Abstract
Introduction and Objectives
Benign prostate-specific antigen (BPSA) and [-2]pro-prostate-specific antigen ([-2]proPSA) have been shown to be predictive of prostate cancer and benign prostatic hyperplasia treatment, but little is known about longitudinal changes in these markers and how they relate to outcomes.
Methods
In 1990, a 25% subsample from a cohort of Caucasian men aged 40–79 years randomly selected from Olmsted County, MN residents completed a detailed clinical examination. BPSA and [-2]proPSA were measured from frozen sera. Subjects were evaluated biennially (median follow-up 7 years; range: 0–8.8 years). Mixed-effects regression models were used to estimate longitudinal changes in BPSA and [-2]proPSA levels overall and by outcomes. Spearman correlations were used to compare these changes with baseline levels and annualized changes in urologic measures.
Results
Median (25th, 75th percentiles) annualized percent change for [-2]proPSA and BPSA were 3.7% (2.5%, 5.2%) and 7.3% (6.8%, 7.7%), respectively. Annualized percent change for both markers were correlated with baseline and annualized changes in PSA and prostate volume. Annualized percent change increased with increasing age decade for [-2]proPSA, but not BPSA. The median (25th, 75th percentiles) rate of increase in [-2]proPSA was significantly greater for men who developed enlarged prostates (3.5% (2.6%, 4.4%)) or prostate cancer (8.1% (6.6%, 9.8%)) compared to those who did not develop enlarged prostates (1.9% (0.9%, 3.0%)) or prostate cancer (3.5% (2.3%, 4.8%)).
Conclusions
BPSA and [-2]proPSA levels increase over time. The annualized percent change in [-2]proPSA increases with age and may be a useful predictor of development of prostate cancer.
Introduction
Prostate-specific antigen (PSA) is a widely used serum marker for the early detection of prostate cancer (CaP). However, it is not specific to CaP since PSA can also be elevated in benign prostatic conditions1,2. Benign prostate-specific antigen (BPSA) and [-2]pro-prostate-specific antigen ([-2]proPSA) have been identified as promising new biomarkers for distinguishing men with benign prostate disease and CaP, respectively3–7. BPSA is associated primarily with benign transition zone tissue exhibiting nodular hyperplasia but not with nonhyperplastic transition zone tissue or benign or cancerous peripheral zone prostatic tissue3. BPSA is elevated in the prostate transition zone and is associated with pathologic benign prostatic hyperplasia (BPH), while [-2]proPSA is associated with prostate tumor3,6. Preliminary studies suggest that [-2]proPSA increases the specificity for detecting prostate cancer over PSA, particularly in the 2–10 ng/ml range5,8 and has also been associated with cancer aggressiveness9. Additionally, we previously showed that men with higher baseline levels of BPSA are at greater risk of receiving BPH treatment and that higher baseline levels of both BPSA and [-2]proPSA are associated with future diagnosis of CaP even beyond serum PSA levels alone10,11.
Longitudinal studies of PSA have shown that serum PSA levels, and the rate of change in PSA levels over time, are associated with a higher risk of acute urinary retention (AUR), CaP, and BPH progression or treatment12–15. However, little is known about changes in BPSA and [-2]proPSA levels over time and the relationship of these changes to longitudinal changes in common urologic measures. The objective of this paper is to describe the distribution of longitudinal changes in BPSA and [-2]proPSA levels in a community-based sample of men, to investigate the association of these changes with longitudinal changes of common clinical urologic measures, and to compare the rates of longitudinal changes among men who do and do not develop enlarged prostates and CaP.
Methods
The Olmsted County Study of Urinary Symptoms and Health Status among Men is a prospective cohort study begun in 1989 which has been described in detail in previous publications16,17. Briefly, an age-stratified random sample was drawn from an enumeration of Caucasian male Olmsted County, Minnesota residents between the ages of 40 and 79 years. Subjects were excluded if they had prior prostate surgery, CaP, or a number of specified medical conditions that would affect normal urinary function (other than BPH). At baseline, 2115 men (of 3874; 55% response rate) were visited in their homes and completed a previously validated self-administered questionnaire which contained questions similar to the American Urological Association Symptom Index (AUASI)18. A random subset of 476 men (of 537; 89%) participated in a full urologic exam, including maximum urinary flow rate assessment, transrectal ultrasonography, digital rectal examination, and a serum PSA determination16,17.
The cohort was actively followed on a biennial basis for fourteen years using a protocol similar to the initial examination. The study design allowed replacement of men who had left the study (n= 158 in the clinic subset) and this paper presents data on the 443 men who had at least one serum measurement during the 4th, 6th, or 8th rounds of the study. This study received approval from the Mayo Clinic and Olmsted Medical Center Institutional Review Boards.
Assays
Stored blood samples from the 4th, 6th, and 8th biennial rounds (1996, 2000, and 2004) were used to measure BPSA and [-2]proPSA levels10,11. Serum samples were obtained prior to any prostatic manipulations, including digital rectal examination and transrectal ultrasound and were frozen at −70°C for latter assays. Measurements of BPSA and [-2]proPSA levels were made using an automated, sequential, two-step immunoenzymatic (“sandwich”) assay developed for use on an Access 2 Immunoassay analyzer (Access® instrument, Beckman Coulter, Brea, CA). The BPSA measurements were run using research-use-only two-site immunoenzymatic reagents and the [-2]proPSA measurements were run using investigational-use-only two-site immunoenzymatic reagents, both provided by Beckman Coulter, Inc. In our laboratory, the intra-assay variation in BPSA ranged from 4.3% to 8.1% while the inter-assay variation ranged from 5.1% to 5.2%. For [-2]proPSA, the intra-assay variation ranged from 2.0% to 3.2% while the inter-assay variation ranged from 3.0% to 9.9%.
Urologic Outcomes
Prostate volume was measured via transrectal ultrasound19 and calculated assuming a prolate ellipsoid shape20. Prostatic enlargement was defined as prostate volume >30 cc21. Additionally, all men were followed through their community medical records. Information on use of medical and surgical lower urinary tract symptom/benign prostatic enlargement treatments were obtained through self-report and through passive follow-up of the community medical records. Dates of biopsy-confirmed CaP were ascertained through detailed medical record review.
Statistical analysis
A natural log transformation was used to normalize variables (BPSA, [-2]proPSA, PSA, prostate volume measurements, and maximum urinary flow rate) with skewed distributions. Mixed-effects regression models were used to estimate longitudinal changes (i.e., annualized percent change over time) in BPSA and [-2]proPSA levels, as well as the other urologic variables. As BPSA and [-2]proPSA measures were only available at the 4th, 6th, and 8th rounds of data collection, the 4th round of follow-up was considered as baseline. Transition zone volume and free PSA were available beginning at the 5th round of data collection. To assess the natural history, observations were censored after subjects received a medical (5-α reductase inhibitor) or surgical BPH treatment or were diagnosed with CaP. Each measure was regressed on time from baseline measurement, and 10-year age groups. An interaction term was included to allow different slopes across age groups. This method estimates group average longitudinal change (fixed effects) while still allowing the longitudinal change from each individual subject to deviate from the group average curve (random effects)22,23. Additional models included a term indicating an enlarged prostate (prostate volume >30 cc) or diagnosis of CaP and an interaction of this term with time from baseline to allow for comparison of slopes in men with and without an enlarged prostate or CaP diagnosis. Prevalent cases of enlarged prostates or CaP were removed from these analyses which resulted in 43 and 65 men with incident CaP and enlarged prostate, respectively. Spearman correlations were used to describe the relationship between annualized percent change in BPSA and [-2]proPSA levels with baseline and annualized changes over time of other urologic measures.
Results
Subjects were evaluated biennially for a median follow-up interval of 7 years (range: 0–8.8 years) with an average of 2.2 BPSA and [-2]proPSA measurements per man. Study participants were Caucasian with an average age at baseline of 58.7 years. Seventy (15.8%) of the men had a family history of prostate cancer and baseline levels of urologic measures are presented in Table 1.
Table 1.
Baselineζ characteristics and Spearman correlations of annualized percent change in BPSA and [-2]proPSA with baseline and annualized changes in urologic measures
| % Change in BPSA | % Change in [-2]proPSA | ||||
|---|---|---|---|---|---|
| Unadjusted | Age-adjusted | Unadjusted | Age-adjusted | ||
| Measure | Median (Q1, Q3)ξ | rs (p) | rs (p) | rs (p) | rs (p) |
| Baseline AUASI score | 6 (3, 11) | 0.17* | 0.08 | 0.25* | 0.08 |
| Baseline maximum flow rate | 18.6 (13.1, 24.7) | −0.11** | −0.04 | −0.26* | −0.16* |
| Baseline prostate volume | 26.7 (21.7, 35.0) | 0.47* | 0.37* | 0.62* | 0.46* |
| Baseline transition zone volume | 11.2 (7.9, 16.3) | 0.47* | 0.36* | 0.60* | 0.41* |
| Baseline PSA | 1.1 (0.7, 1.8) | 0.67* | 0.62* | 0.68* | 0.59* |
| Baseline free PSA | 0.3 (0.2, 0.4) | 0.53* | 0.46* | 0.73* | 0.72* |
| Change in AUASI score | 0.2 (0.1, 0.4) | 0.17* | 0.07 | 0.28* | 0.09 |
| % Change in maximum flow rate | −2.1 (−2.7, −1.4) | −0.17* | 0.03 | −0.50* | −0.16* |
| % Change in prostate volume | 2.1 (1.6, 2.8) | 0.40* | 0.43* | 0.25* | 0.37* |
| % Change in transition zone volume | 4.0 (3.0, 5.1) | 0.38* | 0.45* | 0.09 | 0.21* |
| % Change in PSA | 3.4 (1.3, 5.3) | 0.57* | 0.52* | 0.51* | 0.43* |
| % Change in free PSA | 4.9 (3.5, 6.0) | 0.49* | 0.36* | 0.70* | 0.51* |
p-value <0.001;
p-value <0.05
Baseline measurements correspond to the time of the baseline BPSA or [-2]proPSA measurement, except for transition zone volume and free PSA which were first measured at round 5
Q1: 25th percentile; Q3: 75th percentile
The median (25th, 75th percentile (Q1, Q3)) level of baseline BPSA was 31.6 pg/ml (16.0, 66.3) and baseline [-2]proPSA was 5.6 pg/ml (4.0, 7.7). The overall median (Q1, Q3) annualized percent change in BPSA level was 7.3% (6.8%, 7.7%) per year (Figures 1a and 1b). The overall median (Q1, Q3) annualized percent change in [-2]proPSA level was 3.7% (2.5%, 5.2%) per year (Figures 2a and 2b). The age-adjusted Spearman correlation between baseline levels of BPSA and [-2]proPSA was 0.45 and between annualized changes in BPSA and annualized changes in [-2]proPSA was 0.34.
Figure 1.
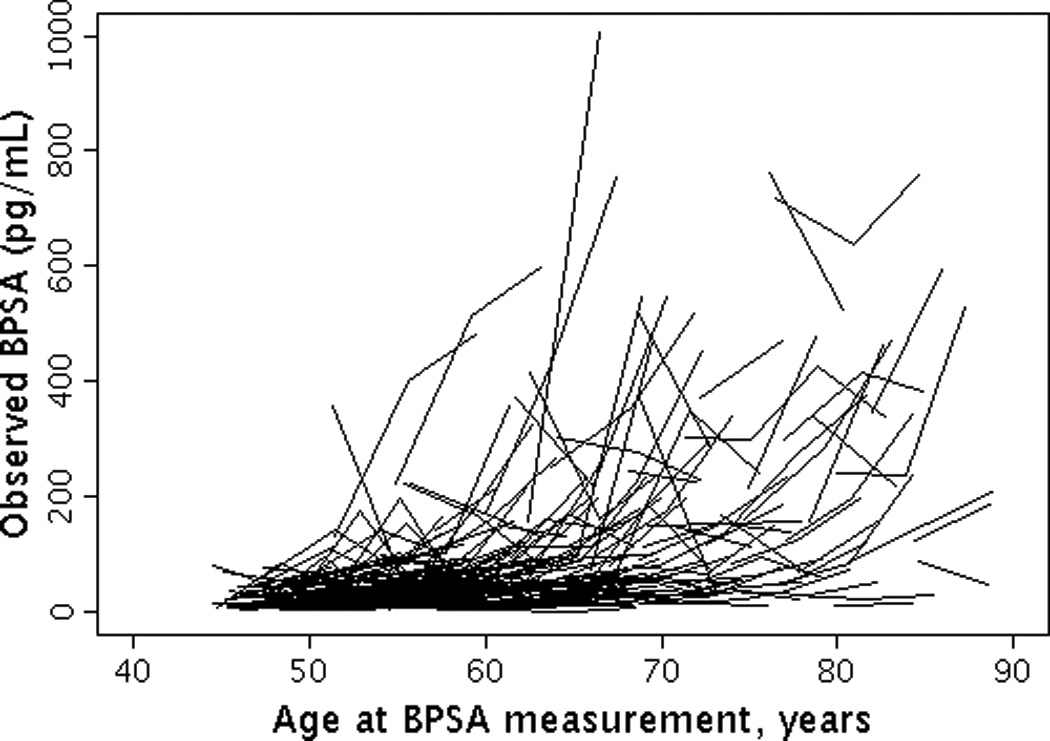
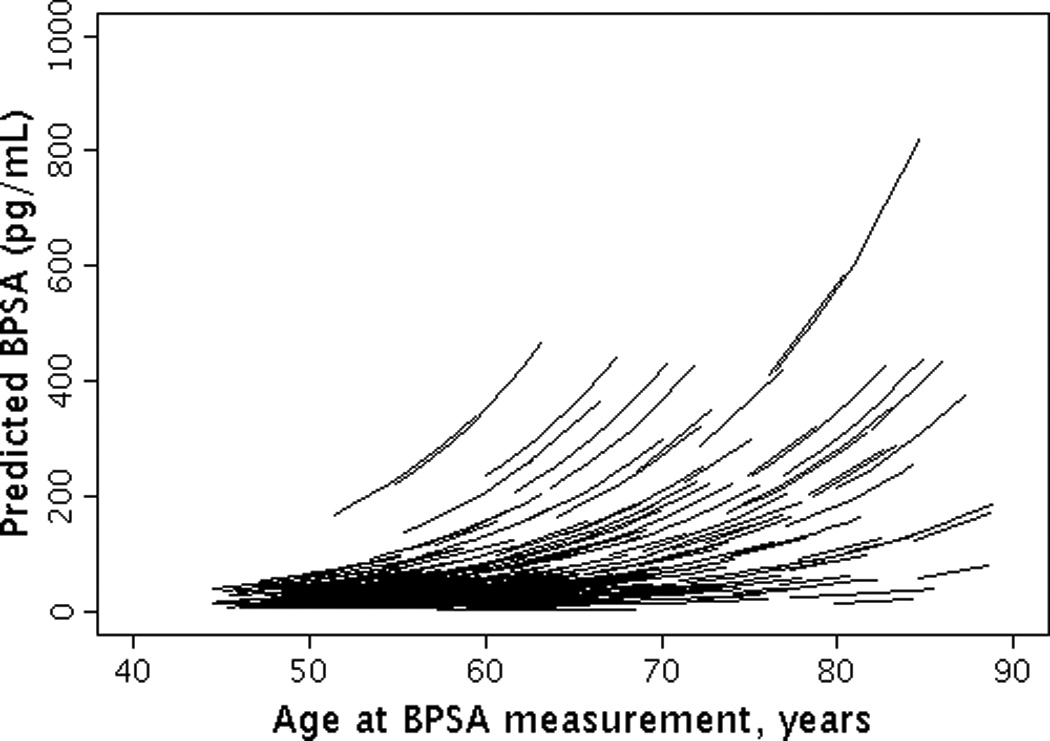
Longitudinal changes in BPSA by age. (1A) Observed changes in BPSA by age; (1B) Predicted changes in BPSA by age.
Figure 2.
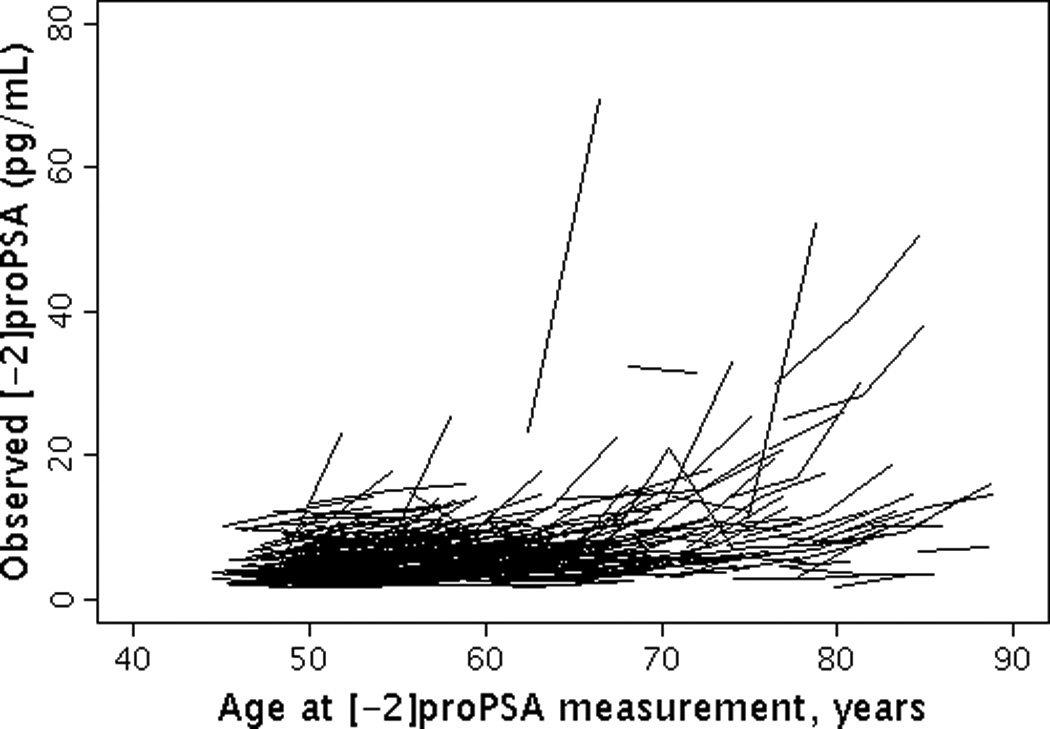
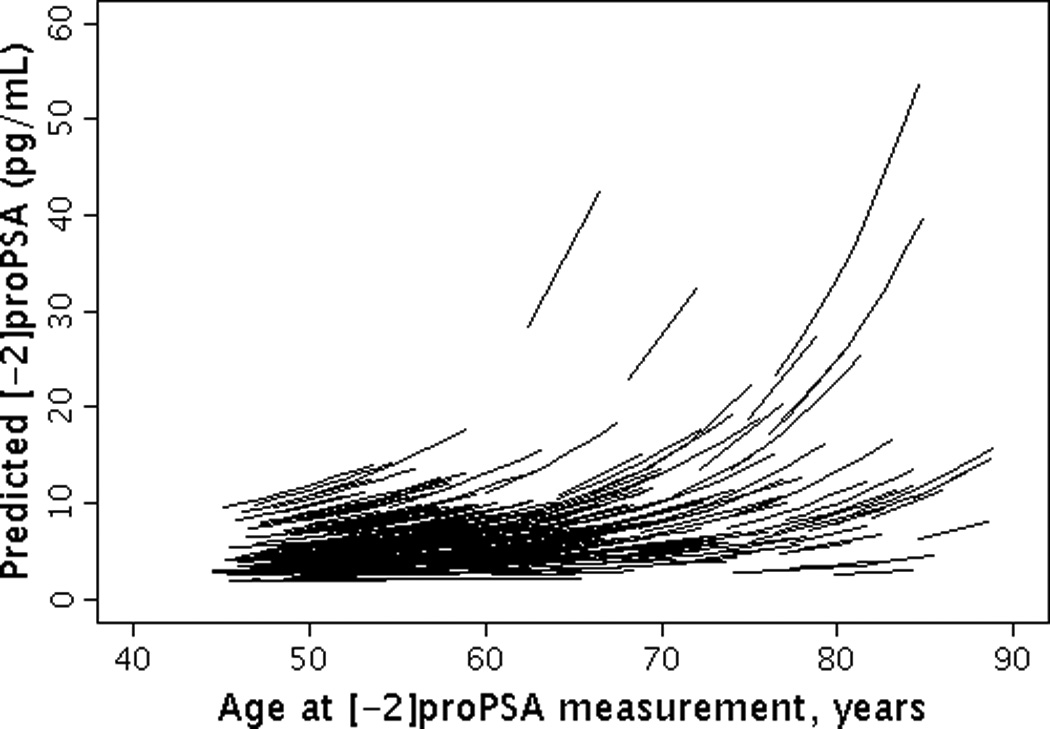
Longitudinal changes in [-2]proPSA by age. (2A) Observed changes in [-2]proPSA by age; (2B) Predicted changes in [-2]proPSA by age.
Annualized percent changes in BPSA and [-2]proPSA levels were modestly correlated with baseline levels and annualized changes in urinary symptoms and urinary flow rates (age-adjusted Spearman correlations (rs) range: −0.16 to 0.09; Table 1). Stronger, positive correlations were observed between annualized changes in BPSA and [-2]proPSA levels with baseline levels and annualized changes in prostate volume, transition zone volume, PSA level, and free PSA level (age-adjusted rs range: 0.21 to 0.72, Table 1).
The annual increase in BPSA level was consistent across age decades. This increase was slightly higher, but not significantly different for men who were diagnosed with CaP compare to men not diagnosed with CaP (Table 2). Similarly, there was a non-significant increased rate of change in BPSA among men who developed as compare to those who did not develop an enlarged prostate (Table 2).
Table 2.
Distribution of annualized percent changes in BPSA and [-2]proPSA stratified by age and urologic conditions
| % Change in BPSA | % Change in [-2]proPSA |
||||
|---|---|---|---|---|---|
| N | Median (Q1, Q3) | p | Median (Q1, Q3) | p | |
| Age | 0.92 | <0.0001 | |||
| 40–49 | 115 | 6.7 (6.5, 7.0) | 2.1 (1.2, 3.0) | ||
| 50–59 | 155 | 7.6 (7.3, 7.9) | 3.4 (2.5, 4.1) | ||
| 60–69 | 98 | 7.5 (7.1, 7.9) | 5.0 (4.0, 5.7) | ||
| 70+ | 75 | 7.2 (6.9, 7.7) | 6.7 (5.5, 7.7) | ||
| Prostate volume* ξ | 0.47 | 0.048 | |||
| ≤30 cc | 132 | 6.2 (5.8, 6.5) | 1.9 (0.9, 3.0) | ||
| >30 cc | 65 | 7.2 (6.9, 7.6) | 3.5 (2.6, 4.4) | ||
| Prostate cancer* ξ | 0.46 | 0.003 | |||
| No | 400 | 7.3 (6.7, 7.7) | 3.5 (2.3, 4.8) | ||
| Yes | 43 | 9.3 (8.9, 9.6) | 8.1 (6.6, 9.8) | ||
Prevalent cases occurring before first assay measurement were removed
Associations adjusted for age
The median annual increase in [-2]proPSA level increased with increasing age decade (p<0.001) and ranged from 2.1% per year for men in their forties to 6.7% per year for men who were 70 years of age and older (Table 2). The annualized rate of increase in [-2]proPSA level among men who developed an enlarged prostate was nearly double the rate of men who did not develop an enlarged prostate (p=0.048; Table 2). The greatest annual rate of increase in [-2]proPSA level was observed among men who developed CaP (median (Q1, Q3)=8.1% (6.6%, 9.8%) per year) (Table 2). This was over twice the rate of men who did not develop CaP (3.5% (2.3%, 4.8%) per year, p=0.003). Annualized changes in [-2]proPSA level for men with and without CaP were similar to the results comparing median (Q1, Q3) changes in PSA level for men with (8.3% (7.3%, 9.4%) per year) and without (3.1% (1.6%, 4.7%) per year) CaP; however, adjusting for baseline PSA level did not alter the rates of change in [-2]proPSA for men with and without CaP (data not shown).
Discussion
In this study, we report important information about longitudinal changes in BPSA and [-2]proPSA levels. Over time, the median annualized percent increase in BPSA level is greater than the median annualized percent increase in [-2]proPSA level. While changes in both measures are correlated with baseline levels and changes in prostate size and PSA measures, these results suggest that annual changes in [-2]proPSA level were significantly greater in men who developed enlarged prostates and men who are diagnosed with CaP.
Annualized changes in both BPSA level and [-2]proPSA level were most strongly correlated with other PSA and prostate size measures. The modest age-adjusted correlations between changes in BPSA and [-2]proPSA levels and changes in symptoms and maximum flow rate are consistent with the correlations among annual changes in urologic measures previously reported in this same cohort24,25. Additionally, cross-sectionally, the associations between BPSA level and presence of lower urinary tract symptoms or depressed urinary flow rate were much lower than the associations with an enlarged prostate10.
The age-adjusted correlation of 0.45 between annualized changes in BPSA level and transition zone volume was greater than the age-adjusted correlation of 0.32 previously observed between changes in PSA level and transition zone volume25. This is consistent with other cross-sectional studies that reported stronger correlations between transition zone volume and BPSA level than with PSA level3,4.
There were differences between the unadjusted and age-adjusted correlations, especially in the correlations between change in maximum flow rate and change in [-2]proPSA level. As these two measures both progress with increasing age, the observed unadjusted association may be due to the confounding effect of age. Alternatively, there could be an association between changes in the two measures which is over-adjusted and removed when adjusting for age.
Annualized changes in BPSA are consistent across age groups, with an overall annual percent change of 7.3% per year. However, as baseline BPSA level increases with increasing age group, absolute levels of BPSA over time will increase across age groups. We had previously found that baseline levels of BPSA were associated with future BPH treatment and diagnosis of CaP10. While changes in BPSA levels over time were slightly higher for men who developed an enlarged prostate or CaP, these differences were not significant. It is possible that men with a higher level of BPSA may be at greater risk of developing CaP or seeking treatment, however the continued rate of increase in BPSA level does not differ between men who do and do not develop CaP or an enlarged prostate.
Annualized percent changes in [-2]proPSA level increased with increasing age and were greatest among men who developed CaP. These findings suggest that age may be an important predictor of the rate of increase in [-2]proPSA level. This is different than the consistent rate of annualized percent change over age decades observed in this cohort for changes in prostate volume, PSA level, and BPSA level19,24,26. Factors other than changes in prostate volume and changes in PSA level may potentially play a role in the age-related increase in [-2]proPSA over time. As [-2]proPSA is a subform of free PSA, age-related increases in free PSA may partially explain the age-related increase in [-2]proPSA over time. Importantly, establishing reference ranges for change in [-2]proPSA as a marker for CaP should account for the age-related increases in annual changes in [-2]proPSA levels. A single cut-point would decrease sensitivity in younger men and decrease specificity which could lead to unnecessary biopsies in older men1.
Several papers have reported improved CaP detection using a single measurement of [-2]proPSA, alone or in combination with other measures5,7–9,27,28. Additionally, we have previously shown that higher baseline levels of [-2]proPSA are associated with future diagnosis of CaP11. In the current study, men who were diagnosed with incident CaP had a median annualized rate of increase in [-2]proPSA level over time which was more than twice the rate of men not diagnosed with CaP, even after adjusting for baseline PSA level. This supports the potential utility of change in [-2]proPSA in distinguishing men with or without CaP.
The rate differences for men with and without CaP were similar for PSA and [-2]proPSA; however there are several findings which point toward the potential usefulness of [-2]proPSA in detecting CaP beyond PSA alone. First, the association between baseline levels of [-2]proPSA and development of CaP11 remained significant after adjustment for PSA level. Similarly, differences in longitudinal rates of change in [-2]proPSA for men with and without CaP remained after adjustment for PSA level. Additionally, age-related increases in [-2]proPSA over time were greater than would be explained by age-related changes in PSA. Further work is needed to determine if development of CaP could partially explain the age-related increase in [-2]proPSA over time.
It is interesting to note that the overall longitudinal estimates of change in BPSA and [-2]proPSA levels were greater than those observed cross-sectionally in this cohort10,11. At baseline, there was an exponential increase of 6.2% and 1.9% per year of age for BPSA and [-2]proPSA levels, respectively, which are equivalent to median rates observed in men with prostate volume ≤30 cc. A diagnosis of CaP and surgical treatment for BPH were two of the baseline study exclusion criteria. This may explain the similarity of cross-sectional rates to rates for men with smaller prostate volumes. Therefore, the slightly higher overall longitudinal estimates may better represent disease progression in the community.
A key strength of this study is the ability to examine longitudinal changes in BPSA and [-2]proPSA levels in a community-based study population. Using a randomly selected community cohort and truncation of observations after treatment or CaP diagnosis provides results which represent a more comprehensive picture of how BPSA and [-2]proPSA change over time in the general population.
There are also several limitations that should be considered. The BPSA and [-2]proPSA levels measured in this community-based cohort are much lower than levels previously reported in patients selected for biopsy, radical prostatectomy or CaP screening10,11. With a maximum of up to three measurements (with approximately 4 years total between the first and last measurements) per subject for BPSA and [-2]proPSA, the high within-subject variability of the urologic measures may result in less stable estimates of change over time and it was not possible to explore nonlinear relationships. With only 43 men who developed CaP, we are unable to simultaneously assess the effect of changes over time in BPSA, [-2]proPSA, and PSA levels with the development of CaP. Additionally, there were not enough prostate cancer cases to permit meaningful analysis with respect to tumor pathology and the majority of the cancer cases were low-grade (Gleason score <7).
While the long follow-up period of this study leads to unavoidable attrition which could bias the results, previous work in this cohort found that dropout was not associated with baseline urologic measures29. Although the preliminary results from this study and earlier reports are promising, further research is needed evaluating both BPSA and [-2]proPSA in the context of a broad spectrum of disease in order to gain a better understanding of the characteristics of these tests. Indeed, as Djavan30 pointed out in a recent editorial, although these new markers are encouraging they are not yet mature enough for general clinical use in a urologic practice. For example, he points out that all of the studies that are investigating these novel biomarkers ultimately base the biopsy decision on PSA values and associated thresholds and not the novel biomarkers themselves. The clinical utility of the predictive models has yet to be determined. Results in the current study predicting prostate cancer in the presence of baseline PSA suggest that these new biomarkers may have a role in the diagnostic workup of patients suspected of BPH or CaP. Clinical studies are needed incorporating BPSA and/or [-2]proPSA into existing protocols (e.g., nomograms) to assess clinical utility. Finally, this study population is limited to Caucasian men and the generalizability of the results to other races or ethnicities may be limited.
Conclusions
Overall these results demonstrate that BPSA and [-2]proPSA levels increase over time. Furthermore, the annual percent change in [-2]proPSA level increases with age and is greater in men who develop enlarged prostates and CaP. These data suggest that rapid increases in [-2]proPSA levels over time may help identify men with CaP.
Acknowledgment
Disclosures
Dr. Klee has received research grants and royalties for unrelated technologies from Beckman Coulter, Inc. Dr. Jacobsen has received research grants from Beckman Coulter, Inc. Thomas Rhodes and Cynthia Girman are employees of Merck Research Laboratories.
The authors thank the men who participate in the Olmsted County Study, the study personnel, and Ms. Tina Condon for her assistance in preparation of this manuscript. This project was supported by research grants from the Public Health Service, National Institutes of Health (Grants DK58859, AG034676, and 1UL1 RR024150-01), and Merck Research Laboratories. Beckman Coulter (Brea, CA) provided the test kits for BPSA and [-2]proPSA free of charge and with no obligation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Oesterling JE, Jacobsen SJ, Chute CG, et al. Serum prostate-specific antigen in a community-based population of healthy men. Establishment of age-specific reference ranges.[see comment] JAMA. 1993;270:860–864. [PubMed] [Google Scholar]
- 2.Catalona WJ, Southwick PC, Slawin KM, et al. Comparison of percent free PSA, PSA density, and age-specific PSA cutoffs for prostate cancer detection and staging. Urology. 2000;56:255–260. doi: 10.1016/s0090-4295(00)00637-3. [DOI] [PubMed] [Google Scholar]
- 3.Mikolajczyk SD, Millar LS, Wang TJ, et al. "BPSA," a specific molecular form of free prostate-specific antigen, is found predominantly in the transition zone of patients with nodular benign prostatic hyperplasia. Urology. 2000;55:41–45. doi: 10.1016/s0090-4295(99)00372-6. [DOI] [PubMed] [Google Scholar]
- 4.Canto EI, Singh H, Shariat SF, et al. Serum BPSA outperforms both total PSA and free PSA as a predictor of prostatic enlargement in men without prostate cancer. Urology. 2004;63:905–910. doi: 10.1016/j.urology.2003.12.037. discussion 910–1. [DOI] [PubMed] [Google Scholar]
- 5.Catalona WJ, Bartsch G, Rittenhouse HG, et al. Serum pro prostate specific antigen improves cancer detection compared to free and complexed prostate specific antigen in men with prostate specific antigen 2 to 4 ng/ml. J Urol. 2003;170:2181–2185. doi: 10.1097/01.ju.0000095460.12999.43. [DOI] [PubMed] [Google Scholar]
- 6.Makarov DV, Isharwal S, Sokoll LJ, et al. Pro-prostate-specific antigen measurements in serum and tissue are associated with treatment necessity among men enrolled in expectant management for prostate cancer. Clin Cancer Res. 2009;15:7316–7321. doi: 10.1158/1078-0432.CCR-09-1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Le BV, Griffin CR, Loeb S, et al. [-2]Proenzyme prostate specific antigen is more accurate than total and free prostate specific antigen in differentiating prostate cancer from benign disease in a prospective prostate cancer screening study. J Urol. 2010;183:1355–1359. doi: 10.1016/j.juro.2009.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Catalona WJ. A Multicenter Study of [-2]Pro-Prostate Specific Antigen Combined With Prostate Specific Antigen and Free Prostate Specific Antigen for Prostate cancer Detection in the 2.0 to 10.0 ng/ml Prostate Specific Antigen Range. Journal of Urology. 2011;185:1650–1655. doi: 10.1016/j.juro.2010.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sokoll LJ, Sanda MG, Feng Z, et al. A prospective, multicenter, National Cancer Institute Early Detection Research Network study of [-2]proPSA: improving prostate cancer detection and correlating with cancer aggressiveness. Cancer Epidemiol Biomarkers Prev. 2010;19:1193–1200. doi: 10.1158/1055-9965.EPI-10-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rhodes T, Jacobson DJ, McGree ME, et al. Benign Prostate-Specific Antigen Distributions and Associatins with Urologic Outcomes in Community-Dwelling Black and White Men. Journal of Urology. doi: 10.1016/j.juro.2011.09.061. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rhodes T, Jacobson DJ, McGree ME, et al. Distribution and Associations of [-2]pro-Prostate-Specific Antigen in Community-Dwelling Black and White Men. Journal of Urology. doi: 10.1016/j.juro.2011.09.060. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roehrborn CG, McConnell JD, Saltzman B, et al. Storage (irritative) and voiding (obstructive) symptoms as predictors of benign prostatic hyperplasia progression and related outcomes. Eur Urol. 2002;42:1–6. doi: 10.1016/s0302-2838(02)00210-5. [DOI] [PubMed] [Google Scholar]
- 13.Crawford ED, Wilson SS, McConnell JD, et al. Baseline factors as predictors of clinical progression of benign prostatic hyperplasia in men treated with placebo. J Urol. 2006;175:1422–1426. doi: 10.1016/S0022-5347(05)00708-1. discussion 1426–7. [DOI] [PubMed] [Google Scholar]
- 14.Jacobsen SJ, Jacobson DJ, Girman CJ, et al. Treatment for benign prostatic hyperplasia among community dwelling men: the Olmsted County study of urinary symptoms and health status. J Urol. 1999;162:1301–1306. [PubMed] [Google Scholar]
- 15.Carter HB, Pearson JD, Metter EJ, et al. Longitudinal evaluation of prostate-specific antigen levels in men with and without prostate disease. Jama. 1992;267:2215–2220. [PMC free article] [PubMed] [Google Scholar]
- 16.Chute CG, Panser LA, Girman CJ, et al. The prevalence of prostatism: a population-based survey of urinary symptoms. J Urol. 1993;150:85–89. doi: 10.1016/s0022-5347(17)35405-8. [DOI] [PubMed] [Google Scholar]
- 17.Jacobsen SJ, Guess HA, Panser L, et al. A population-based study of health care-seeking behavior for treatment of urinary symptoms. The Olmsted County Study of Urinary Symptoms and Health Status Among Men. Arch Fam Med. 1993;2:729–735. doi: 10.1001/archfami.2.7.729. [DOI] [PubMed] [Google Scholar]
- 18.Barry MJ, Fowler FJ, Jr, O'Leary MP, et al. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol. 1992;148:1549–1557. doi: 10.1016/s0022-5347(17)36966-5. discussion 1564. [DOI] [PubMed] [Google Scholar]
- 19.Rhodes T, Girman CJ, Jacobsen SJ, et al. Longitudinal prostate growth rates during 5 years in randomly selected community men 40 to 79 years old. J Urol. 1999;161:1174–1179. [PubMed] [Google Scholar]
- 20.Terris MK, Stamey TA. Determination of prostate volume by transrectal ultrasound. J Urol. 1991;145:984–987. doi: 10.1016/s0022-5347(17)38508-7. [DOI] [PubMed] [Google Scholar]
- 21.Jacobsen SJ, Jacobson DJ, Girman CJ, et al. Natural history of prostatism: risk factors for acute urinary retention. J Urol. 1997;158:481–487. doi: 10.1016/s0022-5347(01)64508-7. [DOI] [PubMed] [Google Scholar]
- 22.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- 23.Feldman HA. Families of lines: random effects in linear regression analysis. J Appl Physiol. 1988;64:1721–1732. doi: 10.1152/jappl.1988.64.4.1721. [DOI] [PubMed] [Google Scholar]
- 24.St Sauver JL, Jacobson DJ, Girman CJ, et al. Tracking of longitudinal changes in measures of benign prostatic hyperplasia in a population based cohort. J Urol. 2006;175:1018–1022. doi: 10.1016/S0022-5347(05)00408-8. discussion 1022. [DOI] [PubMed] [Google Scholar]
- 25.St Sauver JL, Jacobson DJ, Girman CJ, et al. Correlations between longitudinal changes in transitional zone volume and measures of benign prostatic hyperplasia in a population-based cohort. Eur Urol. 2006;50:105–111. doi: 10.1016/j.eururo.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 26.Lieber MM, Rhodes T, Jacobson DJ, et al. Natural history of benign prostatic enlargement: long-term longitudinal population-based study of prostate volume doubling times. BJU Int. 2010;105:214–219. doi: 10.1111/j.1464-410X.2009.08719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chan TY, Mikolajczyk SD, Lecksell K, et al. Immunohistochemical staining of prostate cancer with monoclonal antibodies to the precursor of prostate-specific antigen. Urology. 2003;62:177–181. doi: 10.1016/s0090-4295(03)00138-9. [DOI] [PubMed] [Google Scholar]
- 28.Stephan C, Kahrs AM, Cammann H, et al. A [-2]proPSA-based artificial neural network significantly improves differentiation between prostate cancer and benign prostatic diseases. Prostate. 2009;69:198–207. doi: 10.1002/pros.20872. [DOI] [PubMed] [Google Scholar]
- 29.Gades NM, Jacobson DJ, McGree ME, et al. Dropout in a longitudinal, cohort study of urologic disease in community men. BMC Med Res Methodol. 2006;6:58. doi: 10.1186/1471-2288-6-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Djavan B. Validity and legacy of prostate-specific antigen (PSA) and PSA-based parameters and isoforms in the new millennium. Eur Urol. 57:928–929. doi: 10.1016/j.eururo.2010.03.002. [DOI] [PubMed] [Google Scholar]


