Abstract
Memory is essential for our normal daily lives and our sense of self. Ca2+ influx through the NMDA-type glutamate receptor (NMDAR) and the ensuing activation of the Ca2+ and calmodulin-dependent protein kinase (CaMKII) are required for memory formation and its physiological correlate, long-term potentiation (LTP). The Ca2+ influx induces CaMKII binding to the NMDAR to strategically recruit CaMKII to synapses that are undergoing potentiation. We generated mice with two point mutations that impair CaMKII binding to the NMDAR GluN2B subunit. Ca2+-triggered postsynaptic accumulation is largely abrogated for CaMKII and destabilized for TARPs, which anchor AMPA-type glutamate receptors (AMPAR). LTP is reduced by 50% and phosphorylation of the AMPAR GluA1 subunit by CaMKII, which enhances AMPAR conductance, impaired. The mutant mice learn the Morris water maze (MWM) as well as WT but show deficiency in recall during the period of early memory consolidation. Accordingly, the activity-driven interaction of CaMKII with the NMDAR is important for recall of MWM memory as early as 24 h, but not 1–2 h, after training potentially due to impaired consolidation.
Keywords: AMPA receptor, CaMKII, LTP, memory, NMDA receptor
Introduction
A large body of evidence indicates that memories are encoded by stable increases in the strength of synaptic transmission (i.e., long-term potentiation (LTP)) as a consequence of temporally heightened synaptic activity (Martin et al, 2000; Lee and Silva, 2009; but see Neves et al, 2008 for exceptions). Although LTP can be induced in different brain regions, it is especially robust in the hippocampus. In general, learning, as well as LTP, requires both, Ca2+ influx through the NMDAR (NMDA-type glutamate receptor) and the ensuing CaMKII (calmodulin-dependent protein kinase II) activation (Martin et al, 2000; Collingridge et al, 2004; Malenka and Bear, 2004; Kerchner and Nicoll, 2008; Lisman and Hell, 2008; Kessels and Malinow, 2009). Ca2+ influx stimulates not only CaMKII activity but also CaMKII binding to the NMDAR (Strack and Colbran, 1998; Leonard et al, 1999; Bayer et al, 2006) and CaMKII accumulation at postsynaptic sites (Shen and Meyer, 1999; Bayer et al, 2006; Strack and Hell, 2008). This mechanism supports selective enrichment of CaMKII at synapses that are undergoing potentiation upon repeated glutamate uncaging, a model for LTP (Zhang et al, 2008; Lee et al, 2009).
Work in cultured hippocampal slices indicates that CaMKII binding to GluN2B is important for LTP (Barria and Malinow, 2005). Our previous study on animals expressing an inducible form of the ∼640 residue long GluN2B C-terminus is consistent with this finding (Zhou et al, 2007). However, this mutant mouse must have deficits in addition to disruption CaMKII binding to GluN2B, presumably by affecting binding of other proteins to the GluN2B C-terminus or of CaMKII to other targets, as Morris water maze (MWM) learning in that mouse was severely affected (Zhou et al, 2007) when complete abrogation of the CaMKII–GluN2B interaction in our GluN2B knockin mouse (GluN2B KI) had no effect on this learning at all (see below Figure 8). We created this GluN2B KI mouse by mutating Leu1298 to Ala and Arg1300 to Gln to specifically test the functional role of the CaMKII–GluN2B interaction in vivo. Each mutation individually blocks CaMKII binding to GluN2B as had been elegantly defined by Colbran and coworkers (Strack et al, 2000a).
Results
Activity-driven association of CaMKII with the NMDAR complex is abrogated in GluN2B KI mice
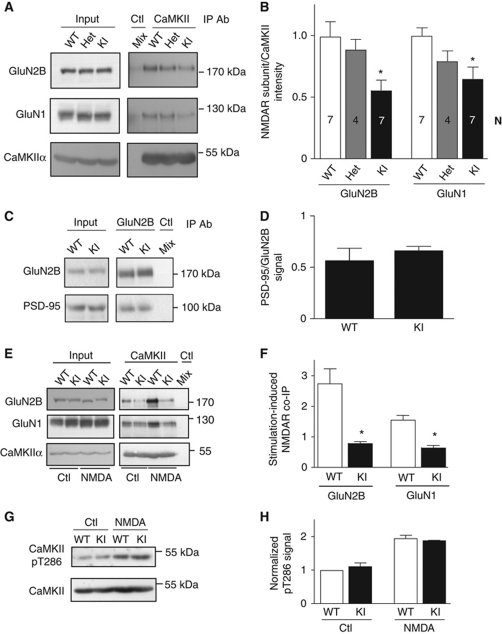
Homozygous GluN2B KI mice showed normal fertility, birth rate, and body weight and normal Mendelian ratio of offspring from heterozygous breeders (Supplementary Figure S5). The amount of GluN1, GluN2B, GluA1, and CaMKIIα present in total brain lysates were as in litter-matched WT mice (Supplementary Figure S1A and B). To biochemically evaluate content of postsynaptic proteins at the postsynaptic density (PSD), we isolated PSDs by differential centrifugation and two sucrose gradient centrifugations, one before and one after extraction of presynaptic and perisynaptic elements with Triton X-100. The content of GluN1, GluN2A, GluN2B, GluA1, PSD-95, and CaMKII in PSD fractions was also comparable between KI and WT mice (Supplementary Figure S1C and D). As expected, co-immunoprecipitation (co-IP) of the NMDAR complex with CaMKII was reduced by 35–40% in KI mice (Figure 1A and B). Co-IP of PSD-95 with GluN2B was unaltered (Figure 1C and D). Accordingly, the KI mutations specifically target CaMKII association with GluN2B without affecting binding of PSD-95 to the very C-terminus of GluN2B. The residual co-IP of the NMDAR with CaMKII was likely due to CaMKII binding to GluN1 (Leonard et al, 1999, 2002; Merrill et al, 2007) as extraction conditions (1% deoxycholate, pH 8.5) were chosen to preserve the overall integrity of the NMDAR complex (Leonard et al, 1999).
Figure 1.
Impaired activity-induced CaMKII binding to the NMDAR in GluN2B KI mice. (A) The GluN2B LR/AQ mutations reduce the association of the NMDAR complex with CaMKII. Membrane fractions from forebrains of WT and heterozygote (Het) and homozygote GluN2B KI (KI) mice were extracted with 1% deoxycholate before removal of insoluble material by ultracentrifugation, IP with CaMKIIα antibodies or isotype-matched control IgG (‘Mix’; each genotype contributed 33% of the extract for this control IP) and IB for NMDAR subunits and CaMKIIα (Leonard et al, 1999). (B) Immunosignals were quantified and NMDAR signals were divided by CaMKIIα signals and normalized to WT values for the corresponding NMDAR subunit. WT values were normalized to the average WT value over all experiments. Bars represent the average values±s.e.m. for each genotype for the indicated number of experiments (n). Asterisks (*) indicate P<0.05 compared with WT (one-way ANOVA). (C) The GluN2B LR/AQ mutation does not reduce the association of the NMDAR complex with PSD-95. Membrane fractions from GluN2B WT and homozygous GluN2B KI mouse forebrains were extracted with 1% deoxycholate before centrifugation and IP with GluN2B antibodies or rabbit control IgG (‘Mix’; each genotype contributed 50% of the extract for this control), and IB for NMDAR subunits and PSD-95. (D) Immunosignals were quantified and PSD-95 signals were divided by GluN2B signals and normalized to WT values for the corresponding NMDAR subunit. WT values were normalized to the average WT value over all experiments. The data represent the average values±s.e.m. for three experiments. Statistical analysis showed no differences between genotypes for the co-IPs (P>0.05; t-test). (E) Activity-driven CaMKII binding to the NMDAR requires its interaction with GluN2B. Forebrain slices were treated with vehicle or NMDA (200 μM, 5 min; plus 1 μM TTX to prevent overexcitation) immediately before extraction, CaMKIIα IP and IB for GluN2B, GluN1, and CaMKIIα, as detailed earlier (Leonard et al, 1999). Mock IP of a mix of 50% WT and 50% KI lysates (Mix) with control IgG showed specificity of CaMKIIα IP. (F) The NMDA-induced increase in NMDAR association with CaMKII was significantly different between WT and GluN2B KI (t-test: *P<0.05; n=4). (G) CaMKII stimulation is not affected in GluN2B KI mice. Acute cortical slices were treated with vehicle or NMDA plus TTX prior to IP with anti-CaMKIIα and IB for phospho-Thr286 (pT286) and total CaMKII. (H) Immunosignals were quantified and pT286 signals were divided by CaMKIIα signals and normalized to WT values. The data represent the average values±s.e.m. for three experiments. Statistical analysis showed no differences between genotypes for the co-IPs (P>0.05; t-test).
We found earlier that Ca2+ influx through the NMDAR increased the NMDAR–CaMKII interaction by about two-fold in acute hippocampal slices from WT rats (Leonard et al, 1999). We treated acute forebrain slices with NMDA in the presence of TTX, the latter preventing overexcitation of the slices, as described earlier (Hell et al, 1996; Leonard et al, 1999). This treatment increased co-IP of the NMDAR with CaMKII in our WT but not KI mice (Figure 1E and F). Hence, CaMKII binding to GluN2B is essential for increased receptor–kinase association following NMDAR activity. To control for potential alterations in access of Ca2+ to CaMKII upon NMDAR stimulation, we determined CaMKIIα T286 autophosphorylation, a measure of CaMKII activation, which did not differ between genotypes under basal conditions and following NMDA stimulation (Figure 1G and H).
Activity-driven postsynaptic accumulation of CaMKII is abolished in GluN2B KI mice
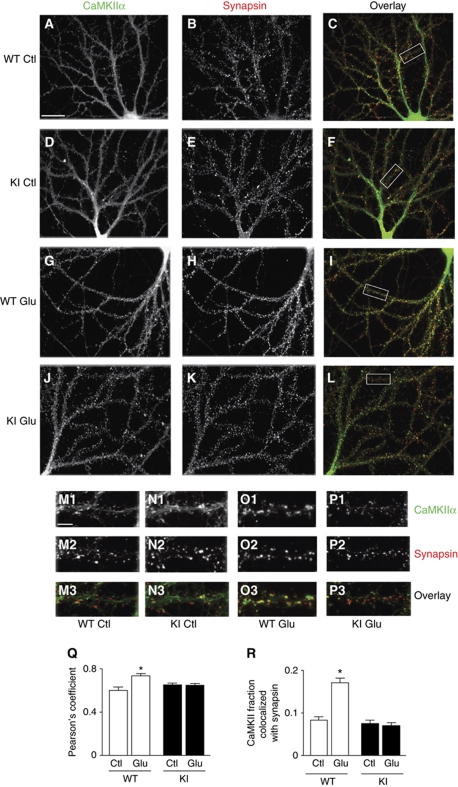
Ca2+ influx through the NMDAR induces clustering of ectopically expressed GFP–CaMKIIα in primary hippocampal rat cultures (Shen and Meyer, 1999; Shen et al, 2000) and organotypic hippocampal slice cultures (Lee et al, 2009; Otmakhov et al, 2004; Zhang et al, 2008). We demonstrated more recently that untagged, endogenous CaMKIIα and CaMKIIβ also cluster upon NMDAR-mediated Ca2+ influx in primary hippocampal cultures (Merrill et al, 2005; Strack and Hell, 2008). Hippocampal cultures were prepared in parallel from littermate WT and KI pubs, treated at 20 DIV with vehicle or glutamate and fixed immediately. Immunofluorescence analysis illustrates that CaMKII was relatively smoothly distributed under control conditions in WT and KI neurons (Figure 2A, D, M1, and N1). Although glutamate induced CaMKII clustering in WT and KI neurons (Figure 2G, J, O1, and P1), double labelling for the synaptic marker synapsin showed that extensive activity-induced CaMKII clustering took place at postsynaptic sites only in WT cultures (Figure 2H, I, O2, and O3). In KI neurons, the lack of increase in Pearson's coefficient as well as in the fraction of CaMKII immunofluorescent pixels that colocalizes with the synaptic marker synapsin (Mander's coefficient) indicates that, contrasting the CaMKII clusters that were present under basal conditions, the numerous newly formed CaMKII clusters were mostly not colocalized with or juxtaposed to synapsin puncta and were, therefore, formed mainly outside synapses (Figure 2K, L, P2, and P3). In fact, such activity-induced CaMKII clustering can occur in dendritic shafts under certain pathological conditions (Hudmon et al, 2005). Colocalization of CaMKII and synapsin indicative of synaptic localization of CaMKII was unaffected in KI neurons under non-stimulated conditions as CaMKII binds to two other major postsynaptic components (densin-180 and α-actinin) and several other postsynaptic proteins without requiring activation by Ca2+ (Colbran, 2004; Robison et al, 2005; Strack and Hell, 2008; Nikandrova et al, 2010). Thus, GluN2B KI mice did not show aberrant targeting of CaMKII under basal conditions that could have otherwise contributed to the reduction in CaMKII binding to postsynaptic NMDARs upon stimulation. Furthermore, the cytoarchitecture was normal throughout the brains of KI mice as illustrated by Nissl staining for Cortex and Hippocampus (Supplementary Figure S2A–H). The GluN2B distribution was also normal indicating that the point mutations in GluN2B did not affect its postsynaptic targeting (Supplementary Figure S2I–R). We conclude that CaMKII binding to GluN2B is the main determinant for activity-driven association of CaMKII with the NMDAR and for enhanced postsynaptic CaMKII accumulation following NMDAR-mediated Ca2+ influx in vivo.
Figure 2.
Loss of activity-driven postsynaptic CaMKII accumulation in GluN2B KI neurons. (A–P) In all, 20 DIV hippocampal cultures from WT and litter-mate KI mice were treated with vehicle (water; A–F, M1–N3) or glutamate (100 μM, 5 min; G–L, O1–P3), immediately fixed (4% paraformaldehyde, 30 min), and stained for CaMKIIα (green in overlay) and synapsin (red in overlay). Areas outlined in C, F, I, and L are shown at larger magnification in (M–P), respectively. Scale bar for (A–L) in (A) is 25 μm and for (M–P) in M1 is 5 μm. (Q, R) Glutamate treatment significantly increased CaMKII colocalization with synapsin in WT but not KI cultures as indicated by Pearson's coefficient (Q), as well as quantification of the fraction of CaMKII clusters colocalized with synapsin clusters determined after thresholding (R; two-way ANOVA, P<0.05; n=3 with 10 neurons analysed per experiment and condition; *P<0.05; error bars: s.e.m.).
Basal synaptic transmission is normal but CA1 LTP reduced in adult GluN2B KI mice
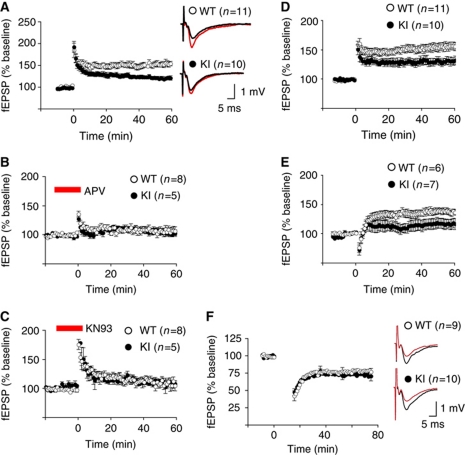
GluN2B KI mice had normal paired-pulse facilitation over the full range of interpulse intervals with maximal facilitation at 50 ms in both genotypes (Figure 3A). Synaptic responses to repetitive stimulation at 10 and 100 Hz were unaffected showing that the totally releasable pool and the readily releasable pool of synaptic vesicles are normal in KI mice (Figure 3B and C). The mEPSC frequency and amplitude were comparable for the two genotypes (Figure 3D). Input–output relationships as determined by plotting initial slope of fEPSPs against fibre volley were virtually identical for the two genotypes (Figure 3E). To evaluate whether postsynaptic NMDAR activity is affected in KI mice, we measured NMDAR-mediated fEPSPs. For this purpose, Mg2+ was removed while CNQX added to inhibit AMPAR (AMPA-type glutamate receptor) and at the same time prevent epileptiform activity due to lack of Mg2+ (Huang et al, 2006; Lu et al, 2007). The resulting input–output relationships were indistinguishable for WT and KI mice (Figure 3F). Also, NMDAR resulting from ectopically expressed GluN1 plus GluN2B with R1330Q/S1303D double mutation, which, like our L1298A/R1300Q double mutation, abrogates CaMKII binding, have normal decay τ and current/voltage relationship (Barria and Malinow, 2005). Accordingly, CaMKII binding to GluN2B does not overtly affect NMDAR properties. These results, together with normal CaMKII activation upon NMDAR stimulation (Figure 1G and H) and normal LTD (Figure 4F), indicate that postsynaptic NMDAR functions are unchanged under basal conditions in KI mice.
Figure 3.
Basal synaptic transmission is normal in adult GluN2B KI mice. (A) Paired-pulse facilitations of fEPSPs for 20–500 ms interstimulus intervals in the CA1 area in acute hippocampal slices were virtually identical for WT and KI mice, suggesting unaltered excitation–exocytosis coupling. (B) Decreases in fEPSPs in response to repetitive stimulations at frequencies of 10 Hz were comparable for WT and KI mice, indicating that the totally releasable pool of synaptic vesicles is unchanged. (C) Decreases in fEPSPs in response to repetitive stimulations at frequencies of 100 Hz were comparable for WT and KI mice, indicating that the readily releasable pool of synaptic vesicles is unchanged. (D) AMPAR-mediated mEPSCs were monitored by whole-cell patch recording from pyramidal cells in CA1 in the presence of 200 nM TTX to block Na+ channels and thereby prevent spontaneous action potentials, 10 μM bicuculline to block GABAA receptors, and 50 μM AP-5 to block NMDARs. Amplitude (middle) and frequency (right) of mEPSCs are statistically not different in neurons from WT and KI mice. Representative traces from WT and KI mice are shown to the left of the bar diagrams. (E, F) Averages of initial fEPSP slopes (±s.e.m.) under standard conditions reflecting AMPAR response strength (E) or with ACSF containing 8 μM CNQX (to block AMPAR) and no Mg2+ indicative of NMDAR response strength (F) are plotted versus presynaptic fibre volley amplitudes (as a measure of presynaptic activation level) obtained with increasing stimulus strengths. Inserts on the right of each panel show sample traces with increasing stimulus strengths for WT (top) and KI (bottom). Each individual panel shows data (±s.e.m.) from n slices obtained from 3 to 5 mice for each genotype.
Figure 4.
LTP is reduced in adult GluN2B KI mice. (A) LTP (2 × 100 Hz/1 s) in CA1 in acute hippocampal slices stabilized at 156±6% (WT) and 121±4% (KI) of baseline (mean±s.e.m.) showing a significant difference between slices from WT and KI mice (t-test: P<0.05). Inserts show fEPSPs sample traces before (black) and 60 min after LTP induction (red) from WT (top) and KI (bottom) mice. (B) LTP is blocked by 100 μM AP5 in both genotypes. Recordings from WT and KI slices plateaued at 107±6% and 104±4%, respectively, 60 min after LTP induction. (C) LTP is blocked by 10 μM KN93 in both genotypes. Recordings from WT and KI slices leveled off at 108±8% and 108±5%, respectively. (D) LTP induced by θ burst stimulation (10 trains of 4 stimuli at 100 Hz; trains were 200 ms apart) in CA1 showed a significant difference (t-test: P<0.05) between slices from WT (156±7%) and KI (131+7%) mice. (E) LTP induced by 10 Hz/15 s stimulation showed a 38±2% increase in fEPSP in slices from WT mice and a 17±2 % increase in slices from KI mice (t-test: P<0.05). (F) LTD (1 Hz/15 min) stabilized at 76±5% (WT) and 72±6% (KI) of baseline (±s.e.m.), revealing no difference between slices from WT and KI mice (t-test: P>0.05). Inserts show fEPSPs sample traces before (black) and 60 min after LTD induction (red) from WT (top) and KI (bottom) mice. Each individual panel shows data from n slices obtained from 3 to 5 mice for each genotype.
Although basal synaptic transmission is normal in GluN2B KI mice, LTP induced by two tetani of 100 Hz/1 s was reduced by about 50% in the KI mice (Figure 4A). The remaining LTP in KI slices was completely abolished by AP5 and KN93 (Figure 4B and C), indicating that the residual LTP is both NMDAR- and CaMK-dependent and not due to compensatory mechanisms that would circumvent CaMKII. We recently showed in young adult mice that LTP induced by a single tetanus is sensitive to blockage of PKA whereas a two-tetanus LTP as measured here is not (Lu et al, 2007). As in WT (Lu et al, 2007), two-tetanus LTP was not sensitive to H-89, which inhibits PKA (Supplementary Figure S3). The residual LTP thus appears to rely on the same main regulatory mechanisms as the LTP in WT mice, that is, Ca2+ influx through NMDAR and the ensuing activation of CaMKII rather than requiring additional support from PKA, which is otherwise important for single-tetanus LTP. These results provide further evidence for the notion that postsynaptic signalling by Ca2+ is normal although the binding deficiency of GluN2B for activated CaMKII results in reduced LTP. Other forms of LTP as triggered by θ burst stimulation and by a 10 Hz/15 s stimulus train were also reduced by about half (Figure 4D and E). Finally, LTD was normal in 2-week-old KI mice (Figure 4F) further supporting that most synaptic properties were unaltered and the reduction in LTP was a highly specific deficit. We conclude that NMDAR and CaMKII play important roles in LTP independent of their interaction but binding of CaMKII to GluN2B is necessary for LTP to fully develop.
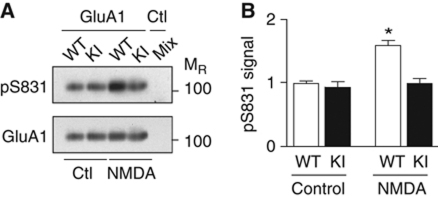
Activity-induced phosphorylation of GluA1 on S831 by CaMKII is abolished in GluN2B KI mice
LTP is, to a large extent, mediated by upregulation of postsynaptic AMPAR activity (Collingridge et al, 2004; Malenka and Bear, 2004; Kerchner and Nicoll, 2008; Lisman and Hell, 2008; Kessels and Malinow, 2009). However, the molecular basis of LTP in general, and specifically how CaMKII upregulates postsynaptic AMPAR activity, remains largely unknown. Because phosphorylation of GluA1 on S831 by CaMKII is thought to contribute to LTP, at least under certain conditions (Lisman and Hell, 2008), we evaluated the impact of reduced CaMKII binding to the NMDAR on S831 phosphorylation. Ca2+ influx through the NMDAR increased S831 phosphorylation in WT but not GluN2B KI slices (Figure 5A and B). Accordingly, the CaMKII–GluN2B interaction is critical for S831 phosphorylation by CaMKII upon Ca2+ influx, perhaps secondarily to correct placement of the kinase. Abrogation of this mechanism likely contributes to some, but likely limited, degree to the reduction of LTP in GluN2B KI mice.
Figure 5.
Deficits in stimulation of GluA1 S831 phosphorylation by CaMKII. (A) Forebrain slices were treated in the presence of GF109203X (10 μM) to inhibit PKC with vehicle or NMDA (100 μM, 5 min) immediately prior to extraction of AMPAR with 1% Triton X-100, ultracentrifugation to remove non-solubilized material, GluA1 IP, and sequential IB for GluA1 pS831 and total GluA1 (Leonard et al, 1999). Mock IP of a mix of WT and KI lysates (Mix) with control IgG showed specificity of GluA1 IP. (B) NMDA significantly increased S831 phosphorylation in WT but not KI slices (shown are mean values±s.e.m.; two-way ANOVA, *P<0.01; n=3).
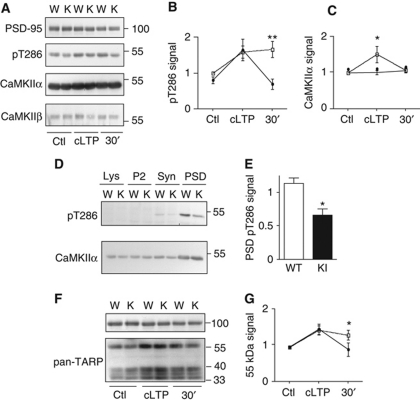
Chemical LTP-induced autophosphorylation of postsynaptic CaMKII on T286 lasts longer in WT than GluN2B KI mice
LTP induction triggers persistent T286 autophosphorylation and thereby activation of CaMKII, which likely contributes to LTP (Fukunaga et al, 1995; Barria et al, 1997; Lee et al, 2000). We thus monitored CaMKII T286 autophosphorylation in acute hippocampal slices following chemically induced LTP (cLTP) using forskolin-induced neuronal stimulation (Makhinson et al, 1999; Lu et al, 2007) (see also Kopec et al, 2006). GluN2B KI and WT mice showed the same relative degree of CaMKII activation (i.e., phospho-T286) in PSD fractions from forebrain slices following cLTP (Figure 6A). However, T286 phosphorylation lasted for at least 30 min only in WT but not in KI slices (Figure 6B). CaMKII content within the PSD relative to PSD-95 increased transiently after cLTP induction in WT slices (Figure 6C). The absence of a similar transient increase in KI slices further supports the above findings that activity-dependent postsynaptic recruitment of CaMKII is impaired by the mutations (Figure 1). Notably, our data do not exclude the possibility of a prolonged increase in total CaMKII within the PSD following LTP in WT mice in parallel to an overall increase in PSD size. In fact, CaMKII content persistently increases in parallel with the size of dendritic spines following LTP (Otmakhov et al, 2004; Zhang et al, 2008; Lee et al, 2009). However, because PSD yields vary substantially, we can only quantify levels of CaMKII relative to a PSD marker protein such as PSD-95 and not changes in total PSD protein content (Ehlers, 2003). Accordingly, we would not be able to detect a more permanent increase in CaMKII content of PSD fractions if PSD-95 content would increase in parallel, as would be expected for stable potentiation.
Figure 6.
Deficits in postsynaptic maintenance of CaMKIIα T286 phosphorylation and of TARP accumulation following chemical LTP in GluN2B KI mice. (A) Forebrain slices underwent control (Ctl), or cLTP treatment (cLTP; sequential incubation with forskolin, followed by increased K+ in the absence of Mg2+ (Lu et al, 2007)), with subsequent 30 min recovery in ACSF if indicated (30′). P2 fractions were isolated by differential centrifugation, presynaptic and perisynaptic elements removed with 0.5% Triton X-100 and crude PSD fractions collected by ultracentrifugation. PSD-95, CaMKIIα pT286, CaMKIIα, and CaMKIIβ were detected in PSD fractions by IB. (B) The ratio CaMKIIα pT286 to total CaMKIIα, normalized to WT control and expressed as mean values±s.e.m., increased in the PSD fractions from WT and KI slices upon cLTP treatment but this increase persisted only in WT but not KI fractions (two-way ANOVA, **P<0.01; n=3). (C) Relative CaMKIIα abundance in the PSD fractions as obtained by normalizing total CaMKIIα signals to PSD-95 signals (expressed as mean values±s.e.m.) is increased after cLTP treatment in WT but not KI slices (two-way ANOVA, *P<0.05; n=3). (D) PSDs were highly purified from WT and KI forebrains by differential and sucrose gradient centrifugation, extraction of PSDs with 0.5% Triton X-100 and a final sucrose gradient centrifugation. Crude lysates (Lys), P2 fraction (P2), synaptosome-enriched fraction (Syn), and highly PSD-enriched fraction (PSD) underwent sequential IB for CaMKIIα pT286 and CaMKIIα. (E) Phosphorylation of CaMKII T286 relative to total CaMKII is significantly reduced in highly purified PSDs from KI mice compared with WT (mean values±s.e.m.; t-test: *P<0.05; n=3). (F) Acute cortical slices were treated and crude PSD fractions isolated as in (A) before IB for PSD-95 and TARPs (stg/γ2, γ3, γ4, γ8). (G) Accumulation of TARP γ-8 (55 kDa) was increased in crude PSD fractions from both WT and KI slices following cLTP treatment but, contrasting WT, did not persist in KI fractions (mean values±s.e.m.; two-way ANOVA: *P<0.05; n=3).
To investigate alterations in long-term effects of the GluN2B mutations in the KI mice in vivo on activation of postsynaptic CaMKII, we evaluated whether phospho-T286 levels are different in GluN2B KI versus WT mice in pure PSD fractions. In these samples, T286 phosphorylation reflects postsynaptic activation of CaMKII due to the normal basal brain activity. Whereas relative CaMKII protein levels were unchanged in the highly enriched PSD fractions from KI mice (Figure 6D; Supplementary Figure S1C and D), phospho-T286 levels were reduced by ∼40% (Figure 6D and E). Given that acute CaMKII activation is not affected in KI mice as determined for the total CaMKII pool (Figure 1G and H), our evidence suggests that persistent activation of CaMKII at postsynaptic sites is impaired in KI mice in vivo. GluN2B association could prolong CaMKII activation through generation of autonomous kinase activity or protection from downregulation by phosphatase activity (Bayer et al, 2001; Lisman and Hell, 2008). Collectively, these extensive molecular analyses demonstrate that long-lasting activation of CaMKII at postsynaptic sites is impaired in GluN2B KI mice.
Lasting accumulation of TARPs following chemical LTP is impaired in GluN2B KI mice
Increased postsynaptic accumulation of AMPAR–TARP complexes contributes to the establishment of LTP. Despite strong electrophysiological and cell biological evidence for such an increase (Collingridge et al, 2004; Malenka and Bear, 2004; Matsuzaki et al, 2004; Tomita et al, 2005; Kerchner and Nicoll, 2008; Lisman and Hell, 2008), biochemical confirmation of this model has proven impossible up to date. This lack of biochemical evidence may be, in part, because PSD isolation requires Triton X-100, which extracts AMPARs to a substantial degree. This extraction is reflected in the reduced enrichment of AMPAR as compared with NMDAR and PSD-95 in PSD preparations (e.g., Supplementary Figure S1C). Thus, AMPAR content is not reliably quantifiable in PSD fractions from forebrain slices. However, TARPs, which target AMPARs to postsynaptic sites, are much more robustly enriched in PSD fractions than AMPARs (Figure 6F), likely because Triton X-100 does not extract them as readily as AMPARs. Also, AMPARs and TARPs are not constitutively associated with each other (Triton X-100 as well as glutamate weakens their interactions (Morimoto-Tomita et al, 2009)). Furthermore, CaMKII-mediated phosphorylations of stargazin (stg/γ2) and potentially other TARPs are important for LTP (Tomita et al, 2005) possibly by increasing attachment sites for AMPARs at the postsynaptic sites or by promoting AMPAR opening (Collingridge et al, 2004; Malenka and Bear, 2004; Kerchner and Nicoll, 2008; Lisman and Hell, 2008; Sumioka et al, 2010). For these reasons, we monitored postsynaptic accumulation of TARPs following cLTP in acute forebrain slices from GluN2B WT and KI mice with an antibody that recognizes all four of the closely related conventional TARPs (stg/γ2, γ3, γ4, γ8; Figure 6F; Supplementary Figure S4). The three bands immediately below the 40-kDa range correspond to stg/γ2, γ3, and γ4, which are similar in MR, and a prominent band around 55 kDa corresponds to the larger γ8 (see Supplementary Figure S4 legend). Immediately following cLTP, relative γ8 (50 kDa band) levels were increased by >50% in WT and KI slices. This increase persisted for at least 30 min only in WT but not KI slices (Figure 6F and G), similarly to the effects seen on cLTP-induced CaMKII activation. The same trend was observed for the other γ isoforms but differences between WT and KI did not reach statistical significance (Figure 6F). This finding illustrates that postsynaptic γ8 targeting can be driven by heightened synaptic activity.
Normal learning but impaired recall of the MWM task by GluN2B mice
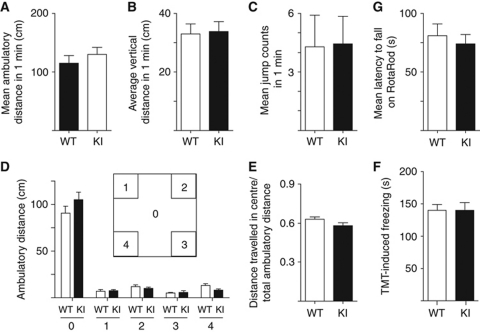
As activity-driven postsynaptic CaMKII recruitment and LTP were impaired in GluN2B KI mice, we conducted behavioural analyses to define potential deficits in learning and memory in these mice. KI mice displayed normal locomotion and motivation as indicated by the absence of any differences in means of ambulatory distance, overall average distance, and jump counts in open field analysis (Figure 7A–C). Basal anxiety levels of KI mice also appear normal as their centre time and centre activity in the open field test were virtually identical to those of WT mice (Figure 7D and E). Their innate fear reactions to trimethyl-thiazoline (TMT; the anxiogenic compound in fox urine) were also indistinguishable from that of WT mice (Figure 7F). Their coordination skills on the RotaRod were as for WT (Figure 7G).
Figure 7.
Open field behaviour and RotaRod balancing is normal in GluN2B KI mice. (A) Open field mean ambulatory distances calculated from averaged distances travelled within 1 min during a 20-min period by individual mice. (B) Open field mean vertical activities calculated from averaged number of beam breaks in the ‘Z’ array (i.e., rearing or jumping) in 1 min during a 20-min period by individual mice. (C) Open field mean jump counts calculated from averaged number of times individual mice left the photocell array (jumping) in 1 min during a 20-min period by individual mice. (D) Mean ambulatory distances travelled within individual zones (the open field was divided into nine equally sized squares; zones 1–4: individual squares in one of each of the four corners; zone 0: remaining area consisting of five squares, that is, centre square plus the four side squares neighbouring the four sides of the centre square). (E) Ratio of mean ambulatory distance travelled within centre (zone 0) to total ambulatory distance. (F) Freezing induced by TMT increased gradually within the first 4 min of mice being placed into the chamber to the same degree for WT and KI mice (Dallapiazza and Hell, data not illustrated). As illustrated, overall freezing during the 6-min observation periods did not differ for KI versus WT mice. (G) Mean latencies to fall from the RotaRod calculated from the averaged latencies of three trials on each day for individual mice across 3 days. All data are mean±s.e.m. from n=9 mice per genotype. None of the above parameters showed any statistically significant difference (t-test).
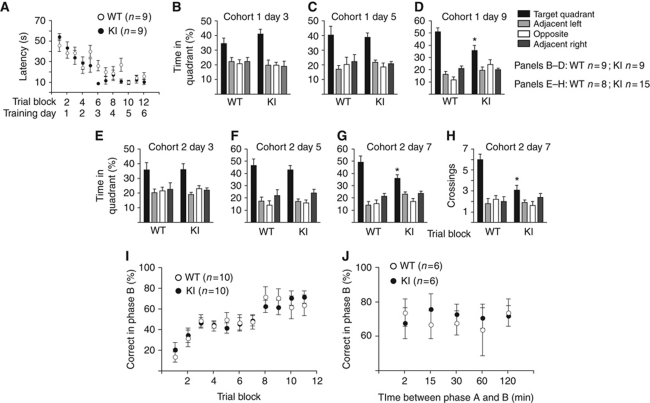
We were surprised to find that the GluN2B KI mice showed also no detectable deficits in spatial learning and short-term memory in the MWM because most manipulations that inhibit or enhance LTP in vivo impair or improve, respectively, this type of learning (Martin et al, 2000; Neves et al, 2008; Lee and Silva, 2009). In detail, latencies for reaching the hidden platform decreased over the 12 training blocks for KI mice as fast as for WT mice (Figure 8A). KI and WT mice spent comparable amounts of time in the target quadrant during probe tests conducted 1 h after the last training session on day 3 (Figure 8B) and day 5 (Figure 8C), further confirming that learning of this task was normal by KI mice. These findings also indicate that short-term memory and its retention upon repetitive task performance is unaltered in KI mice. Yet, when tested 3 days after the last training session on day 6, KI mice spent significantly less time in the target quadrant compared with WT mice (Figure 8D). A second cohort of KI mice also showed normal performance when tested 1 h after the last training session on day 3 (Figure 8E) and day 5 (Figure 8F). However, when tested on day 7, 1 day after the last training session on day 6, time spent in and number of crossings of the target quadrant was significantly reduced in KI versus WT (Figure 8G and H). These results show that GluN2B KI mice learn quite normally but are impaired in the early phases of contextual memory consolidation and maintenance.
Figure 8.
Consolidation but not learning of MWM is impaired in GluN2B KI mice. (A) KI mice learned to escape to a hidden platform to the same extent and as fast as littermate WT mice over 24 practice runs (12 training blocks, each consisting of 2 runs). (B, C) During probe tests 1 h after training sessions on day 3 (B) and on day 5 (C), KI mice searched the target quadrant (TQ) as selectively as WT. (D) During the probe test on day 9, KI mice spent significantly less time in TG than WT mice (F1,16=8.55, *P<0.01). (E–H) A second cohort of KI mice also showed normal performance in probe trials 1 h after training sessions on day 3 (E) and day 5 (F) but spent less time searching in the TQ during probe tests on day 7 than WT mice (G; F1,13=6.87, *P<0.05). The number of TQ crossings (H) and proximity to TQ was also reduced in KI mice (KI: 37.3±1.7 cm; WT: 30.5±1.8 cm; F1,13=6.18, *P<0.05). (I) GluN2B KI mice learned the radial arm maze task with the same time course and to the same degree as litter-matched WT mice. Shown are correct entries as percentage of total entries during phase B into arms that had been closed in phase A over 10 consecutive days. (J) After reaching criterion for learning, the interphase intervals were randomly increased to 15, 30, 60, or 120 min to test short-term spatial memory. Again, there was no significant difference between groups at different intervals (t-test: *P>0.05 at 2, 15, 30, 60, and 120 min intervals). All data are shown as mean values±s.e.m.
We used the delayed win shift radial arm maze task to specifically test working memory in GluN2B KI mice. In this learning paradigm, four of the eight arms of a radial maze are open and provide reward pellets in phase A. In phase B, all eight arms are open but only the four arms that were closed in phase A now provide a reward. Learning as quantified by the ratio of the number of correct arm entries versus total number of arm entries during phase B over a 10-day training period was unchanged in KI versus WT mice (Figure 8I). Statistical analysis revealed an overall significant effect of training days (repeated ANOVA, F(9,13)=8.45, P<0.0001). However, there was no significant difference between genotypes in the percentage of total correct entries made on phase B across training days (repeated ANOVA, F(1,13)=0.009, P>0.05) or an interaction between genotype and training days (repeated ANOVA, F(9,13)=0.38, P>0.05). After 10 days training, all groups reached criterion performance (all four pellets were retrieved in five or fewer choices during phase B for 2 consecutive days). At this point, the performance with increased interphase intervals between phase A and phase B was determined, again with no obvious differences between genotypes (Figure 8J).
Discussion
Role of GluN2B in postsynaptic CaMKII targeting
Non-stimulated CaMKII binds to postsynaptically enriched proteins including densin-180, α-actinin, SAP97, and F-actin (Shen et al, 1998; Walikonis et al, 2001; Colbran, 2004; Robison et al, 2005; Strack and Hell, 2008; Nikandrova et al, 2010), which likely mediate postsynaptic CaMKII localization under basal conditions. Accordingly, its postsynaptic targeting was normal under basal conditions in GluN2B KI mice. In contrast, stimulation-induced postsynaptic CaMKII accumulation is largely if not completely absent in KI mice (Figures 2 and 6A and C), which is surprising given that binding of CaMKII to GluN1 is also activation-dependent (Leonard et al, 1999, 2002; Merrill et al, 2007) and to densin-180 binding is enhanced by CaMKII activation (Strack et al, 2000b). Stimulus-induced association of CaMKII with the NMDAR complex as reflected by co-IP of GluN2B as well as GluN1 with CaMKII is also abrogated in KI mice (Figure 1E and F). This latter finding is, once more, unexpected given the activity-induced CaMKII binding to NR1 in vitro but indicates that the NR1 interaction plays a modest if any role in activation-induced recruiting CaMKII to the NMDAR complex in vivo, including complexes consisting of GluN1/N2A. Given the central role CaMKII plays in LTP and its remarkable abundance (1–2% of total brain protein and 5–10% of postsynaptic protein), it is likely that this GluN2B- and activity-dependent postsynaptic CaMKII accumulation is key to the synapse specificity of LTP. This synapse specificity is critical for LTP to allow effective encoding of extensive memory and, at the same time, for preventing neurons from adopting a state of overexcitability during LTP induction.
Although postsynaptic CaMKII content was unaltered under basal conditions, T286 autophosphorylation of CaMKII in PSD fractions (Figure 6D) but not in the total CaMKII fraction (Figure 1G) was reduced in KI mice. Accordingly, the phosphosignal in PSD samples must constitute a relatively small fraction of the phosphosignal in the total CaMKII population, possibly because overall CaMKII present in the PSD fraction constitutes only a relatively small fraction of total CaMKII. Nevertheless, it reveals a decrease in maintenance of T286 autophosphorylation of postsynaptic CaMKII in KI mice. Such a decrease provides in vivo support for our previous finding that binding to GluN2B fosters autonomous activity of CaMKII (Bayer et al, 2001). GluN2B mediates sustained CaMKII activity by binding to the T-site on CaMKII subunits, which otherwise harbours T286 if this residue is unphosphorylated and if Ca2+/calmodulin is not bound ∼20 residues downstream of T286. Either T286 phosphorylation, binding of Ca2+/calmodulin downstream of T286, or binding of GluN2B to the T-site lead to removal of the pseudosubstrate site, which is straddled by T286 and the calmodulin-binding site, from the catalytic S-site (Bayer et al, 2001; Schulman, 2004; Chao et al, 2010, 2011; Rellos et al, 2010; Hoffman et al, 2011). Although likely not all subunits of dodecameric CaMKII complexes can simultaneously bind to GluN2B, keeping these bound subunits in an active conformation through GluN2B binding to their T-sites allows these subunits to re-phosphorylate neighbouring subunits when dephosphorylated, thereby maintaining a high degree of autophosphorylation of the whole GluN2B-associated CaMKII complex (Lisman et al, 2002).
Repetitive glutamate uncaging causes rather short-lived activation of the bulk of CaMKII in individual spines (t1/2<1 min) as measured with a CaMKII-derived FRET sensor (Lee et al, 2009). As the authors point out, GluN2B-associated CaMKII constitutes only a subpopulation of the total spine population, which could conceivably undergo much longer-lasting activation that is too small for detection within the bulk of CaMKII yet could account for most of the basal activity level of CaMKII at postsynaptic sites under basal condition. This notion is especially conceivable if GluN2B-associated CaMKII is in a privileged position for promoting LTP maintenance as indicated by a previous LTP study (Barria and Malinow, 2005) and our work (Figure 4A, D, and E). In addition, CaMKII might also act by playing a structural role independent of or in addition to prolonged catalytic activity (Okamoto et al, 2007; Pi et al, 2010).
Role of CaMKII binding to GluN2B in synaptic transmission and plasticity
The membrane permeant peptide tatCN21 inhibits CaMKII at 5 μM (Buard et al, 2010). It also displaces CaMKII from GluN2B at 20 but not 5 μM in acute hippocampal slices (Sanhueza et al, 2011). It blocks LTP induction at both concentrations but reverses LTP during its maintenance phase only at 20 μM, consistent with our hypothesis that CaMKII binding to GluN2B is important for LTP to last. However, it also decreases basal synaptic transmission at 20 but not 5 μM (Sanhueza et al, 2011). Thus, we first tested whether GluN2B KI mice have a defect in basal synaptic transmission that could alter LTP by measuring a number of parameters. PPF was normal for all interstimulus intervals. As PPF is sensitive to changes in presynaptic excitation–exocytosis coupling and Ca2+ cycling (Zalutsky and Nicoll, 1990; Schulz et al, 1994; Han et al, 2006; Pelkey et al, 2006), in AMPAR lateral mobility or desensitization or in relief of polyamine block of GluA2-lacking inwardly rectifying AMPAR (Heine et al, 2008; Christie et al, 2010; Stubblefield and Benke, 2010; Savtchouk and Liu, 2011), these parameters appear to be all normal in the KI mice. Unaltered transmitter release was further indicated by lack of changes in the readily and totally releasable synaptic vesicle pools (Figure 3B and C) and in mEPSC frequency (Figure 3D). The mEPSC amplitude and decay τ was also unaffected (Figure 3D), indicating that AMPAR function and composition is not overtly affected. Consistently, input–output relationships showed no changes for AMPAR- and also NMDAR-mediated postsynaptic fEPSPs (Figure 3E and F). Finally, LTD, which does not depend on CaMKII, was normal in KI mice (Figure 4F). Accordingly, synapses in the KI mice are at a level within the dynamic range that is comparable if not identical to WT mice and that can be potentiated and depressed. The lack of defect in KI mice in basal transmission contrasts the decrease in transmission by 20 μM tatCN21 (see above) (Sanhueza et al, 2011), suggesting that this tatCN21 effect might be via a target other than CaMKII binding to GluN2B. In fact, our preliminary results suggest that tatCN21 also disrupts the CaMKII–densin-180 interaction, which can compensate for loss of NMDAR interaction with respect to basal CaMKII targeting (Carlisle et al, 2011). Nevertheless, several forms of LTP were reduced by half in the KI mice, indicating that CaMKII binding to GluN2B is important for maintenance of a portion of but not full LTP (Figure 4A, D, and E).
Frequency of mEPSCs and spine density is reduced in organotypic slice cultures by overexpression of GluN1 with GluN2B with two point mutations that impair CaMKII binding similar to our KI mutations (Gambrill and Barria, 2011). These findings contrast ours that mEPSC frequency and spine density are normal in GluN2B KI mice (Figure 3D and data not shown). The experiments by Gambrill and Barria that implicate loss of CaMKII binding in reduced mEPSC frequency are based on overexpression of combinations of GluN1 with WT and mutant GluN2A and 2B constructs in cultured slices, likely with substantial changes in total NMDAR protein, which was not monitored. Our strictly in vivo KI approach does not alter total NMDAR protein (Figure 1A; Supplementary Figure S1) and appears more specific and subtle than this recent in vitro work. In support of this notion, earlier work with the above approach lead to complete loss of LTP when CaMKII binding deficient GluN2B was overexpressed together with GluN1 (Barria and Malinow, 2005) rather than the partial loss we observe in three different forms of LTP (Figure 4) although it is also possible that the pairing-induced LTP recorded by whole-cell patch clamping in Barria and Malinow (2005) is more sensitive to loss of CaMKII binding than our LTP protocols that are based on less invasive fEPSP recordings. The more dramatic effects following overexpression of NMDAR subunits are thus likely due to the more severe molecular manipulations by Barria and colleagues.
The lack of NMDA-induced phosphorylation of GluA1 on S831 in KI mice (Figure 5) indicates that binding to GluN2B is important for CaMKII to phosphorylate S831. S831 does not constitute a CaMKII consensus site lacking any positively charged residues upstream (and downstream). The exact spatial alignment of S831 with GluN2B-anchored CaMKII might be essential for its effective phosphorylation. S831 is also not a consensus site for PKC but association of PKC with GluA1 via AKAP150 (Tavalin et al, 2002), which is linked to GluA1 through SAP97 (Leonard et al, 1998; Tavalin et al, 2002), strongly promotes S831 phosphorylation by PKC (Tavalin, 2008). Accordingly, this rather unusual phosphorylation site for CaMKII and PKC becomes a reasonably good substrate for these two kinases if they are properly aligned with it. Impaired phosphorylation of S831 by CaMKII in the KI mice could contribute to the reduction in LTP in KI mice. This phosphorylation increases the activity of homomeric GluA1 AMPAR (Oh and Derkach, 2005), which are involved in the early maintenance phase of certain forms of LTP (Plant et al, 2006; Lu et al, 2007; Guire et al, 2008; but see Adesnik and Nicoll, 2007), and the otherwise prevailing GluA1/A2 heteromeric, TARP-associated AMPAR (Kristensen et al, 2011).
CaMKII-mediated phosphorylation of the cytosolic C-terminus of stg/γ2 and potentially other TARPs contributes substantially to standard LTP (Tomita et al, 2005). This phosphorylation is likely promoted upon NMDAR-mediated Ca2+ influx. Ca2+ potently affects electrostatic interactions between membrane proteins and the plasma membrane (Zilly et al, 2011). Accordingly, Ca2+ influx probably decreases the association of the C-terminus of stg with the plasma membrane, thereby rendering it available for phosphorylation by CaMKII. The phosphorylation causes full and long-lasting detachment of the C-terminus of stg from the plasma membrane, thereby promoting its binding of PSD-95 (Sumioka et al, 2010). This interaction is critical for postsynaptic AMPAR targeting (Chen et al, 2000; Schnell et al, 2002). Postsynaptic AMPAR targeting is at least in part mediated by their diffusional trapping by stg binding to PSD-95 (Bats et al, 2007; Opazo et al, 2010) and this trapping depends on the phosphorylation of stg by CaMKII (Opazo et al, 2010). CaMKII can increase the postsynaptic anchoring sites for AMPARs through this mechanism and an increase in postsynaptic AMPARs is thought to underlie LTP (Collingridge et al, 2004; Malenka and Bear, 2004; Kerchner and Nicoll, 2008; Lisman and Hell, 2008). In fact, we find that cLTP augments the content of the prevalent hippocampal stg homologue γ8 and likely of other TARPs in PSD preparations (Figure 6F and G). The correlated loss of persistent CaMKII anchoring and activation with loss of persistent γ8 accumulation upon cLTP in KI mice supports the hypothesis that direct phosphorylation of TARPs by CaMKII increases localization of AMPAR–TARP complexes at the PSD during LTP or at least during its early phases (Hayashi et al, 2000; Tomita et al, 2005; Sumioka et al, 2010).
GluN2B KI mice are defective in recall of MWM during the consolidation phase
Our studies reveal a highly specific impairment in hippocampus-dependent contextual memory in the GluN2B KI mice during the consolidation phase (Figure 8). At the same time, they indicate that fully developed LTP is not required for normal contextual learning, similar to data from GluA1 KO mice and mice in which the two GluA1 phosphorylation sites for CaMKII/PKC (S831) and PKA/PKG (S845) had been mutated to alanine residues (Zamanillo et al, 1999; Lee et al, 2003). Perhaps, a certain amount of LTP that is less than that in WT mice is sufficient to allow apparently normal contextual learning (see Neves et al, 2008 for further discussion and references). Although we cannot exclude minor learning deficits in the GluN2B KI mice that lie beneath the detection threshold, given that KI and WT mice learned the MWM tasks to the same level, it is clear that the main deficit in the NR2B KI mice occurs during consolidation and not initial learning.
These findings are fundamentally different from previous work on CaMKII mutant mice and on mice with an inducible fusion protein of the GluN2B C-terminus, as hippocampus-dependent learning per se was already substantially affected in all of these mice (Silva et al, 1992; Giese et al, 1998; Zhou et al, 2007). The exceptions are heterozygous CaMKIIα knockout mice, which can learn normally (Frankland et al, 2001); however, they show impaired recall of the MWM task 10 days after training, whereas GluN2B KI mice show this deficit much earlier after 1 day following the last day of training. This comparison highlights the relevance of GluN2B anchoring loss of which causes more sever memory deficits than a reduction in overall CaMKIIα abundance. The different behavioural phenotype seen in the GluN2B KI mice as compared with the mice with inducible expression of the GluN2B C-terminus (Zhou et al, 2007) must be due to effects of the C-terminal fusion protein on protein interactions other than that of the association of CaMKII with GluN2B as the latter have clear and strong deficits already in the initial learning phase and in recall immediately after an advanced training session.
Classic studies of HM, who had undergone bilateral medial temporal lobe resection to control epilepsy, demonstrated his inability to acquire lasting declarative memory (Scoville and Milner, 1957; Squire, 2009). Based on his case and many subsequent studies, we now know that declarative learning first occurs in the hippocampus but has to be transferred to other brain regions for consolidation and long-term storage, which takes up to 4 weeks with perhaps most of the transfer occurring in the first week (Takehara-Nishiuchi et al, 2006; Euston et al, 2007; Takehara-Nishiuchi and McNaughton, 2008). A role for NMDARs in this process has been observed but its precise function or other molecular details are unknown (Takehara-Nishiuchi et al, 2006). Our studies now demonstrate a specific requirement for activity-driven binding of CaMKII to the NMDAR during the early phases of hippocampus-dependent memory consolidation, indicating that postsynaptic sites involved in this process must actively recruit CaMKII for continued access (recall) or storage of memories.
Materials and methods
Three point mutations were introduced into the GluN2B gene to obtain the L1298A and R1300Q mutations and a BssHII site for diagnostic purposes. Founder chimeras were backcrossed with EIIa/Cre mice to excise the floxed Neo cassette and with nine generations with C57BL/6J mice. All experiments were conducted with litter-matched WT versus KI mice. Forebrain slices were prepared and biochemically and electrophysiologically analysed as described (Lu et al, 2007). Primary hippocampal cultures were prepared from individual litter-matched WT and KI P0 pups, cultured in Neurobasal medium (Invitrogen) supplemented with NS21, and analysed by immunofluorescence microscopy using established methods as described earlier (Chen et al, 2008). Colocalization of CaMKII and GluN2B with synapsin and bassoon, respectively, was determined using the Image J (Rasband, WS, ImageJ, US. National Institutes of Health, Bethesda, MD, USA; http://imagej.nih.gov/ij/, 1997–2011) plugin JACoP (Bolte and Cordelieres, 2006) to determine colocalization coefficients (Pearson's coefficient and Manders coefficient) for 10 neurons per treatment condition. Pearson's coefficient is an estimate of the fit of the intensity correlation between two channels to a straight line; a value of 0 describes no, 1 complete positive, and –1 complete negative correlation. Mander's coefficient (fraction of CaMKII or GluN2B signals colocalized with synapsin signals) is similar to Pearson's coefficient, but signal intensity is not considered; it describes the fraction of one signal that overlaps with the other.
All animal procedures were approved by the University of Iowa, UC Davis and UCLA Animal Care and Use Committees and followed NIH guidelines. Behavioural tests followed standard procedures. See Supplementary data for more methodological details.
Supplementary Material
Acknowledgments
We thank Drs K Peter Giese (Kings College, London, UK), Ype Elgersma (Erasmus University, Rotterdam, The Netherlands), and Nikolai Otmakhov (Brandeis University, Boston, MA, USA) for critically reading the manuscript. This work was supported by NIH TG32 GM067795 (ARH, RFD), NIH F30 NS054423 (ARH), NIH F31 MH079671 (RFD), Max Planck Society, Munich, Germany (NB), NIH P50-MH077972, 10-3-3-1-2-nsh (YZ), and NSFC 81070881 (YZ) and R01 AG013622 (AJS), NIH R01 NS046450, AG017502, and NS035563 (JWH) and a pilot project grant (JWH) funded by NIH UL1RR024979 to the University of Iowa.
Author contributions: ARH, RFD, YZ, SIS, HQ, NB, AJS, and JWH designed the experiments and analysed the data; ARH, RFD, YZ, SIS, HQ, SJ, and SW performed the experiments; and ARH, RFD, YZ, JAW, NB, AJS, and JWH wrote the manuscript.
Footnotes
The authors declare that they have no conflict of interest.
References
- Adesnik H, Nicoll RA (2007) Conservation of glutamate receptor 2-containing AMPA receptors during long-term potentiation. J Neurosci 27: 4598–4602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barria A, Malinow R (2005) NMDA receptor subunit composition controls synaptic plasticity by regulating binding to CaMKII. Neuron 48: 289–301 [DOI] [PubMed] [Google Scholar]
- Barria A, Muller D, Derkach V, Griffith LC, Soderling TR (1997) Regulatory phosphorylation of AMPA-type glutamate receptors by CaM-KII during long-term potentiation. Science 276: 2042–2045 [DOI] [PubMed] [Google Scholar]
- Bats C, Groc L, Choquet D (2007) The interaction between Stargazin and PSD-95 regulates AMPA receptor surface trafficking. Neuron 53: 719–734 [DOI] [PubMed] [Google Scholar]
- Bayer KU, De Koninck P, Leonard AS, Hell JW, Schulman H (2001) Interaction with the NMDA receptor locks CaMKII in an active conformation. Nature 411: 801–805 [DOI] [PubMed] [Google Scholar]
- Bayer KU, LeBel E, McDonald GL, O'Leary H, Schulman H, De Koninck P (2006) Transition from reversible to persistent binding of CaMKII to postsynaptic sites and NR2B. J Neurosci 26: 1164–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolte S, Cordelieres FP (2006) A guided tour into subcellular colocalization analysis in light microscopy. J Microsc 224: 213–232 [DOI] [PubMed] [Google Scholar]
- Buard I, Coultrap SJ, Freund RK, Lee YS, Dell'Acqua ML, Silva AJ, Bayer KU (2010) CaMKII ‘autonomy’ is required for initiating but not for maintaining neuronal long-term information storage. J Neurosci 30: 8214–8220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlisle HJ, Luong TN, Medina-Marino A, Schenker L, Khorosheva E, Indersmitten T, Gunapala KM, Steele AD, O'Dell TJ, Patterson PH, Kennedy MB (2011) Deletion of Densin-180 results in abnormal behaviors associated with mental illness and reduces mGluR5 and DISC1 in the postsynaptic density fraction. J Neurosci 31: 16194–16207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao LH, Pellicena P, Deindl S, Barclay LA, Schulman H, Kuriyan J (2010) Intersubunit capture of regulatory segments is a component of cooperative CaMKII activation. Nat Struct Mol Biol 17: 264–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao LH, Stratton MM, Lee IH, Rosenberg OS, Levitz J, Mandell DJ, Kortemme T, Groves JT, Schulman H, Kuriyan J (2011) A mechanism for tunable autoinhibition in the structure of a human Ca(2+)/calmodulin-dependent kinase II holoenzyme. Cell 146: 732–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Chetkovich DM, Petralia RS, Sweeney NT, Kawasaki Y, Wenthold RJ, Bredt DS, Nicoll RA (2000) Stargazing regulates synaptic targeting of AMPA receptors by two distinct mechanisms. Nature 408: 936–943 [DOI] [PubMed] [Google Scholar]
- Chen Y, Stevens B, Chang J, Milbrandt J, Barres BA, Hell JW (2008) NS21: re-defined and modified supplement B27 for neuronal cultures. J Neurosci Methods 171: 239–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie LA, Russell TA, Xu J, Wood L, Shepherd GM, Contractor A (2010) AMPA receptor desensitization mutation results in severe developmental phenotypes and early postnatal lethality. Proc Natl Acad Sci USA 107: 9412–9417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbran RJ (2004) Targeting of calcium/calmodulin-dependent protein kinase II. Biochem J 378: 1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingridge GL, Isaac JT, Wang YT (2004) Receptor trafficking and synaptic plasticity. Nat Rev 5: 952–962 [DOI] [PubMed] [Google Scholar]
- Ehlers MD (2003) Activity level controls postsynaptic composition and signaling via the ubiquitin-proteasome system. Nat Neurosci 6: 231–242 [DOI] [PubMed] [Google Scholar]
- Euston DR, Tatsuno M, McNaughton BL (2007) Fast-forward playback of recent memory sequences in prefrontal cortex during sleep. Science 318: 1147–1150 [DOI] [PubMed] [Google Scholar]
- Frankland PW, O'Brien C, Ohno M, Kirkwood A, Silva AJ (2001) Alpha-CaMKII-dependent plasticity in the cortex is required for permanent memory. Nature 411: 309–313 [DOI] [PubMed] [Google Scholar]
- Fukunaga K, Muller D, Miyamoto E (1995) Increased phosphorylation of the Ca2+/calmodulin-dependent protein kinase II and its endogenous substrates in the induction of long term potentiation. J Biol Chem 270: 6119–6124 [DOI] [PubMed] [Google Scholar]
- Gambrill AC, Barria A (2011) NMDA receptor subunit composition controls synaptogenesis and synapse stabilization. Proc Natl Acad Sci USA 108: 5855–5860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giese KP, Fedorov NB, Filipkowski RK, Silva AJ (1998) Autophosphorylation at Thr286 of the alpha calcium-calmodulin kinase II in LTP and learning. Science 279: 870–873 [DOI] [PubMed] [Google Scholar]
- Guire ES, Oh MC, Soderling TR, Derkach VA (2008) Recruitment of calcium-permeable AMPA receptors during synaptic potentiation is regulated by CaM-kinase I. J Neurosci 28: 6000–6009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, Mark MD, Li X, Xie M, Waka S, Rettig J, Herlitze S (2006) RGS2 determines short-term synaptic plasticity in hippocampal neurons by regulating G(i/o)-mediated inhibition of presynaptic Ca(2+) channels. Neuron 51: 575–586 [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Shi SH, Esteban JA, Piccini A, Poncer JC, Malinow R (2000) Driving AMPA receptors into synapses by LTP and CaMKII: requirement for GluR1 and PDZ domain interaction. Science 287: 2262–2267 [DOI] [PubMed] [Google Scholar]
- Heine M, Groc L, Frischknecht R, Beique JC, Lounis B, Rumbaugh G, Huganir RL, Cognet L, Choquet D (2008) Surface mobility of postsynaptic AMPARs tunes synaptic transmission. Science 320: 201–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hell JW, Westenbroek RE, Breeze LJ, Wang KKW, Chavkin C, Catterall WA (1996) N-methyl-D-aspartate receptor-induced proteolytic conversion of postsynaptic class C L-type calcium channels in hippocampal neurons. Proc Natl Acad Sci USA 93: 3362–3367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman L, Stein RA, Colbran RJ, McHaourab HS (2011) Conformational changes underlying calcium/calmodulin-dependent protein kinase II activation. EMBO J 30: 1251–1262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z, Shimazu K, Woo NH, Zang K, Muller U, Lu B, Reichardt LF (2006) Distinct roles of the beta 1-class integrins at the developing and the mature hippocampal excitatory synapse. J Neurosci 26: 11208–11219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudmon A, Lebel E, Roy H, Sik A, Schulman H, Waxham MN, De Koninck P (2005) A mechanism for Ca2+/calmodulin-dependent protein kinase II clustering at synaptic and nonsynaptic sites based on self-association. J Neurosci 25: 6971–6983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerchner GA, Nicoll RA (2008) Silent synapses and the emergence of a postsynaptic mechanism for LTP. Nat Rev 9: 813–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessels HW, Malinow R (2009) Synaptic AMPA receptor plasticity and behavior. Neuron 61: 340–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopec CD, Li B, Wei W, Boehm J, Malinow R (2006) Glutamate receptor exocytosis and spine enlargement during chemically induced long-term potentiation. J Neurosci 26: 2000–2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensen AS, Jenkins MA, Banke TG, Schousboe A, Makino Y, Johnson RC, Huganir R, Traynelis SF (2011) Mechanism of Ca2+/calmodulin-dependent kinase II regulation of AMPA receptor gating. Nat Neurosci 14: 727–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HK, Barbarosie M, Kameyama K, Bear MF, Huganir RL (2000) Regulation of distinct AMPA receptor phosphorylation sites during bidirectional synaptic plasticity. Nature 405: 955–959 [DOI] [PubMed] [Google Scholar]
- Lee HK, Takamiya K, Han JS, Man H, Kim CH, Rumbaugh G, Yu S, Ding L, He C, Petralia RS, Wenthold RJ, Gallagher M, Huganir RL (2003) Phosphorylation of the AMPA receptor GluR1 subunit is required for synaptic plasticity and retention of spatial memory. Cell 112: 631–643 [DOI] [PubMed] [Google Scholar]
- Lee SJ, Escobedo-Lozoya Y, Szatmari EM, Yasuda R (2009) Activation of CaMKII in single dendritic spines during long-term potentiation. Nature 458: 299–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YS, Silva AJ (2009) The molecular and cellular biology of enhanced cognition. Nat Rev 10: 126–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard AS, Bayer K-U, Merrill MA, Lim IA, Shea MA, Schulman H, Hell JW (2002) Regulation of calcium/calmodulin-dependent protein kinase II docking to N-methyl-D-aspartate receptors by calcium/calmodulin and α-actinin. J Biol Chem 277: 48441–48448 [DOI] [PubMed] [Google Scholar]
- Leonard AS, Davare MA, Horne MC, Garner CC, Hell JW (1998) SAP97 is associated with the α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor GluR1 subunit. J Biol Chem 273: 19518–19524 [DOI] [PubMed] [Google Scholar]
- Leonard AS, Lim IA, Hemsworth DE, Horne MC, Hell JW (1999) Calcium/calmodulin-dependent protein kinase II is associated with the N-methyl-D-aspartate receptor. Proc Natl Acad Sci USA 96: 3239–3244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J, Schulman H, Cline H (2002) The molecular basis of CaMKII function in synaptic and behavioral memory. Nat Neurosci 3: 175–190 [DOI] [PubMed] [Google Scholar]
- Lisman JE, Hell JW (2008) Long-term potentiation. In: JW Hell, MD Ehlers (eds). Structural and Functional Organization of the Synapse. Heidelberg: Springer [Google Scholar]
- Lu Y, Allen M, Halt AR, Weisenhaus M, Dallapiazza RF, Hall DD, Usachev YM, McKnight GS, Hell JW (2007) Age-dependent requirement of AKAP150-anchored PKA and GluR2-lacking AMPA receptors in LTP. EMBO J 26: 4879–4890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makhinson M, Chotiner JK, Watson JB, O'Dell TJ (1999) Adenylyl cyclase activation modulates activity-dependent changes in synaptic strength and Ca2+/calmodulin-dependent kinase II autophosphorylation. J Neurosci 19: 2500–2510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka RC, Bear MF (2004) LTP and LTD: an embarrassment of riches. Neuron 44: 5–21 [DOI] [PubMed] [Google Scholar]
- Martin SJ, Grimwood PD, Morris RG (2000) Synaptic plasticity and memory: an evaluation of the hypothesis. Annu Rev Neurosci 23: 649–711 [DOI] [PubMed] [Google Scholar]
- Matsuzaki M, Honkura N, Ellis-Davies GC, Kasai H (2004) Structural basis of long-term potentiation in single dendritic spines. Nature 429: 761–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill MA, Chen Y, Strack S, Hell JW (2005) Activity-driven postsynaptic translocation of CaMKII. Trends Pharmacol Sci 26: 645–653 [DOI] [PubMed] [Google Scholar]
- Merrill MA, Malik Z, Akyol Z, Bartos JA, Leonard AS, Hudmon A, Shea MA, Hell JW (2007) Displacement of alpha-actinin from the NMDA receptor NR1 C0 domain By Ca2+/calmodulin promotes CaMKII binding. Biochemistry 46: 8485–8497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto-Tomita M, Zhang W, Straub C, Cho CH, Kim KS, Howe JR, Tomita S (2009) Autoinactivation of neuronal AMPA receptors via glutamate-regulated TARP interaction. Neuron 61: 101–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neves G, Cooke SF, Bliss TV (2008) Synaptic plasticity, memory and the hippocampus: a neural network approach to causality. Nat Rev 9: 65–75 [DOI] [PubMed] [Google Scholar]
- Nikandrova YA, Jiao Y, Baucum AJ, Tavalin SJ, Colbran RJ (2010) Ca2+/calmodulin-dependent protein kinase II binds to and phosphorylates a specific SAP97 splice variant to disrupt association with AKAP79/150 and modulate alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid-type glutamate receptor (AMPAR) activity. J Biol Chem 285: 923–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh MC, Derkach VA (2005) Dominant role of the GluR2 subunit in regulation of AMPA receptors by CaMKII. Nat Neurosci 8: 853–854 [DOI] [PubMed] [Google Scholar]
- Okamoto K, Narayanan R, Lee SH, Murata K, Hayashi Y (2007) The role of CaMKII as an F-actin-bundling protein crucial for maintenance of dendritic spine structure. Proc Natl Acad Sci USA 104: 6418–6423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opazo P, Labrecque S, Tigaret CM, Frouin A, Wiseman PW, De Koninck P, Choquet D (2010) CaMKII triggers the diffusional trapping of surface AMPARs through phosphorylation of stargazin. Neuron 67: 239–252 [DOI] [PubMed] [Google Scholar]
- Otmakhov N, Tao-Cheng JH, Carpenter S, Asrican B, Dosemeci A, Reese TS, Lisman J (2004) Persistent accumulation of calcium/calmodulin-dependent protein kinase II in dendritic spines after induction of NMDA receptor-dependent chemical long-term potentiation. J Neurosci 24: 9324–9331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelkey KA, Topolnik L, Lacaille JC, McBain CJ (2006) Compartmentalized Ca(2+) channel regulation at divergent mossy-fiber release sites underlies target cell-dependent plasticity. Neuron 52: 497–510 [DOI] [PubMed] [Google Scholar]
- Pi HJ, Otmakhov N, El Gaamouch F, Lemelin D, De Koninck P, Lisman J (2010) CaMKII control of spine size and synaptic strength: role of phosphorylation states and nonenzymatic action. Proc Natl Acad Sci USA 107: 14437–14442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plant K, Pelkey KA, Bortolotto ZA, Morita D, Terashima A, McBain CJ, Collingridge GL, Isaac JT (2006) Transient incorporation of native GluR2-lacking AMPA receptors during hippocampal long-term potentiation. Nat Neurosci 9: 602–604 [DOI] [PubMed] [Google Scholar]
- Rellos P, Pike AC, Niesen FH, Salah E, Lee WH, von Delft F, Knapp S (2010) Structure of the CaMKIIdelta/calmodulin complex reveals the molecular mechanism of CaMKII kinase activation. PLoS Biol 8: e1000426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robison AJ, Bass MA, Jiao Y, Macmillan LB, Carmody LC, Bartlett RK, Colbran RJ (2005) Multivalent interactions of calcium/calmodulin-dependent protein kinase II with the postsynaptic density pr. J Biol Chem 280: 35329–35336 [DOI] [PubMed] [Google Scholar]
- Sanhueza M, Fernandez-Villalobos G, Stein IS, Kasumova G, Zhang P, Bayer KU, Otmakhov N, Hell JW, Lisman J (2011) Role of the CaMKII/NMDA receptor complex in the maintenance of synaptic strength. J Neurosci 31: 9170–9178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savtchouk I, Liu SJ (2011) Remodeling of synaptic AMPA receptor subtype alters the probability and pattern of action potential firing. J Neurosci 31: 501–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell E, Sizemore M, Karimzadegan S, Chen L, Bredt DS, Nicoll RA (2002) Direct interactions between PSD-95 and stargazin control synaptic AMPA receptor number. Proc Natl Acad Sci USA 99: 13902–13907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulman H (2004) Activity-dependent regulation of calcium/calmodulin-dependent protein kinase II localization. J Neurosci 24: 8399–8403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz PE, Cook EP, Johnston D (1994) Changes in paired-pulse facilitation suggest presynaptic involvement in long-term potentiation. J Neurosci 14: 5325–5337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scoville WB, Milner B (1957) Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry 20: 11–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen K, Meyer T (1999) Dynamic control of CaMKII translocation in hippocampal neurons by NMDA receptor stimulation. Science 284: 162–166 [DOI] [PubMed] [Google Scholar]
- Shen K, Teruel MN, Connor JH, Shenolikar S, Meyer T (2000) Molecular memory by reversible translocation of calcium/calmodulin-dependent protein kinase II. Nat Neurosci 3: 881–886 [DOI] [PubMed] [Google Scholar]
- Shen K, Teruel MN, Subramanian K, Meyer T (1998) CaMKIIbeta functions as an F-actin targeting module that localizes CaMKIIalpha/beta heterooligomers to dendritic spines. Neuron 21: 593–606 [DOI] [PubMed] [Google Scholar]
- Silva AJ, Paylor R, Wehner JM, Tonegawa S (1992) Impaired spatial learning in alpha-calcium-calmodulin kinase II mutant mice. Science 257: 206–211 [DOI] [PubMed] [Google Scholar]
- Squire LR (2009) The legacy of patient HM for neuroscience. Neuron 61: 6–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strack S, Colbran RJ (1998) Autophosphorylation-dependent targeting of calcium/calmodulin-dependent protein kinase II by the NR2B subunit of the N-methyl-D-aspartate receptor. J Biol Chem 273: 20689–20692 [DOI] [PubMed] [Google Scholar]
- Strack S, Hell JW (2008) Postsynaptic targeting of kinases and phosphatases. In: JW Hell, MD Ehlers (eds). Structural and Functional Organization of the Synapse. Heidelberg: Springer 459–500 [Google Scholar]
- Strack S, McNeill RB, Colbran RJ (2000a) Mechanism and regulation of calcium/calmodulin-dependent protein kinase II targeting to the NR2B subunit of the N-methyl-D-aspartate receptor. J Biol Chem 275: 23798–23806 [DOI] [PubMed] [Google Scholar]
- Strack S, Robison AJ, Bass MA, Colbran RJ (2000b) Association of calcium/calmodulin-dependent kinase II with developmentally regulated splice variants of the postsynaptic density protein densin-180. J Biol Chem 275: 25061–25064 [DOI] [PubMed] [Google Scholar]
- Stubblefield EA, Benke TA (2010) Distinct AMPA-type glutamatergic synapses in developing rat CA1 hippocampus. J Neurophysiol 104: 1899–1912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumioka A, Yan D, Tomita S (2010) TARP phosphorylation regulates synaptic AMPA receptors through lipid bilayers. Neuron 66: 755–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takehara-Nishiuchi K, McNaughton BL (2008) Spontaneous changes of neocortical code for associative memory during consolidation. Science 322: 960–963 [DOI] [PubMed] [Google Scholar]
- Takehara-Nishiuchi K, Nakao K, Kawahara S, Matsuki N, Kirino Y (2006) Systems consolidation requires postlearning activation of NMDA receptors in the medial prefrontal cortex in trace eyeblink conditioning. J Neurosci 26: 5049–5058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavalin SJ (2008) AKAP79 selectively enhances protein kinase C regulation of GluR1 at a Ca2+-calmodulin-dependent protein kinase II/protein kinase C site. J Biol Chem 283: 11445–11452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavalin SJ, Colledge M, Hell JW, Langeberg LK, Huganir RL, Scott JD (2002) Regulation of GluR1 by the A-kinase anchoring protein 79 (AKAP79) signaling complex shares properties with long-term depression. J Neurosci 22: 3044–3051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita S, Stein V, Stocker TJ, Nicoll RA, Bredt DS (2005) Bidirectional synaptic plasticity regulated by phosphorylation of stargazin-like TARPs. Neuron 45: 269–277 [DOI] [PubMed] [Google Scholar]
- Walikonis RS, Oguni A, Khorosheva EM, Jeng CJ, Asuncion FJ, Kennedy MB (2001) Densin-180 forms a ternary complex with the (alpha)-subunit of Ca2+/calmodulin-dependent protein kinase II and (alpha)-actinin. J Neurosci 21: 423–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalutsky RA, Nicoll RA (1990) Comparison of two forms of long-term potentiation in single hippocampal neurons. Science 248: 1619–1624 [DOI] [PubMed] [Google Scholar]
- Zamanillo D, Sprengel R, Hvalby O, Jensen V, Burnashev N, Rozov A, Kaiser KM, Koster HJ, Borchardt T, Worley P, Lubke J, Frotscher M, Kelly PH, Sommer B, Andersen P, Seeburg PH, Sakmann B (1999) Importance of AMPA receptors for hippocampal synaptic plasticity but not for spatial learning. Science 284: 1805–1811 [DOI] [PubMed] [Google Scholar]
- Zhang YP, Holbro N, Oertner TG (2008) Optical induction of plasticity at single synapses reveals input-specific accumulation of alphaCaMKII. Proc Natl Acad Sci USA 105: 12039–12044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Takahashi E, Li W, Halt A, Wiltgen B, Ehninger D, Li GD, Hell JW, Kennedy MB, Silva AJ (2007) Interactions between the NR2B receptor and CaMKII modulate synaptic plasticity and spatial learning. J Neurosci 27: 13843–13853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilly FE, Halemani ND, Walrafen D, Spitta L, Schreiber A, Jahn R, Lang T (2011) Ca(2+) induces clustering of membrane proteins in the plasma membrane via electrostatic interactions. EMBO J 30: 1209–1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.