Abstract
EMBO J 31 5, 1080–1094 (2012); published online January 13 2012
Have you seen the recent papers that question the traditional role of B-type lamins in DNA replication and transcription, but highlight an entirely novel yet fundamental role for lamin B1 (LB1) in cell proliferation and cellular senescence?
Lamins are the evolutionary progenitors of the intermediate filament superfamily, and constitute the building blocks of the nuclear lamina. They are also a defining feature of metazoan organisms. In vertebrates and higher invertebrates, there are two classes of lamins, namely the B-type lamins (LB1, LB2 and LB3) and the A-type lamins (LMA and LMC) (Hutchison, 2002). For years, dogma has stated that the B-type lamins have fundamental roles in cell physiology and are essential for cell survival (Harborth et al, 2001). This dogma was recently challenged by the astonishing finding that mouse embryonic stem cells devoid of B-type lamins could not only self-renew, but also differentiate (Kim et al, 2011). While the Kim study does not challenge the notion that lamins are essential for embryonic development, it nonetheless questions the reported roles for B-type lamins in basic processes such as DNA replication and transcription, leading the authors to predict a key function for B-type lamins in maintaining tissue homeostasis.
Now an entirely new role for LB1 has been reported, which emphasizes its function in proliferation and longevity of somatic cells and suggests that this ubiquitous protein might indeed have evolved to support tissue homeostasis in metazoans. In this issue of The EMBO Journal, Pascal Bertrand's team describes a novel role for LB1 in entry into an MAPK-mediated state of stress-induced premature senescence (Barascu et al, 2012). Starting from observations in cells obtained from patients with the premature ageing syndrome Ataxia Telangiectasia (A-T), the team showed that increased ROS generation and abnormally shaped nuclei, but not impaired DNA damage repair, were associated with an approximate three-fold increase in LB1 expression levels. The authors went on to show that overexpression of LB1 caused nuclear shape abnormalities and entry into a state of premature senescence in normal cells. In both normal and A-T cells, the increased expression of LB1 appeared to be caused by ROS generation, since pro-oxidant stimulation in normal cells promoted LB1 expression, while anti-oxidant treatment of A-T cells reduced LB1 expression. Finally, the team directly linked ROS-dependent increased expression of LB1 to activation of the senescence-associated p38 MAP kinase, suggesting a novel ATM-independent stress-sensing pathway that causes entry into a state of senescence. Thus, hot on the heels of the key prediction of Kim et al, the paper by Barascu and co-workers appears to have demonstrated a fundamental role for LB1 in maintaining cellular homeostasis in response to oxidative stress.
Alas, cell biology is never simple! A contemporaneous paper from Bob Goldman's laboratory reported that LB1 expression was decreased in fibroblasts that had entered states of either replicative or oncogene-induced senescence (Shimi et al, 2011). This finding was consistent with their earlier report on a dramatic loss of LB1 expression in fibroblasts from an atypical progeria patient that underwent rapid senescence (Taimen et al, 2009). In their current study, Goldman's team showed that siRNA silencing of LB1 slowed proliferation rates and promoted cellular senescence. Silencing of LB1 was associated with increased expression of p53 but not of Rb, nonetheless, entry into a state of senescence following the LB1 silencing was dependent upon the activity of both p53 and Rb. Finally, LB1 silencing was associated with a p53-dependent decrease in the generation of mitochondrial ROS. While at face value the papers by Barascu et al and Shimi et al appear entirely contradictory, there are reasons to believe that both papers reflect convergent pathways that place the lamina as a hub for signals promoting cellular senescence in response to disturbed ROS homeostasis or other cellular stresses.
One clear consistency between the two papers appears to be that silencing of LB1 leads to reduced cellular ROS, while increased expression of LB1 is associated with increased levels of ROS. It is also important to note that the two papers did not investigate the same senescence pathways, with the Bertrand team concentrating on ROS-induced premature senescence, while the Goldman team focussed on replicative and oncogene-induced senescence. Thus, it is entirely plausible that all three pathways feed through LB1 as a signalling hub, but the LB1 response differs depending upon which pathway is activated.
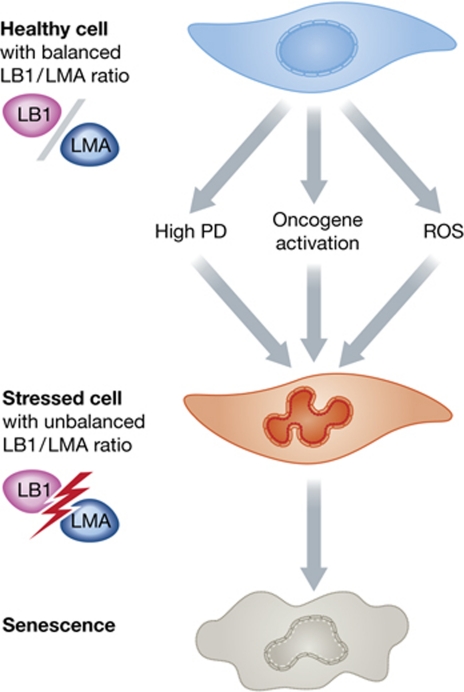
My own group has also recently reported on the role of the nuclear lamina in replicative and stress-induced premature senescence (Pekovic et al, 2011). Our study concentrated on ROS-induced damage to LMA, showing that cysteine residues in the tail domain of the protein were not only major targets of oxidative damage, but that their irreversible oxidation led to loss of LMA function and entry into a state of senescence. While our study did not address the role of LB1 in cellular senescence, it just might provide a clue as to how LB1 operates and why the conclusions of the papers by Barascu and co-workers and Shimi and co-workers at face value appear so contradictory. Whether LB1 increases or decreases, the ratio of LB1 to functional LMA must change dramatically in response to queues that cause senescence, and this in turn must significantly alter the architecture of the nuclear lamina. Indeed, this is evident in the altered shape of nuclei that is common to all senescence pathways (Figure 1). Given that so many stress signalling proteins are tethered to the lamina or to lamin polypeptides, including Oct-1 (Malhas et al, 2009), c-fos (Gonzalez et al, 2008), Rb (Dorner et al, 2006) and now p38 MAPK (Barascu et al, 2012), it is not surprising that disturbances in the architecture of the nuclear lamina would trigger a cascade of adverse signalling events that eventually cause entry into a senescent state. The major impact of the papers under discussion here is that they all demonstrate the significance of lamin polypeptides in maintaining tissue homeostasis, and imply that the entire lamin family is of fundamental importance in longevity.
Figure 1.
The effects of different cellular stresses that lead to senescence on lamin A to lamin B1 ratios. Healthy early passage cells have a balanced LMA to LB1 ratio. In response to senescence-inducing events, LB1 expression is altered and/or LMA is oxidized, dramatically changing the ratio of LB1 to functional LMA, thus leading to altered lamina organization and entry into a state of senescence.
Footnotes
The author declares that he has no conflict of interest.
References
- Barascu A, Le Chalony C, Pennarun G, Genet D, Imam N, Lopez B, Bertrand P (2012) Oxidative stress induces an ATM-independent senescence pathway through p38 MAPK-mediated lamin B1 accumulation. EMBO J 31: 1080–1094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorner D, Vlcek S, Foeger N, Gajewski A, Makolm C, Gotzmann J, Hutchison CJ, Foisner R (2006) Lamina-associated polypeptide 2alpha regulates cell cycle progression and differentiation via the retinoblastoma-E2F pathway. J Cell Biol 173: 83–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González JM, Navarro-Puche A, Casar B, Crespo P, Andrés V (2008) Fast regulation of AP-1 activity through interaction of lamin A/C, ERK1/2, and c-Fos at the nuclear envelope. J Cell Biol 183: 653–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harborth J, Elbashir SM, Bechert K, Tuschl T, Weber K (2001) Identification of essential genes in cultured mammalian cells using small interfering RNAs. J Cell Sci 114: 4557–4565 [DOI] [PubMed] [Google Scholar]
- Hutchison CJ (2002) Lamins: building blocks or regulators of gene expression? Nat Rev Mol Cell Biol 3: 848–858 [DOI] [PubMed] [Google Scholar]
- Kim Y, Sharov AA, McDole K, Cheng M, Hao H, Fan CM, Gaiano N, Ko MS, Zheng Y (2011) Mouse B-type lamins are required for proper organogenesis but not by embryonic stem cells. Science 334: 1706–1710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhas AN, Lee CF, Vaux DJ (2009) Lamin B1 controls oxidative stress responses via Oct-1. J Cell Biol 184: 45–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekovic V, Gibbs-Seymour I, Markiewicz E, Alzoghaibi F, Benham AM, Edwards R, Wenhert M, von Zglinicki T, Hutchison CJ (2011) Conserved cysteine residues in the mammalian lamin A tail are essential for cellular responses to ROS generation. Aging Cell 10: 1067–1079 [DOI] [PubMed] [Google Scholar]
- Shimi T, Butin-Israeli V, Adam SA, Hamanaka RB, Goldman AE, Lucas CA, Shumaker DK, Kosak ST, Chandel NS, Goldman RD (2011) The role of nuclear lamin B1 in cell proliferation and senescence. Genes Dev 25: 2579–2593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taimen P, Pfleghaar K, Shimi T, Möller D, Ben-Harush K, Erdos MR, Adam SA, Herrmann H, Medalia O, Collins FS, Goldman AE, Goldman RD (2009) A progeria mutation reveals functions for lamin A in nuclear assembly, architecture, and chromosome organization. PNAS 106: 20788–20793 [DOI] [PMC free article] [PubMed] [Google Scholar]