Regional Variation in Diagnostic Imaging
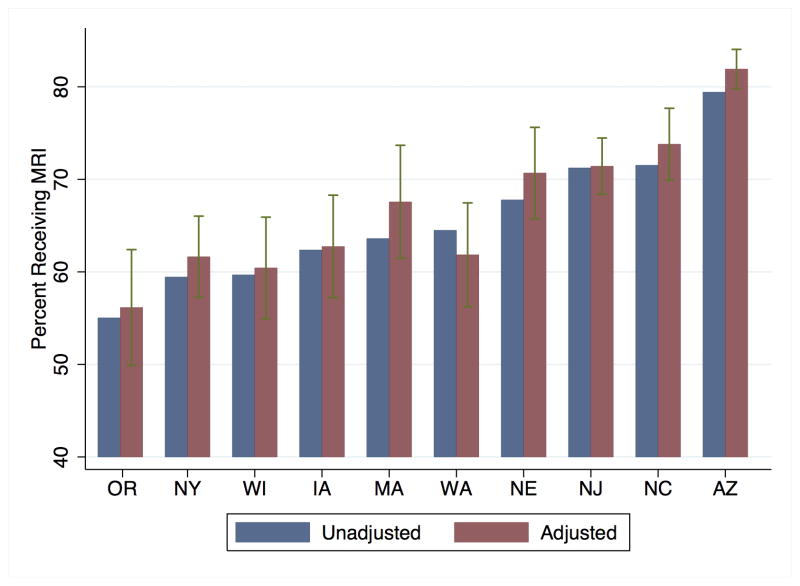
Utilization of MRI by state is illustrated in Figure 2 for 2008, the year for which the broadest cross-section of data was available. In 2008, MRI utilization ranged from a low of 55% in Oregon to a high of 79% in Arizona. Adjustment for age, sex, vascular risk factors, comorbidities and insurance status had minimal impact on the pattern of variation by state. More extreme geographic variation is seen when analyzing MRI utilization by HRR quintile (Table 2). In every year, MRI utilization rates were at least 30 percentage points higher in the highest utilizing quintile compared to the lowest utilizing quintile.
Figure 2. Percent of Stroke Patients Receiving MRI, 2008.
Legend: Dark gray bars represent unadjusted utilization by state. Light gray bars represent utilization adjusted for age, sex, vascular risk factors, comorbidities and insurance status.
Table 2.
MRI Utilization Quintiles by year, defined by Hospital Referral Region (HRR)
| Percent Receiving MRI by HRR utilization Quintile | |||||
|---|---|---|---|---|---|
| Year | 1 (lowest utilizers) | 2 | 3 | 4 | 5 (highest utilizers) |
| 1999 | 7% | 17% | 23% | 29% | 39% |
| 2000 | 10% | 21% | 27% | 35% | 50% |
| 2001 | 17% | 28% | 35% | 42% | 55% |
| 2002 | 22% | 31% | 41% | 51% | 63% |
| 2003 | 23% | 36% | 45% | 57% | 66% |
| 2004 | 31% | 45% | 51% | 64% | 72% |
| 2005 | 34% | 47% | 55% | 66% | 74% |
| 2006 | 34% | 52% | 55% | 62% | 77% |
| 2007 | 36% | 55% | 60% | 66% | 77% |
| 2008 | 43% | 58% | 64% | 70% | 78% |
Established Access to MRI
There was a modest overall increase in the number of hospitals with established MRI access – 74% of hospitals had access in 1999 vs. 87% in 2008 (p< 0.01) (Table 3). The hospitals that gained MRI access over time were mostly lower volume centers, consequently there was less change in the percentage of stroke patients presenting to hospitals with MRI access — 89% in 1999 vs. 96% in 2008 (p < 0.01). This suggests that physician practice regarding MRI is more important than the availability of MRI scanners per se to explain the rising rates of MRI utilization.
Table 3.
Hospital availability of MRI
| Year | % of Hospitals with MRI access | % of Stroke Patients at Hospital with MRI access |
|---|---|---|
| 1999 | 74% | 89% |
| 2000 | 77% | 91% |
| 2001 | 80% | 93% |
| 2002 | 83% | 94% |
| 2003 | 82% | 94% |
| 2004 | 83% | 94% |
| 2005 | 86% | 95% |
| 2006 | 83% | 94% |
| 2007 | 84% | 94% |
| 2008 | 87% | 96% |
Cost Trends
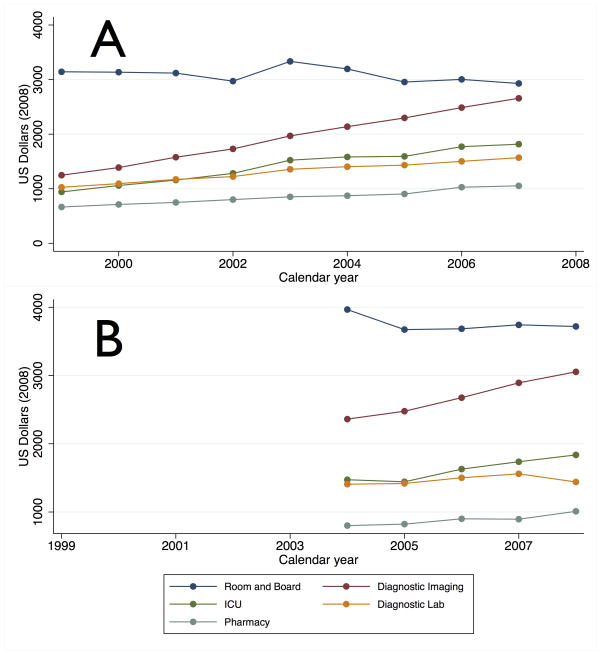
Mean hospital costs increased from $9,058 (SD $11,867) to $12,842 (SD $15,551) in the group of states with most complete cost data (NY, FL, AZ and WA from 1997–2007). Diagnostic imaging, (X-RT, CT, MRI, nuclear medicine, ultrasound, EKG and EEG) was the second largest cost center over this interval behind only Room and Board charges, and underwent the largest percent increase (213%) (Figure 3). MRI costs increased faster than overall Diagnostic Imaging costs, increasing 413% from 1999 to 2007, from 3% of total hospital costs in 1999 to 9% in 2007. CT costs increased by 171% from 6% of total costs in 1999 to 8% in 2007. Similar trends were seen in the group of states with adequate cost data only in the later priod (NY, AZ, WA, NC, and MA from 2004–2008): diagnostic imaging was the second largest cost component and underwent the largest percent increase (129%) with MRI (138%) and CT costs (133%) increasing slightly faster. In 2008, MRI costs accounted for 10% of total hospital costs and CT for 8%.
Figure 3. Trends in Stroke Cost Components.
Legend: Top Panel: Trends in cost components from 1999–2007 in NY, FL, AZ, WA. Bottom Panel: Trends in cost components from 2004–2008 in NY, AZ, WA, NC, MA
Discussion
MRI utilization in ischemic stroke has increased substantially from 1999 to 2008. Growth in MRI utilization was seen in every year in every state in our study population. In high utilizing regions, the rate has plateaued at a level suggesting that nearly all eligible patients are receiving MRI.32, 33 Yet, 95% of patients receiving MRI also received CT; MRI is not replacing CT as the primary stroke neuroimaging study, instead receipt of multiple neuroimaging studies is increasingly common. In addition to an increase in overall MRI utilization, we also observed marked geographic variation. In 2008, 55% of stroke patients underwent MRI in Oregon compared to 79% in Arizona. Partly as a consequence of this widespread increased use, diagnostic imaging has been the fastest growing cost component of stroke care. These data suggest that neuroimaging practices in stroke are neither standardized nor efficient.
The wide geographic variation in MRI utilization demonstrates a potential opportunity to improve stroke care by increasing standardization. This variation was not accounted for by measurable patient characteristics or hospital acquisition of MRI technology. While we cannot directly measure patient preferences, there is little evidence that patients have strong and variable preferences among imaging modalities, independent of the advice of their physicians;34 it is further unclear why those preferences would be so geographically variable so as to explain the observed pattern. Absent a credible alternative explanation, variation in physician practice patterns likely accounts for much of the variation in MRI utilization
The existence of variation in physician practice in this context is not surprising as variation tends be greater when the evidence for a practice is less certain29 and stroke imaging guidelines during the study period did not preferentially recommend CT or MRI.15, 17 Recent guidelines have generally made more favorable recommendations regarding MRI than previous guidelines, but inconsistency between these guidelines exists. Recently, an American Heart Association (AHA) Scientific Statement was the first major guideline to recommend routine use of MRI or CT angiography for stroke patients.35 The recent American Academy of Neurology (AAN) guideline on the role of MRI in stroke36 limits its preferential recommendation of MRI to the period within 12 hours of stroke onset. The most recent European Stroke Organization (ESO) guideline37 calls for stroke patients to receive either CT or MRI. Standardization of neuroimaging practices may improve as guidelines become more consistent, particularly if evidence emerges that MRI leads to improved outcomes or more optimal physician decision-making. The current highly variable use of MRI suggests that there may be community equipoise that would make a randomized trial ethical, feasible and useful.
Not only are stroke neuroimaging practices non-standardized, but our findings suggest stroke neuroimaging may be unnecessarily costly. In our sample, 95% of patients receiving a MRI also received a CT, thus minimizing the use of multiple imaging studies may represent a viable strategy to contain neuroimaging costs. If the practice of obtaining both MRI and CT reflects physician preference for MRI in a context where CT can be obtained more quickly, then several broad strategies for increasing efficiency are possible. First, some patients may be able to safely wait for an imaging study, allowing MRI to be performed without a preceding CT. Patients with delayed presentations, out of the acute treatment window, and stable examinations might meet these criteria and represent a significant proportion of all stroke patients. Up to 36% of stroke patients present more than 12 hours after onset.38 Second, wider dissemination of rapid stroke MRI protocols relying on a selective set of sequences (ie: diffusion-weighted, gradient-echo and T2-weighted sequences) may minimize resource utilization and allow for quicker scanner turnover thus enabling earlier MRI acquisition.39 For MRI to be a viable alternative to CT in the acute stroke period, scan times for MRI must not be greater than CT to minimize time delay for thrombolysis.
This study has several important limitations. First, the use of administrative data to identify stroke is an imprecise process raising the possibility that some of the variability in MRI utilization and cost may be due to imprecision in coding of stroke diagnoses. To minimize this, we have used a previously validated algorithm to identify stroke cases.19 Similarly, revenue code-based identification of imaging studies is also limited by the inability to reliably identify the subcomponents of an imaging study, the number of times an individual patient received a given neuroimaging study which body part was imaged, or the timing of imaging studies. Consequently, we are unable to reliably determine the role of MRA compared to MRI, which portion of studies were focused on the CNS or when imaging studies were obtained during the hospitalization. Because of this limited information, we can not speculate as to why physicians obtained an MRI in an individual case (ie: was MRI obtained to aid thrombolysis decision making, clarify diagnosis or inform secondary prevention strategies?) Additionally, while our dataset was selected to maximize geographic and demographic variability, the southern states and African Americans are underrepresented.
The use of MRI in ischemic stroke has substantially increased over the last decade, with wide geographic variation and increasing contribution to the cost of stroke care. These findings emphasize the importance of future research to define which stroke patients are likely to benefit from MRI, how MRI information should be applied to individuals, and the relationship between MRI and clinical meaningful outcomes. Finally, these findings illustrate the potential to reduce variability and improve the efficiency of neuroimaging in stroke.
Acknowledgments
Dr. Burke had full access to all of the data in the study, performed the primary analyses and takes responsibility for the integrity of the data and the accuracy of the data analysis
The authors would like to thank Colin Cooke, MD MSc, for his assistance in assembling and using the dataset.
Funding/Support: This work was supported by the Robert Wood Johnson Foundation Clinical Scholars Program and an associated Veterans Affairs (VA) Advanced Fellowship to JFB. It was also supported by the U.S. National Institutes of Health via K08 HL091249 to TJI. KAK received research support from NIH grant K23 RR024009 and AHRQ grant R18 HS017690. LBM received research support from NIH grant R01NS038916 and AHRQ grant R18 HS017690.
Footnotes
Financial Disclosures: None of the authors have relevant financial disclosures
IRB: The University of Michigan Institutional Review Board (IRBMED) determined this study to be exempt from formal review as it relied on publicly available data.
Contributor Information
James F Burke, Email: jamesbur@med.umich.edu.
Kevin A Kerber, Email: kakerber@med.umich.edu.
Theodore J Iwashyna, Email: tiwashyn@umich.edu.
Lewis B Morgenstern, Email: lmorgens@med.umich.edu.
References
- 1.Brazzelli M, Sandercock PA, Chappell FM, et al. Magnetic resonance imaging versus computed tomography for detection of acute vascular lesions in patients presenting with stroke symptoms. Cochrane Database Syst Rev. 2009 Jan;1(4):CD007424. doi: 10.1002/14651858.CD007424.pub2. [DOI] [PubMed] [Google Scholar]
- 2.Barber PA, Darby DG, Desmond PM, et al. Identification of major ischemic change. Diffusion-weighted imaging versus computed tomography. Stroke; a journal of cerebral circulation. 1999 Oct 01;30(10):2059–65. doi: 10.1161/01.str.30.10.2059. [DOI] [PubMed] [Google Scholar]
- 3.Fiebach JB, Schellinger PD, Jansen O, et al. CT and diffusion-weighted MR imaging in randomized order: diffusion-weighted imaging results in higher accuracy and lower interrater variability in the diagnosis of hyperacute ischemic stroke. Stroke; a journal of cerebral circulation. 2002 Sep 01;33(9):2206–10. doi: 10.1161/01.str.0000026864.20339.cb. [DOI] [PubMed] [Google Scholar]
- 4.Chalela J, Kidwell C, Nentwich L, Luby M. Magnetic resonance imaging and computed tomography in emergency assessment of patients with suspected acute stroke: a prospective comparison. The Lancet. 2007 January 27;369:293–98. doi: 10.1016/S0140-6736(07)60151-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.González RG, Schaefer PW, Buonanno FS, et al. Diffusion-weighted MR imaging: diagnostic accuracy in patients imaged within 6 hours of stroke symptom onset. Radiology. 1999;210(1):155–62. doi: 10.1148/radiology.210.1.r99ja02155. [DOI] [PubMed] [Google Scholar]
- 6.Morgenstern LB, Hemphill JC, Anderson C, et al. Guidelines for the Management of Spontaneous Intracerebral Hemorrhage: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke; a journal of cerebral circulation. 2010 Aug 30;41(9):2108–29. doi: 10.1161/STR.0b013e3181ec611b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wardlaw JM, Keir SL, Seymour J, et al. What is the best imaging strategy for acute stroke? Health Technol Assess. 2004 Jan 1;8(1):iii, ix–x, 1–180. doi: 10.3310/hta8010. [DOI] [PubMed] [Google Scholar]
- 8.Bryan R, Levy L, Whitlow W, Killian J, Preziosi T, Rosario J. Diagnosis of acute cerebral infarction: comparison of CT and MR imaging. American Journal of Neuroradiology. 1991 Jul 01;12(4):611. [PMC free article] [PubMed] [Google Scholar]
- 9.Schulz UG, Briley D, Meagher T, Molyneux A, Rothwell PM. Diffusion-weighted MRI in 300 patients presenting late with subacute transient ischemic attack or minor stroke. Stroke. 2004 Nov 1;35(11):2459–65. doi: 10.1161/01.STR.0000143455.55877.b9. [DOI] [PubMed] [Google Scholar]
- 10.Barber PA. Is diffusion-weighted imaging helpful in determining the source of stroke? Commentary. Nature Reviews Neurology. 2006;2:424–5. [Google Scholar]
- 11.Gass A, Ay H, Szabo K, Koroshetz WJ. Diffusion-weighted MRI for the “small stuff”: the details of acute cerebral ischaemia. Lancet Neurology. 2004;3(1):39–45. doi: 10.1016/s1474-4422(03)00621-5. [DOI] [PubMed] [Google Scholar]
- 12.Wessels T, Wessels C, Ellsiepen A, et al. Contribution of diffusion-weighted imaging in determination of stroke etiology. AJNR American journal of neuroradiology. 2006;27(1):35–9. [PMC free article] [PubMed] [Google Scholar]
- 13.Albers GW, Lansberg MG, Norbash AM, et al. Yield of diffusion-weighted MRI for detection of potentially relevant findings in stroke patients. Neurology. 2000 Apr 25;54(8):1562–7. doi: 10.1212/wnl.54.8.1562. [DOI] [PubMed] [Google Scholar]
- 14.GAO. Government Accountability Office. Medicare: trends in fees, utilization, and expenditures for imaging services before and after implementation of the Deficit Reduction Act of 2005. Washington, DC: 2008. Contract No.: (GAO-08–1102R.) [Google Scholar]
- 15.Adams HP, Jr, Del Zoppo GJ, Alberts MJ, et al. Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: the American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke. 2007 May 1;38(5):1655–711. doi: 10.1161/STROKEAHA.107.181486. [DOI] [PubMed] [Google Scholar]
- 16.Culebras A, Kase CS, Masdeu JC, et al. Practice guidelines for the use of imaging in transient ischemic attacks and acute stroke. A report of the Stroke Council, American Heart Association. Stroke. 1997 Jul 1;28(7):1480–97. doi: 10.1161/01.str.28.7.1480. [DOI] [PubMed] [Google Scholar]
- 17.Olsen TS, Langhorne P, Diener HC, et al. European Stroke Initiative Recommendations for Stroke Management-update 2003. Cerebrovascular diseases (Basel, Switzerland) 2003;16(4):311–37. doi: 10.1159/000072554. [DOI] [PubMed] [Google Scholar]
- 18.HCUP State Inpatient Databases (SID) Rockville MD: Agency for Healthcare Research and Quality; 1999–2009. [8/31/2011]; Available from: http://www.hcup-us.ahrq.gov/sidoverview.jsp. [Google Scholar]
- 19.Goldstein LB. Accuracy of ICD-9-CM coding for the identification of patients with acute ischemic stroke: effect of modifier codes. Stroke. 1998 Aug 1;29(8):1602–4. doi: 10.1161/01.str.29.8.1602. [DOI] [PubMed] [Google Scholar]
- 20.Tirschwell DL, Longstreth WT. Validating administrative data in stroke research. Stroke; a journal of cerebral circulation. 2002 Oct;33(10):2465–70. doi: 10.1161/01.str.0000032240.28636.bd. [DOI] [PubMed] [Google Scholar]
- 21.HCUP Clinical Classifications Software (CCS) for ICD-9-CM. Rockville, MD: Agency for Healthcare Research and Quality; Available from: http://www.hcup-us.ahrq.gov/toolssoftware/ccs/ccs.jsp. [Google Scholar]
- 22.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Medical care. 2005 Nov 1;43(11):1130–9. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 23.Goldstein LB, Samsa GP, Matchar DB, Horner RD. Charlson Index Comorbidity Adjustment for Ischemic Stroke Outcome Studies. Stroke. 2004 Jun 10;35(8):1941–5. doi: 10.1161/01.STR.0000135225.80898.1c. [DOI] [PubMed] [Google Scholar]
- 24.HCUP Utilization Flags. Rockville, MD: Agency for Healthcare Research and Quality; 2003. Available from: http://www.hcup-us.ahrq.gov/toolssoftware/util_flags/utilflag.jsp. [Google Scholar]
- 25.Dismuke CE. Underreporting of computed tomography and magnetic resonance imaging procedures in inpatient claims data. Medical care. 2005 Jul 1;43(7):713–7. doi: 10.1097/01.mlr.0000167175.72130.a7. [DOI] [PubMed] [Google Scholar]
- 26.Elixhauser ABM, Nisbet J. Development of Utilization Flags for Use with UB-92 Administrative Data. Rockville MD: 2006. Available from: http://www.hcup-us.ahrq.gov/reports/methods.jsp. [Google Scholar]
- 27.Wennberg JECM, editor. The Dartmouth Atlas of Health Care 1999. Chicago: American Hospital Publishing; 1999. [PubMed] [Google Scholar]
- 28.Appendix on the Geography of Health Care in the United States. Lebanon, NH: [cited 2011 2/16]; Available from: http://www.dartmouthatlas.org/downloads/methods/geogappdx.pdf. [Google Scholar]
- 29.Sirovich B, Gallagher PM, Wennberg DE, Fisher ES. Discretionary Decision Making By Primary Care Physicians And The Cost Of U.S. Health Care. Health Affairs. 2008 May 1;27(3):813–23. doi: 10.1377/hlthaff.27.3.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.HCUP Cost-to-Charge Ratio Files (CCR) Rockville, MD: Agency for Healthcare Research and Quality; 2001–2008. Available from: http://www.hcup-us.ahrq.gov/db/state/costtocharge.jsp. [Google Scholar]
- 31.Using Appropriate Price Indices for Analyses of Health Care Expenditures or Income Across Multiple Years. Rockville, MD: Medical Expenditure Panel Survey; [cited 2011 2/16]; Available from: http://www.meps.ahrq.gov/mepsweb/about_meps/Price_Index.shtml-c3. [Google Scholar]
- 32.Singer OC, Sitzer M, du Mesnil de Rochemont R, Neumann-Haefelin T. Practical limitations of acute stroke MRI due to patient-related problems. Neurology. 2004 May 25;62(10):1848–9. doi: 10.1212/01.wnl.0000125320.53244.fa. [DOI] [PubMed] [Google Scholar]
- 33.Hand PJ, Wardlaw JM, Rowat AM, Haisma JA, Lindley RI, Dennis MS. Magnetic resonance brain imaging in patients with acute stroke: feasibility and patient related difficulties. Journal of Neurology, Neurosurgery & Psychiatry. 2005 Nov;76(11):1525–7. doi: 10.1136/jnnp.2005.062539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anthony DL, Herndon MB, Gallagher PM, et al. How much do patients' preferences contribute to resource use? Health affairs (Project Hope) 2009;28(3):864–73. doi: 10.1377/hlthaff.28.3.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Latchaw RE, Alberts MJ, Lev MH, et al. Recommendations for Imaging of Acute Ischemic Stroke. A Scientific Statement From the American Heart Association. Stroke. 2009 Sep;24:1–34. doi: 10.1161/STROKEAHA.108.192616. [DOI] [PubMed] [Google Scholar]
- 36.Schellinger PD, Bryan RN, Caplan LR, et al. Evidence-based guideline: The role of diffusion and perfusion MRI for the diagnosis of acute ischemic stroke: Report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2010 Jul 13;75(2):177–85. doi: 10.1212/WNL.0b013e3181e7c9dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Committee EW. Guidelines for management of ischaemic stroke and transient ischaemic attack 2008. Cerebrovasc Dis. 2008 Jan 1;25(5):457–507. doi: 10.1159/000131083. [DOI] [PubMed] [Google Scholar]
- 38.Majersik JJ, Smith MA, Zahuranec DB, Sánchez BN, Morgenstern LB. Population-based analysis of the impact of expanding the time window for acute stroke treatment. Stroke. 2007 Dec 1;38(12):3213–7. doi: 10.1161/STROKEAHA.107.491852. [DOI] [PubMed] [Google Scholar]
- 39.Schellinger PD, Jansen O, Fiebach JB, et al. Feasibility and Practicality of MR Imaging of Stroke in the Management of Hyperacute Cerebral Ischemia. American Journal of Neuroradiology. 2000 Aug 01;21(7):1184. [PMC free article] [PubMed] [Google Scholar]