Abstract
Drug self-administration is a procedure in which a subject performs a specified response that results in the delivery of a drug injection. This procedure is viewed as a relevant model for the study of human drug-taking behavior. Drug self-administration in primates has several characteristics that resemble drug-taking behavior in humans, and agents commonly abused by humans also generally maintain self-administration behavior in monkeys. Self-administration procedures allow for the study of a variety of drug properties. For instance, they can be used to investigate the abuse potential of new compounds and to study the effects of candidate medications for the treatment of drug addiction. These procedures also can be employed for examining drug reinforcement mechanisms. Described in this unit are procedures for studying intravenous drug self-administration in large primates, such as rhesus macaques, and smaller primates, such as squirrel monkeys.
INTRODUCTION
Drug self-administration is a procedure in which a subject performs a specified response, such as pressing a lever, which results in the delivery of a drug or other chemical substance. This procedure is viewed as a relevant model for the study of human drug-taking behavior. Self-administration in primates has several characteristics that resemble drug-taking behavior in humans, and agents that are commonly abused by humans generally maintain self-administration behavior in monkeys (Schuster and Johanson, 1974). Self-administration procedures allow for the study of a variety of drug properties. For instance, they can be used to investigate the abuse potential of new compounds and to study the effects of candidate medications for the treatment of drug addiction. These procedures also can be employed for examining neurobiological mechanisms underlying drug reinforcement. This unit describes procedures for studying intravenous drug self-administration in large primates, such as rhesus macaques, and in smaller primates such as squirrel monkeys (see Basic Protocol). Descriptions of self-administration techniques in rodents are available from other sources (Weeks, 1972; Caine et al., 1993). In this unit support protocols describe the implantation and testing of intravenous catheters.
Self-administration studies in primates require access to healthy monkeys and the facilities to house them properly. The use of primates, particularly large primates such as macaques, in biomedical research is highly regulated at the institutional, local, and federal levels. The facilities needed to house them are expensive to build and maintain. Animal care staff must, for their own safety and that of the primates, be familiar with the husbandry of large and powerful animals. Monkeys can harbor a variety of diseases that are readily transmitted to humans (e.g., herpes B virus in macaques). Investigations with primates also require access to well-equipped veterinary facilities, operating rooms, expertise in sterile procedure, and experience in basic vascular surgery.
NOTE: All protocols using live animals must first be reviewed and approved by an Institutional Animal Care and Use Committee (IACUC) and must conform to governmental regulations regarding the care and use of laboratory animals.
NOTE: Before testing novel agents, adequate pharmacokinetic studies should be performed to ensure that any self-administration behavior – or lack thereof – can be related to the presence or absence of compound.
STRATEGIC PLANNING
Experimental Chamber
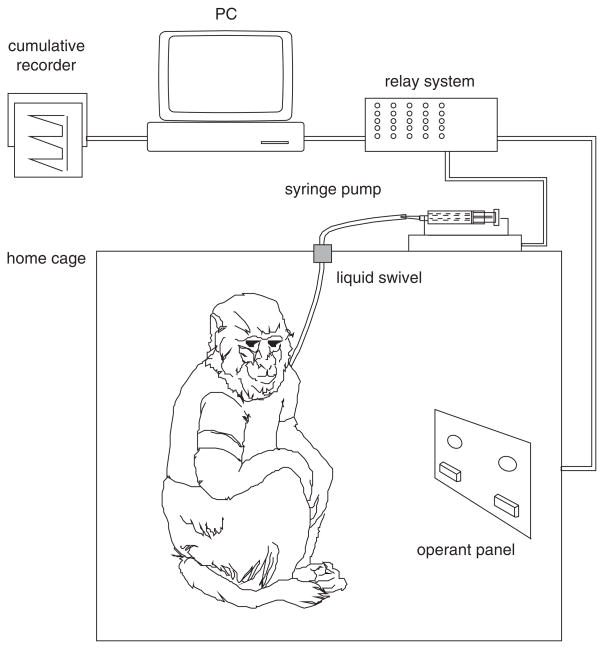
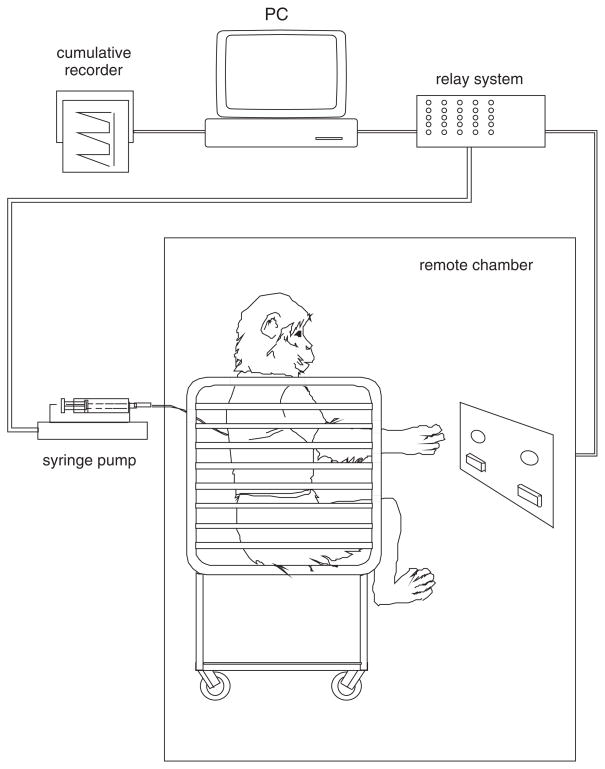
There are two basic types of experimental chambers used to conduct self-administration studies in primates. In one there is a permanent link between the monkey and the self-administration apparatus, making it possible to conduct the studies in the home-cage (Fig. 10.5.1). With this arrangement, one wall of the cage is replaced by an operant panel, allowing the animal access to manipulanda (usually levers) that control drug delivery and, in some cases, food and water delivery (Schuster and Johanson, 1974). A second approach entails removing the subject from its home-cage and placing it in a restraining device (usually a chair to afford minimal restraint consistent with safety), which is transported to a separate self-administration chamber remote from the living quarters (Fig. 10.5.2).
Figure 10.5.1.
Schematic representation of a monkey home cage self-administration apparatus. Note that the monkey lives and works in the same cage.
Figure 10.5.2.
Schematic representation of a monkey remote-chamber self-administration apparatus. Note that the monkey is restrained in a primate restraint chair, which is then transported to the self-administration apparatus.
Each of these procedures has advantages and disadvantages. The home-cage technique allows many animals to be studied concurrently during multiple sessions each day. In addition, less time is needed to habituate an animal to handling and restraint. The experiments are not particularly labor intensive and can, in principle, continue around the clock. The disadvantages of the home-cage technique are increased cost and loss of flexibility. It is expensive to install operant panels and self-administration equipment for all animals. Also, because all of the subjects tend to start and end the experimental session at approximately the same time, with the home-cage approach it is relatively difficult to isolate individual animals from the rest of the colony. Experimental isolation may be useful when it is necessary to work with an individual animal during initial training or if problems arise with self-administration performance during an experiment. It is also less easy to inspect the catheter on a daily basis with the home cage technique, making more difficult the early detection of infection or catheter blockage.
A remote chamber allows more flexibility in experimental scheduling. The procedure is more economical as only one self-administration apparatus is needed to test a number of animals consecutively during the day. However, the procedure is also relatively labor intensive, as each animal must be trained to accept the handling and restraint necessary for transport to the remote site. Depending on the age, size, and temperament of the individual animal, this training can take several days to several weeks. During the initial habituation process, rewarding with food (e.g., raisins, peanuts) successive approximations of progression to the primate restraint chair increases the rapidity with which the animal becomes trained. Because with remote chambers only one self-administration session is normally conducted with each animal daily, experiments may progress at a slower pace than with the home-cage procedure. An advantage of the remote chamber is that the catheter can be inspected daily; making it more likely that catheter failure or infection will be identified at an early stage, thereby prolonging the experimental life of the subject.
Catheters
Catheters for both rhesus monkeys and squirrel monkeys are typically constructed from polyvinyl chloride, Tygon, microrenathane, or silicon microbore tubing. The dimensions of the catheters described in this unit (see Table 10.5.1) are intended as a guide and are not absolute. Other styles and dimensions are available from commercial sources. Individual investigators should modify the dimensions of the catheters so they are most appropriate for the experimental subject.
Table 10.5.1.
Catheter Dimensions for Squirrel and Rhesus Monkeys
| Jugular Vein | Femoral Vein | |
|---|---|---|
| Squirrel monkey | ||
| Gauge | 25 | 25 |
| Tip to cuff (cm) | 4 | 12 |
| Cuff to cuff (cm) | 3.5 | 3.5 |
| Total length (cm) | 35 | 50 |
| Rhesus monkey | ||
| Gauge | 21 | 21 |
| Tip to cuff (cm) | 7 | 18.5 |
| Cuff to cuff (cm) | 7 | 6.5 |
| Total length (cm) | 60 | 80 |
Reinforcement Schedules
Because the effectiveness of an agent in maintaining self-administration behavior depends on the precise scheduling conditions under which it is delivered, it is important that self-administration experiments be conducted using a schedule of reinforcement appropriate for the question being asked (for reviews see Kelleher, 1975; Spealman and Goldberg, 1978). For instance, a simple fixed-ratio schedule may be the best choice if the aim is to determine whether a particular substance maintains self-administration behavior, whereas a progressive-ratio schedule might be better suited to a comparison of the relative effectiveness of different drugs as reinforcers. Second-order schedules, in which persistent behavior is maintained by drug-associated stimuli, are useful for studying the reinforcing effects of widely spaced injections or for investigating the control of drug-taking behavior by environmental stimuli paired with drug injections. Brief descriptions of three reinforcement schedules, as well as choice procedures, commonly used in drug self-administration studies with monkeys are provided below. Table 10.5.2 provides examples of typical parameter values for each of the reinforcement schedules described below used in cocaine self-administration studies with rhesus monkeys.
Table 10.5.2.
Typical Parameter Values Used in Cocaine Self-Administration Studies in Rhesus Monkeysa
| Reinforcement schedule | Reinforcer | Response requirementb | Time out | Session length | Maximum number of rewards |
|---|---|---|---|---|---|
| Fixed-ratio | 0.03 mg/kg/injection | FR 30 | 60 s | 90 min | Determined by monkey |
| Second-order | 0.1 mg/kg/injection | FI 10 min FR 20 |
60 s | 60 min | 5 injections |
| Progressive-ratio | 0.03 mg/kg/injection | IRR 50 | 10 min | After 2 hr without injection | Determined by monkey |
| Choice | 1 g food pellets vs. 0.1 mg/kg/injection | Food: FR 100 Drug: FR 10 |
30 s | 2 hr | 10 reinforcers/component |
Parameter values from: Gerak et al., 2008; Kimmel et al., 2008; Banks and Negus, 2010; Czoty et al., 2010.
FR: fixed-ratio; FI: fixed-interval; IRR: initial response requirement
Fixed-ratio schedules
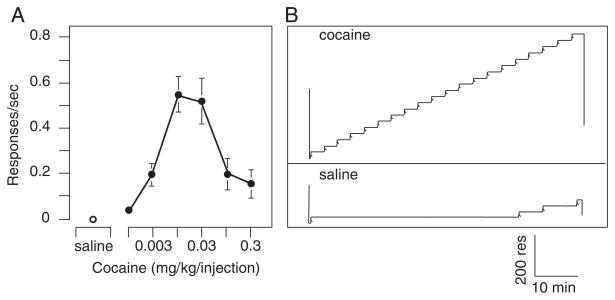
The most frequently employed schedule of reinforcement is the fixed-ratio schedule. In this case, a drug infusion is given each time the subject completes a fixed number of responses. Behavior maintained under fixed-ratio schedules is characterized by an initial brief period of no response, followed by an abrupt transition to a high, steady rate of response that continues until the response requirement is completed and the drug injection delivered (Figure 10.5.3). Many CNS-active drugs from different pharmacological classes have been studied using fixed-ratio schedules, including psychomotor stimulants, opioids, sedatives and hypnotics, and dissociative anesthetics (Spealman and Goldberg, 1978).
Figure 10.5.3.
(A) Effect of cocaine dose on response rate under a 30-response fixed-ratio schedule of intravenous drug delivery. A 3-min timeout period followed each injection and the total session length was 60 min. A maximum of 20 injections could be earned each session. Each point is the mean of the last three sessions at each dose averaged for a group of three rhesus monkeys. Open circle, saline; filled circle, cocaine dose on a logarithmic scale. (B) Representative performance (number of responses versus time) of a monkey under the 30-response fixed-ratio schedule of 0.03 mg/kg/injection of cocaine (top) and saline (bottom). Each reinforcement is a drug injection (indicated by a diagonal stroke at each step up).
One characteristic of fixed-ratio schedules is the direct relationship between the rate of responding and the frequency of injection. Even with short-acting agents, the drug may accumulate rapidly while responding is maintained, causing a change in the rate of response as the session progresses. Thus, responses are influenced not only by the reinforcing effects of the self-administered agent, but also by other pharmacological effects. When using fixed-ratio schedules of reinforcement it is usually advisable to program timeout periods after each injection to limit the rapid accumulation of drug, making it easier to maintain self-administration over more extended periods. The duration of the timeout is mainly determined by the time course of action of the particular agent under study, although it is limited by time considerations. An example of the influence timeout duration can have in determining the shape of the dose-response function, as well as pattern of drug self-administration, comes from Griffiths et al. (1979). In baboons self-administering cocaine under a fixed-ratio schedule with 3-h timeouts between injections, the dose-response is represented by an inverted u-shaped curve, with patterns of self-administration at the highest cocaine doses being erratic and variable. However, when the timeout was increased to 12-h, the dose-response curve was monotonic with no descending limb, and patterns of responding were consistent and stable.
Using fixed-ratio schedules also may allow the investigator to examine whether a drug initiates and maintains self-administration in its own right, or whether it substitutes for an already self-administered training agent. Generally, substitution procedures are used to ascertain abuse liability, although acquisition procedures are sometimes employed instead. Use of these two approaches can highlight differences between test substances. For instance, albeit under a different schedule, a dopamine D3 receptor agonist did not initiate and maintain self-administration in drug-naive monkeys, but did substitute for cocaine and maintain self-administration in animals trained previously to self-administer cocaine (Nader and Mach, 1996).
Second-order schedules
Simple schedules, such as fixed-ratio, may be used as units to form more complex second-order schedules (Goldberg, 1975). Under second-order schedules, the behavioral requirement of one schedule is treated as a unit of responding that is itself reinforced according to a second schedule. Thus, a fixed-interval schedule of drug injection with fixed-ratio units is one in which completion of each fixed ratio produces a brief visual stimulus (previously associated with drug injection), and the first fixed ratio completed after a specified interval of time produces both the brief stimulus and an injection of drug. Under second-order schedules, long and orderly sequences of behavior are maintained with infrequent injections of drug (Figure 10.5.4).
Figure 10.5.4.
(A) Effect of cocaine dose on response rate under a second-order 10-min fixed-interval schedule of ten-response fixed-ratio units. A 1-min timeout followed each injection. Each point is the mean of the last three sessions at a particular dose averaged for a group of twelve squirrel monkeys. Open circle, saline; filled circles, cocaine dose on a logarithmic scale. (B) Representative performance (number of responses versus time) of a squirrel monkey under the second-order schedule of 0.1 mg/kg/injection of cocaine (top) and saline (bottom). Short downward diagonal strokes on the cumulative record indicate presentations of a brief (1-sec) visual stimulus that followed each ten-response fixed-ratio unit. Resetting of the recording pen indicates injection of cocaine or saline.
Progressive-ratio schedules
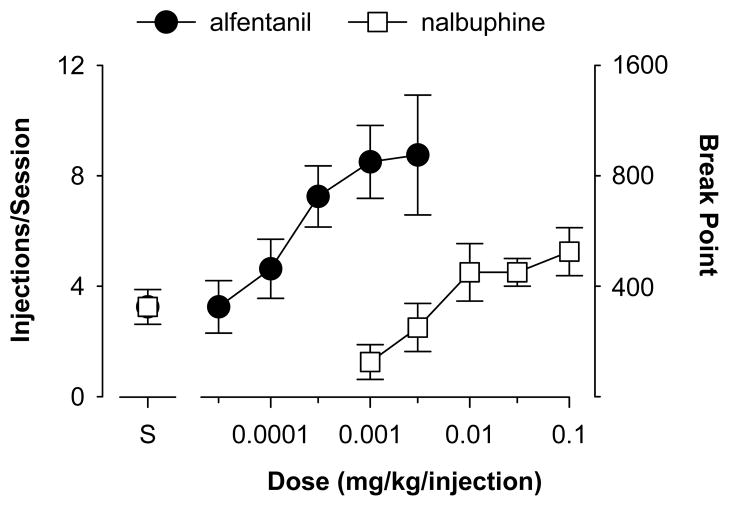
Under progressive-ratio schedules, the number of responses required for each successive injection increases systematically until responding falls below some pre-established level typically coinciding with a cessation of responding across an extended period of time. The response requirement at which this occurs is called the break point. By comparing the break point of different drugs under the same schedule parameters, an estimate of their relative effectiveness in maintaining self-administration can be determined. An example of typical performance maintained under this type of schedule for the high efficacy opioid receptor agonist alfentanil and the low efficacy opioid receptor agonist nalbuphine is shown in Figure 10.5.5. These data demonstrate the positive correlation between in vitro efficacy and the effectiveness of the drugs as reinforcers.
Figure 10.5.5.
Effects of the opioid receptor agonists alfentanil (closed circles) and nalbuphine (open squares) on number of injections/session and break point under a progressive-ratio schedule. A 30-min timeout followed each injection. The initial response requirement was 100, and this requirement doubled every four injections. Each point represents data averaged for a group of four rhesus monkeys. Point over “S” shows performance when saline was available for self-administration.
There are a number of ways in which progressive-ratio experiments are conducted. Most commonly, the required number of responses may be increased systematically after each injection of drug in the experimental session. Alternatively, the subject may be given access to a preset number of injections at one response requirement during a single session. Then, following a long timeout (e.g., 1 hr), the animal is given access to the same number of injections at an increased response requirement. The response requirement is subsequently increased in each session until the break point is reached.
Choice procedures
In choice procedures, animals are given the opportunity to choose between two or more reinforcers; the most common approach being the concurrent availability of drug with a non-drug reinforcer, such as food. The usual primary dependent variable in choice procedures is a measure of the proportion of behavior allocated to each reinforcer. This measure is relatively independent of rates of responding, and dose-response functions for choice tend to be monophasic, even when the functions for response rate are biphasic. Relatively high doses of a drug that tend to suppress response rates below control levels may be chosen over concurrently available food, lower doses of the drug, or saline (Johanson, 1976). Compared to self-administration procedures that have only a single reinforcer available, choice procedures approximate more closely the conditions under which humans take drugs, namely complex environments in which a variety of alternative reinforcers are available. These procedures may be especially useful for the study of factors that promote drug choice and abuse. However, the use of choice procedures is limited by the relative difficulty of training, as well as the long-term nature of the studies. For example, determinations of dose-response functions in choice studies typically require several weeks (but see Negus, 2003 for a relatively rapid choice procedure).
BASIC PROTOCOL
SELF-ADMINISTRATION TRAINING AND TESTING
A number of approaches are utilized to train monkeys to self-administer drugs and other test agents. For example, monkeys can be food restricted and then trained on a food-reinforced operant. Once stable responding for food is achieved, it is replaced by the drug as the reinforcer. Alternatively, monkeys can be trained directly with no prior experience using the method of successive approximations. In this case, their ability to manually operate the infusion pump is contingent on successively closer approximations to the lever press response. Animals acquire self-administration readily using this approach, particularly with highly reinforcing agents such as cocaine.
There are many variants of these training methods to facilitate the acquisition of self-administration behavior. The protocol detailed below is a typical self-administration procedure.
Subjects
Monkeys: male/female ≥ 4-kg rhesus macaques (e.g., Covance Research Products, Denver, PA) or ≥ 0.75-kg squirrel monkeys (e.g., Worldwide Primates, Inc., Miami, FL)
Materials
Raisins or peanuts (optional)
Short length of metal chain (optional)
100 to 200 IU/ml heparinized saline, sterile
Sterile saline
Training compound and vehicle, filter-sterilized
Remote-chamber or home-cage self-administration apparatus (see Strategic Planning) with schedule control apparatus (e.g., Med Associates, Georgia, VT; Lafayette Instrument Company, Lafayette, IN)
Tether system: custom-fitted jacket connected to a flexible stainless steel cable by a fluid swivel (e.g., Lomir Biomedical, Inc., Malone, NY; Alice King Chatham Medical Arts, Hawthorne, CA)
Restraining chair (for remote chamber only: Plas-Labs, Inc. Lansing, MI; Crist Instruments Company, Inc. Hagerstown, MD; Primate Products, Inc., Immokalee, FL for macaques; Med Associates for squirrel monkeys)
Additional reagents and equipment for implanting and testing patency of catheter (see Support Protocols 1 and 2)
Habituate and catheterize animals
-
1
Habituate monkeys ≥ 30 min daily to a tether or to a remote chamber, and to the handling procedures to be used during the experiment. For the remote chamber, restrain the animal in the chair, allow it time to sit quietly, and then move the chair to the ventilated remote chamber where the experiment is to be conducted.
Depending on the monkey, habituation may take several days to several weeks. For the remote chamber, one of the most important considerations is the ease with which the monkey is transferred from its home cage into the restraining chair without inducing stress.
For the home cage method, a suitably constructed tether system permits the monkey to move with relative freedom, but protects the catheter from damage by the monkey. Drug solutions are delivered from an infusion system located outside the cage. The swivel system must turn easily, as resistance to movement not only endangers the catheter, but can result in disruption of the animal’s performance. The protective vest is used for both remote and home-cage systems.
-
2
Place the animal in the experimental chamber and allow it to begin responding. Strategies used to increase the likelihood that the animal will contact the lever include placing raisins or peanuts on it, increasing the size of the lever, or attaching a short length of chain to it to attract the animal’s attention.
These techniques can be used singly or in combination. Alternatively, drug infusions can be used for this initial step. The technique(s) employed will depend on the speed with which the individual monkey is progressing and the experience of the investigator.
This initial response shaping may take several days.
The schedule control apparatus consists of equipment and computer programs that control presentation and record responses (e.g., Med Associates; Lafayette Instrument Company). It is usually purchased as a package and assembled in the experimental room/chamber according to manufacturer’s instructions.
-
3
Once consistent responding is established, eliminate modifications to the lever (food, increased size, or chain).
-
4
Implant a catheter in the jugular or femoral vein (see Support Protocol 1) of each subject.
-
5
During recovery from surgery (typically 7 to 10 days), flush the catheter daily with ~1 ml of 100 to 200 IU/ml heparinized saline if a macaque, or an equivalent volume of sterile saline if a squirrel monkey. The use of heparin as an anticoagulant in squirrel monkeys is not recommended because of an increased likelihood of bleeding problems.
Train animals
-
6a
For remote-chamber method: On the day of the experiment, restrain the monkey in the chair, allow the animal time to sit quietly, and then move the chair to the ventilated, remote chamber. Protect the catheter with the protective vest or jacket and ensure that the animal’s movement is not overly restricted.
-
6b
For home-cage method: Protect the catheter with the tether system and protective vest or jacket, and ensure that the animal’s movement is not restricted.
-
7
Flush ~1 ml sterile saline through the catheter to verify its patency for the remote-chamber method. Connect the catheter to the self-administration apparatus, either directly (for externalized catheter) or by way of a Huber needle (i.e., right angled, non-coring needle) through the skin and into the subcutaneous access port.
At all stages, it is essential that material coming into contact with the catheter remain as sanitary as possible. Strict attention to cleanliness prevents infection and prolongs catheter life.
-
8
Fill a syringe with a filter-sterilized solution of the training agent, and shape behavior of untrained monkeys to respond to an active dose of the training compound (e.g., 0.1 mg/kg/injection cocaine) upon pressing the lever, using a high number of trials (10 to 15 over ~1 hr). With previously lever-trained animals, use a lower dose, such as 0.01 to 0.03 mg/kg/injection of cocaine in 10 to 20 trials over ~1 hr.
With an effective reinforcer such as cocaine, the desired level of responding typically takes 2–4 days to develop.
It is very important that drug self-administration and animal behavior be closely monitored to prevent overdose, especially when high doses are used to facilitate the initial training.
-
9
If the animal does not readily acquire self-administration behavior, prime the animal by administering one or two non-contingent injections of the training drug at the beginning of the next self-administration training session.
The priming approach, combined with the above modifications to the manipulandum, will usually foster self-administration behavior. Depending on the schedule of reinforcement, however, it may take several weeks to maintain stable and consistent patterns of responding day-to-day.
-
10
If the animal still exhibits poor acquisition or inconsistent patterns of self-administration, test catheter patency by performing a ketamine test (see Support Protocol 2).
-
11
Once consistent self-administration of the training compound is established, replace it in the syringe with vehicle to train the animal to respond differentially to the presence versus absence of drug in the injected solution. Repeat training with vehicle until responding is no longer maintained (extinction).
Extinction can take anywhere from 1 – 5 sessions. The extinction time usually decreases with repeated training.
Initially, the animal may respond at a rate equal to, or even greater than, the rate at which it responded to the training compound solution. However, over successive sessions the rate of responding decreases, eventually falling to a level where the monkey takes few or no injections. Training the animal to respond differentially to the presence and absence of drug is one of the most important aspects of initial training and helps ensure orderly dose-dependent results with test compounds or pretreatment with candidate medications targeting drug abuse.
Perform self-administration tests
-
12
When the response rate or number of injections per session for vehicle injection is extinguished, adjust test agent doses for the actual self-administration testing and determine the required schedule of reinforcement (see Strategic Planning and protocol introduction). Determine the effects of vehicle and, for example, four doses of test compound.
For well-characterized compounds such as cocaine or heroin, there is substantial literature regarding appropriate doses (e.g., cocaine: see Table 10.5.2; heroin: Mello and Negus, 1998; Negus 2006; Rowlett et al., 2007; Gerak et al., 2009).
-
13
For remote-chamber method: Following completion of the test session, disconnect the subject’s catheter from the self-administration apparatus. Inject ~1 ml of 100 to 200 IU/ml heparinized saline (macaque) or sterile saline (squirrel monkey) to flush through any residual drug solution.
-
14
Return the monkey to its home environment.
SUPPORT PROTOCOL 1
CATHETER IMPLANTATION
Intravenous self-administration requires surgical implantation of a chronic indwelling venous catheter. Shown in Table 10.5.1 is the approximate catheter dimensions for jugular and femoral veins in squirrel and rhesus monkeys. These lengths are adequate for a monkey of average size. When the monkey is larger or smaller than average, it is possible to “custom-fit” a catheter to the individual during surgery. This is accomplished by measuring the distance from the proximal cuff of the catheter to mid-sternum level of the monkey and cutting the length of catheter based on this estimate. Vascular surgery in primates should be performed only by individuals experienced with the techniques involved. It is essential for the health of the monkey, and the long-term success of the experiment, that catheter implantation is performed using sterile techniques in a well-equipped operating theater. In primates, the femoral and internal jugular veins are the preferred sites for catheter placement, as these vessels are relatively large, protected by an overlying layer of muscle, and yield the longest experimental life (months to years). However, other vessels, such as the external jugular, brachial, and iliac veins, may also be catheterized.
Additional Materials (also see Basic Protocol)
Habituated monkeys (see Basic Protocol, steps 1a and 1b)
Ketamine, veterinary grade (e.g., Butler Schein Animal Health, Melville, NY)
Atropine, veterinary grade (e.g., Butler Schein Animal Health)
Propofol, veterinary grade (e.g., Butler Schein Animal Health)
1% to 1.5% (v/v) isoflurane in oxygen
Povidone iodine (e.g., Butler Schein Animal Health)
70% (v/v) ethanol
Tracheal tube (2.5 – 5.5 mm diameter for macaques, 2 – 3 mm for squirrel monkeys; Rusch Medical/Teleflex Medical, Research Triangle Park, NC)
Intravenous catheter line (angiocatheter; 22-G for macaques, 24-G for squirrel monkeys; Terumo Medical, Co., Somerset, NJ)
Electric razor (Oster; www.Amazon.com)
Sterile surgical instruments
Surgical table (Paragon Medical, Coral Springs, FL)
4-0 Ethibond Excel polyester suture (e.g., Ethicon, Somerville, NJ)
4-0 Surgical gut suture (e.g., Ethicon)
3-0 Ethilon monofilament nylon suture (e.g., Ethicon)
Vascular clips
Vannas scissors (Miltex, Inc., York, PA; Roboz Surgical Instrument, Co., Gaithersburg, MD)
Catheter (see Table 10.5.1)
Sterile stainless steel obturator (optional; Vita Needle Company, Inc., Needham, MA)
Access port (optional; Access Technologies, Inc., Salem, OR)
NOTE: Perform catheter implantations under aseptic conditions in a fully equipped veterinary operating room.
Prepare animal for surgery
-
1
Anesthetize habituated monkeys with a 10- to 20-mg/kg i.m. injection of ketamine.
If using a remote chamber, the animal should be accustomed to leaving the home cage and to sitting in the chair in the remote chamber prior to implantation of the catheter. For the home-cage method, the animal should be habituated to the tether system. For details, see Basic Protocol, step 1.
-
2
Insert intravenous catheter line (angiocatheter) into a saphenous vein and begin an i.v. infusion of normal saline at 5 ml/hr for squirrel monkeys and 20 ml/hr for macaques.
-
3
Administer 0.05 mg/kg atropine by i.m. injection to control salivation.
-
4
Insert a tracheal tube and maintain anesthesia by continuous inhalation of 1% to 1.5% isoflurane.
For squirrel monkeys, propofol (7.5 mg/kg, i.v. via angiocatheter) frequently is administered before intubation to aid anesthesia induction.
-
5
Shave the surgical site and then clean it with three alternating scrubs each of povidone iodine (or equivalent) and 70% (v/v) ethanol.
Isolate vein for catheterization
For internal jugular vein:
-
6a
Place the animal on its back and secure it to a surgical table. Make an incision in the neck ~4 cm lateral to the midline of the trachea.
-
7a
Retract the skin and separate the muscle with blunt dissection to reveal the carotid sheath. Dissect away the internal jugular vein.
For femoral vein:
-
6b
Place the animal on its back and secure its limbs to a surgical table. Make a longitudinal incision ~6 cm long through the skin, parallel to the midline of the inner thigh above the knee (i.e., parallel to and just above the sartorious muscle rostral to the pectineous and gracilis muscle).
-
7b
Dissect away the medial femoral fascia to reveal the femoral vessels. Separate the vein from the artery and clear away excess fascia.
Implant catheter
-
8
Place two 4-0 Ethibond Excel polyester ligatures ~2.0 cm apart around the vein, bracketing the intended catheter insertion site. Tie an overhand knot in each of the ligatures, but do not tighten.
-
9
Place a vascular clip at the proximal end of the dissected vein (i.e., the end closest to the heart) and close it gently, allowing the portion of the vein distal to the clip to fill with blood.
-
10
Tighten the ligature around the distal end of the vein to occlude the vessel, and make a square knot to prevent loosening.
-
11
Make a small incision with Vannas scissors in the top of the vein between the distal ligature and the clip.
A small amount of blood will leak from the incision site when the lumen of the vessel is entered.
-
12
Insert the catheter into the vein until it abuts the vascular clip. Gently tighten the ligature at the proximal end around the vein and the inserted portion of the catheter.
-
13
Remove the vascular clip and insert the catheter until the tip reaches a position just above the right atrium.
If the catheter is inserted too far and enters the right atrium, blood will visibly pulse within the catheter.
-
14
Secure the catheter in the vein by tying the proximal ligature with a square knot, forming a half-hitch behind the proximal cuff of the catheter. Tie the distal ligature behind the cuff with a square knot.
-
15
Make a loop in the distal portion of the catheter and loosely anchor the catheter beneath the surrounding muscle using a 4-0 Ethibond Excel polyester suture tied around the second, distal cuff. Subcutaneously route the distal portion of the catheter with a trocar to an exit area in the mid-scapula region and close the incision with the gut and/or monofilament nylon suture.
Access the catheter
For externalized port:
-
16a
Externalize the catheter through a small incision in the skin and occlude it using a sterile stainless steel obturator until access is required.
The externalized port can be used for both home-cage and remote-cage procedures.
-
17a
Protect the free end of the catheter by placing the monkey in a close-fitting nylon mesh jacket (see Basic Protocol) with a small pocket on the inside to receive the externalized portion of the catheter.
-
18a
Terminate anesthesia and return the animal to the home cage to recover (2 to 4 days).
Animals should be monitored continuously immediately after surgery and until fully alert and moving. Buprenorphine or another suitable analgesic should be administered prior to surgery and b.i.d. for 24 hr after the surgical procedure.
For subcutaneous access port:
-
16b
Attach the distal end of the catheter to a subcutaneous access port. Place the access port into a subcutaneous pocket, prepared by blunt dissection, and use sutures to secure it to underlying tissue using the predrilled holes that encircle the base of the port.
The subcutaneous port is generally used for remote-cage procedures.
-
17b
Suture the incision closed, making certain the sutures do not lie directly over the membrane of the port.
A jacket can be used to protect the wound until it is fully healed. After this time, the jacket can be removed.
Intravenous injections are administered by piercing the overlying skin and membrane of the access port with a noncoring Huber needle attached to an infusion line. This technique has an advantage over the externalized catheter, as there are no portions that the monkey can reach. It also has disadvantages in that the skin must be pierced each time the infusion line is attached and the Huber needle may move around or even disconnect if not secured.
-
18b
Terminate anesthesia and return the animal to the home cage to recover (2 to 4 days).
Animals should be monitored continuously immediately after surgery until fully alert and moving. Buprenorphine, or another suitable analgesic, should be administered prior to surgery and b.i.d. for 24 hr after the surgical procedure.
SUPPORT PROTOCOL 2
DETERMINATION OF CATHETER PATENCY USING THE KETAMINE TEST
The ketamine test is a simple procedure for assessing catheter patency. Ketamine hydrochloride is a short-acting NMDA receptor antagonist that produces anesthesia within seconds when injected intravenously. The ketamine test should be performed on monkeys that exhibit poor acquisition or inconsistent patterns of self-administration, possibly indicating a malfunctioning catheter.
Materials
Catheterized monkey (see Support Protocol 1)
Ketamine, veterinary grade (Butler Schein Animal Health)
-
Sterile saline
Fill a syringe with a volume of ketamine (10 mg/kg) slightly less than the total volume of the catheter.
Fill a second syringe with a sufficient volume of sterile saline to flush the catheter completely.
Attach the ketamine syringe to the catheter and infuse its contents.
-
Remove the ketamine syringe from the catheter, attach the sterile saline syringe to the catheter and infuse the contents.
This procedure ensures that the full dose of ketamine is administered as a bolus, with none remaining in the catheter.
If the catheter is patent, the monkey should lose consciousness almost immediately. Unconsciousness will persist for 3 to 5 min, after which the animal will awaken and be fully alert within an hour. If the catheter is not patent, the monkey did not receive the full dose of ketamine. In that case the animal may be unaffected or may become drowsy after 2 to 3 min. Another indication of catheter malfunction is that the monkey attempts to reach for, or scratch at, the site of catheter implantation or along the catheter tract. This reaction is provoked by irritation caused by the ketamine solution leaking into the subcutaneous space.
COMMENTARY
Background Information
Nonhuman primates have long been considered important experimental animals for studying biomedical disease processes primarily because of their similarities to humans in terms of their genetics, anatomy, physiology, and behavior. In addition, the use of nonhuman primates allows for complex longitudinal experimental designs, incorporating repeated measures that span critical developmental periods or the complex physiological processes that contribute to long-term behavioral- and neuro-adaptation. These and other characteristics of nonhuman primates make them ideal subjects for studies geared toward translating findings from rodents to humans.
Intravenous self-administration of drugs by animals, particularly primates, is viewed as being the most valid animal model of drug taking behavior in humans. This procedure is useful on several levels, including its face, predictive, and construct validities. Self-administration in monkeys has good face validity in that it bears a close physical resemblance to human drug-taking behavior. Additionally, drugs of abuse also maintain self-administration in monkeys, as well as other laboratory animals, providing this model with a high degree of predictive validity. As for construct validity, there is much evidence suggesting that the neurochemical mechanisms that underlie self-administration of drugs in animals overlap with those mediating natural rewards and drug reinforcement in humans.
The use of self-administration procedures in general, and monkey self-administration in particular, can predict the likelihood of whether a test agent will be abused by humans. It can also provide information as to how effective a particular pharmacotherapy may be for treating human addiction. Moreover, drug self-administration in primates also may be used to study the process of drug reinforcement under controlled conditions. This information may then be used to make assumptions as to how drugs alone, and drugs in combination with environmental stimuli associated with the drugs, contribute to the persistent patterns of drug taking observed in humans. This may ultimately allow interventions to be designed to decrease this behavior.
Critical Parameters
Sterile Procedure
Sterile procedure is of vital importance in self-administration studies. All components of the self-administration apparatus must be as clean as possible. All solutions should be filter-sterilized.
Initial Training
The manner in which a monkey is trained has a profound impact on how the animal performs during subsequent testing. During the initial training, it is important that changes in the schedule conditions (e.g., changes in fixed-ratio requirements) be made only when the animal’s performance is adequately controlled by scheduled drug injections.
Another critical parameter in self-administration is the dose of training agent used during the training sessions. For animals with no operant experience, relatively high doses (e.g., 0.1 mg/kg/injection of cocaine) are generally more effective, but care must be taken to prevent overdose. In animals previously trained to lever press, lower training doses can be employed (e.g., 0.01 to 0.03 mg/kg/injection of cocaine). Also, there should be little or no delay between completion of the operant response and injection of the drug. Rapid bolus injections are invariably more effective than slow infusions.
The schedule of reinforcement that is initially used is also an important variable during training. For training purposes, a simple continuous reinforcement schedule (fixed-ratio 1) is usually employed. Under this schedule, monkeys receive a drug infusion each time they operate the lever. Once performance has stabilized under the fixed-ratio 1 schedule, the fixed-ratio requirement can be increased gradually over a few days or weeks. It also is important that the animal be trained to respond differentially in the presence and absence of drug. That is, the behavior must extinguish when the drug solution is replaced with vehicle.
Operant Performance
The operant performance of an animal should be characteristic of the schedule under which self-administration is maintained. Ensuring that animals perform in a manner consistent with the programmed schedule increases the likelihood that subsequent changes in performance will be due to the imposed experimental manipulations and not to inconsistent self-administration behavior.
Defined Questions
It is essential that experimental goals be clearly defined. An appropriate question may be, “Will test agent A maintain self-administration under a fixed-ratio schedule?”. For example, Fantegrossi and colleagues (2002) investigated the degree to which MDMA and its stereoisomers maintained self-administration in rhesus monkeys trained to self-administer cocaine under a fixed-ratio schedule and found that the agents were self-administered but that the active isomer was more potent than either the racemate or inactive isomer. Another question may be “Will test agent B reduce the breakpoint for cocaine under a progressive-ratio schedule?”. Negus and Mello (2004) posed this question when they evaluated the extent to which methadone reduced breakpoints maintained by either cocaine or food pellets. They found that methadone non-selectively decreased breakpoints maintained by both reinforcers. Precisely-defined questions ensure that meaningful data are expeditiously generated, which is an important consideration given the difficulties in maintaining chronic venous catheters.
Dose-Response Functions
By establishing dose-response relationships,, the investigator can define the relevant range of doses over which the reinforcing effects of test agents are observed. Dose-response studies are essential if one is comparing the potency (or efficacy) of reinforcing effects among different compounds. In addition, dose-response data are invaluable for characterizing the manner in which potential therapeutics for drug addiction modify the effects of the self-administered drug. By executing full dose-response experiments, the investigator can observe the effects of the drug over a range of self-administered doses, allowing more informed conclusions about the reinforcing properties. Examples of typical dose-response functions are shown in Figures 10.5.3, 10.5.4 and 10.5.5 (also see Anticipated Results).
Troubleshooting
The troubleshooting guide shown in Table 10.5.3 assumes that catheter implantation and initial training of the monkey were successful.
Anticipated Results
In self-administration experiments, highly effective reinforcers such as cocaine should generate dose-response data similar to those seen in Figures 10.5.3 and 10.5.4. The inverted U-shaped function is characteristic of such studies. Vehicle or low test compound doses maintain little or no responding. As the dose is increased, the response rate and number of infusions increase until self-administration reaches a maximum at some intermediate dose. As the dose per infusion is increased further, the response rate decreases as test compound effects other than those directly associated with reinforcement interfere with responding. It is very important that a range of doses be studied during self-administration experiments. For example, in Figure 10.5.3 the response rate maintained by a 0.003 mg/kg/infusion of cocaine is almost identical to the response rate when a 0.1 mg/kg/infusion of cocaine is employed, even though the two doses define opposite ends of the full dose-effect curve.
Time Considerations
Self-administration studies with monkeys are time-consuming tasks. Monkeys first need to be habituated to the handling procedures associated with the remote-chamber method or to the tether in the home-cage method. Depending on the age, size, and disposition of the individual monkey, this can take from one to several weeks. At this point, the behavior of the monkey is shaped to respond in the operant chamber, either using food reinforcement first, or beginning directly with drug infusions. Again, this process is highly individualistic and can require from 2 to 10 days before monkeys respond consistently. Once animals are at this stage, catheter implantation be performed. This surgery takes between 1.5 and 2.5 hr from initial anesthetization, and 2 to 4 days typically are required for recovery from surgery. During this recovery period it is important that the catheter be flushed with heparinized or sterile saline every day. Following recovery, monkeys are trained to respond to relatively high doses of drug. Again this process is highly individualistic, but naïve animals usually acquire self-administration of an efficacious reinforcer such as cocaine within 2 to 4 days. At this point, the monkeys can be moved gradually to the terminal schedule conditions, which may require a few weeks to a few months depending on the response requirements and the type of schedule selected.
In a typical self-administration study, monkeys are provided access to vehicle or a particular dose of drug for several consecutive days, or until there are no increasing or decreasing trends in response rate or number of injections per session. Therefore, if the effects of vehicle and four doses of drug are to be determined, several weeks may be required before results for the complete dose range are available.
The times provided above are meant to be a guide, as there are any number of factors that can dramatically lengthen the amount of time needed to complete self-administration experiments.
Table 10.5.3.
Problems Encountered with Self-Administration Procedures
| Problem | Possible cause | Solution |
|---|---|---|
| No responding at start of self-administration session | No start signal on operant panel; equipment malfunction | Check apparatus: pump, stimulus lights, lever(s), electrical connections |
| Inconsistent patterns of self-administration following periods of consistent self- administration | Wrong solution or concentration in infusion syringe | Change to appropriate solution |
| Incomplete drug infusion; leak in catheter line | Check links between drug infusion apparatus and catheter | |
| Catheter has become dislodged from vein | Check catheter patency with the ketamine test. If test is negative, re-catheterization is required. | |
| Catheter tract has become infected | Seek veterinary assistance; treat infection with appropriate antibiotic. Check catheter patency with the ketamine test. If test is negative, re-catheterization is required. | |
| Complete or partial catheter block during flushing | Catheter is blocked by a clot or fibrin thrombus | Sometimes possible to free the catheter by forceful flushing with saline. If catheter cannot be unblocked, re-catheterization is required. |
Literature Cited
- Banks ML, Negus SS. Effects of extended access and cocaine withdrawal on choice between cocaine and food in rhesus monkeys. Neuropsychopharmacology. 2010;35:493–504. doi: 10.1038/npp.2009.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caine SB, Lintz R, Koob GF. Intravenous drug self-administration techniques in animals. In: Seghal A, editor. Behavioral Neuroscience: A Practical Approach. Oxford University Press; New York: 1993. pp. 117–139. [Google Scholar]
- Czoty PW, Martelle JL, Carroll FI, Nader MA. Lower reinforcing strength of the phenyltropane cocaine analogs RTI-336 and RTI-177 compared to cocaine in nonhuman primates. Pharmacol Biochem Behav. 2010;96:274–278. doi: 10.1016/j.pbb.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantegrossi WE, Ullrich T, Rice KC, Woods JH, Winger G. 3, 4-methylenedioxymethamphetamine (MDMA, “ecstasy”) and its stereoisomers as reinforcers in rhesus monkeys: Serotonergic involvement. Psychopharmacology. 2002;161:356–364. doi: 10.1007/s00213-002-1021-6. [DOI] [PubMed] [Google Scholar]
- Gerak LR, Galici R, France CP. Self administration of heroin and cocaine in morphine-dependent and morphine-withdrawn rhesus monkeys. Psychopharmacology. 2008;204:403–411. doi: 10.1007/s00213-009-1471-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerak LR, Galici R, France CP. Self administration of cocaine in monkeys receiving LAAM acutely or chronically. Physiol Behav. 2009;93:20–26. doi: 10.1016/j.physbeh.2007.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg SR. Stimuli associated with drug injections as events that control behavior. Pharmacol Rev. 1975;27:325–340. [PubMed] [Google Scholar]
- Griffiths RR, Bradford LD, Brady JV. Progressive ratio and fixed ratio schedules of cocaine-maintained responding in baboons. Psychopharmacology. 1979;65:125–136. doi: 10.1007/BF00433038. [DOI] [PubMed] [Google Scholar]
- Johanson CE. Pharmacological and environmental variables affecting drug preference in rhesus monkeys. Pharmacol Rev. 1976;27:343–355. [PubMed] [Google Scholar]
- Kelleher RT. Characteristics of behavior controlled by scheduled injections of drugs. Pharmacol Rev. 1975;27:307–324. Description of characteristic performance under different schedules of drug self-administration. [PubMed] [Google Scholar]
- Kimmel HL, Negus SS, Wilcox KM, Ewing SB, Stehouwer J, Goodman MM, Votaw JR, Mello NK, Carroll FI, Howell LL. Relationship between rate of drug uptake in brain and behavioral pharmacology of monoamine transporter inhibitors in rhesus monkeys. Pharmacol Biochem Behav. 2008;90:453–462. doi: 10.1016/j.pbb.2008.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello NK, Negus SS. The effects of buprenorphine on self-administration of cocaine and heroin “speedball” combinations and heroin alone by rhesus monkeys. J Pharmacol Exp Ther. 1998;285:444–456. [PubMed] [Google Scholar]
- Nader MA, Mach RH. Self-administration of the dopamine D3 agonist 7-OH-DPAT in rhesus monkeys is modified by prior cocaine exposure. Psychopharmacology Ser (Berl) 1996;125:13–22. doi: 10.1007/BF02247388. [DOI] [PubMed] [Google Scholar]
- Negus SS. Rapid assessment of choice between cocaine and food in rhesus monkeys: Effects of environmental manipulations and treatment with d-amphetamine and flupenthixol. Neuropsychopharmacology. 2003;28:919–931. doi: 10.1038/sj.npp.1300096. [DOI] [PubMed] [Google Scholar]
- Negus SS. Choice between heroin and food in nondependent and heroin-dependent rhesus monkeys: Effects of naloxone, buprenorphine, and methadone. J Pharmacol Exp Ther. 2006;317:711–723. doi: 10.1124/jpet.105.095380. [DOI] [PubMed] [Google Scholar]
- Negus SS, Mello NK. Effects of chronic methadone treatment on cocaine- and food-maintained responding under second-order, progressive-ratio and concurrent-choice schedules in rhesus monkeys. Drug Alcohol Depend. 2004;74:297–309. doi: 10.1016/j.drugalcdep.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Rowlett JK, Platt DM, Yao WD, Spealman RD. Modulation of heroin and cocaine self-administration by dopamine D1- and D2-like receptor agonists in rhesus monkeys. J Pharmacol Exp Ther. 2007;321:1135–1143. doi: 10.1124/jpet.107.120766. [DOI] [PubMed] [Google Scholar]
- Schuster CR, Johanson CE. The use of animal models for the study of drug abuse. In: Gibbins RJ, Israel Y, Kalant H, Popham RE, Schmidt W, Smart RG, editors. Research Advances in Alcohol and Drug Problems. John Wiley & Sons; New York: 1974. pp. 1–31. Description of use of drug self-administration procedures to assess the abuse potential of drugs. [Google Scholar]
- Spealman RD, Goldberg SR. Drug self-administration by laboratory animals: Controls by schedules of reinforcement. Ann Rev Pharmacol Toxicol. 1978;18:313–339. doi: 10.1146/annurev.pa.18.040178.001525. [DOI] [PubMed] [Google Scholar]
- Weeks JR. Long-term intravenous infusion. In: Meyers RD, editor. Methods in Psychobiology. Vol. 2. Academic Press; New York: 1972. pp. 155–168. [Google Scholar]