Abstract
Objective
Children with attention-deficit/hyperactivity disorder (ADHD) have delayed cortical maturation, evidenced by regionally specific slower cortical thinning. However, the relationship between cortical maturation and attention capacities in typically developing children is unknown. This study examines cortical thickness correlates of inattention symptoms in a large sample of healthy children.
Method
Data from 357 healthy subjects (6.0–18.4 years of age) were obtained from the NIH MRI Study of Normal Brain Development. In cross-sectional analysis (first visit, n = 257), Child Behavior Checklist Attention Problems (AP) scores were linearly regressed against cortical thickness, controlling for age, gender, total brain volume, and site. For longitudinal data (up to three visits, n = 357/672 scans), similar analyses were performed using mixed-effects linear regressions. Interactions of AP with age and gender were tested.
Results
A cross-sectional “AP by age” interaction was found in bilateral orbito-frontal cortex, right inferior frontal cortex, bilateral ventromedial prefrontal cortex, bilateral dorsolateral prefrontal cortex, and several additional attention network regions. The interaction was due to negative associations between AP and thickness in younger subjects (6–10 years of age) that gradually disappeared over time secondary to slower cortical thinning. Similar trends were present in longitudinal analyses.
Conclusions
Higher AP scores were associated with thinner cortex at baseline and slower cortical thinning with aging in multiple areas involved in attention processes. Similar patterns have been identified in ADHD, suggesting a dimensional component to the link between attention and cortical maturation. The identified association between cortical maturation and attention in healthy development will help to inform studies of neuroimaging biomarkers of ADHD.
Keywords: attention-deficit/hyperactivity disorder, Child Behavior Checklist, attention, cortical thickness, magnetic resonance imaging
Attention is a crucial cognitive ability that allows efficient processing of environmental information to modulate and control thoughts and behavior.1,2 Fronto-parietal networks involved in attention have been identified primarily with functional imaging of attention-demanding tasks.1,3 One area of weakness in the current literature is a relative lack of data defining the relationship between the development of attentional capacities and structural brain development among typically developing children. General intellectual abilities have been linked to cortical development in healthy children4,5. However, there are fewer data on cortical thickness correlates of specific attention functions. In healthy adults (20–84 years of age), executive control functions as measured by the Attention Network Test were associated with anterior cingulate cortex (ACC), lateral prefrontal cortex, and right inferior frontal gyri cortical thickness, whereas the alerting function was linked to parietal areas cortical thickness.6 In both cases, decreased thickness was associated with poorer test results, and the effect was independent of aging.6 Similar studies have not been done in healthy children, and data on the link between cortical maturation and attention is largely derived from studies of attention-deficit/hyperactivity disorder (ADHD).7 However, the knowledge gap in cortical correlates of attention in normative development limits our capacity to identify meaningful neuroimaging biomarkers of clinical ADHD. We postulate that a better understanding of the link between attention and normal brain development is of major importance in delineating processes that have an impact on attentional functioning in children with a variety of psychiatric disorders.
The clinical syndrome of inattention, impulsivity, hyperactivity, and restlessness has been putatively associated with executive functions deficits and fronto-striatal abnormalities.8–11 Initial imaging studies pointed to reduced total gray matter and frontal lobe volume as potential correlates of ADHD.9,12–14 These findings have been recently refined by large-scale investigations of cerebral cortical thickness. Shaw et al. (2006) initially demonstrated reduced cortical thickness in bilateral dorsolateral prefrontal cortex (DLPFC), right ACC, and right inferior parietal cortex, structures that are demonstrated to be components of the attention network.15–17 Subsequently, in a landmark paper, the same researchers demonstrated that subjects with ADHD have a normal “inverted U” shape gray matter development, but with delayed thickness peaks in many regions (mean peak at 10.5 versus 7.5 years of age), especially in the prefrontal cortex (PFC).18,19 In addition, the growth pattern of the right orbitofrontal cortex (OFC) and inferior frontal cortex (IFC) was found to be abnormal in children with ADHD.20 Deficits in right OFC function have also been linked to impaired inhibition in neuropsychological tasks in children with ADHD.21 Although frontal structures have been most extensively studied and consistently associated with ADHD, the parietal lobe is also potentially implicated.8,17 The two regions share white matter connections that are components of the attention system.22,23 Indeed, genetic–neuroimaging studies report that the DRD4-7-repeat allele, a genetic polymorphism linked to ADHD, is associated with thinner right OFC/inferior frontal and posterior parietal cortex.24
Given that the cortical maturation correlates of attention in typically developing children have not been clarified, it is questionable whether the above-identified neuroimaging correlates of ADHD are specific to clinical populations or whether they are proportional to attention skills even among healthy children. In a recent study, Shaw et al.25 found a significant interaction between age and symptoms of hyperactivity and impulsivity (measured with Conner’s Parent Rating Scale) on cortical thickness in a pooled cohort of children with ADHD and typically developing children and adolescents of 8 to 17 years of age.25 Those with ADHD had a slower rate of thinning of cortical gray matter, but a similar phenomenon was observed in controls proportionally to their Conner’s score, which supported a dimensional view of ADHD. In this study, control subjects were self-selected and not systematically assessed for confounding variables such as substance use.
Using data from the NIH MRI Study of Normal Brain Development, we examined cortical thickness correlates of subclinical attention problems as measured by the Parent Child Behavior Checklist (CBCL) Attention Problems (AP) scale in a large sample of representative healthy children (6.0–18.4 years of age). AP scores provide a noncategorical assessment of attention problems in children and demonstrate an excellent agreement with the clinical diagnosis of ADHD.26–30 We hypothesized that AP scores of healthy children would interact with age to influence cortical thickness development in a similar pattern than what has been demonstrated in subjects with ADHD.25 Brain areas a priori identified as most likely to show this pattern of association were several specific prefrontal structures, including the OFC, IFC, medial PFC, ACC, and DLPFC.
METHOD
Sampling and Recruitment
The NIH MRI Study of Normal Brain Development is a multi-site project providing a normative database to characterize healthy brain maturation in relationship to behavior.31 Subjects were recruited across the United States with a population-based sampling method seeking to minimize biases of selection.32 Based on available U.S. Census 2000 data, a representative healthy sample of subjects was recruited at six pediatric study centers: Children’s Hospital (Boston), Children’s Hospital Medical Center (Cincinnati), University of Texas Houston Medical School (Houston), UCLA Neuropsychiatric Institute and Hospital (Los Angeles), Children’s Hospital of Philadelphia (Philadelphia), and Washington University (St. Louis). Recruitment was monitored continuously to ensure that the sample recruited across all pediatric centers was demographically representative of the U.S. population on the basis of variables that included age, gender, race, ethnicity, and socioeconomic status. Informed consent from parents and child assent were obtained for all subjects. The Objective 1 database (release 4.0) used for this study included 433 children from 4 years 6 months to 18 years 3 months who underwent extensive cognitive, neuropsychological, and behavioral testing, along with repeated MRI brain imaging at up to three visits at 2-year intervals. Given that the goal of this project was to study developmentally healthy typical children, there were extensive exclusion criteria including current or past treatment for an axis 1 disorder, evidence of most axis I disorders including ADHD on structured parent or child interview (Diagnostic Interview for Children and Adolescents) (except for simple phobia, social phobia, adjustment disorder, oppositional defiant disorder, enuresis, encopresis, nicotine dependency), neurological disorders, substance dependence other than nicotine, family history of major axis 1 disorder, family history of inherited neurological disorder or mental retardation due to nontraumatic events, abnormality on neurological examination, gestational age at birth less than 37 weeks or more than 42 weeks, intrauterine exposure to substances known or highly suspected to alter brain structure or function, etc. Structural MRI and clinical/behavioral data were consolidated and analyzed within a purpose-built database at the Data Coordinating Center of the Montreal Neurological Institute (MNI), McGill University.
Child Behavior Checklist
The Child Behavior Checklist (CBCL) is an age-appropriate standardized questionnaire filled by parents.29,33 It has a high test–retest reliability, results are stable over time34 and it has been validated in numerous cultures.35,36 The CBCL/6-18 is divided into eight subscales, including the Attention Problems score (AP).33 At the time of initial enrollment, all children in this study had t scores of less than 70, which is considered to be the pathological threshold. Although no subject with ADHD was included at baseline, subjects would have been kept in the study had they met DSM-IV TR criteria at a follow-up visit. However, only one subject had a t score of more than 70 on follow-up visits at 9 years of age.
MRI Protocol
MR images (1.5-T) were obtained with 1-mm in-plane resolution, 1- to 2-mm slice thickness, whole-brain coverage, and multiple contrasts (T1W, T2W, and PDW).31 A three-dimensional (3D) T1-weighted spoiled gradient recalled (SPGR) echo sequence was selected. Intersite reliability was monitored at all sites with the American College of Radiology phantom. In addition, living phantoms (healthy volunteers) were also scanned repeatedly at regular intervals at each site confirming between-site reliability of cortical thickness measurements.31 Importantly, all data processing was done at a single site (MNI).
Automated Image Processing
Quality-controlled native MR images were processed through the CIVET automated pipeline that includes the CLASP algorithm for generating cortical thickness measurements at 40,962 vertices per hemisphere.37–41 Cortical thickness is calculated as the distance between the “outer CSF–gray matter” and the “gray matter– white matter” interface. Subjects with missing values (either anatomical measures or AP scores) were eliminated and a visual quality control (blinded as to the AP score of the subjects) of the native cortical thickness images was implemented to ensure that there were no aberrations in values for a given subject.5 Subjects less than 6 years of age who were assessed with the CBCL/1.5–5 were eliminated, as were subjects more than 18 years of age, because attention problems were assessed with a self-report measure as opposed to the Parent CBCL. Out of this blinded process, 672 of 955 scans were kept for statistical analyses.
Data Analysis
Statistical analyses were implemented using SurfStat (http://www.math.mcgill.ca/keith/surfstat/), a statistical toolbox created for MATLAB (MathWorks, Natick, MA). Analyses were performed both for cross-sectional data (first visit only) and for longitudinal data (pooled data from all three visits).
For cross-sectional analyses (n = 257, 141 female and 116 male), each subject’s absolute native-space cortical thickness was linearly regressed against CBCL AP scores at each cortical point after accounting for the effects of age, total brain volume, gender and MRI scanner (as different scanners were used at the six sites). This was done both in first-order (Y = 1 + β1AP + β2Age + β3Gender + β4TotalBrainVolume + β5Scanner) and in higher-order (quadratic and cubic) models. The “AP by age” (Y = 1 + β1AP + β2Age + β3Age*AP + β4Gender + β5TotalBrainVolume + β6Scanner), “AP by gender,” and “AP by age by gender” interactions were also analyzed. IQ and handedness were tested as potential confounding variables. To account for multiple comparisons across the whole brain, a p ≤ .05 false discovery rate (FDR) was applied. An uncorrected p ≤ .005 threshold was selected to identify trends in associations. Significant “AP by age” interactions were decomposed across all age levels by centering.42
For longitudinal analyses (n = 357, 672 total scans; 368 female and 304 male), a similar strategy was implemented, but using a mixed-effects linear regression model that accounts for some subjects having repeated measurements over time.25
RESULTS
Demographics
Table 1 shows basic descriptive statistics of the two analyzed samples (cross-sectional and longitudinal). Males had higher mean CBCL AP scores in the cross-sectional sample (males 1.94 ± 0.21, females 1.43 ± 0.16, t = 1.97, df = 255, p = .05), and in the longitudinal data (males 1.98 ± 0.14, females 1.59 ± 0.11, t = 2.23, df = 670, p = .026). Although there were fewer AP raw scores over 8 in older children, the average AP score remained stable over time.
TABLE 1.
Age, Child Behavior Checklist (CBCL) Attention Problems Scores, Gender Distribution, and Hand Used to Write in the Two Analyzed Samples
| Cross-Sectional n = 257 | Longitudinal n = 357 (672 scans) | |
|---|---|---|
| Age (y) | 11.8 ± 0.22 (6.0–18.3) | 12.0 ± 0.12 (6.0–18.4) |
| CBCL Attention Problems Score | 1.66 ± 0.13 (0–11) | 1.76 ± 0.09 (0–14) |
| Gender | Female = 141 (54.9%) Male = 116 (45.1%) |
Females = 368 scans (54.8%) Males = 304 scans (45.2%) |
| Hand used to write | Right = 232 (90.3%) Left = 25 (9.7%) |
Right = 601 (89.4%) Left = 71 (10.6%) |
Cross-Sectional Analysis
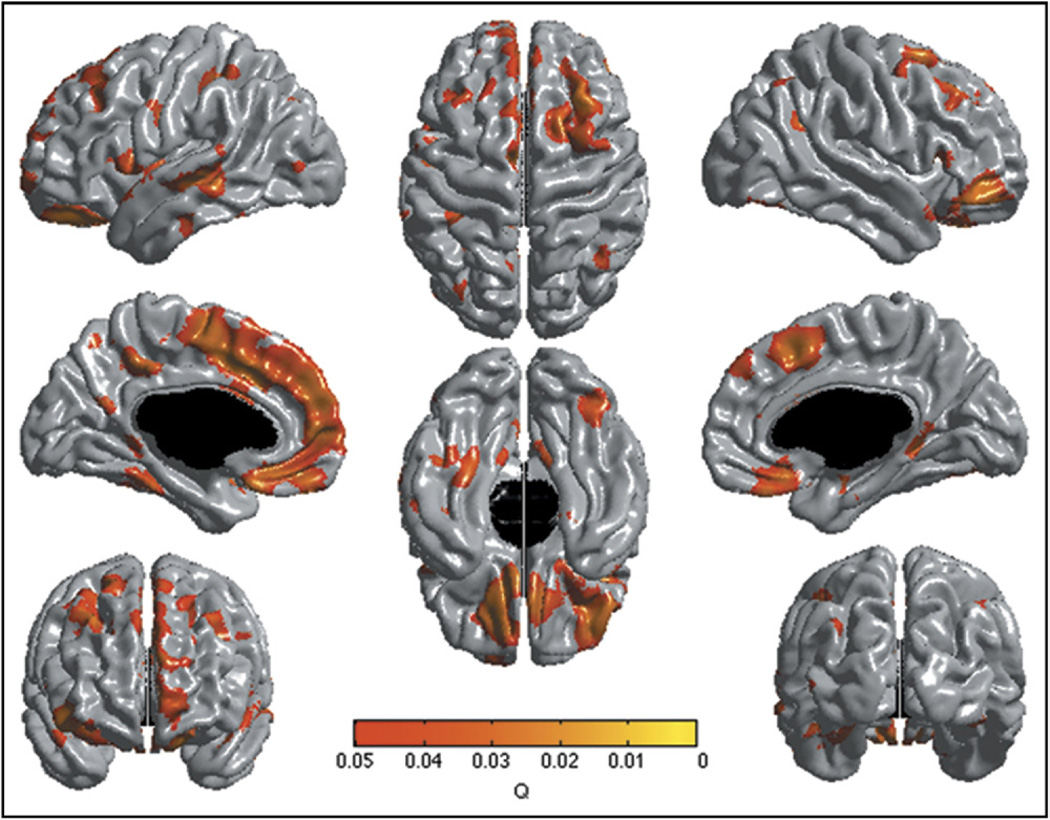
There were no direct first-order or higher-order associations between AP scores and cortical thickness. However, the “AP by age” interaction revealed multiple areas of association with cortical thickness (Figure 1, p ≤ .05, FDR corrected). The strongest association was located in the right lateral OFC and right IFC. There were also significant associations in the left OFC, bilateral ventromedial prefrontal cortex (vmPFC), bilateral premotor/supplementary motor cortex, bilateral DLPFC, left medial prefrontal cortex (mPFC), left dorsal anterior midcingulate cortex (dorsal ACC), left posterior cingulate cortex, left medial temporal gyrus, left parietal somatosensory association cortex and a few other small temporo-parietal areas. These findings were independent of all control variables, including IQ and handedness. There were no “AP by gender” or three-way “AP by age by gender” interactions, indicating the absence of gender specific effect on this maturational pattern despite the presence of a slight difference in mean AP scores between sexes (see unthresholded t map in Figure S1, available online).
FIGURE 1.
Brain areas where local cortical thickness is associated with the “Child Behavior Checklist Attention Problems by Age” interaction in the cross-sectional analysis (n = 257). Note: The figure is shown at q ≤ 0.05 with a false discovery rate correction. Controlled for age, gender, total brain volume, and scanner.
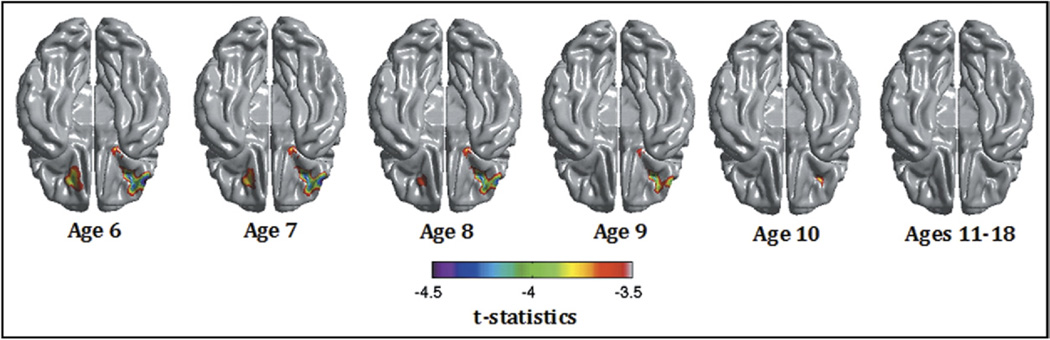
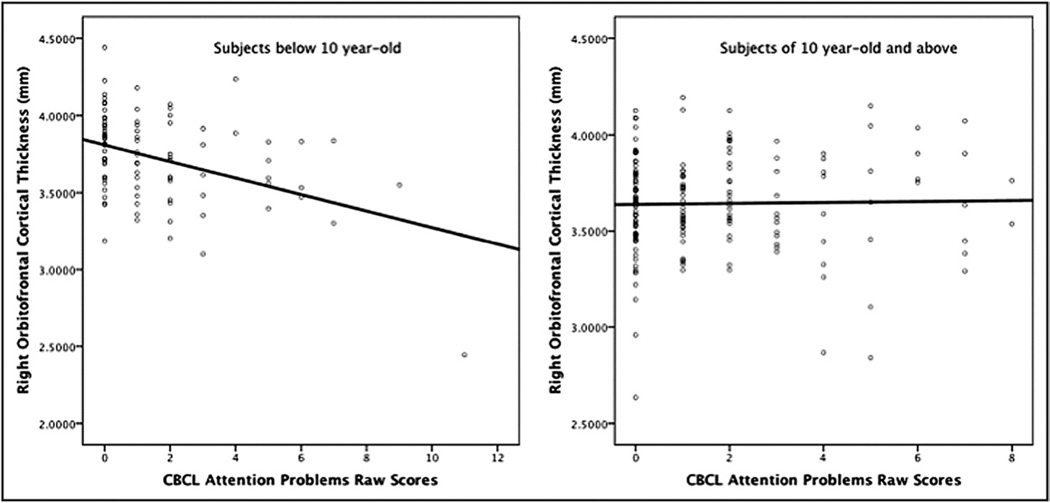
The interaction was explained by negative associations between AP and cortical thickness in the younger subjects up to the age of 10 years. The association then disappeared as children aged (positive trends at 17 and 18 years). As an illustration, Figure 2 shows the uncorrected t statistic map (focused on the right OFC/IFC) of the relationship between AP and thickness centered at ages 6 to 18 years. Scatterplots of the relationship between AP and right OFC cortical thickness in subjects below and above age 10 years are shown in Figure 3, demonstrating the negative association in younger subject and the absence of association above age 10. To further illustrate the impact of age, linear regression models of the mean cortical thickness of the right lateral OFC cluster were performed with SPSS version 18.0 (SPSS Inc., Chicago, IL) after dividing groups into lower AP scores (0 or 1) and higher AP scores (≥2). The analysis revealed a much stronger negative effect of age in the lower group (standardized β = −0.340), compared with the higher group (standardized β = 0.022) (see scatterplots in Figure S2, available online). In other words, subjects with higher AP scores started with thinner cortices, but this difference disappeared over time because of slower cortical thinning.
FIGURE 2.
t Scores map (df = 246) of the association between Child Behavior Checklist Attention Problems and cortical thickness centered from age 6 to 18 years in the cross-sectional sample (n = 257). Note: The brain is shown from below, and the main association is located in the right lateral orbitofrontal cortex. Controlled for age, gender, total brain volume, and scanner.
FIGURE 3.
Scatterplots of the right lateral orbitofrontal cortical thickness against Child Behavior Checklist (CBCL) Attention Problems raw scores in subjects less than age 10 on the left and of 10 years and older subjects on the right. Note: The negative relationship on the left scatterplot remained significant even when removing the outlier (subject with cortical thickness below 1 standard deviation).
Longitudinal Analysis
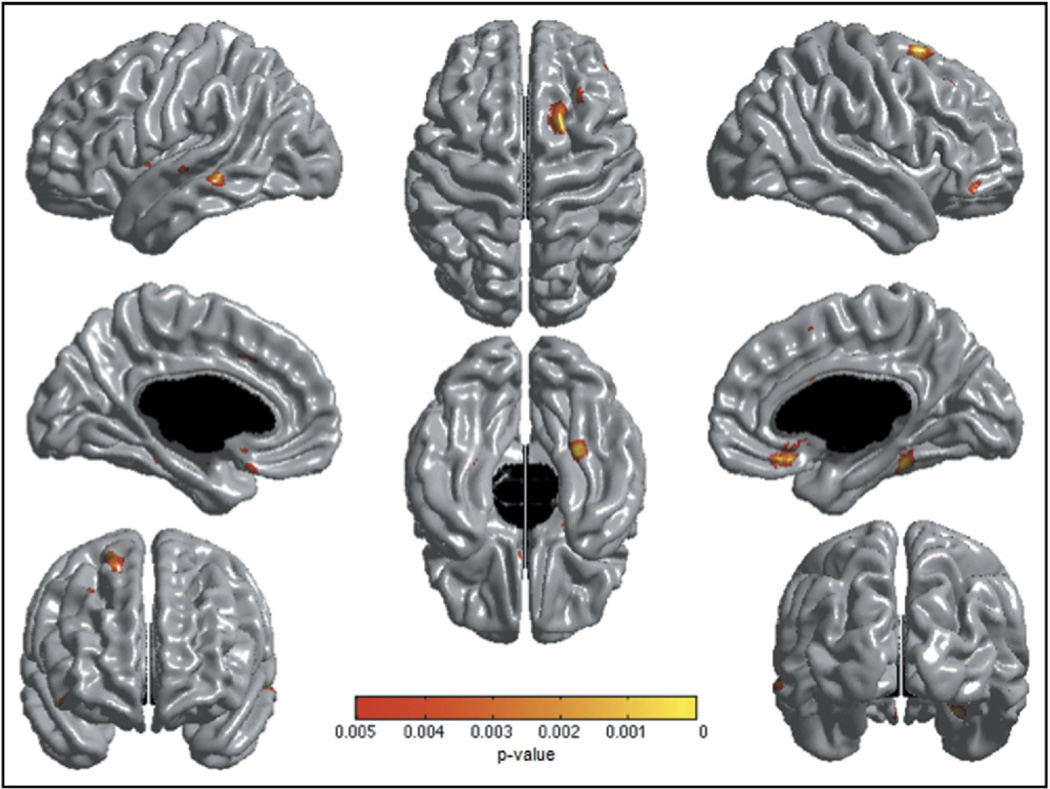
No significant association was found between AP scores and cortical thickness. The “Age by AP” interaction showed trends of association in similar areas than those identified in the crosssectional analyses, including the right IFC, bilateral vmPFC, right supplementary motor area, right DLPFC, left mPFC, and left medial/superior temporal gyri (Figure 4). However, these associations did not survive the whole-brain FDR correction.
FIGURE 4.
Brain areas where local cortical thickness is associated with the “Child Behavior Checklist Attention Problems by Age” interaction in a mixed-effects linear regression in the longitudinal sample (n = 357, 672 scans). Note: The figure is shown at p ≤.005 (uncorrected). Trends of associations were not statistically significant after a whole-brain false discovery rate correction. Controlled for age, gender, total brain volume, and scanner.
DISCUSSION
In this large neuroanatomical study of healthy children, a cross-sectional association between the “CBCL AP by age” interaction and cortical thickness was identified in various components of the attention network.3,17 Associations were found in the right IFC, bilateral OFC, bilateral vmPFC, bilateral premotor/supplementary motor cortex, bilateral DLPFC, left mPFC, left dorsal anterior midcingulate cortex (dorsal ACC), left posterior cingulate cortex, left medial temporal gyrus, and a few other temporo-parietal areas. Analysis by age groups revealed a negative association between AP and cortical thickness in the above areas in children 6 to 9 years of age, followed by a decrease in the strength of the association at age 10 and a disappearance of any association in teenage years. Further analysis revealed that children with higher AP scores had thinner cortices at age 6, but this difference was lost over time in the context of slower cortical thinning. This parallels the Shaw et al.25 results in which children with ADHD had thinner cortex than controls at baseline but eventually reached a similar average thickness in later teenage years through a slower thinning process.25 Our results in healthy children show a similar tendency with subclinical attention problems in preteen years being associated with thinner cortices at baseline and slower thinning over the years. These results are compatible with both developmental rates of cortical maturation and cortical thinning being proportional to inattention symptoms, even in typically developing children without ADHD.
The presence of associations between attention problems and prefrontal cortical thickness across the developmental spectrum is biologically plausible, given that these regions have been consistently found to be hypoactive in functional magnetic resonance imaging studies of ADHD.43,44 In addition, the strongest association in this healthy population was found in the right lateral OFC/IFC, which has been shown to have both impaired functioning on neuropsychological testing and an abnormal development pattern in children with ADHD.20,21 The involvement of the premotor/supplementary motor areas is also logical, given the motoric component of hyperactivity.45 Importantly, a similar analysis of a different CBCL externalizing scale, the aggressive behavior scores, showed no interaction with age and cortical thickness, and demonstrated results with different anatomical distributions, which supports the specificity of the associations between cortical thickness and AP.46 Anxiety disorders have also been related to attentional problems and altered cortical thickness.7,47 Given that some anxiety disorders were not exclusion criteria in this study (simple phobia and social phobia, only if CBCL Anxious/Depressed t scores were below clinical threshold), anxiety levels could have been a confounding factor in the CBCL AP results. However, the analysis of the CBCL Anxious/Depressed scores showed an age by score interaction with a different cortical thickness distribution only circumscribed to the right ventromedial prefrontal cortex. In addition, controlling for CBCL Anxious/Depressed scores in the linear regression of CBCL AP against cortical thickness did not change the above-described results.
Although a similar trend was observed in the longitudinal analysis, the “AP by age” interaction was not significant after the FDR correction. This can most likely be explained by the fact that the strongest component of the interaction was the negative association in younger subjects. In the longitudinal sample, adding data points from visits 2 and 3, which are respectively taken 2 and 4 years later, shifted the sample toward older subjects that have a tendency toward smaller variance. This had the impact of “diluting” the impact of the youngest subjects. In addition, it is important to mention that children in this study were strictly screened at entry for any type of psychopathology, meaning that the range for any behavioral measurement is restricted. This limits the statistical power to detect associations, as opposed to the Shaw et al. study, in which both typically developing children and children with ADHD were analyzed.25
The impact and etiology of this cortical development pattern cannot be determined with this type of observational study. One hypothesis would be that the gradual disappearance of the relationship between cortical thickness and AP could be a demonstration of healthy developmental patterns, potentially related to typically observed improvements in attention as children get older. In that regard, previous research has suggested that persistence of decreased thickness in both the left ACC and parietal areas was associated with ADHD symptoms in later age.15,48 However, it was not possible to replicate this finding in this very healthy cohort because the mean AP scores (low at baseline) did not significantly change over time. Further studies examining longitudinal follow-up of cortical thickness in children with ADHD would be interesting to determine whether cortical changes are related to the persistence of symptoms in adulthood. Ultimately, we argue that performing neurobiological research across the continuum of severity (from well to severely ill) is crucial to inform the pursuit of understanding dimensional considerations of mental disorders, as defined in the Research Domain Criteria (RDoC) agenda (http://www.nimh.nih.gov/research-funding/nimh-research-domain-criteria-rdoc.shtml).49
The limitations of this study include the fact that, although AP scores correlate well with diagnostic criteria of ADHD,28,30 the Parent CBCL provides only an estimate of the complex attention skills phenotypes and includes items that are not part of ADHD. However, the CBCL DSM ADHD scale, which is a variant of the AP score focusing on the ADHD criteria, gave results identical results to those presented in this article. By analyzing attention in a large numbers of healthy children in isolation, this study provides a normative framework against which neuroimaging studies of ADHD can be more meaningfully compared. Understanding the impact of subclinical attention problems on cortical development is crucial in order to further investigate delayed cortical maturation and slower gray matter thinning as potential biomarkers of ADHD. Although this constitutes valuable information for researchers and clinicians in terms of neurobiological conceptualization, excluding all children with ADHD limits the generalizability of these findings to clinical populations. In terms of strength, it is worth mentioning the large sample size for a pediatric imaging study and the fact that subjects were demographically representative of the U.S. population. Moreover, we used a precise cortical thickness measurement at more than 80,000 points for each brain. Finally, results were analyzed with whole-brain statistical corrections for multiple comparisons.
Recent neuroimaging studies focusing on ADHD have increasingly demonstrated alterations in attention related networks; however there have been limitations to conclusions drawn, as we have little knowledge about normal brain development associated with attentional abilities. Clearly it is crucial to understand brain development in typically developing children and the relationship with various behaviors to identify meaningful correlates of disorders such as ADHD. This is even more important, given the results from this study and previous reports, which support at least some dimensional aspect to the neurobiology of this disorder.25 Data of this type could be integrated into the NIMH RDoC initiative in which one of the primary goals is to “develop, for research purposes, new ways of classifying mental disorders based on dimensions of observable behavior and neurobiological measures.”49 Future research should attempt to correlate cortical development with long-term longitudinal clinical outcomes in adulthood, in addition to searching for other factors (genetics, environmental, anatomical, electrophysiological, etc.) that might be additive to an abnormal cortical developmental pattern in the etiology of ADHD and its various comorbidities.
Supplementary Material
Acknowledgments
This project was funded in whole or in part with federal funds from the National Institute of Child Health and Human Development, the National Institute on Drug Abuse, the National Institute of Mental Health, and the National Institute of Neurological Disorders and Stroke (contract nos. N01-HD02-3343, N01-MH9-0002, and N01-NS-9-2314, -2315, -2316, -2317, -2319, and -2320).
The authors express special thanks to the National Institutes of Health contracting officers for their support.
Appendix
Brain Development Cooperative Group: Key personnel from the six pediatric study centers are as follows: Children’s Hospital Medical Center of Cincinnati: Principal Investigator: William S. Ball, M.D.; Investigators: Anna Weber Byars, Ph.D., Mark Schapiro, M.D., Wendy Bommer, R.N., April Carr, B.S., April German, B.A., Scott Dunn, R.T.; Children’s Hospital Boston: Principal Investigator: Michael J. Rivkin, M.D.; Investigators: Deborah Waber, Ph.D., Robert Mulkern, Ph.D., Sridhar Vajapeyam, Ph.D., Abigail Chiverton, B.A., Peter Davis, B.S., Julie Koo, B.S., Jacki Marmor, M.A., Christine Mrakotsky, Ph.D., M.A., Richard Robertson, M.D., Gloria McAnulty, Ph.D; University of Texas Health Science Center at Houston: Principal Investigators: Michael E. Brandt, Ph.D., Jack M. Fletcher, Ph.D., Larry A. Kramer, M.D.; Investigators: Grace Yang, M.Ed., Cara McCormack, B.S., Kathleen M. Hebert, M.A., Hilda Volero, M.D.; Washington University in St. Louis: Principal Investigators: Kelly Botteron, M.D., Robert C. McKinstry, M.D., Ph.D.; Investigators: William Warren, Tomoyuki Nishino, M.S., C. Robert Almli, Ph.D., Richard Todd, Ph.D., M.D. (deceased), John Constantino, M.D.; University of California Los Angeles: Principal Investigator: James T. McCracken, M.D.; Investigators: Jennifer Levitt, M.D., Jeffrey Alger, Ph.D., Joseph O’Neil, Ph.D., Arthur Toga, Ph.D., Robert Asarnow, Ph.D., David Fadale, B.A., Laura Heinichen, B.A., Cedric Ireland B.A.; Children’s Hospital of Philadelphia: Principal Investigators: Dah-Jyuu Wang, Ph.D., and Edward Moss, Ph.D.; Investigators: Robert A. Zimmerman, M.D.; and Research Staff Brooke Bintliff, B.S., Ruth Bradford, Janice Newman, M.B.A.; Data Coordinating Center at McGill University: Principal Investigator: Alan C. Evans, Ph.D.; Investigators: Rozalia Arnaoutelis, B.S., G. Bruce Pike, Ph.D., D. Louis Collins, Ph.D., Gabriel Leonard, Ph.D., Tomas Paus, M.D., Alex Zijdenbos, Ph.D.; and Research Staff Samir Das, B.S., Vladimir Fonov, Ph.D., Luke Fu, B.S., Jonathan Harlap, Ilana Leppert, B.E., Denise Milovan, M.A., Dario Vins, B.C.; Georgetown University: Thomas Zeffiro, M.D., Ph.D., and John Van Meter, Ph.D; Harvard University/McLean Hospital: Nicholas Lange, Sc.D., and Michael P. Froimowitz, M.S., work with data coordinating center staff and all other team members on biostatistical study design and data analyses; Clinical Coordinating Center at Washington University: Principal Investigator: Kelly Botteron, M.D.; Investigators: C. Robert Almli, Ph.D., Cheryl Rainey, B.S., Stan Henderson, M.S., Tomoyuki Nishino, M.S., William Warren, Jennifer L. Edwards, M.SW., Diane Dubois R.N., Karla Smith, Tish Singer, and Aaron A. Wilber, M.S.; Diffusion Tensor Processing Center at the National Institutes of Health: Principal Investigator: Carlo Pierpaoli, M.D., Ph.D.; Investigators: Peter J. Basser, Ph.D., Lin-Ching Chang, Sc.D., Chen Guan Koay, Ph.D., and Lindsay Walker, M.S.; Principal Collaborators at the National Institutes of Health: Lisa Freund, Ph.D. (National Institute of Child Health and Human Development), Judith Rumsey, Ph.D. (National Institute of Mental Health [NIMH]), Lauren Baskir, Ph.D. (NIMH), Laurence Stanford, PhD. (National Institute on Drug Abuse [NIDA]), Karen Sirocco, Ph.D. (NIDA), Katrina Gwinn-Hardy, M.D. (National Institute of Neurological Disorders and Stroke [NINDS]) and Giovanna Spinella, M.D. (NINDS); Spectroscopy Processing Center at the University of California Los Angeles: Principal Investigator: James T. McCracken, M.D.; Investigators: Jeffry R. Alger, Ph.D., Jennifer Levitt, M.D., Joseph O’Neill, Ph.D.
Footnotes
The views expressed herein do not necessarily represent the official views of the National Institute of Child Health and Human Development, the National Institute on Drug Abuse, the National Institute of Mental Health, the National Institute of Neurological Disorders and Stroke, the National Institutes of Health, the U.S. Department of Health and Human Services, or any other agency of the United States Government.
Disclosure: Drs. Ducharme and Nguyen receive financial support from the Canadian Institutes of Health Research with a Master’s Award: Frederick Banting and Charles Best Canada Graduate Scholarship. Dr. Hudziak has received funding from the National Institute of Mental Health (NIMH) and the National Institute of Diabetes and Digestive and Kidney Disease. He serves as a consultant to Erasmus University in Rotterdam and Avera Institute of Human Behavioral Genetics in Sioux Falls, South Dakota. Dr. Botteron has received funding from the National Institutes of Health, NIMH, National Institute of Child Health and Human Behavior, the National Institute of Neurological Disorders and Stroke, Communities Healing Adolescent Depression and Suicide Foundation, the McDonnell Foundation, the Simons Foundation, and Autism Speaks. Dr. Karama has received funding from the Fonds de Recherche en Santé du Québec. Dr. Evans is the founder and director of Biospective Inc. He serves as a consultant and receives stock options and licensing fees from the company. Dr. Albaugh reports no biomedical financial interests or potential conflicts of interest.
REFERENCES
- 1.Corbetta M, Shulman G. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- 2.Driver J. A selective review of selective attention research from the past century. Br J Psychol. 2001;92:53–78. [PubMed] [Google Scholar]
- 3.Posner M, Petersen S. The attention system of the human brain. Annu Rev Neurosci. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- 4.Shaw P, Greenstein D, Lerch J, et al. Intellectual ability and cortical thickness development in children and adolescents. Nature. 2006;440:676–679. doi: 10.1038/nature04513. [DOI] [PubMed] [Google Scholar]
- 5.Karama S, Ad-Dab’bagh Y, Haier R, et al. Positive association between cognitive ability and cortical thickness in a representative US sample of healthy 6 to 18 year-olds. Intelligence. 2009;37:145–155. doi: 10.1016/j.intell.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Westlye L, Grydeland H, Walhovd K, Fjell A. Associations between regional thickness and attentional networks as measured by the attention network test. Cereb Cortex. 2011;21:345–356. doi: 10.1093/cercor/bhq101. [DOI] [PubMed] [Google Scholar]
- 7.Biederman J, Faraone S. Attention-deficit hyperactivity disorder. Lancet. 2005;366:237–248. doi: 10.1016/S0140-6736(05)66915-2. [DOI] [PubMed] [Google Scholar]
- 8.Cherkasova M, Hechtman L. Neuroimaging in attention-deficit hyperactivity disorder: beyond the frontostriatal circuitry. Can J Psychiatry. 2009;54:651–664. doi: 10.1177/070674370905401002. [DOI] [PubMed] [Google Scholar]
- 9.Valera E, Faraone S, Murray K, Seldman L. Meta-analysis of structural imaging findings in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2007;61:1361–1369. doi: 10.1016/j.biopsych.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 10.Makris N, Biederman J, Valera E, et al. Cortical thinning of the attention and executive function networks in adults with attention-deficit/hyperactivity disorder. Cereb Cortex. 2007;17:1364–1375. doi: 10.1093/cercor/bhl047. [DOI] [PubMed] [Google Scholar]
- 11.Casey B, Castellanos F, Giedd J, et al. Implication of the right frontostriatal circuitry in response inhibition and attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 1997;36:374–383. doi: 10.1097/00004583-199703000-00016. [DOI] [PubMed] [Google Scholar]
- 12.Sowell E, Thompson P, Welcome S, Henkenius A, Toga A, Peterson B. Cortical abnormalities in children and adolescents with attention-deficit hyperactivity disorder. Lancet. 2003;362:1699–1707. doi: 10.1016/S0140-6736(03)14842-8. [DOI] [PubMed] [Google Scholar]
- 13.Castellanos F, Giedd J, Marsh W, et al. Quantative brain magnetic resonance imaging in attention-deficit hyperactivity disorder. Arch Gen Psychiatry. 1996;53:607–616. doi: 10.1001/archpsyc.1996.01830070053009. [DOI] [PubMed] [Google Scholar]
- 14.Castellanos F, Lee P, Sharp W, et al. Developmental trajectories of brain volume abnormalities in children and adolescents with attention-deficit/hyperactivity disorder. JAMA. 2002;288:1740–1748. doi: 10.1001/jama.288.14.1740. [DOI] [PubMed] [Google Scholar]
- 15.Shaw P, Lerch J, Greenstein D, et al. Longitudinal mapping of cortical thickness and clinical outcome in children and adolescent with attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2006;63:540–549. doi: 10.1001/archpsyc.63.5.540. [DOI] [PubMed] [Google Scholar]
- 16.Wager T, Jonides J, Reading S. Neuroimaging studies of shifting attention: a meta-analysis. NeuroImage. 2004;22:1679–1693. doi: 10.1016/j.neuroimage.2004.03.052. [DOI] [PubMed] [Google Scholar]
- 17.Bush G. Cingulate, frontal, and partietal cortical dysfunction in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2011;69:1160–1167. doi: 10.1016/j.biopsych.2011.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shaw P, Eckstrand K, Sharp W, et al. Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proc Natl Acad Sci USA. 2007;104:19649–19654. doi: 10.1073/pnas.0707741104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giedd J, Blumenthal J, Jeffries N, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nature Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- 20.Shaw P, Lalonde F, Lepage C, et al. Development of cortical asymmetry in typically developing children and its disruption in attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2009;66:888–896. doi: 10.1001/archgenpsychiatry.2009.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rubia K, Cubillo A, Smith A, Woolley J, Heyman I, Brammer M. Disorder-specific dysfunction in right inferior prefrontal cortex during two inhibition tasks in boys with attention-deficit hyperactivity disorder compared with boys with obsessive-compulsive disorder. Hum Brain Mapp. 2010;31:287–299. doi: 10.1002/hbm.20864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petrides M, Pandya D. Projections to the frontal cortex from the posterior parietal region in the rhesus monkey. J Compar Neurol. 1984;228:105–116. doi: 10.1002/cne.902280110. [DOI] [PubMed] [Google Scholar]
- 23.Makris N, Buka S, Biederman J, et al. Attention and executive systems abnormalities in adults with childhood ADHD: a DTMRI study of connections. Cereb Cortex. 2008;18:1210–1220. doi: 10.1093/cercor/bhm156. [DOI] [PubMed] [Google Scholar]
- 24.Shaw P, Gornick M, Lerch J, et al. Polymorphisms of the dopamine D4 receptor, clinical outcomes, and cortical structure in attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2007;64:921–931. doi: 10.1001/archpsyc.64.8.921. [DOI] [PubMed] [Google Scholar]
- 25.Shaw P, Gilliam M, Liverpool M, et al. Cortical development in typically developing children with symptoms of hyperactivity and impulsivity: support for a dimensional view of attention deficit hyperactivity disorder. Am J Psychiatry. 2011;168:143–151. doi: 10.1176/appi.ajp.2010.10030385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hudziak J, Copeland W, Stanger C, Wadsworth M. Screening for DSM-IV externalizing disorders with the Child Behavior Checklist: a receiver-operating characteristic analysis. J Child Psychol Psychiatry. 2004;45:1299–1307. doi: 10.1111/j.1469-7610.2004.00314.x. [DOI] [PubMed] [Google Scholar]
- 27.Hudziak J, Derks E, Althoff R, Rettew D, Boomsma D. The genetic and environmental contributions to attention deficit hyperactivity disorder as measured by the Conners’ rating scales-revised. Am J Psychiatry. 2005;162:1614–1620. doi: 10.1176/appi.ajp.162.9.1614. [DOI] [PubMed] [Google Scholar]
- 28.Chen W, Faraone S, Biederman J, Tsuang M. Diagnostic accuracy of the Child Behavior Checklist scales for attention-deficit hyperactivity disorder: a receiver-operating characteristics analysis. J Consult Clin Psychol. 1994;62:1017–1025. doi: 10.1037/0022-006X.62.5.1017. [DOI] [PubMed] [Google Scholar]
- 29.Achenbach T. Manual for the Child Behavior Checklist/4–18. Burlington, VT: University of Vermont; 1991. [Google Scholar]
- 30.Biederman J, Faraone S, Doyle A, et al. Convergence of the Child Behavior Checklist with structured interview-based psychiatric diagnoses of ADHD children with and without comorbidity. J Child Psychol Psychiatry. 1993;34:1241–1251. doi: 10.1111/j.1469-7610.1993.tb01785.x. [DOI] [PubMed] [Google Scholar]
- 31.Evans A Brain Development Cooperative Group. The NIH MRI study of normal brain development. NeuroImage. 2006;30:184–202. doi: 10.1016/j.neuroimage.2005.09.068. [DOI] [PubMed] [Google Scholar]
- 32.Waber D, De Moor C, Forbes P, et al. The NIH MRI study of normal brain development: performance of a population based sample of healthy children aged 6 to 18 years on a neuropsychological battery. J Int Neuropsychol Soc. 2007;13:729–746. doi: 10.1017/S1355617707070841. [DOI] [PubMed] [Google Scholar]
- 33.Achenbach T, Ruffle T. The Child Behavior Checklist and related forms for assessing behavioral/emotional problems and competencies. Pediatr Rev. 2000;21:265–271. doi: 10.1542/pir.21-8-265. [DOI] [PubMed] [Google Scholar]
- 34.Achenbach T, Rescorla L. Manual for the ASEBA school-age forms and profiles. Burlington, VT: University of Vermont: Research Center for Children, Youth & Families; 2001. [Google Scholar]
- 35.Crijnen A, Achenbach T, Verlhuist F. Problems reported by parents of children in multiple cultures: the Child Behavior Checklist syndrome constructs. Am J Psychiatry. 1999;156:569–574. doi: 10.1176/ajp.156.4.569. [DOI] [PubMed] [Google Scholar]
- 36.Heubeck B. Cross-cultural generalizability of CBCL syndromes across three continents: from the USA and Holland to Australia. J Abnorm Child Psychol. 2000;28:439–450. doi: 10.1023/a:1005131605891. [DOI] [PubMed] [Google Scholar]
- 37.Lyttelton O, Boucher M, Robbins S, Evans A. An unbiased iterative group registration template for cortical surface analysis. NeuroImage. 2007;34:1535–1544. doi: 10.1016/j.neuroimage.2006.10.041. [DOI] [PubMed] [Google Scholar]
- 38.Ad-Dab’bagh Y, Lyttelton O, Muehlboeck J, Lepage C, Einarson D, Mok K. The CIVET image-processing environment: a fully automated comprehensive pipeline for anatomical neuroimaging research. In: Corbetta M, editor. Proceedings of the 12th Annual Meeting of the Organization for Human Brain Mapping; Florence, Italy. 2006. 2006. [Google Scholar]
- 39.Kim J, Singh V, MacDonald D, Lee J, Kim S, Evans A. Automated 3D extraction and evaluation of the outer cortical surface using a Laplacian map and partial volume effect classification. NeuroImage. 2005;27:210–221. doi: 10.1016/j.neuroimage.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 40.MacDonald D, Kabani N, Avis D, Evans A. Automated 3D extraction of inner and outer surfaces of cerebral cortex from MRI. NeuroImage. 2000;13:340–356. doi: 10.1006/nimg.1999.0534. [DOI] [PubMed] [Google Scholar]
- 41.Collins D, Holmes C, Peters T, Evans A. Automatic 3D model-based neuroanatomical segmentation. Hum Brain Mapp. 1995;3:190–208. [Google Scholar]
- 42.Aiken L, West S. Multiple regression: testing and interpreting interactions. Thousand Oaks, CA: Sage Publications; 1991. [Google Scholar]
- 43.Rubia K, Overmeyer S, Taylor E, et al. Hypofrontality in attention deficit hyperactivity disorder during higher-order motor control: a study with functional MRI. Am J Psychiatry. 1999;156:891–896. doi: 10.1176/ajp.156.6.891. [DOI] [PubMed] [Google Scholar]
- 44.Dickstein S, Bannon K, Castellanos F, Milham M. The neural correlates of attention deficit hyperctivity disorder: an ALE meta-analysis. J Child Psychol Psychiatry. 2006;47:1051–1062. doi: 10.1111/j.1469-7610.2006.01671.x. [DOI] [PubMed] [Google Scholar]
- 45.Nachev P, Kennard C, Husain M. Functional role of the supplementary and pre-supplementary motor areas. Nature Reviews Neuroscience. 2008;9:856–869. doi: 10.1038/nrn2478. [DOI] [PubMed] [Google Scholar]
- 46.Ducharme S, Hudziak J, Botteron K, et al. Right anterior cingulate cortical thickness and bilateral striatal volume correlate with Child Behavior Checklist aggressive behavior scores in healthy children. Biol Psychiatry. 2011;70:283–290. doi: 10.1016/j.biopsych.2011.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van Tol M, van der Wee N, van den Heuvel A, et al. Regional brain volume in depression and anxiety. Arch Gen Psychiatry. 2010;67:1002–1011. doi: 10.1001/archgenpsychiatry.2010.121. [DOI] [PubMed] [Google Scholar]
- 48.Makris N, Seidman L, Valera E, et al. Anterior cingulate volumetric alterations in treatment-naïve adults with ADHD: a pilot study. J Atten Disord. 2010;13:407–413. doi: 10.1177/1087054709351671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miller G. Beyond DSM: seeking a brain-based classification of mental illness. Science. 2010;327:1437. doi: 10.1126/science.327.5972.1437. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.