Abstract
The requirement of Akt for cell proliferation and oncogenesis is mammalian target of rapamycin complex 1 (mTORC1) dependent. SV40 large T expression in Akt-deficient cells restores cell proliferation rate, but is insufficient for exiting contact inhibition and oncogene-induced anchorage-independent growth, because of a failure to promote Skp2 mRNA translation. Skp2 mRNA and protein are induced upon exiting contact inhibition, which enables entry into mitosis. While Skp2 mRNA is induced in Akt-deficient cells, it is not translated, preventing entry into mitosis. Restoring Skp2 expression in Akt-deficient cells is sufficient to restore exit from contact inhibition and oncogenesis. Skp2 mRNA translation is dependent on mTORC1 and the eukaryotic translation initiation factor 4E (eIF4E). Thus, the requirement of Akt for exiting contact inhibition is mediated by the induction of Skp2 mRNA translation in eIF4E-dependent mechanism. These results provide a new insight into the role of the Akt/mTORC1/eIF4E axis in tumourigenesis. Akt-dependent Skp2 mRNA translation is also required for mitotic clonal expansion (MCE)—the earliest event in adipogenesis. Skp2 re-expression in Akt-deficient preadipocytes, which are impaired in adipogenesis, is sufficient to restore adipogenesis. These results uncover the mechanism by which Akt mediates adipogenesis.
Keywords: adipogenesis, Akt, eIF4E, oncogenesis, Skp2
Introduction
Akt is perhaps the most frequently activated oncoprotein in human cancers. Hyperactivation of Akt contributes to the genesis of cancer through multiple mechanisms, including inhibition of apoptosis, increased cell metabolism, abrogation of cell-cycle checkpoint, and increased proliferation (Bhaskar and Hay, 2007; Robey and Hay, 2009). A large number of substrates that can potentially be phosphorylated by Akt and contribute to the genesis of cancer (Lawlor and Alessi, 2001). However, it is still unclear which targets are the most critical for Akt's role in cell proliferation and oncogenic transformation. We had previously shown that Akt is required for entry into cell cycle and for normal cell proliferation, as well as for oncogenic transformation, independently of its anti-apoptotic function (Skeen et al, 2006). Previously, we showed that the most critical downstream effector of Akt that is required for cell proliferation and oncogenic transformation, is the mammalian target of rapamycin complex 1 (mTORC1; Skeen et al, 2006). We showed that when mTORC1 is hyperactivated, the requirement of Akt for cell proliferation and oncogenic transformation is diminished. However, it is not known how mTORC1 executes Akt's function in cell proliferation and oncogenic transformation. mTORC1 contributes to cancer development and tumourigenesis through multiple mechanisms most notably through its effect on protein synthesis and lipid metabolism (Laplante and Sabatini, 2009). The most well-known targets of mTORC1 are S6 kinase 1 (S6K1) and the eukaryotic translation initiation factor 4E-binding proteins (4E-BPs), both of which are implicated in mRNA translation (Hay and Sonenberg, 2004). In particular, phosphorylation of 4E-BPs by mTORC1 alleviates the inhibitory activity of 4E-BPs on mRNA translation by facilitating its dissociation from eukaryotic translation initiation factor 4E (eIF4E), enabling eIF4E to interact with eIF4G to initiate mRNA translation (Hay and Sonenberg, 2004). Thus, the phosphorylation of 4E-BPs by mTORC1 may constitute a rate-limiting step in mRNA translation. In general, eIF4E is required for 5′-cap-dependent mRNA translation and specifically for the translation of mRNAs with a long and structured 5′ untranslated region (UTR). S6 kinase contributes to mRNA translation by phosphorylating translation factors (Hay and Sonenberg, 2004), as well as by affecting the levels of eIF4A (Dorrello et al, 2006). In addition, as a kinase, S6K1 can phosphorylate other target proteins, which might be required for cell-cycle progression and oncogenic transformation. However, as was recently shown, eIF4E plays a predominant role in cell proliferation downstream of mTORC1 (Dowling et al, 2010).
Because we found that Akt is required for pRB phosphorylation during entry into cell cycle (Skeen et al, 2006), we expressed SV40 large T (LT) to sequester and impair pRB function with the aim of restoring normal cell proliferation in Akt1 and Akt2 doubly deficient cells. Indeed, LT restored normal cell proliferation of Akt-deficient cells under normal growth conditions. Surprisingly, however, LT was not sufficient to restore oncogenic transformation of Akt-deficient cells as assessed by anchorage-independent growth. In addition, LT was sufficient to drive wild-type cells out of contact inhibition but not Akt-deficient cells. Thus, Akt is required for exit from contact inhibition and anchorage-independent growth—two hallmarks of cancer cells, independently of pRB. As demonstrated in our current studies, this activity of Akt is also dependent on mTORC1 and its downstream effector, eIF4E. We show here that Akt is required for the translation of S phase kinase-associated protein 2 (Skp2) mRNA during exit from contact inhibition. Skp2 is an F-box binding protein, which is required for the degradation of p21 and p27. p21 and p27 degradation is required for the activation of cyclin-dependent kinase 1 (CDK1), facilitating cell-cycle progression through the G2 phase and entry into mitosis even in the absence of pRb (Nakayama and Nakayama, 2005; Frescas and Pagano, 2008). Therefore, ectopic expression of Skp2 in Akt-deficient cells is sufficient to restore the ability to exit from contact inhibition and promote anchorage-independent growth. Thus, our studies not only uncovered a new mechanism by which Akt contributes to cell proliferation and oncogenic transformation, but also a new mechanism by which eIF4E contributes to these processes.
Finally, we showed that the role of the Akt/mTORC1/eIF4E axis in mediating Skp2 mRNA translation is also required for adipocyte differentiation. Our results showed that Akt is required for the expression of all the transcriptional regulators, whose expression is dependent on mitotic clonal expansion (MCE). MCE is a cell division process, which is a prerequisite for the induction of the adipogenesis transcriptional cascade (Tang et al, 2003). This process was shown to be dependent on Skp2 and the lack of Skp2 impairs adipogenesis in vivo and in vitro (Cooke et al, 2007; Sakai et al, 2007). Thus, the function of the Akt/mTORC1/eF4E axis in cell proliferation and Skp2 expression is also required for adipocyte differentiation.
Results
SV40 LT restores a normal cell proliferation rate for Akt1/2 DKO cells but is not sufficient to restore oncogenic transformation and promote exit from contact inhibition
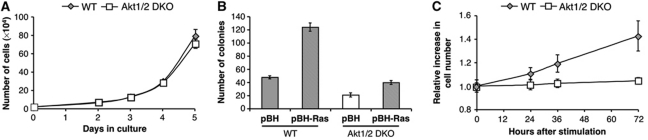
We previously showed that mouse embryo fibroblasts (MEFs) derived from Akt1 KO or Akt1/2 double knockout (DKO) mice are impaired in their ability to enter the S phase of the cell cycle, and in the phosphorylation and inactivation of pRb. Therefore, we expressed SV40 LT, which neutralizes pRb in Akt1/2 DKO MEFs. The expression of LT was sufficient to promote a similar proliferation rate of Akt1/2 DKO cells to that of WT cells (Figure 1A). Surprisingly, however, LT was not sufficient to restore Ras-oncogenic transformation of Akt1/2 DKO cells (Figure 1B). In addition, while LT could promote exit from contact inhibition of WT cells, it could not promote exit from contact inhibition of Akt1/2 DKO cells (Figure 1C). Taken together, the results suggest: first, in addition to its role in G1/S progression, Akt is required for exit from contact inhibition, through a mechanism, which cannot be compensated for by LT. Second, the role of Akt in the exit from contact inhibition is coupled to its role in oncogenic transformation and anchorage-independent growth.
Figure 1.
SV40 large T neither restores oncogenic transformation of Akt1/2 DKO cells, nor promotes exit from contact inhibition. (A) WT-MEFs or Akt1/2 DKO MEFs were immortalized with SV40 large T antigen and cell proliferation rate was measured by counting number of cells in the plates for 4 consecutive days. The experiment was performed in triplicate at least three times. Error bars represent ±s.e. (B) WT (LT) and Akt1/2 DKO (LT) MEFs expressing H-Rasvaline12 were plated in 0.35% agarose-containing medium, as described in Materials and methods, and allowed to grow with twice-weekly media changes for ∼3 weeks. The bar graph represents the quantitation of soft agarose colonies. The experiment was performed in triplicate at least three times. Error bars represent ±s.e. (C) Two days post confluency, cells were induced with 20% FBS and cell number was measured at 0, 24, 36, and 72 h after stimulation. The relative increase in cell number compared with time 0 is depicted. The results represent the average ±s.e. of three independent experiments.
Akt1/2 DKO (LT) cells fail to reduce p21 and p27 proteins and elevate Skp2 protein during exit from contact inhibition
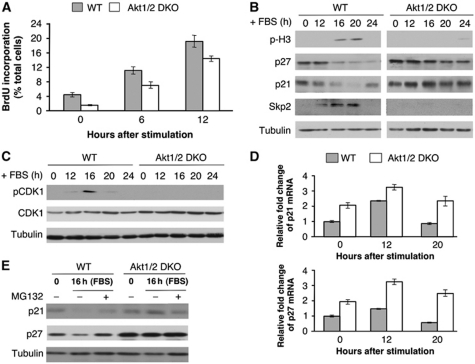
To determine why Akt1/2 DKO cells are impaired in exit from contact inhibition, we first verified whether LT could drive Akt1/2 DKO cells through the S phase of the cell cycle. As expected, we found that LT is sufficient to drive both WT and Akt-deficient cells through the S phase of the cell cycle as measured by BrdU incorporation 12 h following induction of exit from contact inhibition (Figure 2A). However, Akt-deficient cells were markedly inhibited in their entry into mitosis as measured by phospho histone H3 (pH3), a marker of mitosis (Figure 2B). Both p21 and p27 protein levels decreased during exit from contact inhibition in WT cells but were maintained at a relatively high levels in Akt-deficient cells even 24 h after induction of exit from contact inhibition (Figure 2B). Importantly, expression of Skp2, which targets p21 and p27 for degradation, was elevated in WT cells but not in Akt-deficient cells (Figure 2B). Thus, it appears that Akt is required for Skp2 expression during exit from contact inhibition and for the downregulation of p21 and p27. Since LT could drive Akt-deficient cells through G1/S but not through mitosis, we concluded that high p21 and p27 protein levels impair progression through the G2 phase of the cell cycle and entry into mitosis. Elevated levels of p21 and p27 could thus inhibit CDK1 activation, which is required for G2 progression and entry into mitosis. Indeed, CDK1 phosphorylation at Thr 161 is impaired in Akt1/2 DKO (LT) cells after induction of exit from contact inhibition (Figure 2C). These results raised the possibility that the impaired expression of Skp2 in Akt-deficient cells prevents proteasomal degradation of p21 and p27. This possibility is consistent with previous observations showing that Skp2 is mainly required during the G2 phase of cell cycle to downregulate p27 (Nakayama et al, 2004; Nakayama and Nakayama, 2005).
Figure 2.
SV40 large T is sufficient to promote DNA synthesis in Akt1/2 DKO cells following induction of exit from contact inhibition, but is insufficient to promote entry into mitosis. (A) BrdU incorporation in contact inhibited WT (LT) and Akt1/2 DKO (LT) MEFs following addition of 20% FBS. BrdU incorporation was measured by counting at least 300 cells from at least 5 fields of view. The average of triplicate plates is presented. Error bars represent ±s.e. (B) Immunoblot analysis following addition of 20% FBS to contact inhibited WT (LT) and Akt1/2 DKO (LT) MEFs. Cell lysates of WT (LT) and Akt1/2 DKO (LT) MEFs were prepared at 0, 12, 16, 20, and 24 h post FBS stimulation and subjected to immunoblotting with anti-phospho-H3, p27, p21, Skp2, and tubulin antibodies. (C) The same lysates prepared in (B) were subjected to immunoblotting with anti-phospho-CDK1 (Thr 161), CDK1, and tubulin antibodies. (D) RNA isolated from WT and Akt1/2 DKO cells at 0, 12, and 20 h after FBS stimulation were analysed by quantitative RT–PCR with primers for p21, p27, and β-actin. Results are expressed relative to β-actin and represent the average±s.e. of three independent experiments. (E) WT and Akt1/2 DKO cells were grown to confluence and stimulated with FBS for 16 h in the presence or absence of the proteasomal inhibitor, MG132 (10 μM) for 3 h prior to harvesting cell lysates. Cell lysates were analysed by immunoblotting with anti-p21, p27, and tubulin antibodies.
To verify this possibility, we analysed the mechanism by which p21 and p27 proteins are downregulated during exit from contact inhibition. Surprisingly, p21 and p27 mRNA levels were elevated during exit from contact inhibition in both WT and Akt1/2 DKO cells, albeit not to the same extent (Figure 2D). The higher levels of p21 and p27 mRNAs in Akt1/2 DKO cells, are probably due to impaired phosphorylation of FoxO transcription factors (Nogueira et al, 2008), increasing FoxOs’ activity and thereby elevating its transcriptional targets, p21 and p27. Thus, there is no direct correlation between p21 and p27 mRNA levels and their protein levels. Indeed, during exit from contact inhibition, the p27 protein level is largely regulated by proteasomal degradation as treatment with the proteasome inhibitor, MG132, elevated p21 and p27 protein levels in WT cells but not in Akt1/2 DKO cells (Figure 2E). These results are consistent with the notion that during exit from contact inhibition, downregulation of p21 and p27 proteins is largely dependent on Skp2-mediated proteasomal degradation.
Ectopic expression of Skp2 in Akt1/2 DKO (LT) cells is sufficient to downregulate p21 and p27 to promote exit from contact inhibition, and to restore oncogenic transformation
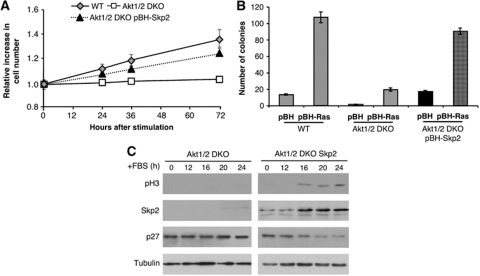
To determine whether the inability of Akt1/2 DKO (LT) cells to downregulate p21 and p27 proteins, with subsequent failure to exit from contact inhibition and promote oncogenic transformation is due to their failure to induce Skp2 expression, we ectopically expressed Skp2 in these cells. Ectopic expression of Skp2 in Akt1/2 DKO (LT) cells was sufficient to promote both exit from contact inhibition (Figure 3A) and anchorage-independent growth, induced by activated Ras (Figure 3B). The expression of Skp2 in Akt1/2 DKO (LT) cells promotes entry into mitosis concomitant with p27 downregulation (Figure 3C). We therefore concluded that Akt1/2 DKO (LT) cells are prohibited from exiting contact inhibition and are resistant to oncogenic transformation because they fail to express sufficient amounts of Skp2 protein.
Figure 3.
Restoring Skp2 expression in Akt1/2 DKO (LT) MEFs is sufficient to restore exit from contact inhibition, entry into mitosis, and oncogenic transformation. (A) Ectopic expression of Skp2 in Akt1/2 DKO MEFs is sufficient to promote exit from contact inhibition. Results represent the average ±s.e. of three independent experiments. (B) Ectopic expression of Skp2 in Akt1/2 DKO (LT) MEFs is sufficient to promote anchorage-independent growth. Results represent the average ±s.e. of three independent experiments. (C) Cell lysates of Akt1/2 DKO (LT) and Akt1/2 DKO (LT) expressing Skp2 MEFs were prepared at 0, 12, 16, 20, and 24 h post FBS stimulation and subjected to immunoblotting with anti-phospho-H3, Skp2, p27, and tubulin antibodies.
Akt is required for Skp2 mRNA translation in an mTORC1 and eIF4E-dependent manner during exit from contact inhibition
Previous studies suggested that Akt affects Skp2 function and stability through direct phosphorylation (Gao et al, 2009; Lin et al, 2009), but subsequent studies did not support these conclusions (Bashir et al, 2010; Boutonnet et al, 2010; Wang et al, 2010). Since ectopic expression of Skp2 in Akt1/2 DKO (LT) cells was sufficient to phenocopy WT (LT) cells, it suggests that Akt does not regulate Skp2 function during exit from contact inhibition in a post-translational mechanism, such as phosphorylation. Furthermore, ectopically expressed Skp2 protein has a similar half-life in both WT and Akt1/2 DKO cells (Supplementary Figure S1). Thus, our results are consistent with previous observations showing that Akt may not regulate Skp2 function through a direct phosphorylation or a post-translational mechanism (Bashir et al, 2010; Boutonnet et al, 2010; Wang et al, 2010).
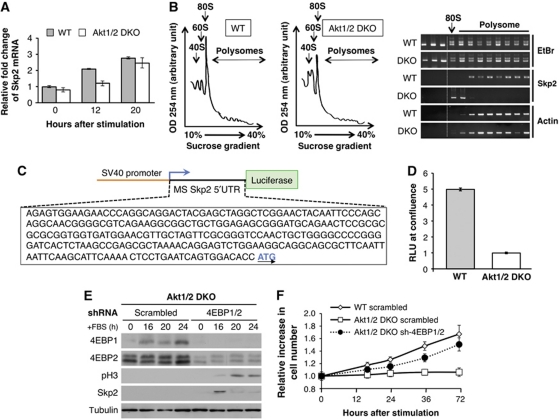
To understand the mechanism by which Akt mediates Skp2 protein expression and promote subsequent downregulation of p21 and p27 proteins during exit from contact inhibition, we examined Skp2 mRNA levels during exit from contact inhibition. Surprisingly, Skp2 mRNA was induced to a similar extent in both WT and Akt1/2 DKO cells (Figure 4A), suggesting that Akt is required for Skp2 mRNA translation during exit from contact inhibition.
Figure 4.
Skp2 mRNA translation is induced in WT (LT) MEFs during exit from contact inhibition, but not in Akt1/2 DKO (LT) MEFs due to the inhibition by 4E-BPs. (A) RNA isolated from WT and Akt1/2 DKO cells at 0, 12, and 20 h after FBS stimulation were analysed by quantitative RT–PCR with primers for Skp2 and β-actin. Results are expressed relative to β-actin and represent the average ±s.e. of three independent experiments. (B) (Left panels) Representative absorption profiles of ribosomes from WT and Akt1/2 DKO cells treated for 16 h with 20% FBS. 40S and 60S denote the corresponding ribosomal subunits and 80S the monosome. (Right panel) RNA was visualized by ethidium bromide (EtBr). Distribution of Skp2 and β-actin mRNAs was determined by semi-quantitative RT–PCR (representative results of four independent experiments). (C) Schematic of 5′ UTR of mouse Skp2-Luciferase reporter construct. (D) WT and Akt1/2 DKO MEFs were co-transfected with Renilla and pGL3 Skp2 5′ UTR luciferase vector as described in Materials and methods. The cells were then grown to confluence and analysed with the Dual Luciferase Reporter Assay system. Relative light units (RLUs) are depicted in the graph. Results are expressed relative to Renilla luciferase luminescence and represent the average±s.e. of four independent experiments. (E) Akt1/2 DKO (LT) MEFs stably expressing a scrambled or 4EBP1 and 4EBP2-specific shRNA (sh-4EBP1/2) were grown to confluency. Two days post confluency, medium was replaced with medium containing 20% FBS and cell lysates of Akt1/2 DKO scrambled control and sh-4EBP1/2 MEFs were prepared at 0, 16, 20, and 24 h post FBS stimulation and subjected to immunoblotting with anti-4EBP1, 4EBP2, phospho-H3, Skp2, and tubulin antibodies. (F) In a parallel experiment, cell number was measured at 0, 16, 24, 36, and 72 h after FBS stimulation. The relative increase in cell number (compared with time 0) is depicted. Results represent the average ±s.e. of three independent experiments.
To directly determine whether Skp2 mRNA translation is dependent on Akt during exit from contact inhibition, we isolated polysomes from WT (LT) or Akt1/2 DKO (LT) cells during exit from contact inhibition to assess Skp2 mRNA translation. As shown in Figure 4B, Skp2 mRNA was found associated with the polysomes isolated from WT cells but not with polysomes isolated from Akt1/2 DKO cells, clearly showing that Skp2 mRNA translation during exit from contact inhibition is Akt dependent.
Akt regulates mRNA translation through its effect on mTORC1 and its downstream effector, eIF4E. Translation of certain mRNAs is more dependent on eIF4E than others. The subset of mRNAs whose translation is highly dependent on eIF4E are mRNAs that possess highly structured 5′ UTRs (Hay and Sonenberg, 2004). Analysis of mouse and human Skp2 mRNA revealed highly structured 5′ UTRs (Supplementary Figure S2). Therefore, we cloned the 5′ UTR of mouse Skp2 mRNA upstream of the luciferase gene as a reporter for mRNA translation. The reporter plasmid was driven by an SV40 promoter to uncouple transcription from translation (Figure 4C). The Skp2 5′ UTR reporter plasmid was co-transfected with the Renilla luciferase reporter plasmid into WT and Akt1/2 DKO cells, prior to subjecting the cells to exit from contact inhibition. The Renilla luciferase construct is constitutively expressed and serves as a transfection control. Luciferase activity was quantified after induction of exit from contact inhibition. To control for transfection efficiency, the ratio of firefly to Renilla luciferase activity was taken as the relative light units (RLUs), and used in our comparisons between WT and DKO Skp2 5UTR luciferase activity. RLU was about five-fold higher in WT cells compared with Akt1/2 DKO cells (Figure 4D). These results are consistent with the notion that Akt is required for Skp2 mRNA translation during exit from contact inhibition in an mTORC1- and eIF4E-dependent manner. To directly determine that eIF4E is a downstream effector of Akt, which is required for the expression of Skp2 and for exiting contact inhibition, we knocked down 4E-BP1 and 4E-BP2 in Akt1/2 DKO cells (Figure 4E). The knockdown of 4E-BP1/2 in Akt1/2 DKO cells restored Skp2 expression upon serum stimulation of contact inhibited cells (Figure 4E), and promoted exit from contact inhibition (Figure 4F). Taken together, these results show that eIF4E activation is required and sufficient for Akt-mediated exit from contact inhibition.
The deletion of Tsc2 or the deletion of 4E-BP1/2 is sufficient to induce exit from contact inhibition
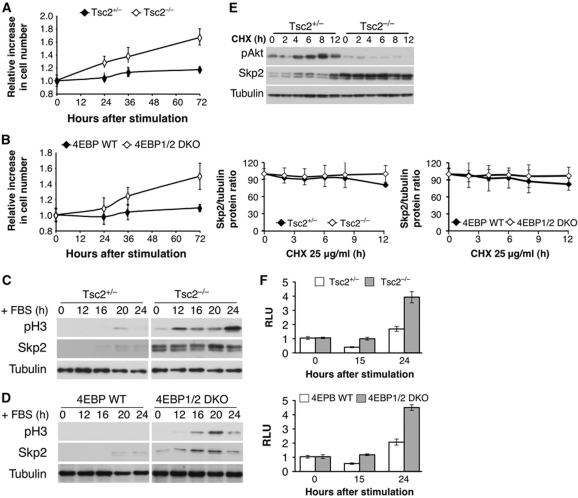
The results described above imply that when mTORC1 or eIF4E is hyperactivated, they could promote exit from contact inhibition. We therefore subjected Tsc2−/− cells, in which mTORC1 is hyperactivated, and 4E-BP1/2 DKO, in which eIF4E is hyperactivated, to exit from contact inhibition and found that both Tsc2−/− and 4E-BP1/2 DKO cells were able to exit contact inhibition while the control cells could not (Figure 5A and B), which is also manifested by entry into mitosis, as measured by pH3 (Figure 5C and D). Notably, the onset of pH3 detection occurs much earlier in Tsc2−/− cells (Figure 5C). Consistently, we noticed that Tsc2−/− cells are continuously dividing and are resistant to contact inhibition. We also found that Skp2 is expressed at higher levels in Tsc2−/− and 4E-BP1/2 DKO cells than in control cells (Figure 5C and D). Importantly, the half-life of Skp2 protein in Tsc2+/− and Tsc2−/− is similar, despite the low Akt activity in Tsc2−/− cells (Figure 5E). Similarly, the higher levels of Skp2 in 4E-BP1/2 DKO cells are not due to the higher stability of Skp2 in these cells (Figure 5E). Finally, we subjected Tsc2−/− and 4E-BP1/2 DKO cells, to a luciferase assay using the Skp2 5′ UTR reporter plasmid, during exit from contact inhibition. The results in Figure 5F clearly indicate that Skp2 mRNA translation is markedly elevated in Tsc2 KO and 4E-BP1/2 DKO cells during exit from contact inhibition. Taken together, these results strongly suggest that Akt is required for oncogenic transformation through its effect on eIF4E to mediate Skp2 mRNA translation.
Figure 5.
Tsc2−/− and 4E-BP1/2 DKO cells are capable of exiting contact inhibition and entering into mitosis with concomitant high Skp2 protein expression. (A, B) Two days post confluency, Tsc2+/−, Tsc2−/− MEFs (A) and 4E-BP WT, 4E-BP1/2 DKO MEFs (B) medium was replaced with medium containing 20% FBS, and cell number was measured at 0, 24, 36, and 72 h after stimulation. The relative increase in cell number (compared with time 0) is depicted. Results represent the average ±s.e. of three independent experiments. (C, D) Cell lysates of Tsc2+/−, Tsc2−/− MEFs (C) and 4E-BP WT, 4E-BP1/2 DKO MEFs (D) were prepared at 0, 12, 16, 20, and 24 h post FBS stimulation and subjected to immunoblotting with anti-phospho-H3, Skp2, and tubulin antibodies. (E) Tsc2+/−, Tsc2−/−, 4E-BP WT, and 4E-BP1/2 DKO MEFs were incubated for 0, 2, 4, 6, 8, and 12 h with CHX (25 μg/ml) and cell lysates were prepared and subjected to immunoblotting with anti-Akt, Skp2, and tubulin antibodies. Quantification of at least three experiments was performed and expressed as Skp2 over tubulin protein ratio. Results represent the average ±s.e. of three independent experiments. (F) Tsc2+/−, Tsc2−/−, 4E-BP WT, and 4E-BP1/2 DKO MEFs were co-transfected with Renilla and pGL3 Skp2 5′ UTR luciferase vector as described in Materials and methods. The cells were then grown to confluence and after 2 days medium was replaced with medium containing 20% FBS. At 0, 15, and 24 h post addition of 20% FBS, cells were harvested and analysed with the Dual Luciferase Reporter Assay system. RLU is depicted in the graph. Results are expressed relative to Renilla luciferase luminescence and to time 0 and represent the average ±s.e. of four independent experiments.
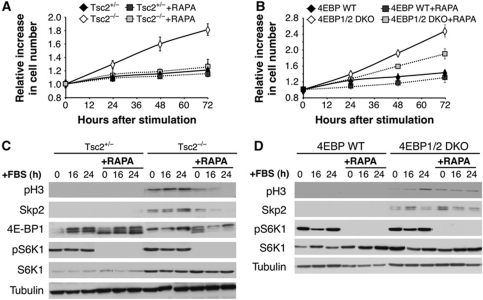
If the expression of Skp2 protein is largely dependent on eIF4E, it is expected that inhibition of mTORC1 activity in Tsc2−/− cells should impair exit from contact inhibition while the inhibition of mTORC1 in 4E-BP1/2 DKO cells should not affect exit from contact inhibition. To examine this prediction, we inhibited mTORC1 activity with rapamycin (Figure 6A). While inhibition of mTORC1 inhibits exit from contact inhibition of Tsc2−/− cells, mTORC1 inhibition failed to inhibit exit from contact inhibition of 4E-BP1/2 DKO cells (Figure 6B). 4E-BP1/2 DKO cells were still capable of exiting contact inhibition, regardless of mTORC1 inhibition. The inhibition of mTORC1 in Tsc2−/− cells prohibited Skp2 protein expression and histone H3 phosphorylation following induction of exit from contact inhibition (Figure 6C). In contrast, mTORC1 inhibition in 4E-BP1/2 DKO cells still maintains relatively high levels of Skp2 protein expression and histone H3 phosphorylation after induction of exit from contact inhibition (Figure 6D). We therefore concluded that eIF4E activity is critical for exit from contact inhibition. This activity of eIF4E is mediated through the induction of Skp2 mRNA translation. Consistently, Skp2 null cells expressing SV40 LT cannot exit contact inhibition (Supplementary Figure S3A), even if they express activated Akt (Supplementary Figure S3B). In Skp2 null cells, histone H3 is not phosphorylated, and p21 and p27 are not downregualted (Supplementary Figure S3C), even if the cells express activated Akt (Supplementary Figure S3D).
Figure 6.
Inhibition of mTORC1 activity inhibits exit from contact inhibition of Tsc2−/− cells but not of 4E-BP1/2 DKO cells. (A, B) Two days post confluency, Tsc2+/−, Tsc2−/− MEFs (A) and 4E-BP WT, 4E-BP1/2 DKO MEFs (B) were incubated with rapamycin (20 nM) 3 h prior to being induced with 20% FBS. Cells were then harvested and counted at 0, 24, 48, and 72 h post stimulation. The relative increase in cell number (compared with time 0) is depicted. Results represent the average ±s.e. of three independent experiments. (C, D) Cell lysates of cells treated as in (A) and (B) were prepared at 0, 16, and 24 h post addition of 20% FBS, and subjected to immunoblotting with anti-phospho-S6K, S6K, 4E-BP1, phospho-H3, Skp2, and tubulin antibodies.
The requirement of Akt for adipocyte differentiation is mediated by Skp2 mRNA translation
Previously, we had shown that Akt1/2 DKO mice display an almost complete impairment of adipogenesis (Peng et al, 2003). MEFs derived from these mice are impaired in their ability to differentiate into adipocytes in vitro (Peng et al, 2003). These results indicate that Akt is likely the most critical downstream signalling effector required for executing insulin action in adipogenesis. The role of Akt in human adipogenesis is underscored by the discovery of a family that carries an Akt2 mutation and display lipoatrophy. This Akt2 mutation acts in a dominant manner and inhibits adipogenesis in vitro (George et al, 2004). However, the mechanism by which Akt exerts its effect on adipogenesis is not fully understood.
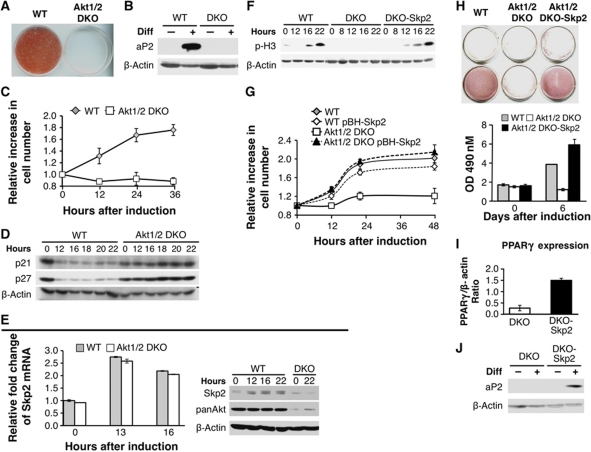
To further characterize the effects and the molecular pathways influenced by Akt during adipogenesis in vitro, we isolated preadipocytes from WT and Akt1/2 DKO 18.5 day embryos. Preadipocytes were immortalized using SV40 LT, subjected to differentiation, and assessed for their ability to differentiate into adipocytes as previously described (Klein et al, 2002). Compared with WT, Akt1/2 DKO preadipocytes were almost completely impaired in their ability to differentiate and fail to accumulate lipid as assessed by Oil-Red-O (ORO) staining and quantification of lipid extracted from stained plates (Figure 7A). Six days after induction of differentiation, we assessed leptin expression and secretion as one of the markers of adipocyte differentiation and function. Compared with WT cells, leptin expression and secretion were nearly undetectable in Akt1/2 DKO preadipocytes (Supplementary Figure S4A). Further, adipocyte markers of differentiation such as the fatty acid binding protein, aP2, fail to get expressed in Akt1/2 DKO preadipocytes (Figure 7B). On the other hand, even in the absence of differentiation inducing agents, expression of activated Akt was sufficient to induce differentiation as well as leptin expression, as a marker of fully differentiated cells (Supplementary Figure S4B).
Figure 7.
Akt1/2 DKO preadipocytes fail to undergo MCE and adipogenesis because of the inability to induce Skp2 protein expression. (A) SV40 large T-immortalized preadipocytes, isolated from WT and Akt1/2 DKO mice, were subjected to differentiation in vitro. Differentiation was assessed by Oil-Red-O staining and quantification as described in Materials and methods. (B) Assessment of WT and Akt1/2 DKO preadipocytes by the expression of aP2 using immunoblotting. (C) Akt1/2 DKO preadipocytes fail to undergo MCE. Two days post confluency, cells were induced to differentiate and cell number was measured at 0, 12, 24, and 36 h after induction of differentiation. Results represent the average ±s.e. of three independent experiments. (D) Akt1/2 DKO preadipocytes fail to downregulate p21 and p27 protein expression after induction of differentiation. Two days post confluency, cells were induced to differentiate. Protein extracts were isolated at 0, 12, 16, 18, 20, and 22 h after induction of differentiation and were subjected to immunoblotting using anti-p21 and anti-p27 antibodies. (E) Left panel: Skp2 mRNA levels are induced to the same extent in WT and Akt1/2 DKO preadipocytes immediately after induction of differentiation. Two days post confluency, cells were induced to differentiate and mRNAs were extracted at 0, 13, and 16 h post induction of differentiation. mRNAs were quantified by quantitative RT–PCR. Results represent the average ±s.e. of three independent experiments. Right panel: Skp2 protein is elevated in WT but not in Akt1/2 DKO preadipocytes immediately after induction of differentiation. Two days post confluency, cells were induced to differentiate and proteins were extracted at 0, 12, 16, and 22 h post induction of differentiation. Protein extracts were subjected to immunoblotting, using anti-Skp2, anti-pan Akt, and anti-β actin antibodies. (F) Ectopic expression of Skp2 in Akt1/2 DKO preadipocytes is sufficient to restore entry into mitosis, as measured by the phosphorylation of histone H3, following induction of differentiation. (G) Ectopic expression of Skp2 in Akt1/2 DKO preadipocytes is sufficient to restore MCE. Results represent the average ±s.e. of three independent experiments. (H) Ectopic expression of Skp2 in Akt1/2 DKO preadipocytes is sufficient to restore adipocyte differentiation, as measured by Oil-red-O staining and quantification. Results represent the average ±s.e. of three different experiments. (I) Ectopic expression of Skp2 in Akt1/2 DKO preadipocytes is sufficient to restore the induction of PPARγ expression, as measured by quantitative RT–PCR 5 days after induction of differentiation. Results represent the average ±s.e. of three different experiments. (J) Ectopic expression of Skp2 in Akt1/2 DKO preadipocytes is sufficient to restore the induction of aP2 expression, as measured by immunoblotting, 5 days post induction of differentiation.
Consistent with the lack of lipid accumulation, Akt1/2 DKO preadipocytes fail to upregulate transcription factors essential for driving adipogenesis such as the CAAT/enhancer binding protein α (cEBPα), and peroxisome proliferator-activated receptor γ (PPARγ), the master regulator of adipogenesis (Supplementary Figure S4C). PPARγ had been shown to be necessary and sufficient to drive adipogenesis (Rosen et al, 1999; Rosen and Spiegelman, 2001). Since PPARγ failed to be upregulated in Akt1/2DKO preadipocytes, we wished to determine whether the defect in adipogenesis could stem from failure to induce PPARγ. To test this, ectopic PPARγ was stably expressed in DKO cells (Supplementary Figure S4D). Ectopic PPARγ induced cEBPα expression as expected (Supplementary Figure S4D), since PPARγ and cEBPα regulate each other's expression (Wu et al, 1999), PPARg expression was sufficient to drive adipogenesis of Akt1/2 DKO preadipocytes, as assessed by lipid accumulation (Supplementary Figure S4E), and the expression of differentiation markers such as aP2 (Supplementary Figure S4F).
Multiple upstream transcriptional regulators regulate PPARγ expression (Rosen and MacDougald, 2006). One of the most important classical regulators of PPARγ is the adipocyte determination and differentiation factor ADD1 (also known as sterol regulatory binding protein 1c, SREBP1c), which transactivates PPARγ gene transcription by binding to an E-box motif in its promoter (Fajas et al, 1999; Miard and Fajas, 2005). We therefore assessed SREBP1c expression in WT and Akt1/2 DKO cells by RT–PCR. SREBP1c mRNA was elevated in WT cells on day 2 after being induced to differentiate, and increased progressively until day 6, but in Akt1/2 DKO cells there was a complete failure in the induction of SREBP1c (Supplementary Figure S5A).
To examine whether restoration of SREBP1c expression in Akt1/2 DKO preadipocytes would be able to rescue the differentiation defect and PPARγ expression, we used retroviral mediated gene transfer to express a constitutively active form of SREBP1c (Kim et al, 1998) that always accumulates in the nucleus (Supplementary Figure S5B). Even though SREBP1c was expressed to wild-type levels in the DKO cells, it failed to induce PPARγ expression and differentiation (Supplementary Figure S5B, and data not shown).
We then studied the expression of other transcription factors, which have been shown to regulate PPARγ expression during adipocyte differentiation (Rosen et al, 2000). Both upstream regulators, PGC1α and PGC1β, failed to be induced in the DKO cells (Supplementary Figure S5C), as well as cEBPβ, which is expressed at approximately the same level in both WT and Akt1/2 DKO preadipocytes but fails to be further induced in Akt1/2 DKO preadipocytes (Supplementary Figure S5D). Finally, there was a failure to downregulate multiple repressors of differentiation and PPARγ transcription such as GATA2 and Wnt10a in the Akt1/2 DKO preadipocytes (Supplementary Figure S5E). These results clearly show that Akt regulates PPARγ expression and subsequently adipocyte differentiation by affecting the expression of multiple upstream PPARγ regulators.
Because Akt affected all the tested upstream regulators of adipocyte differentiation, we wished to examine whether Akt is required for the earliest event in adipocyte differentiation, which is MCE. MCE has been shown to be a prerequisite for the induction of the adipogenic gene expression program (Tang et al, 2003). MCE is characterized by hormone-induced re-entry into the cell cycle from contact inhibition (Patel and Lane, 2000). Since the WT and Akt1/2 DKO preadipocytes were immortalized by SV40 LT, we thought that like the exit from contact inhibition, Akt is also required for the entry into mitosis during MCE. Indeed, we found that Akt1/2 DKO preadipocytes are impaired in MCE (Figure 7C). The inability to undergo MCE is accompanied by the inability to downregulate p21 and p27 proteins (Figure 7D). The same as we observed during exit from contact inhibition there is no direct correlation between the levels of p21 and p27 mRNAs and their protein levels during MCE (Supplementary Figure S6) and the downregulation of p27 protein is mediated by proteasomal degradation (Supplementary Figure S7). The higher levels of p21 and p27 mRNAs in Akt1/2 DKO preadipocytes are likely due to the activation of FoxO in these cells (Supplementary Figure S8A). Furthermore, expression of dominant-negative FOXO (DN-FOXO) in Akt1/2 DKO preadipocytes decreased p27 mRNA levels following induction of differentiation (Supplementary Figure S8B), but did not substantially reduce p27 protein levels (Supplementary Figure S8C). DN-FOXO accelerated entry into S phase following induction of differentiation (Supplementary Figure S8D), but was insufficient to induce completion of MCE (Supplementary Figure S8E) and adipocyte differentiation (Supplementary Figure S8F). Similarly to what we observed during exit from contact inhibition, while Skp2 mRNA level is elevated to a similar extent in both WT and Akt1/2 DKO preadipocytes following induction of differentiation, Skp2 protein level was elevated only in WT preadipocytes (Figure 7E). These results are consistent with our results showing that Akt mediates exit from contact inhibition through its effect on Skp2 mRNA translation (Figures 1, 2, 3 and 4). These results are also consistent with previous results showing that Skp2 is required for adipocyte differentiation and that in the absence of Skp2, preadipocytes arrest in the G2 phase of the cell cycle and fail to undergo MCE (Auld et al, 2007a, 2007b).
To determine whether Skp2 is a downstream effector of Akt, which is required for MCE and adipogenesis, we ectopically expressed Skp2 in Akt1/2 DKO preadipocytes. As shown in Figure 7F, the restoration of Skp2 expression in Akt1/2 DKO preadipocytes was able to promote entry into mitosis, as measured by the phosphorylation of histone H3, and the completion of MCE (Figure 7G). Furthermore, Skp2 was also able to rescue differentiation of Akt1/2 DKO preadipocytes as assessed by lipid accumulation via ORO staining and quantification (Figure 7H) and by the induction of PPARγ (Figure 7I) and aP2 expression (Figure 7J). Taken together, these results clearly show that Skp2 is the major downstream effector of Akt required for adipogenesis.
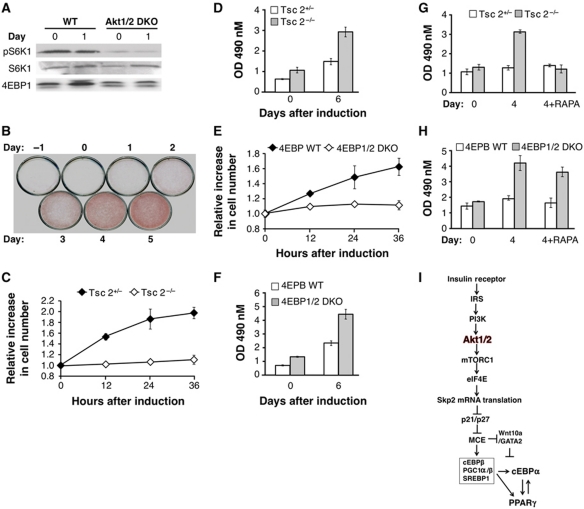
As shown in Figure 4, the ability of Akt to elevate Skp2 expression is dependent on mRNA translation downstream of mTORC1 and eIF4E. mTORC1 and eIF4E activation, as measured by the phosphorylation of S6K1 and 4E-BP1 mobility shift, is impaired in Akt1/2 DKO preadipocytes (Figure 8A). Consistent with the requirement of mTORC1 activation for MCE, inhibition of mTORC1 with rapamycin inhibits adipocyte differentiation only if the preadipocytes are treated in the first 2 days but not if treated later than 2 days post induction of differentiation (Figure 8B). Moreover, Tsc2−/− cells in which Akt activity is low (Skeen et al, 2006) undergo MCE better than control Tsc2-proficient cells (Figure 8C), and consistent with previous results (Zhang et al, 2009) differentiate robustly into adipocytes as compared with control Tsc2-proficient cells (Figure 8D). These results suggest that when mTORC1 is hyperactivated Akt is no longer required for Skp2 expression, MCE, and induction of differentiation. The ability of Akt to elevate Skp2 protein expression is dependent on eIF4E, downstream of mTORC1 (Figure 6B and C). Indeed, MEFs derived from 4E-BP1/2 DKO mice, in which eIF4E is hyperactivated, undergo MCE (Figure 8E) and adipogenesis much better than control 4E-BP-proficient cells (Figure 8F), consistent with previous results (Le Bacquer et al, 2007). Furthermore, while the differentiation of Tsc2−/− cells is sensitive to rapamycin (Figure 8G), the differentiation of 4E-BP1/2 DKO cells is relatively resistant to rapamycin treatment (Figure 8H). Taken together, our results showed that mTORC1 and its downstream effector eIF4E, which regulate Skp2 mRNA translation, are the most critical downstream effector of Akt required for adipocyte differentiation (Figure 8I).
Figure 8.
Akt promotes MCE and adipocyte differentiation by activating mTORC1 and eIF4E. (A) mTORC1 activity, as measured by S6K1 phosphorylation and 4E-BP1 mobility shift, is induced upon induction of differentiation in WT but not in Akt1/2 DKO preadipocytes. Protein extracts were isolated from cells at the indicated time points and subjected to immunoblotting using anti-pS6K1, S6K1, and 4E-BP1 antibodies. (B) Rapamycin inhibits adipocyte differentiation only if the cells are exposed to rapamycin within the first 2 days post induction of differentiation. WT preadipocytes were subjected to the adipocyte differentiation protocol and were exposed to rapamycin at the indicated days post induction of differentiation. Differentiation was assessed by Oil-red-O staining. (C) mTORC1 activation is sufficient to promote MCE following induction of differentiation. Tsc2−/−p53−/− and Tsc2+/−p53−/− MEFs were grown to confluency and induced to differentiate. Cell numbers were quantified at the indicated time points post induction of differentiation. Results represent the average ±s.e. of three independent experiments. (D) mTORC1 activation accelerates adipocytes differentiation. Tsc2−/−p53−/− and Tsc2+/−p53−/− were subject to adipocyte differentiation protocol. Differentiation was quantified after Oil-red-O staining and measuring absorbance at 490 nm. Results represent the average ±s.e. of three independent experiments. (E) Activation of eIF4E is sufficient to promote MCE following induction of differentiation. WT (p53−/−) and 4E-BP1/2 (p53−/−) DKO MEFs were grown to confluency and induced to differentiate. Cell numbers were quantified at the indicated time points post induction of differentiation. Results represent the average ±s.e. of three independent experiments. (F) Activation of eIF4E accelerates adipocyte differentiation. Cells were subjected to adipocyte differentiation protocol. Differentiation was quantified after Oil-red-O staining and measuring absorbance at 490 nm. Results represent the average ±s.e. of three independent experiments. (G) Adipocyte differentiation of Tsc2−/− cells is sensitive to rapamycin. Experiments were done as in (D) except that the cells were also treated with rapamycin for the period of differentiation. (H) Adipocyte differentiation of 4E-BP1/2 DKO cells is resistant to rapamycin. Experiments were done as in (F) except that the cells were also treated with rapamycin for the period of differentiation. (I) A schematic illustration showing the mechanism by which Akt regulates adipogenesis. Through the activation of mTORC1 and the phosphorylation of 4E-BP, Akt elevates eIF4E activity, which in turn elevates Skp2 mRNA translation. Elevated Skp2 protein promotes MCE through the degradation of p21 and p27 proteins. MCE enables the initiation of a transcriptional cascade that governs adipocyte differentiation.
Discussion
Akt is perhaps the most frequently activated oncoprotein in human cancers (Bhaskar and Hay, 2007; Robey and Hay, 2009). Akt is required for the survival and proliferation of cancer cells as well as for accelerated glucose metabolism and other anabolic pathways observed in cancer cells. Previously, we showed that in addition to its role in cell survival, Akt is required for both normal cell proliferation and oncogenic transformation (Skeen et al, 2006). These effects are largely, if not exclusively, mediated through its downstream effector mTORC1 (Skeen et al, 2006). When mTORC1 is hyperactivated, Akt is no longer required for cell proliferation or susceptibility to oncogenic transformation. As was previously shown, Akt is required for pRb phosphorylation, which promotes entry into the S phase of the cell cycle (Skeen et al, 2006). Indeed, expression of SV40 LT, which sequesters pRb, in Akt-deficient cells restored normal cell proliferation. The expression of SV40 LT in WT cells promotes exit from contact inhibition. Surprisingly, however, Akt1/2 DKO cells are impaired in exit from contact inhibition, despite the expression of SV40 LT. Since exit from contact inhibition is one hallmark of tumourigenic cells and is required for oncogenic transformation, as measured by anchorage-independent growth, Akt1/2 DKO cells are relatively resistant to oncogenic transformation despite the expression of SV40 LT. Thus, in addition to its effect on G1/S progression, Akt is required for exit from contact inhibition and anchorage-independent growth in a mechanism, which is independent of pRb phosphorylation and inactivation by the G1/S CDKs. This additional mechanism was unveiled in the current study.
In contact inhibited cells, the high levels of the cyclin-dependent kinase inhibitors, p21 and p27, prohibit re-entry into the cell cycle and exit from contact inhibition. p21 and p27 attenuates the phosphorylation of pRb by the G1/S CDKs, which are activated upon addition of growth factors (Weinberg, 1995). Expression of SV40 LT alleviates the inhibition of pRb phosphorylation by p21 and p27 and promotes exit from contact inhibition. Interestingly, we found that Skp2 protein levels are elevated after addition of growth factors to contact inhibited cells, which led to subsequent downregulation p21 and p27 protein levels. We showed that downregulation of p21 and p27 protein levels is required for exit from contact inhibition even if pRb activity is abrogated by SV40 LT. High levels of p21 and p27 in the G2 phase of the cell cycle inhibit CDK1 activation, which is required for G2 progression prior to entry into mitosis (Nakayama et al, 2004; Pagano, 2004). Like in WT cells, DNA synthesis, and therefore entry into S phase, is induced upon addition of growth factors to contact inhibited Akt1/2 DKO cells expressing SV40 LT. However, unlike in WT cells, Skp2 protein is not sufficiently elevated in contact inhibited Akt1/2 DKO cells expressing SV40 LT, following addition of growth factors. Subsequently, p21 and p27 proteins remain elevated and prevent entry into mitosis.
Our results showed that Akt is not required for the induction of Skp2 mRNA following addition of growth factors to contact inhibited cells, but rather is required for the translation of Skp2 mRNA under these conditions. Akt mediates its effect on Skp2 mRNA translation through mTORC1 and eIF4E. These results are consistent with our previous observation showing that when mTORC1 is hyperactivated, high Akt activity is no longer required for oncogenic transformation as quantified by anchorage-independent growth. The activation of eIF4E, via inhibition of 4E-BP activity, was shown to have a predominant role downstream of mTORC1 in enhancing cell proliferation and oncogenic transformation (Petroulakis et al, 2009; Dowling et al, 2010). It was shown that eIF4E activation accelerates the recruitment of certain mRNAs that could impact cell proliferation, such as of ODC and cyclin D3, to the polysomes (Dowling et al, 2010). Our results showed that upon exit from contact inhibition Skp2 mRNA is recruited to polysomes in an Akt-dependent manner. Our results indicate that activation of eIF4E by Akt is required for Skp2 mRNA translation and that activation of eIF4E is sufficient to promote exit from contact inhibition. Our results are consistent with previous results showing that silencing of eIF4G, whose interaction with eIF4E is required for mRNA translation, inhibits the recruitment of Skp2 mRNA to polysomes (Ramirez-Valle et al, 2008). It should be noted, however, that in 4E-BP1/2 DKO cells, S6K1 activity is also elevated, and since S6K1 was shown to increase the level of eIF4A (Dorrello et al, 2006), we cannot completely exclude the possibility that S6K1 can also contributes to Skp2 mRNA translation.
Our results are inconsistent with previous reports, which were subsequently challenged by others (Bashir et al, 2010; Boutonnet et al, 2010; Wang et al, 2010), showing that Akt regulates Skp2 function through direct phosphorylation (Gao et al, 2009; Lin et al, 2009). Although we cannot completely exclude the possibility that Akt regulates Skp2 function, it is unlikely the case because ectopic expression of Skp2 in Akt1/2 DKO cells was sufficient to recapitulate Skp2 function in WT cells. Thus, our results are consistent with the reports showing that the phosphorylation of Serine 72 on Skp2, which was reported to be phosphorylated by Akt, does not affect Skp2 function (Bashir et al, 2010; Boutonnet et al, 2010; Wang et al, 2010).
In summary, the results presented here showed that Skp2 is a downstream effector of Akt. However, Akt does not affect Skp2 function, but rather its protein accumulation in cells via the induction of its mRNA translation, which is dependent on eIF4E. The results also showed that Skp2 is required for Akt-dependent entry into mitosis during exit from contact inhibition and anchorage-independent growth. Skp2 was also shown to mediate G1/S progression through the degradation of p21 and p27. Thus, in principle, the elevation of Skp2 mRNA translation by eIF4E could also explain the requirement of mTORC1 downstream of Akt for G1/S progression. However, during G1/S transition there are alternative ubiquitin ligases, which could induce the proteasomal degradation of p21 and p27 (Nakayama and Nakayama, 2005).
Thus, we discovered a new mechanism by which Akt and eIF4E affect tumourigenicity. Like the activation of Akt, Skp2 expression is elevated in a wide range of human cancers (Frescas and Pagano, 2008), and therefore high levels of Skp2 protein in cancer cells could reflect hyperactivation of Akt and eIF4E. Inhibition of eIF4E activity was shown to attenuate tumourigenicity induced by Akt activation due to Pten deficiency (Furic et al, 2010; Ueda et al, 2010). Thus, based on our results Skp2 could be a critical downstream effector; ablation of which could be used preferentially for the therapy of tumours, which exhibit Akt, mTORC1, or eIF4E hyperactivation.
A similar paradigm exists during adipocyte differentiation as we showed that Akt is required to induce the transcriptional cascade leading to adipogenesis by promoting MCE. The promotion of MCE is dependent on the ability of Akt to activate mTORC1 and eIF4E, which are required for Skp2 mRNA translation during MCE. In turn, Skp2 protein promotes MCE largely through the degradation of p21 and p27 proteins. These results are consistent with results showing that the lack Skp2 impairs adipogenesis in vivo and in vitro (Cooke et al, 2007; Sakai et al, 2007) and that the deficiency of the cyclin kinase inhibitors, p21 and p27, mediates adipocyte hyperplasia in vivo and in vitro, as well as obesity in vivo (Naaz et al, 2004). Thus, the function of Akt/mTORC1/eIF4E axis in cell proliferation is coupled to adipocyte differentiation. However, our results cannot exclude the possibility that additional functions of Akt are required for the full functionality of mature adipocytes.
Materials and methods
Antibodies and reagents
See Supplementary data.
Retroviral vectors and cells
The following retroviral vectors were used: pBabe-Puro-SV40 LT antigen; pBabe-Hygro and pBabe-Hygro H-Rasvaline12; pBabe-Hygro Skp2; pBabe-Puro myristoylated Akt (mAkt)-ER. PPARγ and CA-SREBP1c expression vector were obtained from Bruce Spiegelman laboratory (Harvard Medical School), and were subcloned into pBabe-Hygro retroviral vector. pBabe-GFP retroviral vectors expressing mAkt or DN-FOXO were previously described (Nogueira et al, 2008). For 4E-BP1 and 4E-BP2 knockdown, lentiviral vectors were purchased from Sigma (St Louis, MO). shRNA vector accession numbers are mouse 4E-BP1 (Sigma: TRCN0000075612), mouse 4E-BP2 (Sigma: TRCN0000075614), and scrambled shRNA Control (Sigma: SHC002).
WT, Akt1/2 DKO, Skp2+/−, and Skp2−/− primary fibroblasts were immortalized with SV40 LT. Skp2+/− and Skp2−/− primary fibroblast were generated from embryos of Skp2−/− mice obtained from Paul Cooke, University of Illinois, Urbana-Champaign and Keiichi Nakayama, Kyushu University. For Skp2 overexpression, polyclonal cell lines were generated by infecting SV40 LT-immortalized MEFs with pBabe-Hygro or pBabe-Hygro-Skp2 retrovirus. To generate cell lines expressing activated Akt, polyclonal cell lines were generated by retroviral infection with mAkt-ER. Following selection, mAkt was expressed by addition of 300 nM 4-hydroxytamoxifen (4-OHT) to culture media for 48 h. Tsc2+/−, Tsc2−/−, 4E-BP WT, and 4E-BP1/2 DKO MEFs were previously described (Bhaskar et al, 2009; Dowling et al, 2010).
Preadipocytes were isolated from 18.5 day embryos following the established protocol (Klein et al, 2002). For details, see Supplementary data.
Cell culture and proliferation
WT, Akt1/2 DKO, Akt1/2 DKO pBabe-Hygro-Skp2, Skp2+/−, Skp2−/−, Tsc2+/−, Tsc2−/−, 4E-BP WT, and 4E-BP1/2 DKO MEFs were grown in DMEM supplemented with 10% FBS and 1% Penicillin/Streptomycin. Cell proliferation experiments were performed by seeding cells at low density (25 × 103 cells per 6 cm plate). To determine the proliferation rate, cells were trypsinized at the time points indicated (phosphate-buffered saline solution (PBS) containing 0.05% trypsin and 0.02% EDTA), re-suspended in 1 ml of media and counted using a hemocytometer. Cell number for each time point was determined in triplicate.
To determine whether the cells underwent cell doubling during induction with serum, cells were seeded at high density (50 × 105 cells per 6 cm plate) grown to confluence, and 48 h post confluency, medium was replaced with DMEM containing 20% FBS. Cells were counted at 0, 24, 36, 48, and 72 h post stimulation to assess ability to exit from contact inhibition. Where indicated, 4-OHT was used to induce activation of mAkt-ER. To induce Akt activity, 4-OHT (300 nM) was added to culture media when cells reached confluency (48 h prior to stimulation). To generate stable knockdowns of 4E-BP1 and 4E-BP2 in Akt1/2 DKO cells, the cells were infected with a lentivirus carrying 4E-BP1 shRNA. The next day, cells were re-infected with lentivirus carrying 4E-BP2 shRNA and selected with puromycin for 48 h (2.5 μg/ml).
Anchorage-independent growth
Anchorage-independent growth was performed as previously described (Skeen et al, 2006). In brief, cells (15 × 103) were re-suspended in 10% FBS in DMEM containing 0.35% agarose and plated onto a layer of 0.7% agarose-containing medium in a 6-well dish. For Ras transformation, polyclonal cell lines were generated by infecting SV40 LT-immortalized MEFs with pBabe-Hygro or pBabe-Hygro-H-Rasvaline12 retrovirus followed by selection with 300 mg/ml hygromycin. Soft agar colonies from the entire plate were counted 19 days after cells were plated. Experiments were performed three times in triplicate.
BrdU incorporation
BrdU incorporation was performed as previously described (Skeen et al, 2006). For details, see Supplementary data.
Adipocyte differentiation
Differentiation of SV40 LT-immortalized preadipocytes was performed by growing the cells to confluence in 10% DMEM supplemented with 20 nM insulin and 1 nM Triiodothyronine (differentiation medium). Confluent cells were incubated for 48 h in differentiation medium further supplemented with 0.5 mM isobutylmethylxanthine, 0.5 μM dexamethasone, and 0.125 mM indomethacin (induction medium). Subsequently, the cells were maintained in differentiation medium for 4 more days for complete differentiation into mature adipocytes. Differentiation of MEFs was performed after growing the cells to confluency to induce contact inhibition. The cells were induced to differentiate with a cocktail containing troglitazone 5 μM, insulin 830 nM, isobutylmethylxanthine 0.5 mM, and dexamethasone 1 μM. Two days post induction, media were changed and supplemented with insulin and cultured for another subsequent 4 days with media change every 2 days.
RNA extraction and QRT–PCR
See Supplementary data.
Cloning of mouse Skp2 5′ UTR
The 480-bp 5′ UTR of Mouse Skp2 5′ UTR (ensembl, ENSMUST00000096482) was amplified with the following forward and reverse primers: forward 5′-CCTTAAGCTTCAGTCAATCGGCTGACATTTC-3′ and reverse 5′-CCTTCCATGGAGCAACATTCGCGGCCTAAAA-3′ from genomic DNA of mouse embryonic fibroblast (MEF) cells isolated from C57Bl6 mice. The resultant PCR fragment and target vector, the PGL3 promoter vector (Promega, Madison, USA) were digested with HindIII and NcoI restriction enzymes, gel purified and ligated, creating the pGL3 Skp2 5′ UTR vector that was used in transfection assays during contact inhibition experiments.
Transfection and luciferase assays
Tsc+/−, Tsc−/−, 4E-BP WT, and DKO cells were plated in 12-well dishes (8 × 104/well) and 24 h later (65% confluence) were co-transfected with 10 ng of the Renilla luciferase vector and 100 ng of the pGL3 Skp2 5′ UTR luciferase vector, along with empty luciferase control vector pGL3 (Promega) using Lipofectamine 2000 reagent (Life Technologies, Inc.). At 48 h post transfection, confluent and contact inhibited cells were stimulated with DMEM containing 20% serum and luciferase assays were performed. Cells were assayed at various times points: 0 h (just prior stimulation), 16 and 22 h post stimulation. A non-stimulated control was included. Cells were harvested at each time point using the passive lysis and analysed with the Dual Luciferase Reporter Assay system (Promega). WT, Akt12 DKO cells were subjected to a different transfection method. In brief, 1 × 105 cells were seeded in 6-well plates and reporter constructs were co-transfected using the PolyMag Neo-magnetofection reagent in a 1:3 ratio of DNA to magnetofection reagent (Boca scientific). Following transfection, cells were grown to confluency prior to being harvested and analysed with the Dual Luciferase Reporter Assay system. All transfections were performed in triplicate and standard error mean values of four independent experiments were calculated.
Polysomes fractionation and analysis
Polysomes fractionation was performed as described previously (Dowling et al, 2010). For details, see Supplementary data.
Supplementary Material
Acknowledgments
We thank Pankaj Malhotra, Priya Katwala, Shriya Gandhi, and Kevin Martinez for technical assistance and discussion. This work was supported by NIH grants CA090764, AG016927, and AG025953, by the Chicago Biomedical Consortium with support from The Searle Funds at The Chicago Community, and by grant P60DK20595 to the Diabetes Research and Training Center, University of Chicago to NH, and by NIH training grant T32 DK07739 to JMK.
Author contributions: VN, DS, JMK, and NH designed, performed the experiments, and wrote the manuscript. XDP and NSa helped with the experiments and provided research materials. NS provided research materials and consultation.
Footnotes
The authors declare that they have no conflict of interest.
References
- Auld CA, Caccia CD, Morrison RF (2007a) Hormonal induction of adipogenesis induces Skp2 expression through PI3K and MAPK pathways. J Cell Biochem 100: 204–216 [DOI] [PubMed] [Google Scholar]
- Auld CA, Fernandes KM, Morrison RF (2007b) Skp2-mediated p27(Kip1) degradation during S/G2 phase progression of adipocyte hyperplasia. J Cell Physiol 211: 101–111 [DOI] [PubMed] [Google Scholar]
- Bashir T, Pagan JK, Busino L, Pagano M (2010) Phosphorylation of Ser72 is dispensable for Skp2 assembly into an active SCF ubiquitin ligase and its subcellular localization. Cell Cycle 9: 971–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaskar PT, Hay N (2007) The two TORCs and Akt. Dev Cell 12: 487–502 [DOI] [PubMed] [Google Scholar]
- Bhaskar PT, Nogueira V, Patra KC, Jeon SM, Park Y, Robey RB, Hay N (2009) mTORC1 hyperactivity inhibits serum deprivation-induced apoptosis via increased hexokinase II and GLUT1 expression, sustained Mcl-1 expression, and glycogen synthase kinase 3beta inhibition. Mol Cell Biol 29: 5136–5147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutonnet C, Tanguay PL, Julien C, Rodier G, Coulombe P, Meloche S (2010) Phosphorylation of Ser72 does not regulate the ubiquitin ligase activity and subcellular localization of Skp2. Cell Cycle 9: 975–979 [DOI] [PubMed] [Google Scholar]
- Cooke PS, Holsberger DR, Cimafranca MA, Meling DD, Beals CM, Nakayama K, Nakayama KI, Kiyokawa H (2007) The F box protein S phase kinase-associated protein 2 regulates adipose mass and adipocyte number in vivo. Obesity (Silver Spring) 15: 1400–1408 [DOI] [PubMed] [Google Scholar]
- Dorrello NV, Peschiaroli A, Guardavaccaro D, Colburn NH, Sherman NE, Pagano M (2006) S6K1- and betaTRCP-mediated degradation of PDCD4 promotes protein translation and cell growth. Science 314: 467–471 [DOI] [PubMed] [Google Scholar]
- Dowling RJ, Topisirovic I, Alain T, Bidinosti M, Fonseca BD, Petroulakis E, Wang X, Larsson O, Selvaraj A, Liu Y, Kozma SC, Thomas G, Sonenberg N (2010) mTORC1-mediated cell proliferation, but not cell growth, controlled by the 4E-BPs. Science 328: 1172–1176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fajas L, Schoonjans K, Gelman L, Kim JB, Najib J, Martin G, Fruchart JC, Briggs M, Spiegelman BM, Auwerx J (1999) Regulation of peroxisome proliferator-activated receptor gamma expression by adipocyte differentiation and determination factor 1/sterol regulatory element binding protein 1: implications for adipocyte differentiation and metabolism. Mol Cell Biol 19: 5495–5503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frescas D, Pagano M (2008) Deregulated proteolysis by the F-box proteins SKP2 and beta-TrCP: tipping the scales of cancer. Nat Rev Cancer 8: 438–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furic L, Rong L, Larsson O, Koumakpayi IH, Yoshida K, Brueschke A, Petroulakis E, Robichaud N, Pollak M, Gaboury LA, Pandolfi PP, Saad F, Sonenberg N (2010) eIF4E phosphorylation promotes tumorigenesis and is associated with prostate cancer progression. Proc Natl Acad Sci USA 107: 14134–14139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao D, Inuzuka H, Tseng A, Chin RY, Toker A, Wei W (2009) Phosphorylation by Akt1 promotes cytoplasmic localization of Skp2 and impairs APCCdh1-mediated Skp2 destruction. Nat Cell Biol 11: 397–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- George S, Rochford JJ, Wolfrum C, Gray SL, Schinner S, Wilson JC, Soos MA, Murgatroyd PR, Williams RM, Acerini CL, Dunger DB, Barford D, Umpleby AM, Wareham NJ, Davies HA, Schafer AJ, Stoffel M, O’Rahilly S, Barroso I (2004) A family with severe insulin resistance and diabetes due to a mutation in AKT2. Science 304: 1325–1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay N, Sonenberg N (2004) Upstream and downstream of mTOR. Genes Dev 18: 1926–1945 [DOI] [PubMed] [Google Scholar]
- Kim JB, Wright HM, Wright M, Spiegelman BM (1998) ADD1/SREBP1 activates PPARgamma through the production of endogenous ligand. Proc Natl Acad Sci USA 95: 4333–4337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein J, Fasshauer M, Klein HH, Benito M, Kahn CR (2002) Novel adipocyte lines from brown fat: a model system for the study of differentiation, energy metabolism, and insulin action. Bioessays 24: 382–388 [DOI] [PubMed] [Google Scholar]
- Laplante M, Sabatini DM (2009) mTOR signaling at a glance. J Cell Sci 122: 3589–3594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlor MA, Alessi DR (2001) PKB/Akt: a key mediator of cell proliferation, survival and insulin responses? J Cell Sci 114: 2903–2910 [DOI] [PubMed] [Google Scholar]
- Le Bacquer O, Petroulakis E, Paglialunga S, Poulin F, Richard D, Cianflone K, Sonenberg N (2007) Elevated sensitivity to diet-induced obesity and insulin resistance in mice lacking 4E-BP1 and 4E-BP2. J Clin Invest 117: 387–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HK, Wang G, Chen Z, Teruya-Feldstein J, Liu Y, Chan CH, Yang WL, Erdjument-Bromage H, Nakayama KI, Nimer S, Tempst P, Pandolfi PP (2009) Phosphorylation-dependent regulation of cytosolic localization and oncogenic function of Skp2 by Akt/PKB. Nat Cell Biol 11: 420–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miard S, Fajas L (2005) Atypical transcriptional regulators and cofactors of PPARgamma. Int J Obes (Lond) 29(Suppl 1): S10–S12 [DOI] [PubMed] [Google Scholar]
- Naaz A, Holsberger DR, Iwamoto GA, Nelson A, Kiyokawa H, Cooke PS (2004) Loss of cyclin-dependent kinase inhibitors produces adipocyte hyperplasia and obesity. FASEB J 18: 1925–1927 [DOI] [PubMed] [Google Scholar]
- Nakayama K, Nagahama H, Minamishima YA, Miyake S, Ishida N, Hatakeyama S, Kitagawa M, Iemura S, Natsume T, Nakayama KI (2004) Skp2-mediated degradation of p27 regulates progression into mitosis. Dev Cell 6: 661–672 [DOI] [PubMed] [Google Scholar]
- Nakayama KI, Nakayama K (2005) Regulation of the cell cycle by SCF-type ubiquitin ligases. Semin Cell Dev Biol 16: 323–333 [DOI] [PubMed] [Google Scholar]
- Nogueira V, Park Y, Chen CC, Xu PZ, Chen ML, Tonic I, Unterman T, Hay N (2008) Akt determines replicative senescence and oxidative or oncogenic premature senescence and sensitizes cells to oxidative apoptosis. Cancer Cell 14: 458–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano M (2004) Control of DNA synthesis and mitosis by the Skp2-p27-Cdk1/2 axis. Mol Cell 14: 414–416 [DOI] [PubMed] [Google Scholar]
- Patel YM, Lane MD (2000) Mitotic clonal expansion during preadipocyte differentiation: calpain-mediated turnover of p27. J Biol Chem 275: 17653–17660 [DOI] [PubMed] [Google Scholar]
- Peng XD, Xu PZ, Chen ML, Hahn-Windgassen A, Skeen J, Jacobs J, Sundararajan D, Chen WS, Crawford SE, Coleman KG, Hay N (2003) Dwarfism, impaired skin development, skeletal muscle atrophy, delayed bone development, and impeded adipogenesis in mice lacking Akt1 and Akt2. Genes Dev 17: 1352–1365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petroulakis E, Parsyan A, Dowling RJ, LeBacquer O, Martineau Y, Bidinosti M, Larsson O, Alain T, Rong L, Mamane Y, Paquet M, Furic L, Topisirovic I, Shahbazian D, Livingstone M, Costa-Mattioli M, Teodoro JG, Sonenberg N (2009) p53-dependent translational control of senescence and transformation via 4E-BPs. Cancer Cell 16: 439–446 [DOI] [PubMed] [Google Scholar]
- Ramirez-Valle F, Braunstein S, Zavadil J, Formenti SC, Schneider RJ (2008) eIF4GI links nutrient sensing by mTOR to cell proliferation and inhibition of autophagy. J Cell Biol 181: 293–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robey RB, Hay N (2009) Is Akt the ‘Warburg kinase’?-Akt-energy metabolism interactions and oncogenesis. Semin Cancer Biol 19: 25–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen ED, MacDougald OA (2006) Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol 7: 885–896 [DOI] [PubMed] [Google Scholar]
- Rosen ED, Sarraf P, Troy AE, Bradwin G, Moore K, Milstone DS, Spiegelman BM, Mortensen RM (1999) PPAR gamma is required for the differentiation of adipose tissue in vivo and in vitro. Mol Cell 4: 611–617 [DOI] [PubMed] [Google Scholar]
- Rosen ED, Spiegelman BM (2001) PPARgamma: a nuclear regulator of metabolism, differentiation, and cell growth. J Biol Chem 276: 37731–37734 [DOI] [PubMed] [Google Scholar]
- Rosen ED, Walkey CJ, Puigserver P, Spiegelman BM (2000) Transcriptional regulation of adipogenesis. Genes Dev 14: 1293–1307 [PubMed] [Google Scholar]
- Sakai T, Sakaue H, Nakamura T, Okada M, Matsuki Y, Watanabe E, Hiramatsu R, Nakayama K, Nakayama KI, Kasuga M (2007) Skp2 controls adipocyte proliferation during the development of obesity. J Biol Chem 282: 2038–2046 [DOI] [PubMed] [Google Scholar]
- Skeen JE, Bhaskar PT, Chen CC, Chen WS, Peng XD, Nogueira V, Hahn-Windgassen A, Kiyokawa H, Hay N (2006) Akt deficiency impairs normal cell proliferation and suppresses oncogenesis in a p53-independent and mTORC1-dependent manner. Cancer Cell 10: 269–280 [DOI] [PubMed] [Google Scholar]
- Tang QQ, Otto TC, Lane MD (2003) Mitotic clonal expansion: a synchronous process required for adipogenesis. Proc Natl Acad Sci USA 100: 44–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda T, Sasaki M, Elia AJ, Chio II, Hamada K, Fukunaga R, Mak TW (2010) Combined deficiency for MAP kinase-interacting kinase 1 and 2 (Mnk1 and Mnk2) delays tumor development. Proc Natl Acad Sci USA 107: 13984–13990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Cui J, Bauzon F, Zhu L (2010) A comparison between Skp2 and FOXO1 for their cytoplasmic localization by Akt1. Cell Cycle 9: 1021–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg RA (1995) The retinoblastoma protein and cell cycle control. Cell 81: 323–330 [DOI] [PubMed] [Google Scholar]
- Wu Z, Rosen ED, Brun R, Hauser S, Adelmant G, Troy AE, McKeon C, Darlington GJ, Spiegelman BM (1999) Cross-regulation of C/EBP alpha and PPAR gamma controls the transcriptional pathway of adipogenesis and insulin sensitivity. Mol Cell 3: 151–158 [DOI] [PubMed] [Google Scholar]
- Zhang HH, Huang J, Duvel K, Boback B, Wu S, Squillace RM, Wu CL, Manning BD (2009) Insulin stimulates adipogenesis through the Akt-TSC2-mTORC1 pathway. PLoS One 4: e6189. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.